Abstract
Background
Associations between subtle changes in cardiac and cerebral structure and function are not well understood, with some studies suggesting that subclinical cardiac changes may be associated with markers of vascular brain insult.
Methods and Results
Data from the ARIC (Atherosclerosis Risk in Communities) Study (5th ARIC visit; 2011‐2013; N=1974) were used to explore relationships between abnormalities of cardiac structure/function and subclinical brain disease and to test specific associations between those cardiac and vascular brain changes that share a common mechanism. In adjusted models white matter hyperintensities were 0.66 cm3 greater (95% confidence interval [CI] 0.08‐1.25) for every 1‐mm increase in left ventricular LV wall thickness and 0.64 cm3 greater (95% CI 0.19‐1.08) for every 10 g/m2 increase in LV mass index, both markers of LV structure. Odds of brain infarction also increased with greater LV wall thickness (odds ratio 1.11, 95% CI 1.01‐1.23 per 1 mm) and larger LV mass (odds ratio 1.08, 95% CI 1.00‐1.17 per 10 g/m2). Higher ejection fraction (per 5%), a marker of systolic function, was significantly associated with decreased odds of overall infarct (odds ratio 0.85, 95% CI0.77‐0.95), but not with cortical infarction (odds ratio 0.92, 95% CI0.78‐1.08).
Conclusions
Among elderly participants in a large cohort study, subclinical markers of LV structure and LV systolic dysfunction were associated with increased odds of brain infarction and more white matter hyperintensities, independent of other vascular risk factors. This suggests end‐organ dysfunction occurs in the heart and brain in parallel, with further studies needed to determine causality.
Keywords: brain infarction, cardiology, echocardiography, white matter disease
Subject Categories: Echocardiography, Cerebrovascular Disease/Stroke, Ischemic Stroke, Vascular Disease
Clinical Perspective
What Is New?
Subclinical changes in cardiac structure and function occur in parallel with markers of cerebrovascular brain insult; specifically, subclinical changes in left ventricular mass were associated with greater odds of infarction and size of white matter hyperintensities even when controlling for hypertension.
What Are the Clinical Implications?
These findings emphasize the importance of control of shared risk factors and may suggest a direct link between cardiac function and potential for neurologic sequelae.
Introduction
Heart failure (HF) is a significant public health problem, affecting over 5 million individuals with the estimated cost of nearly $35 billion.1 Both the prevalence and incidence of HF are greatest among the elderly. Subclinical cardiac dysfunction (ACC/AHA Stage B HF) is present in up to 44% of elderly people, predominantly occurs despite preserved left ventricular ejection fraction (LVEF), and is associated with a higher risk of incident HF or death.2 This large burden of subclinical cardiac dysfunction may also be associated with brain changes and risk for subclinical brain injury in the elderly, ranging from subclinical stroke to more chronic changes such as white matter injury or white matter hyperintensities (WMH). Mechanisms of these brain changes in patients with clinical or subclinical HF include strokes from reduced systolic function, a secondary arrhythmia, or possibly subtle hypoperfusion even in the setting of only modestly reduced ejection fraction (EF). Importantly, co‐occurrence of cardiac and brain dysfunction could result from shared cardiovascular risk factors. For example, systemic hypertension can result in increased left ventricular (LV) mass and left atrial (LA) volume due to chronically increased cardiac afterload load,3, 4, 5 and is also associated with an increased incidence of lacunar infarction and WMH.6, 7
We hypothesize that there are shared mechanisms by which subclinical alterations in cardiac structure and function associate with subclinical brain lesions. Specifically, we hypothesize that echocardiographic features indicative of chronically increased cardiac load are associated with vascular changes in the brain such as lacunar infarcts and WMH. We believe that these associations will be independent of other vascular risk factors, including the presence of hypertension. In contrast, we hypothesize that echocardiographic indices of abnormal LV function indicate increased embolic risk and thus will more strongly associate with embolic‐appearing brain infarcts. In this study we define the cross‐sectional associations of cardiac structure, systolic function, and diastolic function, and subclinical brain markers in nearly 2000 elderly participants in the community‐based ARIC (Atherosclerosis Risk in Communities) Study attending the fifth study visit.
Methods
The ARIC Study initially recruited 15 792 people in 1987‐1989 from 4 US communities: Washington County, MD; Forsyth County, NC; the suburbs of Minneapolis, MN; and the suburbs of Jackson, MS. Participants have been studied in person for a total of 5 visits, with the most recent visit (ARIC visit 5; 2011‐2013) including the ARIC‐NCS (ARIC Neurocognitive Study), which provided detailed cognitive assessment, brain magnetic resonance imaging (MRI) in a subset of participants (N=1974), as well as echocardiography (N=6118).8 As reported previously,9 participants for brain MRI were selected from the larger ARIC‐NCS cohort on the basis of having no MRI contraindications and any of the following: having had a prior research brain MRI in the ARIC Study, having impaired cognition, or being chosen from a sample of normals. The prevalence of dementia in the ARIC‐NCS Study was 9.0%.8 Of the participants who then had a research brain MRI, 5.4% (N=114) had a diagnosis of dementia. The sampling frame and weights for the ARIC Study have also been previously described.10 The study was approved by the institutional review boards at all institutions involved in the study, and informed consent was obtained from all participants. The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure in accordance with ARIC policies.
