Abstract
Background
Patients with tetralogy of Fallot (TOF) remain at risk for cardiovascular events despite successful repair. Some of the current risk stratification tools require advanced imaging and invasive studies, and hence are difficult to apply to routine patient care. A recent study showed that QRS fragmentation (QRS‐f) is predictive of mortality in patients with TOF. The current study aims to validate this result by assessing whether severity of QRS‐f could predict all‐cause mortality in a different TOF population.
Methods and Results
The authors reviewed the Mayo Adult Congenital Heart Disease database for patients with TOF who had ECG from 1990–2017. QRS‐f was defined as notches in QRS complex in ≥2 contiguous leads on ECG, not related to bundle branch block, and classified as none, mild (≤3 leads), moderate (4 leads), or severe (≥5 leads). Of 465 patients (age 37±14 years) in the study, QRS‐f was present in 161 (35%): mild (n=43, 9%), moderate (n=77, 17%), and severe (n=41, 9%). There were 55 deaths (12%) during 13.6±8.2 years of follow‐up. Severity of QRS‐f remained an independent predictor of all‐cause mortality after adjustment for other ECG parameters, patient demographics, and atrial and ventricular arrhythmia (hazard ratio, 1.74 per class; 95% confidence interval, 1.08–2.93 [P=0.041]).
Conclusions
The presence of severe QRS‐f may be used as complementary data to the usual clinical indices to determine whether interventions such as invasive electrophysiology study should be performed in patients with nonsustained ventricular tachycardia or to proceed with pulmonary valve replacement in patients with severe pulmonary regurgitation with ventricular volumes below the guideline‐directed threshold for intervention.
Keywords: electrocardiography, mortality, QRS fragmentation, risk stratification, tetralogy of Fallot
Subject Categories: Congenital Heart Disease
Clinical Perspective
What Is New?
QRS fragmentation is an independent risk factor for mortality in adults with tetralogy of Fallot.
The severity of QRS fragmentation provides a good assessment of incremental risk for mortality in this population.
What Are the Clinical Implications?
The presence of severe QRS fragmentation may be used as complementary data to the usual clinical indices to determine whether interventions such as invasive electrophysiology study should be performed in patients with nonsustained ventricular tachycardia or to proceed with pulmonary valve replacement in patients with severe pulmonary regurgitation with ventricular volumes below the guideline‐directed threshold for intervention.
Introduction
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease,1 and despite improvement in medical and surgical management of this disease, adults with repaired TOF remain at risk for cardiovascular morbidity and mortality.2, 3, 4 Patients with repaired TOF have reduced survival compared with the general population, with a 30‐year survival of ≈85% to 90%.2, 4, 5 As a result, risk stratification is important in this population to identify and treat patients at high risk for cardiovascular death.
Several studies have described risk factors for mortality in adults with repaired TOF.3, 5, 6, 7, 8, 9, 10 The proposed risk factors include patient demographics such as age at time of TOF repair, left and right ventricular (RV) dysfunction, RV fibrosis, RV hypertrophy, ventricular arrhythmia, and increased left ventricular end‐diastolic pressure.3, 5, 6, 7, 8, 9, 10 Unfortunately, some of the available risk scores include variables derived from magnetic resonance imaging (MRI) and cardiac catheterization, and such tests are not routinely performed during annual evaluation. ECG, on the other hand, is inexpensive, noninvasive, readily available, and routinely performed during annual evaluation of patients with TOF.11 In a recent study, Bokma et al12 reported that QRS fragmentation (QRS‐f), assessed using a standard 12‐lead ECG, could reliably predict mortality in this population. The purpose of our study was to determine whether QRS‐f derived from a standard 12‐lead ECG could independently predict mortality in a different population of adult patients with TOF.
Methods
Patient Selection
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. The data will be made available for requests made to corresponding author via e‐mail. We reviewed the Mayo Adult Congenital Heart Disease database and identified patients (age ≥18 years) with repaired TOF that had at least 1 ECG in the electronic health records between January 1, 1990, and December 31, 2017. The patients with pulmonary atresia were excluded. The Mayo Clinic's institutional review board approved this study and waived informed consent for patients who provided research authorization.
Data Collection
The following electronic health records were reviewed in detail: clinical notes, ECG, Holter monitor, transthoracic echocardiogram, cardiopulmonary exercise test, surgical records, and cardiac MRI. The first standard 12‐lead ECG (25 mm/s, 10 mm/mV) obtained during the study period was considered the baseline ECG and was used for the assessment of QRS‐f. The clinical data obtained within 12 months of the baseline ECG were analyzed as the baseline characteristics of the cohort. All‐cause mortality was ascertained using the Mayo Clinic registration database and Accurint, an institutionally approved location service.
