Abstract
Background
Diminished peak lung function in young adulthood is a risk factor for future chronic obstructive pulmonary disease. The association between lung disease and cardiovascular disease later in life is well documented. Whether peak lung function measured in young adulthood is associated with risk of future cardiovascular events is unknown.
Methods and Results
CARDIA (The Coronary Artery Risk Development in Young Adults) study is a prospective, multicenter, community‐based, longitudinal cohort study including 4761 participants aged 18 to 30 years with lung function testing we investigated the association between lung health in young adulthood and risk of subsequent cardiovascular events. We performed Cox proportional hazards regression to test the association between baseline and years 10 and 20 pulmonary function with incident cardiovascular events. Linear and logistic regression was performed to explore the associations of lung function with development of risk factors for cardiovascular disease as well as carotid intima‐media thickness and coronary artery calcified plaque. At baseline, mean age (±SD) was 24.9±3.6 years. Baseline forced expiratory volume in 1 second (hazard ratio) per −10‐unit decrement in percent predicted forced expiratory volume in 1 second (hazard ratio, 1.18; 95% CI, 1.06–1.31 [P=0.002]) and FVC per −10‐unit decrement in percent predicted FVC (hazard ratio, 1.19; 95% CI, 1.06–1.33 [P=0.003]) were associated with future cardiovascular events independent of traditional cardiovascular risk factors. Baseline lung function was associated with heart failure and cerebrovascular events but not coronary artery disease events.
Conclusions
Lung function in young adulthood is independently associated with cardiovascular events into middle age. This association appears to be driven by heart failure and cerebrovascular events rather than coronary heart disease.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00005130.
Keywords: cardiac disease, heart failure, lung, pulmonary, pulmonary heart disease
Subject Categories: Cardiovascular Disease, Aging, Epidemiology
Clinical Perspective
What Is New?
Lung function measured in young adulthood at a mean age of 25 years (range 18–30 years) is associated with greater risk of future cardiovascular disease events over subsequent 29 years of follow‐up independent of traditional cardiovascular risk factors such as sex, race, smoking, body mass index, total cholesterol, blood pressure, and diabetes mellitus.
What Are the Clinical Implications?
This association, which appears to be independent of atherosclerotic heart disease, adds to the growing body of evidence exploring the importance of peak lung health attained in young adulthood on the coevolution of heart and lung disease across the adult lifespan.
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with an increased risk of cardiovascular disease (CVD).1, 2, 3, 4, 5, 6, 7, 8 While some of the concurrence of lung and heart disease may be attributable to common risk factors such as cigarette smoking and age, CVD burden is greater in people with COPD than the general population independent of these shared risk factors.4, 9 Furthermore, this association does not appear to be simply driven by the common risk factor of cigarette smoking. Even in the absence of lung disease (including in nonsmokers), reduced lung function is associated with risk of future CVD. We have previously shown the association between lung function measured in early adulthood and the development of hypertension later in life.10 In the Framingham Heart Study, forced vital capacity (FVC) at a mean age of 40 to 45 years was associated with risk of subsequent CVD.11 This age range, however, is older than when most people attain peak lung function (typically around age 25–35 years)12 and at an age when lung disease starts to become clinically apparent.13, 14
Most reports of associations between chronic respiratory disease and CVD have been cross‐sectional studies of individuals with either established lung disease or in older populations at risk for lung disease.5, 6, 8, 15, 16 However, it was recently reported in a study of 3 independent cohorts that an abnormal (<80% of the predicted value) forced expiratory volume in 1 second (FEV1) was associated with accelerated development of respiratory, cardiovascular, and metabolic comorbidities.17 In a prior analysis, it was also noted that an abnormal FEV1 early in life is associated with increased risk of future COPD independent of rate of decline18 suggesting that peak lung health in young adulthood is an important risk factor for a variety of future health consequences. These findings replicate and reinforce those previously reported in the CARDIA (Coronary Artery Risk Development in Young Adults) study of young adults aged 18 to 30 years recruited in 198510, 19, 20, 21, 22, 23 which was included in the 3 cohort study described above, but only included an intermediate‐term follow‐up interval of 20 years (to a mean age of 45 years).17
Although of interest, testing the association between only those with abnormal lung function (<80% predicted) and future comorbid conditions does not acknowledge the full potential impact of measurements of peak lung function. The association of overall respiratory health in young adulthood and future comorbid conditions likely operates on a continuum in which lung function ranging from ideal to abnormal is associated with overall health outcomes later in life. Given the considerable interest in both the respiratory and cardiovascular communities in identifying distinct patient phenotypes (in COPD for example, recognizing that people with lower peak lung function in early adulthood who have COPD are different from those with accelerated decline), expanding our understanding of how peak lung function is associated with different phenotypic expressions of CVD is critical to more precisely understand the concurrent evolution of heart and lung conditions.24 In the present article, we utilized the complete CARDIA study events data to test the association between lung health in young adulthood (mean age 25 years, range 18–30 years) and cardiovascular events (including coronary heart disease [CHD] events, stroke, and heart failure) over nearly 30 years of follow‐up. We hypothesized that lung health in young adulthood is an independent risk factor for future cardiovascular events and subclinical atherosclerosis.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The CARDIA study has a formal centralized process for requesting data and study materials, which can be accessed through its website: www.cardia.dopm.uab.edu.
Study Design and Sample
CARDIA is a prospective cohort study of the evolution of CVD risk factors in young adults.25 Briefly, from 1985 to 1986, 5115 black and white individuals aged 18 to 30 years were examined in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. At the Birmingham, Minneapolis, and Chicago sites, participants were randomly selected from total communities or from specific census tracts. In Oakland, participants were recruited from the Kaiser Permanente Medical Care Program membership. Recruitment achieved nearly equal numbers at each site of race, sex, education (more than high school or high school or less), and age (18–24 years or 25–30 years). Fifty percent of invited individuals contacted were examined (47% of blacks and 60% of whites). In‐person examinations occurred at 2, 5, 7, 10, 15, 20, 25, and 30 years. Participants were contacted twice yearly, and periodic National Death Index searches were conducted. This analysis includes data collected through 348 months of follow‐up (median follow‐up of 29.8 years); 89.2% of surviving participants were contacted at or within 2 years of the 348‐month contact. The study was approved by the institutional review boards of each CARDIA field center and written informed consent was obtained from all participants at each examination.