Brain MRI
All 4 field centers included brain imaging with a 3‐T MRI scanner with the following sequences: MP RAGE, Axial T2* GRE, Axial T2 FLAIR, and Axial DTI. All scans were evaluated for protocol compliance and scan quality by a trained MRI image analyst, and these data were tracked through the coordinating center. MP RAGE images were preprocessed to correct intensity inhomogeneity and gradient nonlinearity using methods developed by the Mayo Aging and Dementia Imaging Research Laboratory and previously published.11 For rating of cerebrovascular lesions, MRI images were read centrally at the Mayo Aging and Dementia Imaging Research Laboratory by experienced image analysts with confirmation by neuroradiologists. Studies were evaluated for presence, size (in multiple axes), and location of infarcts in the central gray matter, hemispheric cortical infarctions, and hemispheric white matter lacunar infarctions (Figure). Infarcts were classified as cortical if they extended to the cortical surface (cortical gray and could include underlying white matter) and were hyperintense on fluid‐attenuated inversion recovery sequences as previously described.9 Lacunar infarcts were defined as subcortical infarcts between 3 and 20 mm in maximum diameter, as has been defined previously in ARIC.9 Infarcts were either present or absent, and the number of each infarct type was considered in a separate analysis. Quantitative measures of WMH volume were derived from the axial FLAIR images using a semiautomated algorithm developed by the Mayo Aging and Dementia Imaging Research Laboratory.12 Following segmentation, WMH volumes were calculated in different anatomic compartments using an atlas‐based parcellation technique. Estimated total intracranial volume was quantified from the MPRAGE pulse sequences using Freesurfer (version 5.1). All analyses including the WMH data included estimated total intracranial volume as a covariate in statistical models.
Figure 1.
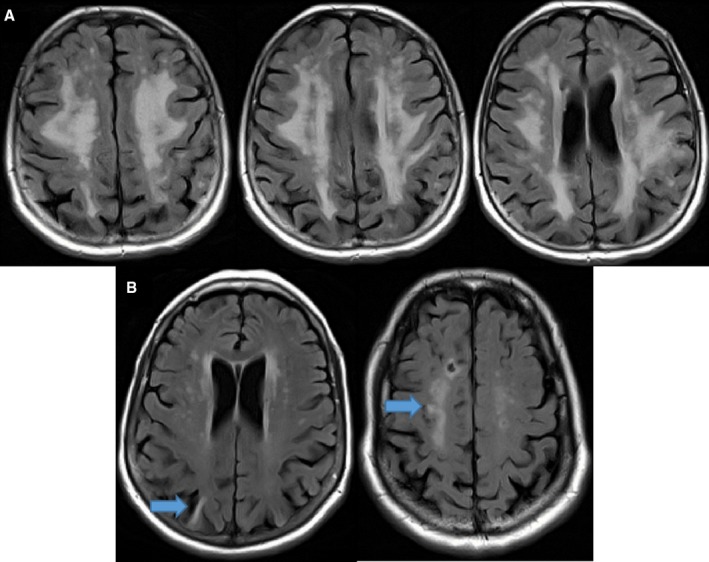
A, Sample MRI demonstrating white matter hyperintensity (WMH) in a participant. The total calculated WMH volume demonstrated in this axial fluid‐attenuated inversion recovery (FLAIR) is 101.2 cm3, and the total percentage of normal white matter that includes WMH is 19.36%. B, Sample participant's axial FLAIR sequences demonstrating cortical infarction (left arrow) and lacunar infarction (right arrow) as defined by study criteria. MRI indicates magnetic resonance imaging.
Echocardiography
Echocardiograms were performed during ARIC Visit 5 (2011‐2013). All echocardiograms were performed using dedicated Philips iE33 ultrasound systems (Bothell, WA) with Vision 2011 and X5‐1 xMatrix transducer for 2‐dimensional, Doppler, and 3‐dimensional data acquisition, purchased specifically for use in the ARIC Study. Details of the design and procedures for echocardiography at the fifth ARIC visit have been previously published, including reproducibility metrics. Coefficients of variation for measures of LV structure, systolic function, and diastolic function were consistently ≤10%.13 LV dimensions and wall thickness were measured from the parasternal long‐axis view according to the recommendations of the American Society of Echocardiography.14 LV mass was calculated from LV linear dimensions and indexed to body surface area as recommended by the American Society of Echocardiography guidelines. All tissue Doppler imaging was acquired with the following ARIC protocol defaults: sweep speed 100 cm/s; sample volume length 5 mm; and filter setting 100 Hz. The parameters chosen for our analysis were indicative of (1) LV structure: LV end‐diastolic diameter (cm), mean LV wall thickness (mm), and LV mass index (per 10 g/m2); (2) LV systolic function: LV ejection fraction (per 5%), and longitudinal strain (%); and (3) LV diastolic function: tissue Doppler‐based early diastolic lateral mitral annular relaxation velocity (cm/s), Early diastolic transmitral flow velocity to early diastolic lateral mitral annular relaxation velocity (E‐Em lateral ratio) (cm/s), and LA volume index (per 5 mL/m2). LV mass was also indexed to height (per 10 g/m2.7) (Tables S1 and S2).