All ECGs were analyzed by 2 observers (N.M., M.F.) blinded to patients’ characteristics and clinical data. Standard ECG voltages and intervals were collected and analyzed. In cases of discordant assessments, a third observer (A.D.) provided adjudication. The severity of QRS‐f was assessed using similar methods as described by Bokma et al.12 QRS‐f was defined as an additional R wave (R′) or notch in the nadir of the S wave in ≥2 contiguous leads (right‐sided/septal: aVR, V1, V2; anterior: V2–V5; lateral: I, aVL, V5, V6; or inferior: II, aVF, III) in patients with QRS duration <120 ms. In patients with right bundle branch block, QRS‐f was defined as ≥3 R waves/notches in the R/S complex (more than typical 2 in right bundle branch block) in ≥2 contiguous leads. In patients with paced QRS and premature ventricular complexes, QRS‐f was defined as ≥3 notches in the R/S complex (Figure 1). The severity of QRS‐f was classified based on the number of leads with QRS‐f: none, mild (≤3 leads), moderate (4 leads), and severe (≥5 leads) (Figure S1). The severity of QRS‐f (number of leads with QRS‐f) was verified in a randomly selected sample (50% of the cohort) by one of the investigators (A.C.E.). To assess for the progression of QRS‐f, we reviewed ECGs performed in patients who had at least 5 years of follow‐up (ie, ECGs performed 5 years from the baseline ECG).
Figure 1.
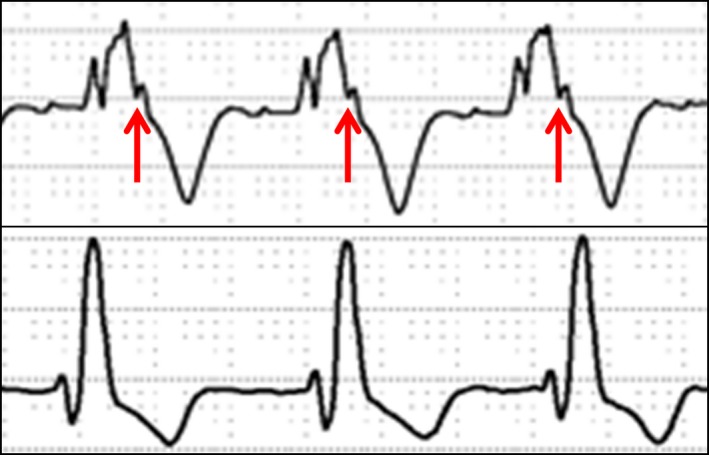
Top, Right bundle branch block with QRS fragmentation. The arrows are pointing at the third R‐wave/notch. Bottom, Right bundle branch block without QRS fragmentation.
Statistical Analysis
Categorical variables were reported as counts (percentages), and continuous variables were reported as means±SDs or medians (interquartile ranges) for skewed data. Intraclass correlation coefficient was used to determine interobserver variability in the observed number of leads with QRS‐f. Survival curves were constructed by the Kaplan–Meier method and compared by log‐rank test using the time of baseline ECG as “time zero.” Incremental Cox proportional hazards models were used to assess the relationship between severity of QRS‐f and all‐cause mortality, and the strength of association is expressed as hazard ratio (HR) and 95% CI. The Schoenfeld residual method was used for testing proportional hazard assumption. Incremental models were constructed as follows: model 1 was a univariable analysis showing the association between QRS‐f and all‐cause mortality (unadjusted risk). Model 2 included all variables in model 1 plus adjustment for the following ECG parameters: QRS duration, JT interval, and rhythm (sinus, paced rhythm, atrial fibrillation, and atrial flutter/tachycardia). Model 3 included all variables in model 2 plus adjustment for patient demographic characteristics (current age, sex, age at the time of TOF repair, and type of TOF repair [transannular patch versus nontransannular patch]). Model 4 included all variables in model 3 plus adjustment for history of sustained ventricular tachycardia and/or defibrillator implantation, moderate RV dysfunction by qualitative assessment, and left ventricular ejection fraction <40%.