Spirometry
Lung function was measured at baseline and at the year 2, year 5, year 10, and year 20 examinations. Standard spirometry procedures as recommended by the American Thoracic Society were followed at each examination in which lung function was measured.26, 27 Lung function measurements including FVC (the volume of air that can be forcibly exhaled from the lungs after taking the deepest breath possible), FEV1 (the volume of air exhaled in the first second of a forced exhalation maneuver), and FEV1/FVC ratio were calculated. The FVC can serve as a marker of lung health that encompasses total lung volume as well as respiratory muscle strength. The FEV1 can serve as a marker of airway disease in the lung. The FEV1/FVC ratio is the diagnostic marker used to define obstructive airway disease.
CVD Events
Definitions of all‐cause mortality and cardiovascular events have been previously described.28, 29, 30, 31, 32, 33, 34 Participants were contacted annually to inquire about interim hospitalizations or outpatient vascular procedures. Vital status was assessed at 6‐month intervals. For each event, medical records were obtained and adjudicated by 2 physician members of the adjudication committee. Disagreements were resolved by full committee review.
For CVD events, we included:
nonfatal myocardial infarction or stroke.
hospitalization for acute coronary syndrome not resulting in infarction, heart failure, or transient ischemic attack.
revascularization for or demonstration of obstruction of carotid arteries or peripheral arterial disease on angiographic or ultrasonographic findings.
underlying cause of death of fatal atherosclerotic CHD, stroke, atherosclerotic disease other than coronary or stroke, or nonatherosclerotic cardiac disease (eg, hypertensive cardiomyopathy).
At the time of this analysis, there were 233 CVD events recorded over ≈29 years of follow‐up in the analytic cohort of 4761 participants. Of the 5115 participants originally enrolled, 354 were excluded from the analysis to arrive at the analytic cohort of 4761 participants; 1 patient withdrew consent, 254 patients were missing baseline pulmonary function data, and 99 patients were missing baseline covariate data (Figure S1).
We further categorized the first cardiovascular events into 3 mutually exclusive groups: cerebrovascular disease events (fatal and nonfatal stroke, transient ischemic attacks, and carotid artery disease) (n=57); CHD events (nonmyocardial infarction acute coronary syndrome, myocardial infarction, and death caused by atherosclerotic CHD) (n=112); and heart failure events (congestive heart failure, and death caused by nonatherosclerotic cardiac disease) (n=56). Because peripheral arterial disease events have a pathogenesis not consistent with the 3 categories we defined, event times for 8 participants with peripheral arterial disease were treated as censored. Furthermore, 8 participants had multimorbid first CVD events on the same day, guidelines laid out by the CARDIA study for addressing these are as follows: myocardial infarction and nonmyocardial infarction acute coronary syndrome take precedence over both congestive heart failure and stroke, transient ischemic attack, or carotid artery disease as first event. Stroke, transient ischemic attack, or carotid artery disease take precedence over congestive heart failure as first event. Any event takes precedence over death (with the idea being that death follows other events as the proximate cause of death).
Participants free of CVD events were censored at the date of non‐CVD death (n=268), last contact (n=4260), or August 31, 2015, whichever occurred first.
Coronary Artery Calcuim Score
The Agatson coronary artery calcium (CAC) score was measured in 2978 of the 4761 participants with a multidetector computed tomography system in accordance with standard protocols at year 25 examination, as previously described.35
Carotid Intima‐Media Thickness
Carotid intima‐media thickness (CIMT) was measured in 3046 of the 4761 patients at year 20 examination in the CARDIA study with high‐resolution B‐mode ultrasound in accordance with standard procedures.36, 37 Measurement of maximum average common CIMT and average maximum internal CIMT were included in the analysis.
Statistical Analysis
Age‐, race‐, and sex‐adjusted rates of CVD events per 1000 patient‐years were computed across quintiles of FEV1, FVC, and FEV1/FVC ratio. Cox proportional hazards regression analysis was used to assess the association of baseline lung function measures and incident CVD events (sample size: 4761, events: 233). Covariates in the analysis included baseline age, race‐sex group (model 1), baseline body mass index (BMI), smoking status (current, former, or never), and highest education level (model 2). In a third model, designed to assess the association between lung function and cardiovascular events independent of classic CVD risk factors, diabetes mellitus (defined as fasting glucose ≥126 mg/dL or use of diabetic medication), systolic blood pressure (BP), use of antihypertensive medications, total cholesterol, and high‐density lipoprotein cholesterol (note that no participants were taking cholesterol medication at the baseline examination) were added as covariates. Similar analysis was completed using lung function measurements at the year 10 (sample size: 3627, events: 186) and year 20 (sample size: 3295, events: 102) examinations as the main predictor variables.
Linear regression was used to test the association between baseline lung function and CIMT at year 20. Covariates included in these analyses included age, race‐sex group, highest education level, diabetes mellitus, BMI, total cholesterol, high‐density lipoprotein cholesterol, systolic BP, BP medication use, and smoking status (current, former, or never). The prevalence of CAC >0 Agatston units and >100 Agatston units adjusted for age, race, and sex was computed across quintiles of lung function including FEV1, FVC, and FEV1/FVC ratio. Logistic regression was used to model the association of baseline lung function and CAC >0 and CAC >100, with inclusion of the same covariates as in the CIMT models.
Logistic regression was used to test the associations of lung function measured at the year 10 examination with incident cardiovascular risk factors (defined as present at year 20 and not present at year 10) including hypertension (defined as a systolic BP ≥130 mm Hg, diastolic BP ≥80 mm Hg, or use of BP medications), hypercholesterolemia (defined as total cholesterol ≥200 mg/dL or use of cholesterol‐lowering medications), and diabetes mellitus (fasting blood glucose >126 mg/dL or use of diabetic medications). Covariates in this analysis included age, race‐sex groups, BMI at year 10, change in BMI defined as year 20 minus year 10, and smoking status (current, former, or never) at year 10.