Covariate Assessment
Covariates were defined based on status at the time of the 2011‐2013 visit. Blood pressure was measured using a random‐zero sphygmomanometer, with the average of the second and third of 3 measured values used in analysis. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, antihypertensive drug use, or prior history of hypertension. Diabetes mellitus was defined as self‐report of a physician diagnosis of diabetes mellitus, fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or use of medications for diabetes mellitus. Body mass index was measured as the weight (kilograms) divided by the square of measured height (meters), and hypercholesterolemia was defined as a measured total cholesterol >200 mg/dL. Smoking status was self‐reported. Race was self‐reported at the ARIC baseline visit, and a combined race‐center variable was defined based on a combination of racial group and field center. Level of educational attainment was defined at ARIC baseline, as was sex.
Statistical Analysis
Baseline characteristics were examined by the presence of white or black race. Categorical variables were reported as percentages, and continuous variables were recorded as mean (SD). The χ2 method was used to compare differences for categorical variables, and the equivalence t test was used for continuous variables. Due to right skewness, WMH volume was modeled by generalized linear models with γ families and identity links; thus, results are presented as increases (or decreases) per 1‐unit increase in each predictor variable. Likewise, logistic regression was used to estimate the odds ratio (OR) of the presence of infarcts. Data were weighted using ARIC sampling fractions to weight back to the visit 5 cohort. Modeling of the numbers of cortical and lacunar infarcts was done with negative binomial regression with mean dispersion and log links, and results are reported as rate ratios (RR). Interaction terms were included where appropriate to examine racial disparities, and linear combinations of these models were constructed to give race‐specific estimates. Participants with heart failure (N=59) or atrial fibrillation (N=105) were excluded for the primary outcomes of interest: WMH, infarction, and cortical/lacunar infarcts. All models were adjusted for age, sex, estimated total intracranial volume (for WMH analyses), education, hypertension, diabetes mellitus, low‐density lipoprotein, smoking, alcohol, body mass index, and history of myocardial infarction. Participants with a history of myocardial infarction (N=80) were also excluded in a sensitivity analysis (Tables S3 and S4). Covariates were chosen on the basis of clinical relevance and prior knowledge of their potential to confound associations rather than solely on univariate associations with the outcome of interest. The models were structured to evaluate each variable in a separate model with inclusion of the covariates but not with inclusion of other cardiac markers due to concern for collinearity. A sensitivity analysis with different modeling strategies was performed (Table S5). All models were checked for linearity and homoscedasticity in primary predictors, and neither of these assumptions was violated. All statistical analyses were performed with Stata v14.1 (StataCorp, College Station, TX).
Results
Demographics
The analysis sample included 1829 ARIC participants with brain MRI imaging and available echocardiographic data who did not have heart failure or atrial fibrillation (Table 1). Echocardiographic measures were grouped according to those reflective of LV structure and systolic and diastolic function. The majority of the participants had a preserved LV ejection fraction, normal LA volume index and normal LV mass index (LVMi).
Table 1.
Demographic Features of Study Participants
| Overall (N=1829) | |
|---|---|
| Age (y), mean (SD) | 76.42 (5.35) |
| Female | 1180 (60%) |
| Education | |
| < High school | 285 (14%) |
| High school or vocational school | 809 (41%) |
| College, graduate or professional school | 878 (45%) |
| History of hypertension | 1334 (68%) |
| History of diabetes mellitus | 565 (29%) |
| Body mass index (kg/m2), mean (SD) | 28.47 (5.65) |
| Current smoking | 102 (5%) |
| Current alcohol use | 887 (45%) |
| History of heart failure | 59 (3%) |
| History of myocardial infarction | 102 (5%) |
| WMH (adj TIV) (cm3), mean (SD) | 17.58 (2.80) |
| Cortical infarcts | 205 (10%) |
| Lacunar infarcts | 366 (19%) |
| Microhemorrhages | 478 (24%) |
| Myocardial infarction | 80 (5%) |
| Ejection fraction, mean (SD) | 65.68 (6.40) |
| LVEF <35% | 2 (<1%) |
| Mean LV wall thickness (cm), mean (SD) | 0.99 (0.14) |
| LV mass index (g/m2), mean (SD) | 78.67 (19.54) |
| Average peak longitudinal strain | −18.00 (2.46) |
| Lateral early diastolic myocardial velocity (cm/s), mean (SD) | 7.01 (2.07) |
| LA volume index (mL/m2), mean (SD) | 26.20 (8.71) |
Values are N (%) unless otherwise specified. adj TIV indicates adjusted total intracranial volume; LA, left atrial; LV, left ventricular; LVEF, LV ejection fraction; WMH, white matter hyperintensities.