We also performed separate analyses using the severity of QRS‐f as a categorical variable. Univariable analyses were performed using the patients without QRS‐f as the reference group to compare the risk of all‐cause mortality for the mild, moderate, and severe QRS‐f groups. Multivariable analyses were then performed for each QRS‐f group by adjusting for all of the potential confounders described above. All statistical analyses were performed with JMP software (version 13.0; SAS Institute Inc). P<0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 465 patients were selected for the study (Figure S2). The age at the time of baseline ECG was 37±14 years, age at the time of TOF repair was 5 (3–10) years, and 223 (48%) were men. The baseline characteristics and hemodynamic data of the cohort are shown in Tables 1 and 2, respectively. There were 41 (9%) patients with pacemakers, 58 (13%) patients with defibrillators, and 100 (22%) patients with history of ventricular tachycardia.
Table 1.
Baseline Characteristics
| All (N=465) | Alive (n=410) | Dead (n=55) | P Value | |
|---|---|---|---|---|
| Age at the beginning of study, y | 37±14 | 35±13 | 47±15 | <0.001 |
| Men, No. (%) | 223 (48) | 186 (45) | 37 (67) | 0.004 |
| Body mass index, kg/m2 | 27±6 | 27±6 | 28±6 | 0.168 |
| Body surface area, m2 | 1.9±0.3 | 1.8±0.2 | 1.9±0.2 | 0.475 |
| Age at TOF repair, y | 5 (3–10) | 5 (2–8) | 14 (5–34) | <0.001 |
| Prior palliative shunt, No. (%) | 181 (39) | 148 (37) | 33 (60) | 0.004 |
| Comorbidities, No. (%) | ||||
| Atrial fibrillation | 118 (25) | 82 (20) | 36 (55) | <0.001 |
| Atrial flutter/tachycardia | 100 (22) | 79 (19) | 21 (38) | 0.002 |
| Hypertension | 125 (27) | 102 (25) | 23 (42) | 0.004 |
| Hyperlipidemia | 193 (42) | 162 (40) | 31 (56) | 0.060 |
| Coronary artery disease | 57 (12) | 38 (9) | 19 (35) | 0.011 |
| Current or prior smoker | 93 (20) | 77 (18) | 18 (32) | 0.064 |
| Diabetes mellitus | 72 (16) | 59 (15) | 13 (23) | 0.117 |
| Sleep apnea | 130 (28) | 109 (27) | 21 (38) | 0.155 |
| Prior stroke | 41 (9) | 33 (8) | 8 (15) | 0.943 |
| NYHA class III/IV | 73 (16) | 59 (15) | 14 (25) | 0.002 |
| Down syndrome | 9 (2) | 8 (2.0) | 1 (1.8) | 0.914 |
| 22q11 deletion | 7 (1.5) | 6 (1.5) | 1 (1.8) | 0.943 |
| Laboratory tests | ||||
| Hemoglobin, g/dL | 14.1±2.5 | 14.0±2.6 | 13.4±2.1 | 0.018 |
| Creatinine, mg/dL | 1.0±0.3 | 0.9±0.2 | 1.3±0.5 | <0.001 |
| NT‐proBNP, pg/mL | 255 (123–720) | 223 (112–813) | 313 (146–481) | 0.348 |
| Medications, No. (%) | ||||
| Diuretics | 84 (18) | 63 (15) | 21 (38) | <0.001 |
| β‐Blockers | 106 (23) | 76 (19) | 30 (54) | <0.001 |
| Calcium channel blockers | 59 (13) | 39 (10) | 20 (36) | <0.001 |
| RAAS antagonist | 87 (19) | 68 (17) | 19 (34) | 0.002 |
| Warfarin | 41 (9) | 29 (7) | 12 (21) | 0.001 |
| Direct oral anticoagulants | 3 (0.7) | 2 (0.5) | 1 (0.7) | 0.330 |
| Aspirin | 116 (25) | 97 (24) | 19 (33) | 0.118 |
Values are expressed as mean±SD or median (interquartile range) unless otherwise indicated. NT‐proBNP indicates N‐terminal pro b‐type natriuretic peptide; NYHA, New York Heart Association; RAAS, renin‐angiotensin‐aldosterone system; TOF, tetralogy of Fallot.
Table 2.