Results
A total of 4761 participants who had lung function testing and were not missing baseline covariates at the baseline examination were included in the analysis. Baseline characteristics of the sample, stratified by presence or absence of cardiovascular events, are shown in Table 1. CVD event rates per 1000 patient‐years adjusted for age, sex, and race across quintiles of lung function are shown in Figure S2A through S2C. In the cohort as a whole, the mean age was 24.9±3.6 years, over half (55.7%) reported being never‐smokers, and lung function was generally normal (FEV1 97.8±11.8% predicted) with only 166 (3.49%) having an FEV1/FVC ratio of <70. The incidence of obstructive airway disease, defined as an FEV1/FVC ratio of <70, remained low at both the year 10 (n=227, 6.26%) and year 20 (n=281, 8.53%) examinations.
Table 1.
Baseline Participant Characteristics Stratified by the Presence or Absence of a Cardiovascular Event Over 29 y of Follow‐Up
| Characteristic | No Events (n=4528) | Cardiovascular Event (n=233) |
|---|---|---|
| Age, y | 24.8±3.6 | 26.2±3.5 |
| Women, No. (%) | 2462 (54.4) | 90 (38.6) |
| Black, No. (%) | 2270 (50.1) | 144 (61.8) |
| Smoking status, No. (%) | ||
| Never | 2556 (56.4) | 95 (40.8) |
| Former | 622 (13.7) | 25 (10.7) |
| Current | 1350 (29.8) | 113 (48.5) |
| Highest education level attained (y of education) | 15±3 | 14±2 |
| BMI, kg/m2 | 24.5±4.9 | 26.7±6.5 |
| Total cholesterol, mg/dL | 176±33 | 192±37 |
| HDL cholesterol, mg/dL | 53±13 | 49±13 |
| Hypercholesterolemia, No. (%) | 1006 (22.2) | 91 (39.1) |
| Systolic BP, mm Hg | 110±11 | 116±12 |
| Diastolic BP, mm Hg | 68±9 | 72±12 |
| Hypertension, No. (%) 2017 ACC/AHA guideline definition | 615 (13.6) | 70 (30.0) |
| Fasting glucose, mg/dL | 82.3±12.7 | 91.0±43.6 |
| Diabetes mellitus, No. (%) | 20 (0.4) | 10 (4.3) |
| FEV1, % predicted | 98.0±11.8 | 94.7±12.1 |
| FVC, % predicted | 100.6±11.5 | 97.8±11.9 |
| FEV1/FVC, % | 83.1±6.5 | 82.0±6.8 |
Values are expressed as mean±SD unless otherwise indicated. ACC/AHA indicates American College of Cardiology/American Heart Association; BMI, body mass index; BP, blood pressure; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HDL, high‐density lipoprotein.
Association Between Baseline Lung Function and Cardiovascular Events
Lower baseline FEV1 and FVC were both associated with greater risk of cardiovascular events (Table 2). These associations between lung function and cardiovascular events were similar in models adjusted for traditional cardiovascular risk factors including BP, total cholesterol, and diabetes mellitus determined at the same time as lung function. The hazard ratio per each −10‐unit decrement in FEV1 percent predicted was 1.18 (95% CI, 1.06–1.31) and the hazard ratio per −10‐unit decrement FVC percent predicted was 1.19 (95% CI, 1.06–1.33) (Table 2, Figure 1A,D).
Table 2.
Association Between Baseline (Mean Age 25 y, Range 18–30 y) Lung Function and Cardiovascular Events Over 29 y of Follow‐Up (n=4761)
| Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | |
|---|---|---|---|
| FEV1, % predicted | 1.25 (1.13–1.39) | 1.21 (1.09–1.34) | 1.18 (1.06–1.31) |
| FVC, % predicted | 1.25 (1.12–1.40) | 1.23 (1.10–1.38) | 1.19 (1.06–1.33) |
| FEV1/FVC, % | 1.05 (0.95–1.16) | 1.00 (0.91–1.11) | 1.03 (0.93–1.14) |
Hazard ratios (HRs) are expressed per −10‐unit decrement in the percent predicted value or −5‐unit decrement in forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC). Model 1 covariates: baseline age and race‐sex group. Model 2 covariates: model 1+maximal educational attainment, baseline body mass index, and smoking status. Model 3 covariates: model 2+baseline diabetes mellitus (defined as fasting glucose ≥126 mg/dL or use of diabetic medication), systolic blood pressure, use of antihypertensive, total cholesterol, and high‐density lipoprotein cholesterol. No participants were taking cholesterol‐lowering medication at the baseline examination.
Figure 1.
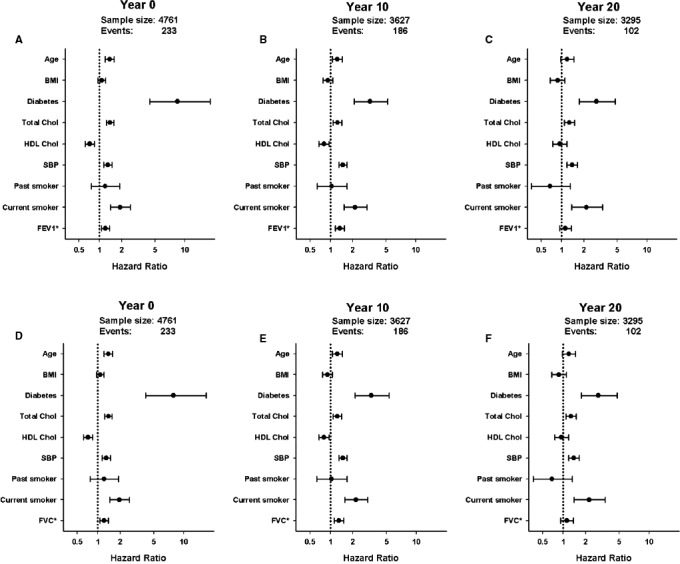
Association of lung function at multiple time points with first fatal and nonfatal cardiovascular disease (CVD) events (A, B, and C report forced expiratory volume in 1 second [FEV1], and D, E, and F report forced vital capacity [FVC]). A, Baseline (year 0) FEV 1 is associated with increased risk of CVD events independent of year 0 covariates: age, race‐sex group, maximum education, diabetes mellitus, body mass index (BMI), total cholesterol, high‐density lipoprotein (HDL) cholesterol, systolic blood pressure (SBP), blood pressure medication use, and smoking status. B, With adjustment for year 10 covariates, year 10 FEV 1 remains independently associated with first fatal and nonfatal CVD events. C, With adjustment for year 20 covariates, year 20 FEV 1 no longer shows a statistically significant association with events. D, Baseline (year 0) FVC is associated with increased risk of CVD events independent of year 0 covariates: age, race‐sex group, maximum education, diabetes mellitus, BMI, total cholesterol, HDL cholesterol, SBP, blood pressure medication use, and smoking status. E, With adjustment for year 10 covariates, year 10 FVC remains independently associated with first fatal and nonfatal CVD events. F, With adjustment for year 20 covariates, year 20 FVC no longer shows a statistically significant association with events. Hazard ratios are expressed per −1 SD decrement in percent predicted FEV 1 or FVC.