Cardiac Structure and Function and White Matter Hyperintensities
Greater LVMi was significantly associated with greater WMH, an association driven by greater LV wall thickness but not greater LV chamber size (Table 2). Every 10 g/m2 increase in LVMi was associated with 0.64 cm3 (95% confidence interval [CI] 0.19‐1.08) greater WMH, and every 1‐mm increase in mean LV LVMi wall thickness was associated with 0.66 cm3 (95% CI 0.08‐1.25) greater WMH. LV mass was also indexed to height to account for obesity‐induced bias with similar results (Table S1). Worse longitudinal strain, but not LVEF, was suggestive of greater WMH (β=0.35, 95% CI 0.07‐0.64) but did not achieve statistical significance (P=0.08). Markers of LV diastolic function were not significantly associated with WMH. The association of LA volume index with WMH was the only cardiac predictor that could potentially be modified by race, with a larger effect seen in blacks (0.85 cm3, 95% CI 0.05‐1.65) than in whites (0.01 cm3, 95% CI −0.49 to 0.47) per 5 mL/m2 increase, at P<0.1 (Table S6).
Table 2.
Cardiac Predictors of Brain Structures (N=1829)
| WMH β Coefficientsa | 95% CI | P Values | |
|---|---|---|---|
| LV structure | |||
| End‐diastolic left ventricular diameter, cm | 1.47 | −0.31 to 3.24 | 0.11 |
| Mean LV wall thickness, mm | 0.66 | 0.08‐1.25 | 0.03 |
| LV mass index (10 g/m2) | 0.64 | 0.19‐1.08 | <0.01 |
| LV systolic function | |||
| Ejection fraction (per 5%) | −0.25 | −0.80 to 0.30 | 0.38 |
| Average peak longitudinal strain, % | 0.31 | −0.04 to 0.65 | 0.08 |
| LV diastolic function | |||
| Lateral early diastolic myocardial velocity, cm/s | −0.08 | −0.39 to 0.23 | 0.61 |
| E‐Em lateral ratio, cm/s | 0.15 | −0.07 to 0.36 | 0.18 |
| LA volume index (5 mL/m2) | 0.18 | −0.25 to 0.61 | 0.42 |
| Presence of Infarct Odds Ratiosa | 95% CI | P Values | |
|---|---|---|---|
| End‐diastolic left ventricular diameter, cm | 1.21 | 0.88‐1.68 | 0.24 |
| Mean LV wall thickness, mm | 1.11 | 1.01‐1.23 | 0.04 |
| LV mass index, 10 g/m2 | 1.08 | 1.00‐1.17 | 0.04 |
| LV systolic function | |||
| Ejection fraction, per 5% | 0.85 | 0.77‐0.95 | <0.01 |
| Average peak longitudinal strain, % | 1.04 | 0.98‐1.10 | 0.24 |
| LV diastolic function | |||
| Lateral early diastolic myocardial velocity, cm/s | 0.94 | 0.87‐1.01 | 0.08 |
| E‐Em lateral ratio, cm/s | 1.00 | 0.97‐1.04 | 0.82 |
| LA volume index, 5 mL/m2 | 1.06 | 0.97‐1.16 | 0.19 |
BMI indicates body mass index; CI, confidence interval; E‐Em lateral ratio, early diastolic transmitral flow velocity to early diastolic lateral mitral annular tissue velocity; LA, left atrial; LDL, low‐density lipoprotein; LV, left ventricular.
Adjusted for age, sex, estimated total intracranial volume, education, hypertension, diabetes mellitus, smoking, alcohol, BMI, LDL, and history of myocardial infarction. Participants with heart failure or atrial fibrillation were excluded.
Cardiac Structure and Function and Cerebral Infarction
Greater LVMi and greater mean LV wall thickness were both associated with higher odds of having any brain infarct (OR 1.08, 95% CI 1.00‐1.17 per 10 g/m2 and OR 1.11, 95% CI 1.01‐1.23] per 1‐mm increase in LV wall thickness, respectively) (Table 2). When indexed to height, the effect estimate for LVMi was similar (OR 1.14, 95% CI 0.98‐1.33) but did not achieve statistical significance (P=0.09). No association was noted with LV chamber size. Average peak longitudinal strain, a marker of LV systolic function, was not associated with brain infarcts; however, a higher EF (per 5% increase) was associated with a decreased odds of infarction (OR 0.85, 95% CI 0.77‐0.95). The association of a higher EF with decreased odds of infarction remained significant when there was adjustment for a history of myocardial infarction (Table 2). The effect estimate minimally changed when excluding participants with myocardial infarction and also remained statistically significant (Table S3; OR 0.87, 95% CI [0.78, 0.96]). Race did not significantly modify these associations (Table S6).
Cardiac Structure and Function and Subtype of Infarct
Markers of LV structure, LV diastolic function, or LV systolic function were not associated with cortical or lacunar infarcts, with the exception of EF (Table 3). Higher EF (per 5%) was associated with decreased rates of lacunar infarcts (RR 0.89, 95% CI [0.80, 1.00]) but no association was found with cortical infarcts. This association was not modified by race (Table S7), and the direction and size of the effect estimate remained similar when excluding those with prior history of myocardial infarction, although decreased power resulted in widening of the CI and loss of statistical significance (Table S4; RR 0.92, 95% CI 0.82‐1.03). Although average peak longitudinal strain was not associated with cortical infarction in the study sample overall, race significantly modified this association such that a higher percentage strain was associated with higher rates of cortical infarction (RR 1.18, 95% CI 1.03‐1.35) in blacks compared with whites (RR 0.93, 95% CI 0.82‐1.05; P interaction=0.01).