Hemodynamic Data
| Echocardiography | All (N=465) | Alive (n=410) | Dead (n=55) | P Value |
|---|---|---|---|---|
| ≥Moderate RV enlargement, No. (%)* | 318 (68) | 278 (68) | 40 (72) | 0.887 |
| ≥Moderate RV systolic dysfunction, No. (%)* | 137 (30) | 113 (28) | 24 (43) | 0.025 |
| ≥Moderate tricuspid regurgitation, No. (%)* | 92 (20) | 75 (18) | 17 (31) | 0.042 |
| Tricuspid regurgitation velocity, m/s | 3.1±0.7 | 3.1±0.7 | 3.2±0.8 | 0.786 |
| Pulmonary valve peak velocity, m/s | 2.5±0.9 | 2.5±0.9 | 2.5±1.0 | 0.218 |
| TAPSE, cm | 18±4 | 18±4 | 18±3 | 0.793 |
| FAC, % | 40±10 | 39±6 | 33±11 | 0.048 |
| RV S′, cm/s | 10±2 | 11±8 | 9±3 | 0.210 |
| Medial E/e′ | 10±4 | 10±3 | 10±4 | 0.788 |
| Lateral E/e′ | 7±3 | 10±3 | 10±4 | 0.788 |
| LV ejection fraction, % | 58±8 | 58±8 | 54±10 | 0.020 |
| Moderate LV dysfunction (<40%), No. (%) | 62 (13) | 50 (12) | 12 (22) | 0.031 |
| Magnetic resonance imaging (n=157) | ||||
| RVEDV index, mL/m2 | 140±48 | 142±47 | 150±78 | 0.808 |
| RVESV index, mL/m2 | 80±37 | 81±37 | 89±49 | 0.693 |
| RV ejection fraction, % | 44±10 | 44±10 | 36±10 | 0.101 |
| Catheterization (n=148) | ||||
| Right atrial pressure, mm Hg | 11±6 | 10±5 | 16±7 | <0.001 |
| RVEDP, mm Hg | 14±5 | 13±5 | 19±7 | 0.004 |
| Mean PA pressure, mm Hg | 24±9 | 23±8 | 30±11 | 0.005 |
| LVEDP, mm Hg | 16±5 | 15±5 | 21±7 | 0.003 |
| Mean arterial pressure, mm Hg | 88±13 | 89±13 | 83±14 | 0.103 |
| Cardiac index, L/min×m2 | 2.3±0.6 | 2.3±0.7 | 2.1±0.4 | 0.072 |
| PVR index (WU×m2) | 4.5±3.0 | 4.3±3.1 | 5.3±2.4 | 0.129 |
| Cardiopulmonary exercise test (n=177) | ||||
| Peak VO2, mL/kg per min | 22±7 | 22±7 | 22±8 | 0.995 |
| Peak VO2, % predicted | 63±17 | 63±16 | 64±23 | 0.956 |
| VE/VCO2 nadir | 28±4 | 28±4 | 30±4 | 0.377 |
Values are expressed as mean±SD unless otherwise indicated.
FAC indicates fractional area change; LV, left ventricular; LVEDP, left ventricular end‐diastolic pressure; PA, pulmonary artery; PVR, pulmonary vascular resistance; RV, right ventricular; RVEDP, right ventricular end‐diastolic pressure; RVEDV, right ventricular end‐diastolic volume; RVESV, right ventricular end‐systolic volume; TAPSE, tricuspid annular plane systolic excursion; VE/VCO2, ventilatory equivalent for carbon dioxide; VO2, oxygen consumption; WU×m2, Wood units times meters squared.
*Quantitative assessment.
Electrocardiography
The baseline ECG showed the following rhythm: sinus, 407 (88%); atrial fibrillation, 17 (4%); atrial flutter/tachycardia, 20 (4%); and atrial and/or ventricular paced rhythm, 21 (5%). Heart rate was 74±9 beats per minute, QRS duration was 148±33 ms, and QRS morphology were as follows: normal, 9 (2%); incomplete right bundle branch block, 102 (22%) and right bundle branch block, 354 (76%). QRS‐f was present in 161 (35%) patients, and severity of QRS‐f was as follows: mild (QRS‐f in ≤3 leads) in 43 (9%), moderate (QRS‐f in 4 leads) in 77 (17%), and severe (QRS‐f in ≥5 leads) in 41 (9%). The interobserver intraclass correlation coefficient for QRS‐f was 0.81 (95% CI, 0.68–0.90) (Table S1). The patients with QRS‐f had longer QRS durations (167±31 versus 126±24 ms, P=0.001) and were older at the time of TOF repair (7±6 versus 4±3 years, P=0.023) and at the beginning of the study period (40±14 versus 32±11 years, P<0.001). Of the 465 patients, 371 (80%) had ECG at 5 years of follow‐up. Of these 371 patients, 39 (11%) showed progression of QRS‐f, and these progressions were from no QRS‐f to mild QRS‐f in 34 patients and mild QRS‐f to moderate QRS‐f in 5 patients.