The fully adjusted models were repeated using year 10 (mean age 35 years, range 28–40) and year 20 (mean age 45 years, range 38–50) lung function as the predictor variables of interest. Decrements in year 10 FEV1 (Figure 1B) and FVC (Figure 1E) (hazard ratio reported per −1 SD decrement of both percent predicted FEV1 and FVC) were both associated with greater risk of incident cardiovascular events independent of traditional cardiovascular risk factors. By contrast, year 20 FEV1 and FVC (Figure 1C,F) were not associated with cardiovascular events.
In secondary analysis of specific adjudicated cardiovascular event subtypes, significant associations were found between both baseline FEV1 and FVC with cerebrovascular events. On the other hand, FVC but not FEV1 was associated with risk of heart failure events and neither FEV1 or FVC were significantly associated with CAD events (Table 3).
Table 3.
Association Evaluated Using Cause‐Specific Cox Regression Between Baseline Lung Function (Mean Age 25 y, Range 18–30 y) and Adjudicated Categories of Cardiovascular Events Over 29 y of Follow Up
| Cerebrovascular Event (n=57) HR (95% CI) | Coronary Event (n=112) HR (95% CI) | Heart Failure Event (n=56) HR (95% CI) | |
|---|---|---|---|
| FEV1, % predicted | 1.30 (1.06–1.59) | 1.11 (0.95–1.30) | 1.20 (0.98–1.48) |
| FVC, % predicted | 1.32 (1.06–1.65) | 1.06 (0.89–1.25) | 1.29 (1.03–1.62) |
| FEV1/FVC, % | 1.04 (0.85–1.28) | 1.08 (0.93–1.24) | 0.93 (0.75–1.16) |
Hazard ratios (HRs) are expressed per −10‐unit difference in the percent predicted value for forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) or −5‐unit difference in the FEV1/FVC. Covariates: race‐sex group, maximum educational attainment, baseline age, body mass index, diabetes mellitus (defined as fasting glucose ≥126 mg/dL or use of diabetic medication), systolic blood pressure, use of an antihypertensive, total cholesterol, high‐density lipoprotein cholesterol, and smoking status.
Association Between Baseline Lung Function and Subclinical Atherosclerosis
Given prior literature showing an association between COPD and CHD38 we assessed the association between baseline lung function and the presence of both common CIMT and maximum internal CIMT at year 20 as well as CAC at year 25. We found that baseline FEV1 and FEV1/FVC ratio but not FVC were inversely associated with mean maximal common CIMT. No association was noted between any baseline lung function measurements and mean maximal internal CIMT (Table 4). We also found no association between baseline FEV1, FVC, or FEV1/FVC ratio with either presence of CAC at year 25 nor with presence of CAC score >100 at year 25 after adjustment for covariates including BMI, diabetes mellitus, BP, cholesterol, and smoking status (Table 4), The prevalence of CAC >0 Agatston units and CAC >100 Agatston units across quintiles of baseline lung function is shown in Figure S2D through S2F.
Table 4.
Association Between Baseline Lung Function (Mean Age 25 y, Range 18–30 y) and CIMT and CAC Measured 20 and 25 y Later, Respectively
| FEV1, % Predicted | FVC, % Predicted | FEV1/FVC, % | ||||
|---|---|---|---|---|---|---|
| Beta | P Value | Beta | P Value | Beta | P Value | |
| CIMT (year 20) | ||||||
| Common carotid, mm | 0.006 | <0.001 | 0.002 | 0.23 | 0.006 | <0.001 |
| Internal carotid, mm | 0.001 | 0.83 | −0.001 | 0.81 | 0.002 | 0.49 |
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
|---|---|---|---|---|---|---|
| CAC (year 25) | ||||||
| CAC >0 AU | 1.02 | 0.95–1.10 | 1.07 | 0.99–1.16 | 0.94 | 0.88–1.02 |
| CAC >100 AU | 0.99 | 0.89–1.11 | 1.01 | 0.90–1.13 | 0.99 | 0.90–1.10 |
For carotid intima‐media thickness (CIMT), beta coefficients reflect the covariate‐adjusted difference in CIMT per −10 units of the percent predicted forced expiratory volume in 1 second (FEV1) or forced vital capacity (FVC) or −5 units of FEV1/FVC. For coronary artery calcium (CAC), the association is similarly expressed as an adjusted odds ratio (OR) per −10 units in the FEV1 or FVC and −5 units in FEV1/FVC. Covariates: race‐sex group, maximum educational attainment, baseline age, body mass index, diabetes mellitus (defined as fasting glucose ≥126 mg/dL or use of diabetic medication), systolic blood pressure, use of an antihypertensive, total cholesterol, high‐density lipoprotein cholesterol, and smoking status. AU indicates Agatston units.
Association Between Year 10 Lung Function and Incident Cardiovascular Risk Factors
To explore why baseline and year 10 lung function were both associated with future cardiovascular events but year 20 was not, we tested whether year 10 lung function was associated with the development of CVD risk factors at year 20. We found that year 10 FEV1 (odds ratio, 1.09; 95% CI, 1.00–1.19), but not FVC or FEV1/FVC, was associated with incident hypertension at year 20. No year 10 lung function measurement was associated with hypercholesterolemia at year 20. Both FEV1 (odds ratio, 1.18; 95% CI, 1.03–1.36) and FVC (odds ratio, 1.27; 95% CI, 1.10–1.48) at year 10 were associated with greater risk of diabetes mellitus at year 20 (Table S1).