Table 3.
Cardiac Predictors of Rates of Cortical and Lacunar Infarcts (N=1829)
| Cortical Infarcts Rate Ratiosa | 95% CI | P Values | |
|---|---|---|---|
| LV structure | |||
| End‐diastolic left ventricular diameter, cm | 1.35 | 0.83‐2.21 | 0.23 |
| Mean LV wall thickness, mm | 1.12 | 0.97‐1.28 | 0.12 |
| LV mass index, 10 g/m2 | 1.08 | 0.99‐1.17 | 0.09 |
| LV systolic function | |||
| Ejection fraction, per 5% | 0.92 | 0.78‐1.08 | 0.29 |
| Average peak longitudinal strain, % | 1.00 | 0.91‐1.10 | 0.97 |
| LV diastolic function | |||
| Lateral early diastolic myocardial velocity, cm/s | 0.99 | 0.89‐1.09 | 0.77 |
| E‐Em lateral ratio, cm/s | 1.00 | 0.94‐1.05 | 0.9 |
| LA volume index (5 mL/m2) | 1.03 | 0.91‐1.17 | 0.66 |
| Lacunar Infarcts Rate Ratiosa | 95% CI | P Values | |
|---|---|---|---|
| LV structure | |||
| End‐diastolic left ventricular diameter, cm | 0.96 | 0.68‐1.36 | 0.83 |
| Mean LV wall thickness, mm | 1.05 | 0.94‐1.17 | 0.4 |
| LV mass index, 10 g/m2 | 1.02 | 0.94‐1.11 | 0.65 |
| LV systolic function | |||
| Ejection fraction, per 5% | 0.89 | 0.80‐1.00 | 0.04 |
| Average peak longitudinal strain, % | 1.03 | 0.97‐1.10 | 0.27 |
| LV diastolic function | |||
| Lateral early diastolic myocardial velocity, cm/s | 0.96 | 0.90‐1.03 | 0.28 |
| E‐Em lateral ratio, cm/s | 0.99 | 0.95‐1.03 | 0.73 |
| LA volume index, 5 mL/m2 | 1.07 | 0.99‐1.16 | 0.09 |
CI indicates confidence interval; E‐Em lateral ratio, early diastolic transmitral flow velocity to early diastolic lateral mitral annular tissue velocity; LA, left atrial; LV, left ventricular.
Adjusted for age, sex, estimated total intracranial volume, education, hypertension, diabetes mellitus, smoking, alcohol, body mass index, low‐density lipoprotein, and history of myocardial infarction. Participants with heart failure or atrial fibrillation were excluded.
Discussion
Our study defined the vascular neurologic correlates, as detected on MRI, of subclinical alterations in cardiac structure and function assessed by comprehensive echocardiography in a cohort of elderly people in the community. Greater LVMi, indicative of chronically increased afterload, was associated with greater WMH and prevalence of infarction. Importantly, this association was driven by the association of greater LV wall thickness with these brain phenotypes and not by greater LV chamber size. Additionally, higher participant EF was associated with a decreased odds of infarction. These associations were, importantly, found in a cohort excluding participants with prevalent HF or atrial fibrillation and accounting for sampling strategy so that they might be reflective of the population.
White matter changes in the brain are associated with long‐standing hypertension,15 which itself is one of the most powerful risk factors for HF. Similarly, cardiac changes that are common in long‐standing hypertension, including LV hypertrophy, are known to be independently associated with new atherothrombotic brain infarction16 as well as silent cerebrovascular disease.17 Addition of LVMi has been shown to improve traditional cardiovascular disease risk prediction models.18 When cardiac MRI has been used, LV mass was found to be associated with incident coronary heart disease but was no longer significant when adjustment was made for blood pressure.19 The association between greater LVMi and greater WMH and higher odds of infarct has been previously reported, with this study confirming these prior results while differing in the magnitude of the association, potentially suggesting a higher risk than previously cited. Our work also supports prior work in demonstrating that the odds of infarction are increased among those with changes in LV structure while data are controlled for hypertension. It is still likely that, despite the persistence of results after adjustment for hypertension and other risk factors, the observed associations are due to shared risk factors. However, it is possible that there are other subtle forms of injury to the brain that result more directly from these cardiac abnormalities, due to either hypoperfusion or microemboli; our data do not allow us to speculate further about the likelihood of these other mechanisms. We acknowledge that our findings may not directly translate to similar relationships in individuals with clinical HF, but these findings suggest that such associations may exist among people with even subtle cardiac structural changes.