Outcomes
There were 55 deaths (12%) during a follow‐up period of 13.6±8.2 years. Among the 55 patients who died, the cause of death was congestive heart failure, 13 (24%); arrhythmic/sudden death, 11 (20%); postoperative death after cardiac surgery, 3 (6%); multisystem organ failure caused by sepsis, 4 (7%); malignancy, 5 (9%); gastrointestinal bleeding, 1 (2%); stroke, 2 (4%); and unknown/mixed, 16 (29%). The 20‐year survival differed based on the severity of QRS‐f (Figure 2). The 20‐year survival in the patients without QRS‐f was 97%, and using the group without QRS‐f as the reference, the 20‐year survival was similar in patients with mild QRS‐f (97% versus 93%, P=0.643) but significantly lower in the groups with moderate QRS‐f (97% versus 82%, P=0.031) and severe QRS‐f (97% versus 33%, P<0.001), respectively. The severity of QRS‐f was a risk factor (unadjusted risk) for all‐cause mortality (HR, 2.72 per class [ie, degree of severity]; 95% CI, 2.11–3.56 [P<0.001]). The severity of QRS‐f remained an independent predictor of all‐cause mortality after adjustment for other ECG parameters, patient demographics, ventricular function, and history of atrial or ventricular arrhythmia (HR, 1.74 per class; 95% CI, 1.08–2.93 [P=0.041]) (Table 3). Separate models were constructed for subgroup analyses for patients with cardiac MRI data (Table S2) and patients with cardiac catheterization data (Table S3).
Figure 2.
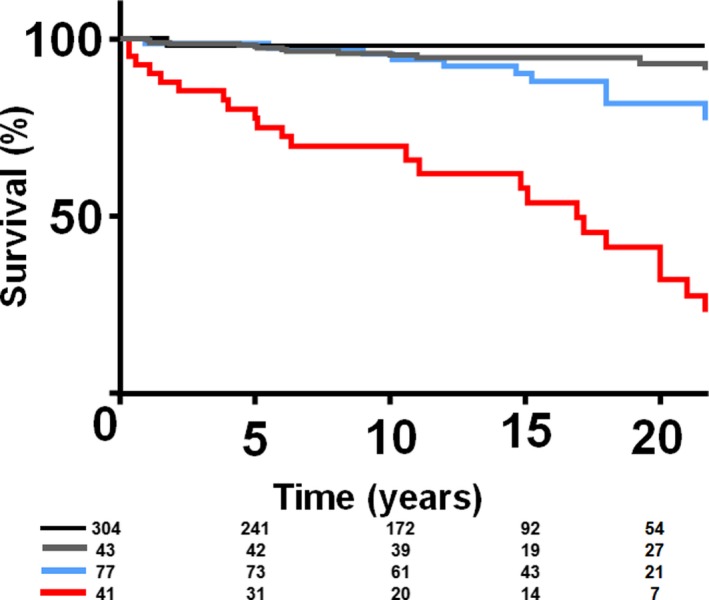
Kaplan–Meier curves comparing survival between patients without QRS fragmentation (QRS‐f) (dark blue), mild QRS‐f (red), moderate QRS‐f (light blue), and severe QRS‐f (green).
Table 3.
Incrementally Adjusted Risk for All‐Cause Mortality
| HR (95% CI) | P Value | |
|---|---|---|
| Model 1 | 2.72 (2.11–3.56) | <0.001 |
| Model 2 | 2.67 (2.06–3.64) | <0.001 |
| Model 3 | 2.22 (1.68–2.97) | 0.014 |
| Model 4 | 1.74 (1.08–2.93) | 0.041 |
Model 1: unadjusted all‐cause mortality risk based on the extent of QRS fragmentation (QRS‐f). Model 2: includes all variables in model 1 plus adjustment for the following ECG parameters: QRS duration, JT interval, and rhythm (sinus, paced rhythm, atrial fibrillation, and atrial flutter/tachycardia). Model 3: includes all variables in model 2 plus adjustment for patient demographic characteristics (current age, sex, age at the time of tetralogy of Fallot [TOF] repair and type of TOF repair [transannular patch vs nontransannular patch]). Model 4: includes all variables in model 3 plus adjustment for history of sustained ventricular tachycardia and/or defibrillator implantation, moderate right ventricular dysfunction by qualitative assessment, and left ventricular ejection fraction <40%. Hazard ratio (HR) is expressed per incremental risk per class/extent of QRS‐f (none, mild, moderate, severe).