Discussion
In this community‐based study of generally healthy young adults followed for nearly 30 years, we found that baseline lung function measured at a mean age of 25 years (range 18–30 years) is associated with greater risk of future CVD events over the subsequent 29 years. This association was independent of sex, race, smoking, BMI, total cholesterol, BP, and diabetes mellitus. These findings add to a growing body of evidence that peak lung function in young adulthood has important implications for future respiratory and overall health. The implication of these findings is that lung function testing in early adulthood may provide an early window into cardiovascular health at a time when the evolution of potential heart and lung disease is in its beginning stages.
Established lung diseases such as COPD are associated with CVD and poor health outcomes.3 Even among patients who do not meet spirometric criteria for COPD, reduced lung function (defined as the lowest quintile of FEV1) is associated with cardiovascular death.5 Furthermore, low lung function and obstructive lung physiology have been linked with greater risk of incident heart failure.39, 40 Most of these reports focus on measurements of lung function in middle age. Little is known about the association between lung function measured around the time it peaks in young adulthood and future cardiovascular outcomes. We have previously observed in the CARDIA study an association between FVC decline and incident hypertension.10 Furthermore, we have also described in this cohort that patterns of loss of lung health are associated with distinct echocardiographic phenotypes in middle age.41 The results of both of these studies are notable in that the majority of participants still had lung function that would typically be considered in the normal range (ie, >80% of their predicted value for FEV1 or FVC). This highlights the importance of evaluating lung function in young adulthood as a continuum (rather than using cutoff levels for disease) as overall lung health is associated with cardiovascular risk even before the onset of clinically overt lung disease. In the current analysis, we show that measurements of lung function occurring as early as in an individual's 20s are associated with future CVD events. This finding mirrors recent work showing that abnormal lung function in early adulthood is an important risk factor for the development of future COPD.19, 42 The aggregate of these findings highlights the importance of the measurement of lung function in early adulthood around the time lung function peaks not only as an important predictor of the development of future lung disease but as a key parameter to understand the concurrent evolution of lung and heart disease.
The mechanism of association between lung function in young adults and CVD events is unknown. Given the known association between lung disease and CHD events later in life,4, 8 one striking finding from our study was the lack of association between baseline lung function and indicators of atherosclerotic disease.43, 44 We found that baseline lung function was not associated with CHD events such as myocardial infarction and acute coronary syndrome in the CARDIA study. Furthermore, year 10 lung function was not associated with year 20 elevated total cholesterol levels nor did we see any association between baseline lung function and the presence of future coronary artery calcified plaque. When we examined the association between lung health and CIMT measurements, no association was noted with measurement of internal carotid artery intima‐media thickness, which is associated with vascular plaque formation and predictive of CHD events.37, 45, 46 These findings suggest that the hypothesis that common exposures such as tobacco smoke, likely by triggering inflammatory pathways, leads to both lung injury with subsequent lung function decline and eventually lung disease and activation of atherosclerotic pathways leading to the development of CHD may not tell the whole story.47 Our findings suggest that in addition to exposure‐driven pathways in the development of COPD and CHD, peak lung health attained in young adulthood is predictive of unique mechanisms that contribute to the coevolution of heart and lung disease.
We did find that baseline lung health was associated with both cerebrovascular events and heart failure events. Furthermore, year 10 FEV1 was predictive of both elevated year 20 BP and common carotid artery intima‐media thickness. As opposed to internal carotid artery intima‐media thickness, common carotid artery intima‐media thickness has been associated with increased sheer stress, hypertension, cerebrovascular events, and pathologically more diffuse vascular changes rather than focal plaque formation.37, 48, 49 Furthermore, we noted an association between year 10 lung function and incident diabetes mellitus at year 20. This finding of the association between lung function and diabetes mellitus risk has been previously reported with many potential mechanisms being proposed ranging from the impact of lung function on decreased physical activity influencing diabetes mellitus risk to activation of common inflammatory pathways which impact both lung health and insulin sensitivity.20, 50, 51 Our findings imply that the association between lung function in young adulthood with hypertension, diabetes mellitus, and eventually heart failure and cerebrovascular events may lie more along metabolic pathways that impact vascular compliance. Indeed, several studies in older populations have shown an inverse cross‐sectional relationship between lung function and markers of arterial compliance, but whether this is a consequence of low peak lung function or accelerated decline in lung function cannot be discerned in these studies.2, 52, 53, 54, 55
We have previously noted in the CARDIA study that different patterns of lung function decline from young adulthood are associated with distinct echocardiographic phenotypes in middle age. When FVC and FEV1 declined in conjunction with one another leaving a preserved FEV1/FVC ratio, a pattern that could be a precursor of restrictive lung physiology, the echocardiographic phenotype was hypertrophy and diastolic dysfunction. When FEV1 declined with a preserved FVC such that the FEV1/FVC ratio dropped, a pattern that could be a precursor to obstructive lung physiology, the echocardiographic phenotype was an underfilled left heart with low output state.41 In the current article, we found similarly that baseline FVC is associated heart failure events while FEV1 was not and that FEV1 was associated with CIMT while FVC was not. Although, in general, FVC and FEV1 measurements are highly correlated, this does raise an interesting question related to whether a pattern of airway abnormalities (diminished FEV1) versus overall lung restriction (diminished FVC), which is established in young adulthood, influences future cardiovascular phenotypes. Prior work from our group has pointed toward systemic inflammation being associated with lung restriction and sustained inflammation is associated with heart failure, in particular heart failure with preserved ejection fraction.21, 56 One could speculate that the diminished FEV1 out of proportion to FVC (the airway abnormality pattern) further implies something beyond just a generalized systemic inflammatory response that increases risk of carotid atherosclerotic disease. This idea is reinforced by the fact that there is a well‐established association between COPD (the classic established airway disease) and atherosclerosis.4
Study Strengths and Limitations
A notable strength is that the CARDIA cohort provides a unique opportunity to study heart lung interactions since it includes multiple measurements of lung function in early adulthood in a well‐characterized group with long‐term reporting of CVD events. This allows for prospectively studying the relationship of lung health to the development of CVD rather than focusing on the relationship of the heart and lung after disease is established, thus avoiding the potential for reverse causation bias. A limitation inherent to any large epidemiologic study is the inability to study mechanistic pathways of disease. Although our findings related to cardiovascular risk factors, CAC, and CIMT in relation to lung function are intriguing, suggesting that lung health influences cardiovascular events independent of coronary artery disease, without a prospective study specifically designed to study pathways of disease evolution, the true mechanistic link between lung health and cardiovascular risk remains to be determined.