EF plays a central role in the stratification and management of patients with HF, and increased rates of stroke and cardiovascular death are well documented in patients with decreased EF.20 We found an association between higher EF and decreased odds of overall infarction but did not find an association when we looked specifically at the rates of cortical infarcts. It is worth noting that the strongest relationship with stroke is with EF ≤15%,20 and an LVEF <50% is rare in our cohort, which might limit our ability to detect any possible associations of EF with stroke subtype. We acknowledge that our findings are not reflective of the lower range of EF but rather EF across a relatively narrow and healthy range. Lacunar infarcts, in contrast, have traditionally been considered a disease of a single penetrating artery and are more susceptible to the effects of chronic hypertension. As a result, markers of increased cardiac load (LV mass) would be hypothesized to be more closely associated with increased rates of lacunar disease. These findings may be reflective of decreased power, or it may be that several other disease processes may also affect the cerebral small vessels and lead to lacunae, perhaps explaining our lack of association with these measures. There has been prior work demonstrating a high prevalence of subclinical cerebral infarction in patients with HF with a preserved EF,21 but this question is distinct from our work, as our population was mostly healthy, with mild degrees of cardiac impairment.
We did not find an association between odds of infarction and longitudinal strain, which some might consider a more sensitive marker for systolic dysfunction. LVEF has formed the foundation for establishing and diagnosing heart failure and remains an important clinical measure. There is growing recognition of the importance of echocardiography with strain, in addition to LVEF, in particular in understanding beat‐to‐beat perturbation, early valve disease, and subclinical LV systolic function.22 LVEF and longitudinal strain are importantly reflective of different entities and should be used together in assessing LV systolic function. Although LVEF is an integral part of the assessment of LV function, this measure can be influenced by variability secondary to image quality, the axis of the image, and measurement error.23 Echocardiography with strain uses speckle‐tracking technology to examine myocardial fiber deformation, and longitudinal strain in particular represents shortening and lengthening of the subendocardial and subepicardial myocardium, which are thought to be more sensitive to subendocardial ischemia.24 A particular advantage to global longitudinal strain, therefore, is using it for prognosis when LVEF is normal or nearly normal.23 The ARIC Study, a cohort of mostly healthy older adults, is ongoing and provides an opportunity to reexamine these relationships in the future.
The biracial structure of our cohort allows for examination of relationships stratified by race. We did find differences in some cardiac parameters by race, although generally these did not have differential effects on WMH or infarcts. The variables suggestive of significance for effect modification by race, at a P<0.1, were 2 measures of LV diastolic dysfunction and 1 measure of LV systolic function. Specifically, increasing LA volume index, which is a marker of chronic LV diastolic pressure, appeared to be associated with a greater increase in WMH in blacks than in whites. There also appeared to be differences in the rates of cortical infarction between whites and blacks per unit increase in lateral early diastolic myocardial velocity. It is notable that longitudinal strain, a marker of LV systolic function, was not associated with a greater odds of infarction and did not impact the prevalence of cortical infarction among our participants until we accounted for potential differences in race with suggestion of increased rates of cortical infarcts in blacks compared with whites. Racial differences have been previously explored with prior work also suggesting a modification of the association between cardiac and cerebrovascular subclinical disease,17, 25 and we suggest that such differences may also have an impact on infarction outside of traditional cardiovascular risk factors. Our large sample size uniquely allows for separate analyses in whites and blacks, although the effect sizes between whites and blacks were similar, without evidence of effect modification by race, so we interpret any slight differences cautiously.
We have shown that mild decreases in cardiac function are associated with neuroimaging changes, even when data are controlled for independently associated factors such as hypertension. Our findings demonstrate that participants with mild cardiac structure/function changes, considered “normal” by standard clinical definitions, have brain parenchyma damage evidenced by WMH and infarcts. It is likely that the association between brain and subclinical cardiac dysfunction is at least partly due to shared risk for end‐organ damage from hypertension and other underlying risk factors, although we attempted to reduce some of this potential confounding in our adjustment for these risk factors. Given the association between these imaging findings and cognitive impairment, and an increase in mortality in HF accompanied by cognitive impairment,26 there is a need for further research to continue to elucidate the clinical meaning behind these findings in a cohort without clinical HF and to determine if there is a more direct causal relationship between cardiac dysfunction and these cerebrovascular lesions.
There are several limitations to our study. Our assessments of cardiac function were made using echocardiography. It is known that echocardiography has a limited accuracy, particularly for LV mass, but our large sample size helps mitigate the risk of a small systematic error and variability.27, 28 Parameters that were felt to be the best indicators of LV structure, LV systolic function, and LV diastolic function, were included in the model, but the variables we considered were not comprehensive of all possible cardiac markers. Additionally, our models considered 1 cardiac predictor with the adjustment covariates of interest rather than all cardiac predictors together or with predictors from the other groups. This was a decision made based on concerns for collinearity among cardiac predictors, but such statistical considerations may influence results (Table S5). Our covariate model adjusted for a number of potential confounders, but residual confounding remains a possibility, particularly given the cross‐sectional design of the study. Strengths of the study include the cohort (ARIC), a biracial prospective study in which all echocardiograms and MRI analyses were rigorously standardized and ascertained by an independent adjudication committee, with the analysis accounting for cohort sampling. The reasonably large sample size allows us to estimate interactions by race. In addition, we were able to look at a range of echocardiographic markers and tested hypotheses specific to likely mechanisms by which those cardiac changes might lead to brain vascular changes. Our data support other work evaluating cardiac markers in association with brain MRI findings,21, 29, 30 further emphasizing the importance of future studies of subtle cardiac dysfunction or structural abnormality in evaluating mechanisms not only of vascular disease in the brain but possibly also those of vascular cognitive impairment.