Using the group without QRS‐f as the reference group, there was a significant difference in the unadjusted risk for all‐cause mortality for the patients in the moderate QRS‐f group (HR, 2.63; 95% CI, 1.73–3.64) and for the patients in the severe QRS‐f group (HR, 3.29; 95% CI, 2.55–3.94) but not in the mild QRS‐f group (HR, 1.65; 95% CI, 0.89–3.16). After multivariable adjustments, having moderate QRS‐f (HR, 1.62; 95% CI, 1.13–2.98) and severe QRS‐f (HR, 3.03; 95% CI, 1.96–5.11) remained significant risk factors for all‐cause mortality.
Discussion
In this study we reviewed a cohort of 465 adult patients with TOF and showed that the severity of QRS‐f, assessed using a standard 12‐lead ECG, was an independent predictor of mortality. The importance of QRS duration in the risk stratification of patients with repaired TOF was described more than 2 decades ago, and QRS duration >180 ms is, almost universally, adopted as a risk factor for sudden death in the TOF population.7, 8 In the recent study, Bokma et al12 reported that QRS‐f was superior to QRS duration in the prediction of all‐cause mortality in patients with TOF. We sought to validate this result by testing the ability of QRS‐f to predict all‐cause mortality in a different population of adult patients with TOF. The current study shows that QRS‐f remained an independent risk factor for all‐cause mortality, even after adjusting for QRS duration, patient demographics, and arrhythmia. The patients with moderate (or greater) QRS‐f had reduced 20‐year survival, and there was a 2‐fold increase in the risk of all‐cause mortality per unit increase in the severity of QRS‐f (mild versus moderate versus severe fragmentation).
QRS Fragmentation as a Marker of Ventricular Dysfunction
Previous studies have shown that QRS‐f involving the left precordial leads was associated with myocardial scar and left ventricular aneurysm in patients with coronary artery disease.13, 14 One of the studies evaluated 479 patients with coronary artery disease who underwent nuclear stress test and showed that the presence and severity of QRS‐f correlated with the presence and extent of myocardial scar.13 Similarly, QRS‐f in the anterior leads has been shown to be associated with RV dysfunction and RV outflow tract aneurysm in patients with TOF.15, 16 It is postulated that QRS‐f is a marker of myocardial fibrosis based on the observation of late gadolinium enhancement on cardiac MRI in patients with TOF and QRS‐f.16 In a retrospective study of 37 patients with repaired TOF who underwent cardiac MRI, QRS‐f was present in 20 of the patients, and the presence and severity of QRS‐f (number of leads involved) correlated with extent of late gadolinium enhancement and RV dilation and dysfunction.16
Myocardial scar/fibrosis is known to occur in patients with repaired TOF, and the presence of fibrosis is associated with RV dysfunction and poor cardiovascular outcomes such as heart failure, arrhythmia, and death.17, 18 Myocardial fibrosis results in deterioration of myocardial contractility and relaxation, and may serve as a substrate for arrhythmias.17, 18 The accurate identification of myocardial fibrosis by cardiac MRI is therefore important in risk stratification of patients with TOF. Previous studies suggest that QRS‐f can be used as a surrogate for myocardial fibrosis based on the correlation between the extent and location of myocardial fibrosis and extent and location of QRS‐f.13, 14, 17, 18 The current study shows that the presence and extent of QRS‐f, a surrogate for myocardial fibrosis, was predictive of all‐cause mortality even after adjustment for other ECG parameters, patient demographics, and arrhythmia history. Our finding is consistent with, and to a large extent replicates, the results of the recent study by Bokma et al12 despite differences in the demographic characteristics of both populations. In comparison to the Bokma et al cohort, the Mayo Clinic cohort had older patients with more comorbidities, which may explain the higher all‐cause mortality (6% versus 12%). In spite of these differences in cohort characteristics and outcomes (mortality), both studies show that the severity of QRS‐f was predictive of all‐cause mortality in different populations of patients with TOF. Additionally, there was good interobserver correlation in the identification and quantification of the severity of QRS‐f in both studies, which suggests that this technique can easily be integrated into daily clinical practice.