Conclusions
Lower lung function in early adulthood is independently associated with cardiovascular events into middle age. We show that it is not only lung disease in young adulthood (defined as FEV1 or FVC <80% predicted) that is associated with lung and cardiac comorbidities later in life, rather the full continuum of lung health measured when individuals are in their mid‐20s is associated with cardiovascular events over 29 years of follow‐up. This association is noted at an age when lung function measurements are within the normal ranges and occurs independent of known cardiovascular risk factors and atherosclerotic heart disease. Our findings add to the growing body of evidence exploring the importance of peak lung health attained in young adulthood on the coevolution of heart and lung disease across the adult lifespan. A greater understanding of the physiologic links between measurements of lung function and cardiovascular health across the lifespan is needed to define preventive health strategies focused on the concurrent evolution of common cardiovascular and respiratory conditions.
Sources of Funding
The CARDIA study is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI); the Intramural Research Program of the National Institute on Aging (NIA); and an intra‐agency agreement between the NIA and NHLBI (AG0005) and grant R01 HL122477 (to Kalhan). The CARDIA study is funded by the NHLBI, which had input into the overall design and conduct of our study and was represented on the publications committee that approved this article.
Disclosures
Dr Kalhan reports grants from the NHLBI during the conduct of the study and grants and personal fees from Boehringer Ingelheim, grants from PneumRx (BTG), grants from Spiration, grants and personal fees from AstraZeneca, personal fees from CVS Caremark, personal fees from Aptus Health, grants and personal fees from GlaxoSmithKline, and personal fees from Boston Scientific outside the submitted work. Dr Dransfield reports grants from the NHLBI during the conduct of the study and grants from the Department of Defense, personal fees and other from Boehringer Ingeheim, personal fees and other from GlaxoSmithKline, other from Novartis, personal fees and other from AstraZeneca, other from Yungjin, other from PneumRx/BTG, other from Pulmonx, personal fees from Genentech, and personal fees and other from Boston Scientific outside the submitted work. Dr Washko reports grants and other from Boehringer Ingelheim, other from GSK, other from PulmonX, other from Genentech, other from Janssen, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Table S1. Association Between Lung Function Measured at Examination Year 10 (Mean Age 35 y, Range 28–40 y) and the Development of Incident Cardiovascular Risk Factors by the Year 20 Examination
Figure S1. Number of participants included in the analytic cohorts based on available data. CAC indicates coronary artery calcium; CC, common carotid IMT; IC, internal carotid IMT.
Figure S2. Cardiovascular disease (CVD) event rate and prevalence of coronary artery calcium (CAC) across lung function quintiles. A, CVD event rate per 1000‐person years across quintiles of forced expiratory volume in 1 second (FEV1) adjusted for age, race, and sex. FEV1 percent predicted ranges for quintiles; 1: 46.2230 to 88.4222, 2: 88.4268 to 95.0391, 3: 95.0394 to 100.6025, 4: 100.6254 to 107.4562, and 5: 107.4596 to 152.0148. B, CVD event rate per 1000‐person years across quintiles of forced vital capacity (FVC) adjusted for age, race, and sex. FVC percent predicted ranges for quintiles; 1: 50.3075 to 90.8605, 2: 90.8622 to 97.3808, 3: 97.3907 to 102.8751, 4: 102.8984 to 109.8769, and 5: 109.8868 to 169.2371. C, CVD event rate per 1000‐person years across quintiles of FEV1/FVC ratio adjusted for age, race, and sex. FEV1/FVC ratio ranges for quintiles; 1: 41.9795 to 78.1533, 2: 78.1734 to 82.0513, 3: 82.0588 to 85.0467, 4: 85.0485 to 88.2637, and 5: 88.2716 to 100.00. D, Prevalence of CAC (closed circle CAC score >0 Agatston units [AU], triangle CAC score >100 AU) across quintiles of FEV1. FEV1 percent predicted ranges for quintiles; 1: 46.2230 to 88.4222, 2: 88.4268 to 95.0391, 3: 95.0394 to 100.6025, 4: 100.6254 to 107.4562, and 5: 107.4596 to 152.0148. E, Prevalence of CAC (closed circle CAC score >0 AU, triangle CAC score >100 AU) across quintiles of FVC. FVC percent predicted ranges for quintiles; 1: 50.3075 to 90.8605, 2: 90.8622 to 97.3808, 3: 97.3907 to 102.8751, 4: 102.8984 to 109.8769, and 5: 109.8868 to 169.2371. F, Prevalence of CAC (closed circle CAC score >0 AU, triangle CAC score >100 AU) across quintiles of FEV1/FVC ratio. FEV1/FVC ratio ranges for quintiles; 1: 41.9795 to 78.1533, 2: 78.1734 to 82.0513, 3: 82.0588 to 85.0467, 4: 85.0485 to 88.2637, and 5: 88.2716 to 100.00.
(J Am Heart Assoc. 2018;7:e010672 DOI: 10.1161/JAHA.118.010672.)