In conclusion, we have demonstrated that among participants in a large community‐based cohort study, subclinical changes in LV mass were associated with greater odds of infarction and size of WMH even when controlling for hypertension. Such associations persisted in a cohort without prevalent heart failure or AF. These findings are likely at least partly due to end‐organ sequelae from shared risk factors but may also represent a direct impact of cardiac disease on the brain. Further understanding of these changes may help to better delineate the link between other possible neurologic sequelae from subclinical cardiac changes, such as the potential for cognitive ramifications, and how this might relate to those individuals with clinical HF.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive and MRI data are collected by the National Institute of Health (U01 HL096812, HL096814, HL096899, HL096902, HL096917) with previous brain MRI examinations funded by the National Institute of Health (R01‐HL70825). Dr Gottesman also receives funding from the National Institute on Aging (K24 AG052573).
Disclosures
R.F. Gottesman, MD, PhD, is an associate editor for Neurology (modest). The remaining authors have no disclosures to report.
Supporting information
Table S1. Cardiac Predictors of Brain Structure With LV Mass Indexed to Height (N=1829)
Table S2. Cardiac Predictors of Rates of Cortical and Lacunar Infarcts With LV Mass Indexed to Height (N=1829)
Table S3. Sensitivity Analysis Considering the Association of Cardiac Predictors With Brain Structure When Excluding Participants With History of Myocardial Infarction (N=1673)
Table S4. Sensitivity Analysis Considering Cardiac Predictors of Rates of Cortical and Lacunar Infarcts When Excluding Participants With History of Myocardial Infarction (N=1673)
Table S5. Sensitivity Analysis Considering the Impact of Modeling Approaches on the Association of Cardiac Predictors With Brain Structure
Table S6. Cardiac Predictors of Brain Structures by Race
Table S7. Cardiac Predictors of Rates of Cortical and Lacunar Infarcts by Race
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions.
(J Am Heart Assoc. 2018;7:e008992 DOI: 10.1161/JAHA.118.008992)
References
- 1. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd‐Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. [DOI] [PubMed] [Google Scholar]
- 2. Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska‐Newton A, Sueta CA, Mosley TH, Wright JD, Coresh J, Heiss G, Folsom AR, Solomon SD. Heart failure stages among older adults in the community: the Atherosclerosis Risk in Communities Study. Circulation. 2017;135:224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu TY, Sun JP, Lee AP, Yang XS, Ji L, Zhang Z, Li Y, Yu CM, Wang JG. Left atrial function as assessed by speckle‐tracking echocardiography in hypertension. Medicine (Baltimore). 2015;94:e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wachtell K, Palmieri V, Gerdts E, Bella JN, Aurigemma GP, Papademetriou V, Dahlof B, Aalto T, Ibsen H, Rokkedal JE, Devereux RB. Prognostic significance of left ventricular diastolic dysfunction in patients with left ventricular hypertrophy and systemic hypertension (the LIFE Study). Am J Cardiol. 2010;106:999–1005. [DOI] [PubMed] [Google Scholar]
- 5. Cao G, Chen C, Lin Q, Chen Y, Zhen Z, Zou Y, Liu J, Wu M, Wang R, Liu M, Zhao C, Lu S, Ng MY, Tse HF, Yiu KH. Prevalence, clinical characteristics and echocardiography parameters of non‐resistant, resistant and refractory hypertension in Chinese. Postgrad Med. 2017;129:187–192. [DOI] [PubMed] [Google Scholar]
- 6. Chen X, Wen W, Anstey KJ, Sachdev PS. Prevalence, incidence, and risk factors of lacunar infarcts in a community sample. Neurology. 2009;73:266–272. [DOI] [PubMed] [Google Scholar]
- 7. Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small‐vessel disease burden. Neurology. 2014;83:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider AL, Hengrui S, Alonso A, Coresh J, Albert MS, Mosley TH Jr. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC‐NCS). Alzheimers Dement (Amst). 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, Jack CR Jr, Graff‐Radford J, Schneider AL, Windham BG, Coker LH, Albert MS, Mosley TH Jr; ARIC Neurocognitive Investigators . Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: Atherosclerosis Risk in Communities‐Neurocognitive Study. Stroke. 2015;46:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators . Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11. Jack CR, Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM, Schuff N, Krueger G, Killiany RJ, DeCarli CS. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement. 2010;6:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raz L, Jayachandran M, Tosakulwong N, Lesnick TG, Wille SM, Murphy MC, Senjem ML, Gunter JL, Vemuri P, Jack CR. Thrombogenic microvesicles and white matter hyperintensities in postmenopausal women. Neurology. 2013;80:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community‐dwelling elderly persons: the Atherosclerosis Risk in Communities Study. Circ Cardiovasc Imaging. 2014;7:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 15. Longstreth WT Jr, Arnold AM, Beauchamp NJ Jr, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O'Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. [DOI] [PubMed] [Google Scholar]