Clinical Impact and Future Directions
Several risk scores have been proposed for identifying patients with TOF at high risk for cardiovascular adverse events, and some of these risk scores have good predictive value, at least in the population from which these variables were derived.3, 5, 6, 7, 8, 9, 10 Unfortunately, some of the variables required to calculate these risk scores involve performing cardiac MRI and, sometimes, cardiac catheterization. Some of these tests are not performed during annual evaluation, making it difficult to provide ongoing risk stratification, which is important since patients’ risk changes over time. ECG, on the other hand is inexpensive, readily available, easy to interpret by a general cardiologist (without any advanced training), and is recommended as part of routine annual evaluation in adults with congenital heart disease.11, 19 QRS‐f from a standard ECG can therefore be applied for ongoing risk stratification in our patients, and may potentially be a trigger to prompt further investigation and treatment if progression of QRS‐f is observed on serial ECGs.
Limitations
The major limitation of this study was that we used all‐cause mortality instead of cardiovascular mortality as the primary end point. If QRS‐f is postulated to be caused by myocardial fibrosis, which subsequently results in ventricular dysfunction and arrhythmia, then QRF‐f should predict cardiovascular mortality without much association with noncardiovascular mortality. Unfortunately, the cause of death could not be reliably ascertained in ≈29% of the cohort because of the retrospective study design, and the small sample size prohibited more robust subgroup analysis. Another study limitation was that we did not correct for the length of time each patient had QRS‐f before the initial ECG (thereby introducing lead time bias). In spite of these study limitations, findings from the current study and the prior study by Bokma et al show that the severity of QRS‐f could independently predict all‐cause mortality in different TOF populations.
Conclusions
The assessment of the presence and severity of QRS‐f was feasible in all patients with TOF, was reproducible based on good interobserver correlation, and was independently predictive of all‐cause mortality. A standard 12‐lead ECG is inexpensive, easy to interpret, readily available, and routinely obtained during annual cardiac evaluation for patients with repaired TOF. Putting all of these together, QRS‐f can provide readily accessible risk stratification for patients with TOF that may be complementary to existing risk scores, especially when the other risk scores may not be feasible because of lack of data. Further studies are required to evaluate temporal changes in the extent of QRS‐f, and whether timely intervention (medical or surgical) based on changes in extent of QRS‐f will improve outcomes.
Sources of Funding
Dr Egbe is supported by National Heart, Lung, and Blood Institute grant K23 HL141448‐01.
Disclosures
None.
Supporting information
Table S1. Interobserver Correlation for Diagnosis of QRS‐f
Table S2. Incrementally Adjusted Risk for All‐Cause Mortality for Patients With Cardiac MRI (N=157)
Table S3. Incrementally Adjusted Risk for All‐Cause Mortality for Patients With Cardiac Catheterization Data (N=148)
Figure S1. Twelve‐lead ECG showing different severities of QRS fragmentation (QRS‐f).
Figure S2. Flowchart showing cohort selection.
Acknowledgments
The authors thank Mohamad Fahim.
(J Am Heart Assoc. 2018;7:e010274 DOI: 10.1161/JAHA.118.010274.)
References
- 1. Egbe A, Uppu S, Stroustrup A, Lee S, Ho D, Srivastava S. Incidences and sociodemographics of specific congenital heart diseases in the United States of America: an evaluation of hospital discharge diagnoses. Pediatr Cardiol. 2014;35:975–982. [DOI] [PubMed] [Google Scholar]
- 2. Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM, McGoon DC, Kirklin JW, Danielson GK. Long‐term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993;329:593–599. [DOI] [PubMed] [Google Scholar]
- 3. Valente AM, Gauvreau K, Assenza GE, Babu‐Narayan SV, Schreier J, Gatzoulis MA, Groenink M, Inuzuka R, Kilner PJ, Koyak Z, Landzberg MJ, Mulder B, Powell AJ, Wald R, Geva T. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nollert G, Fischlein T, Bouterwek S, Bohmer C, Klinner W, Reichart B. Long‐term survival in patients with repair of tetralogy of Fallot: 36‐year follow‐up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol. 1997;30:1374–1383. [DOI] [PubMed] [Google Scholar]