References
- 1. Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the first national health and nutrition examination survey and follow‐up. Int J Tuberc Lung Dis. 2005;9:613–621. [PubMed] [Google Scholar]
- 2. Duprez DA, Hearst MO, Lutsey PL, Herrington DM, Ouyang P, Barr RG, Bluemke DA, McAllister D, Carr JJ, Jacobs DR Jr. Associations among lung function, arterial elasticity, and circulating endothelial and inflammation markers: the multiethnic study of atherosclerosis. Hypertension. 2013;61:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ford ES, Wheaton AG, Mannino DM, Presley‐Cantrell L, Li C, Croft JB. Elevated cardiovascular risk among adults with obstructive and restrictive airway functioning in the United States: a cross‐sectional study of the National Health and Nutrition Examination Survey from 2007–2010. Respir Res. 2012;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E Jr, She D. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. [DOI] [PubMed] [Google Scholar]
- 5. Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population‐based study and a systematic review of the literature. Chest. 2005;127:1952–1959. [DOI] [PubMed] [Google Scholar]
- 6. Hole DJ, Watt GC, Davey‐Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and paisley prospective population study. BMJ. 1996;313:711–715; discussion 715–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schunemann HJ, Dorn J, Grant BJ, Winkelstein W Jr, Trevisan M. Pulmonary function is a long‐term predictor of mortality in the general population: 29‐year follow‐up of the buffalo health study. Chest. 2000;118:656–664. [DOI] [PubMed] [Google Scholar]
- 8. Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser permanente medical care program. Chest. 2005;128:2068–2075. [DOI] [PubMed] [Google Scholar]
- 9. Al Rifai M, DeFilippis AP, McEvoy JW, Hall ME, Acien AN, Jones MR, Keith R, Magid HS, Rodriguez CJ, Barr GR, Benjamin EJ, Robertson RM, Bhatnagar A, Blaha MJ. The relationship between smoking intensity and subclinical cardiovascular injury: the Multi‐Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2017;258:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jacobs DR Jr, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG, Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: the coronary artery risk development in young adults study. Hypertension. 2012;59:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kannel WB, Hubert H, Lew EA. Vital capacity as a predictor of cardiovascular disease: the Framingham Study. Am Heart J. 1983;105:311–315. [DOI] [PubMed] [Google Scholar]
- 12. Beck GJ, Doyle CA, Schachter EN. A longitudinal study of respiratory health in a rural community. Am Rev Respir Dis. 1982;125:375–381. [DOI] [PubMed] [Google Scholar]
- 13. Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination survey, 1988–1994. Arch Intern Med. 2000;160:1683–1689. [DOI] [PubMed] [Google Scholar]
- 14. Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. Respir Care. 2002;47:1184–1199. [PubMed] [Google Scholar]
- 15. Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, Shahar E, Smith LJ, Watson KE. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anthonisen NR, Connett JE, Enright PL, Manfreda J; Lung Health Study Research Group . Hospitalizations and mortality in the lung health study. Am J Respir Crit Care Med. 2002;166:333–339. [DOI] [PubMed] [Google Scholar]
- 17. Agusti A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5:935–945. [DOI] [PubMed] [Google Scholar]
- 18. Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez‐Camblor P, Meek P, Owen CA, Petersen H, Pinto‐Plata V, Schnohr P, Sood A, Soriano JB, Tesfaigzi Y, Vestbo J. Lung‐function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. [DOI] [PubMed] [Google Scholar]
- 19. Kalhan R, Arynchyn A, Colangelo LA, Dransfield MT, Gerald LB, Smith LJ. Lung function in young adults predicts airflow obstruction 20 years later. Am J Med. 2010;123:468.e1–468.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lazarus R, Sparrow D, Weiss ST. Baseline ventilatory function predicts the development of higher levels of fasting insulin and fasting insulin resistance index: the Normative Aging Study. Eur Respir J. 1998;12:641–645. [DOI] [PubMed] [Google Scholar]
- 21. Kalhan R, Tran BT, Colangelo LA, Rosenberg SR, Liu K, Thyagarajan B, Jacobs DR Jr, Smith LJ. Systemic inflammation in young adults is associated with abnormal lung function in middle age. PLoS One. 2010;5:e11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moualla M, Qualls C, Arynchyn A, Thyagarajan B, Kalhan R, Smith LJ, Carr JJ, Jacobs DR, Sood A. Rapid decline in lung function is temporally associated with greater metabolically active adiposity in a longitudinal study of healthy adults. Thorax. 2017;72:1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins M, Keller JB, Wagenknecht LE, Townsend MC, Sparrow D, Jacobs DR Jr, Hughes G. Pulmonary function and cardiovascular risk factor relationships in black and in white young men and women. The CARDIA Study. Chest. 1991;99:315–322. [DOI] [PubMed] [Google Scholar]
- 24. Reyfman PA, Washko GR, Dransfield MT, Spira A, Han MK, Kalhan R. Defining impaired respiratory health. A paradigm shift for pulmonary medicine. Am J Respir Crit Care Med. 2018;198:440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. Cardia: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 26. Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 27. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 28. Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall‐Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. [DOI] [PubMed] [Google Scholar]
- 29. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Glob Heart. 2012;7:275–295. [DOI] [PubMed] [Google Scholar]
- 30. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 31. Madden KP, Karanjia PN, Adams HP Jr, Clarke WR. Accuracy of initial stroke subtype diagnosis in the toast study. Trial of org 10172 in acute stroke treatment. Neurology. 1995;45:1975–1979. [DOI] [PubMed] [Google Scholar]
- 32. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. [DOI] [PubMed] [Google Scholar]
- 33. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah RV, Murthy VL, Colangelo LA, Reis J, Venkatesh BA, Sharma R, Abbasi SA, Goff DC Jr, Carr JJ, Rana JS, Terry JG, Bouchard C, Sarzynski MA, Eisman A, Neilan T, Das S, Jerosch‐Herold M, Lewis CE, Carnethon M, Lewis GD, Lima JA. Association of fitness in young adulthood with survival and cardiovascular risk: the coronary artery risk development in young adults (CARDIA) study. JAMA Intern Med. 2016;176:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carr JJ, Nelson JC, Wong ND, McNitt‐Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population‐based studies: standardized protocol of Multi‐Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 36. O'Leary DH, Polak JF, Kronmal RA, Savage PJ, Borhani NO, Kittner SJ, Tracy R, Gardin JM, Price TR, Furberg CD. Thickening of the carotid wall. A marker for atherosclerosis in the elderly? Cardiovascular health study collaborative research group. Stroke. 1996;27:224–231. [DOI] [PubMed] [Google Scholar]