- 16. Aronow WS, Ahn C, Kronzon I, Gutstein H, Schoenfeld MR. Association of extracranial carotid arterial disease, prior atherothrombotic brain infarction, systemic hypertension, and left ventricular hypertrophy with the incidence of new atherothrombotic brain infarction at 45‐month follow‐up in 1,482 older patients. Am J Cardiol. 1997;79:991–993. [DOI] [PubMed] [Google Scholar]
- 17. Nakanishi K, Jin Z, Homma S, Elkind MS, Rundek T, Tugcu A, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR. Left ventricular mass‐geometry and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) Study. Am Heart J. 2017;185:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Desai CS, Bartz TM, Gottdiener JS, Lloyd‐Jones DM, Gardin JM. Usefulness of left ventricular mass and geometry for determining 10‐year prediction of cardiovascular disease in adults aged >65 years (from the Cardiovascular Health Study). Am J Cardiol. 2016;118:684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) Study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Tullio MR, Qian M, Thompson JL, Labovitz AJ, Mann DL, Sacco RL, Pullicino PM, Freudenberger RS, Teerlink JR, Graham S, Lip GY, Levin B, Mohr JP, Buchsbaum R, Estol CJ, Lok DJ, Ponikowski P, Anker SD, Homma S; WARCEF Investigators . Left ventricular ejection fraction and risk of stroke and cardiac events in heart failure: data from the warfarin versus aspirin in reduced ejection fraction trial. Stroke. 2016;47:2031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cogswell RJ, Norby FL, Gottesman RF, Chen LY, Solomon S, Shah A, Alonso A. High prevalence of subclinical cerebral infarction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2017;10:1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Januzzi JL Jr, Chandrashekhar Y. Strain echocardiography: the new gold standard for imaging ventricular function? J Am Coll Cardiol. 2017;70:955–957. [DOI] [PubMed] [Google Scholar]
- 23. Szymanski C, Levy F, Tribouilloy C. Should LVEF be replaced by global longitudinal strain? Heart. 2014;100:1655–1656. [DOI] [PubMed] [Google Scholar]
- 24. Zhang KW, French B, May Khan A, Plappert T, Fang JC, Sweitzer NK, Borlaug BA, Chirinos JA, St John Sutton M, Cappola TP, Ky B. Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. J Am Heart Assoc. 2014;3:e000550 DOI: 10.1161/JAHA.113.000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kishi S, Reis JP, Venkatesh BA, Gidding SS, Armstrong AC, Jacobs DR Jr, Sidney S, Wu CO, Cook NL, Lewis CE, Schreiner PJ, Isogawa A, Liu K, Lima JA. Race‐ethnic and sex differences in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc. 2015;4:e001264 DOI: 10.1161/JAHA.114.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zuccala G, Pedone C, Cesari M, Onder G, Pahor M, Marzetti E, Lo Monaco MR, Cocchi A, Carbonin P, Bernabei R. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am J Med. 2003;115:97–103. [DOI] [PubMed] [Google Scholar]
- 27. Bois JP, Geske JB, Foley TA, Ommen SR, Pellikka PA. Comparison of maximal wall thickness in hypertrophic cardiomyopathy differs between magnetic resonance imaging and transthoracic echocardiography. Am J Cardiol. 2017;119:643–650. [DOI] [PubMed] [Google Scholar]
- 28. Crean AM, Maredia N, Ballard G, Menezes R, Wharton G, Forster J, Greenwood JP, Thomson JD. 3D echo systematically underestimates right ventricular volumes compared to cardiovascular magnetic resonance in adult congenital heart disease patients with moderate or severe RV dilatation. J Cardiovasc Magn Reson. 2011;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Russo C, Jin Z, Liu R, Iwata S, Tugcu A, Yoshita M, Homma S, Elkind MS, Rundek T, Decarli C, Wright CB, Sacco RL, Di Tullio MR. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) Study. JACC Cardiovasc Imaging. 2013;6:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russo C, Jin Z, Homma S, Elkind MS, Rundek T, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR. Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) Study. Circulation. 2013;128:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cardiac Predictors of Brain Structure With LV Mass Indexed to Height (N=1829)
Table S2. Cardiac Predictors of Rates of Cortical and Lacunar Infarcts With LV Mass Indexed to Height (N=1829)
Table S3. Sensitivity Analysis Considering the Association of Cardiac Predictors With Brain Structure When Excluding Participants With History of Myocardial Infarction (N=1673)
Table S4. Sensitivity Analysis Considering Cardiac Predictors of Rates of Cortical and Lacunar Infarcts When Excluding Participants With History of Myocardial Infarction (N=1673)
Table S5. Sensitivity Analysis Considering the Impact of Modeling Approaches on the Association of Cardiac Predictors With Brain Structure
Table S6. Cardiac Predictors of Brain Structures by Race
Table S7. Cardiac Predictors of Rates of Cortical and Lacunar Infarcts by Race