- 5. Katz NM, Blackstone EH, Kirklin JW, Pacifico AD, Bargeron LM Jr. Late survival and symptoms after repair of tetralogy of Fallot. Circulation. 1982;65:403–410. [DOI] [PubMed] [Google Scholar]
- 6. Khairy P, Harris L, Landzberg MJ, Viswanathan S, Barlow A, Gatzoulis MA, Fernandes SM, Beauchesne L, Therrien J, Chetaille P, Gordon E, Vonder Muhll I, Cecchin F. Implantable cardioverter‐defibrillators in tetralogy of Fallot. Circulation. 2008;117:363–370. [DOI] [PubMed] [Google Scholar]
- 7. Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995;92:231–237. [DOI] [PubMed] [Google Scholar]
- 8. Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD, Redington AN. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. [DOI] [PubMed] [Google Scholar]
- 9. Ghai A, Silversides C, Harris L, Webb GD, Siu SC, Therrien J. Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of Fallot. J Am Coll Cardiol. 2002;40:1675–1680. [DOI] [PubMed] [Google Scholar]
- 10. Diller GP, Kempny A, Liodakis E, Alonso‐Gonzalez R, Inuzuka R, Uebing A, Orwat S, Dimopoulos K, Swan L, Li W, Gatzoulis MA, Baumgartner H. Left ventricular longitudinal function predicts life‐threatening ventricular arrhythmia and death in adults with repaired tetralogy of fallot. Circulation. 2012;125:2440–2446. [DOI] [PubMed] [Google Scholar]
- 11. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Page RL, Riegel B, Tarkington LG, Yancy CW; American College of Cardiology, American Heart Association Task Force on Practice Guidelines, American Society of Electrocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography, Interventions and Society of Thoracic Surgeons . ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Circulation. 2008;118:e714–e833. [DOI] [PubMed] [Google Scholar]
- 12. Bokma JP, Winter MM, Vehmeijer JT, Vliegen HW, van Dijk AP, van Melle JP, Meijboom FJ, Post MC, Zwinderman AH, Mulder BJ, Bouma BJ. QRS fragmentation is superior to QRS duration in predicting mortality in adults with tetralogy of Fallot. Heart. 2017;103:666–671. [DOI] [PubMed] [Google Scholar]
- 13. Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. [DOI] [PubMed] [Google Scholar]
- 14. Reddy CV, Cheriparambill K, Saul B, Makan M, Kassotis J, Kumar A, Das MK. Fragmented left sided QRS in absence of bundle branch block: sign of left ventricular aneurysm. Ann Noninvasive Electrocardiol. 2006;11:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shanmugam N, Yap J, Tan RS, Le TT, Gao F, Chan JX, Chong D, Ho KL, Tan BY, Ching CK, Teo WS, Tan JL, Liew R. Fragmented QRS complexes predict right ventricular dysfunction and outflow tract aneurysms in patients with repaired tetralogy of Fallot. Int J Cardiol. 2013;167:1366–1372. [DOI] [PubMed] [Google Scholar]
- 16. Park SJ, On YK, Kim JS, Park SW, Yang JH, Jun TG, Kang IS, Lee HJ, Choe YH, Huh J. Relation of fragmented QRS complex to right ventricular fibrosis detected by late gadolinium enhancement cardiac magnetic resonance in adults with repaired tetralogy of fallot. Am J Cardiol. 2012;109:110–115. [DOI] [PubMed] [Google Scholar]
- 17. Oosterhof T, Mulder BJ, Vliegen HW, de Roos A. Corrected tetralogy of Fallot: delayed enhancement in right ventricular outflow tract. Radiology. 2005;237:868–871. [DOI] [PubMed] [Google Scholar]
- 18. Babu‐Narayan SV, Diller GP, Gheta RR, Bastin AJ, Karonis T, Li W, Pennell DJ, Uemura H, Sethia B, Gatzoulis MA, Shore DF. Clinical outcomes of surgical pulmonary valve replacement after repair of tetralogy of Fallot and potential prognostic value of preoperative cardiopulmonary exercise testing. Circulation. 2014;129:18–27. [DOI] [PubMed] [Google Scholar]
- 19. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke‐Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E; Task Force on the Management of Grown‐up Congenital Heart Disease of the European Society of C, Association for European Paediatric C and Guidelines ESCCfP . ESC Guidelines for the management of grown‐up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Interobserver Correlation for Diagnosis of QRS‐f
Table S2. Incrementally Adjusted Risk for All‐Cause Mortality for Patients With Cardiac MRI (N=157)
Table S3. Incrementally Adjusted Risk for All‐Cause Mortality for Patients With Cardiac Catheterization Data (N=148)
Figure S1. Twelve‐lead ECG showing different severities of QRS fragmentation (QRS‐f).
Figure S2. Flowchart showing cohort selection.