- 37. Polak JF, Person SD, Wei GS, Godreau A, Jacobs DR Jr, Harrington A, Sidney S, O'Leary DH. Segment‐specific associations of carotid intima‐media thickness with cardiovascular risk factors: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Stroke. 2010;41:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65:956–962. [DOI] [PubMed] [Google Scholar]
- 39. Agarwal SK, Heiss G, Barr RG, Chang PP, Loehr LR, Chambless LE, Shahar E, Kitzman DW, Rosamond WD. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Heart Fail. 2012;14:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macchia A, Rodriguez Moncalvo JJ, Kleinert M, Comignani PD, Gimeno G, Arakaki D, Laffaye N, Fuselli JJ, Massolin HP, Gambarte J, Romero M, Tognoni G. Unrecognised ventricular dysfunction in COPD. Eur Respir J. 2012;39:51–58. [DOI] [PubMed] [Google Scholar]
- 41. Cuttica MJ, Colangelo LA, Shah SJ, Lima J, Kishi S, Arynchyn A, Jacobs DR Jr, Thyagarajan B, Liu K, Lloyd‐Jones D, Kalhan R. Loss of lung health from young adulthood and cardiac phenotypes in middle age. Am J Respir Crit Care Med. 2015;192:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lange P, Celli B, Agusti A. Lung‐function trajectories and chronic obstructive pulmonary disease. N Engl J Med. 2015;373:1575. [DOI] [PubMed] [Google Scholar]
- 43. Roversi S, Roversi P, Spadafora G, Rossi R, Fabbri LM. Coronary artery disease concomitant with chronic obstructive pulmonary disease. Eur J Clin Invest. 2014;44:93–102. [DOI] [PubMed] [Google Scholar]
- 44. Mullerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144:1163–1178. [DOI] [PubMed] [Google Scholar]
- 45. Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, Kuller LH, Cushman M. Association of carotid artery intima‐media thickness, plaques, and C‐reactive protein with future cardiovascular disease and all‐cause mortality: the Cardiovascular Health Study. Circulation. 2007;116:32–38. [DOI] [PubMed] [Google Scholar]
- 46. Naqvi TZ, Lee MS. Carotid intima‐media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–1038. [DOI] [PubMed] [Google Scholar]
- 47. Boschetto P, Beghe B, Fabbri LM, Ceconi C. Link between chronic obstructive pulmonary disease and coronary artery disease: implication for clinical practice. Respirology. 2012;17:422–431. [DOI] [PubMed] [Google Scholar]
- 48. Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate‐risk individuals. JAMA. 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, Shear CL, Duggan WT, Vicari RM, Grobbee DE, Kastelein JJ. Torcetrapib and carotid intima‐media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double‐blind trial. Lancet. 2007;370:153–160. [DOI] [PubMed] [Google Scholar]
- 50. Zaigham S, Nilsson PM, Wollmer P, Engstrom G. The temporal relationship between poor lung function and the risk of diabetes. BMC Pulm Med. 2016;16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Engstrom G, Hedblad B, Nilsson P, Wollmer P, Berglund G, Janzon L. Lung function, insulin resistance and incidence of cardiovascular disease: a longitudinal cohort study. J Intern Med. 2003;253:574–581. [DOI] [PubMed] [Google Scholar]
- 52. Jankowich MD, Taveira T, Wu WC. Decreased lung function is associated with increased arterial stiffness as measured by peripheral pulse pressure: data from NHANES III. Am J Hypertens. 2010;23:614–619. [DOI] [PubMed] [Google Scholar]
- 53. Brunner EJ, Shipley MJ, Witte DR, Singh‐Manoux A, Britton AR, Tabak AG, McEniery CM, Wilkinson IB, Kivimaki M. Arterial stiffness, physical function, and functional limitation: the Whitehall II Study. Hypertension. 2011;57:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zureik M, Benetos A, Neukirch C, Courbon D, Bean K, Thomas F, Ducimetiere P. Reduced pulmonary function is associated with central arterial stiffness in men. Am J Respir Crit Care Med. 2001;164:2181–2185. [DOI] [PubMed] [Google Scholar]
- 55. Bolton CE, Cockcroft JR, Sabit R, Munnery M, McEniery CM, Wilkinson IB, Ebrahim S, Gallacher JE, Shale DJ, Ben‐Shlomo Y. Lung function in mid‐life compared with later life is a stronger predictor of arterial stiffness in men: the Caerphilly Prospective Study. Int J Epidemiol. 2009;38:867–876. [DOI] [PubMed] [Google Scholar]
- 56. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Association Between Lung Function Measured at Examination Year 10 (Mean Age 35 y, Range 28–40 y) and the Development of Incident Cardiovascular Risk Factors by the Year 20 Examination
Figure S1. Number of participants included in the analytic cohorts based on available data. CAC indicates coronary artery calcium; CC, common carotid IMT; IC, internal carotid IMT.
Figure S2. Cardiovascular disease (CVD) event rate and prevalence of coronary artery calcium (CAC) across lung function quintiles. A, CVD event rate per 1000‐person years across quintiles of forced expiratory volume in 1 second (FEV1) adjusted for age, race, and sex. FEV1 percent predicted ranges for quintiles; 1: 46.2230 to 88.4222, 2: 88.4268 to 95.0391, 3: 95.0394 to 100.6025, 4: 100.6254 to 107.4562, and 5: 107.4596 to 152.0148. B, CVD event rate per 1000‐person years across quintiles of forced vital capacity (FVC) adjusted for age, race, and sex. FVC percent predicted ranges for quintiles; 1: 50.3075 to 90.8605, 2: 90.8622 to 97.3808, 3: 97.3907 to 102.8751, 4: 102.8984 to 109.8769, and 5: 109.8868 to 169.2371. C, CVD event rate per 1000‐person years across quintiles of FEV1/FVC ratio adjusted for age, race, and sex. FEV1/FVC ratio ranges for quintiles; 1: 41.9795 to 78.1533, 2: 78.1734 to 82.0513, 3: 82.0588 to 85.0467, 4: 85.0485 to 88.2637, and 5: 88.2716 to 100.00. D, Prevalence of CAC (closed circle CAC score >0 Agatston units [AU], triangle CAC score >100 AU) across quintiles of FEV1. FEV1 percent predicted ranges for quintiles; 1: 46.2230 to 88.4222, 2: 88.4268 to 95.0391, 3: 95.0394 to 100.6025, 4: 100.6254 to 107.4562, and 5: 107.4596 to 152.0148. E, Prevalence of CAC (closed circle CAC score >0 AU, triangle CAC score >100 AU) across quintiles of FVC. FVC percent predicted ranges for quintiles; 1: 50.3075 to 90.8605, 2: 90.8622 to 97.3808, 3: 97.3907 to 102.8751, 4: 102.8984 to 109.8769, and 5: 109.8868 to 169.2371. F, Prevalence of CAC (closed circle CAC score >0 AU, triangle CAC score >100 AU) across quintiles of FEV1/FVC ratio. FEV1/FVC ratio ranges for quintiles; 1: 41.9795 to 78.1533, 2: 78.1734 to 82.0513, 3: 82.0588 to 85.0467, 4: 85.0485 to 88.2637, and 5: 88.2716 to 100.00.


