Abstract
Background
Beyond their potent LDL (low‐density lipoprotein) cholesterol (LDL‐C)–lowering efficacy (50–60%), PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors also reduce Lp(a) (lipoprotein[a]) levels by 25% to 30%, suggesting a 2:1 response ratio. We aimed to characterize the relationship between LDL‐C and Lp(a) lowering by evolocumab, a PCSK9 inhibitor, in a large clinical trial population and to determine the prevalence of concordant/discordant LDL‐C and Lp(a) responses to PCSK9 inhibition.
Methods and Results
Data were analyzed from 4 randomized, 12‐week, multicenter, phase 3 evolocumab trials. Patients with familial hypercholesterolemia, nonfamilial hypercholesterolemia, or statin intolerance participated in the trials. The main measure was the degree of concordance or discordance of LDL‐C and Lp(a) in response to PCSK9 inhibition; concordant response was defined as LDL‐C reduction >35% and Lp(a) reduction >10%. The study cohort comprised 895 patients (438 female; median age: 59.0 years [interquartile range: 51–66 years]). Baseline mean level of LDL‐C was 133.6 mg/dL (SE: 1.7) and median Lp(a) level was 46.4 mg/dL (interquartile range: 18.4–82.4 mg/dL). A discordant response was observed in 165 (19.7%) patients. With these cutoffs, the prevalence of discordance was higher when considering baseline Lp(a) concentrations >30 mg/dL (26.5%) or >50 mg/dL (28.6%).
Conclusions
We demonstrate high prevalence of discordance in LDL‐C and Lp(a) reduction in response to evolocumab, particularly when considering higher baseline Lp(a) concentrations, indicating the possibility of alternative pathways beyond LDLR (LDL receptor)–mediated clearance involved in Lp(a) reduction by evolocumab.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifiers: NCT01763827, NCT01763866, NCT01763905, NCT01763918.
Keywords: lipid‐lowering therapy, lipoprotein[a], low‐density lipoprotein cholesterol, proprotein convertase subtilisin/kexin type 9
Subject Categories: Atherosclerosis, Coronary Artery Disease, Vascular Disease
Short abstract
See Editorial by Nestel
Clinical Perspective
What Is New?
Although it is known that PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors lower plasma LDL (low‐density lipoprotein) cholesterol and Lp(a) (lipoprotein[a]), the relationship between LDL cholesterol and Lp(a) lowering in response to such therapy is complex and likely governed by multiple factors.
What Are the Clinical Implications?
The results of this analysis suggest that Lp(a) may not always or exclusively utilize LDLR (low‐density lipoprotein receptor) as a clearance receptor and, thus, alternative pathways of Lp(a) reduction beyond LDLR‐mediated clearance may be affected by PCSK9 blockade.
Despite its low plasma abundance, PCSK9 (proprotein convertase subtilisin/kexin type 9) acts as a master regulator of LDL (low‐density lipoprotein) metabolism by binding to the LDLR (low‐density lipoprotein receptor) and targeting it for lysosomal degradation.1 In 2015, 2 therapeutic monoclonal antibodies targeting PCSK9 were approved by the US Food and Drug Administration to treat patients with established atherosclerotic cardiovascular disease and/or familial hypercholesterolemia requiring additional LDL cholesterol (LDL‐C) lowering. In 2017, the first large randomized controlled outcome trial with a PCSK9 inhibitor, evolocumab, demonstrated statistically significant reductions in myocardial infarction and stroke rates over 2.2 years in patients with preexisting vascular disease above optimized statin therapy.2 Similarly, positive results from the other antibody (alirocumab) large randomized controlled outcome trial were recently published.3
PCSK9 inhibitors are highly efficacious lipid‐lowering drugs, with LDL‐C reductions generally ranging from 50% to 60% in 12‐week interventional studies.4, 5, 6 Both antibodies targeting PCSK9 have also consistently demonstrated significant (25–30%) reductions in Lp(a) (lipoprotein[a]).7, 8 Although the potent reduction in LDL‐C achieved by PCSK9 inhibition is mediated through its profound effect on LDLR preservation, the mechanism of Lp(a) lowering is unknown. Although some suggest that Lp(a) reduction achieved with PCSK9 inhibition is also secondary to the increase in LDLR expression,9 alternative pathways beyond LDLR‐mediated clearance may be involved in Lp(a) reduction.2, 7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 If PCSK9 antagonism lowers Lp(a) solely through LDLR‐mediated clearance, then Lp(a) and LDL‐C lowering should be proportional, with all individuals achieving the 2:1 ratio.
The range of Lp(a) responses and the relationship of LDL‐C to Lp(a) lowering in a clinical cohort of patients receiving PCSK9 inhibitor therapy was recently characterized under standard‐of‐care protocols, with cutoffs for response to therapy of LDL‐C reduction >35% and Lp(a) reduction >10%.13 In this study, it was hypothesized that if normal clearance of Lp(a) were mediated only through upregulation of LDLR, then most patients would have concordant reductions in both particle levels. Although moderate correlation between LDL‐C and Lp(a) was reported in the cohort, similar to that in a large pooled analysis of 4 phase 2 clinical trials of evolocumab,8 38% of patients (10 of 26) had Lp(a) reduction <10% despite robust LDL‐C lowering. This observation suggests that PCSK9 inhibition may activate alternative mechanisms and/or additional factors beyond LDLR that ultimately determine the degree of Lp(a) reduction. The major limitations of this work, however, were small study size, retrospective design, and use of 2 different antibodies.
In this analysis, we assess the relationship of LDL‐C and Lp(a) lowering in response to 1 PCSK9 antibody, evolocumab. Although there is no widely accepted set of lipid cutoffs for such analyses, we used the same criteria (response to therapy defined as LDL‐C reduction >35% and Lp(a) reduction >10%) used in a prior study.13 We also performed the analyses with response to therapy defined as LDL‐C reduction >0% and Lp(a) reduction >0%. The present study provides an analysis from a larger cohort from the PROFICIO (Program to Reduce LDL‐C and Cardiovascular Outcomes Following Inhibition of PCSK9 in Different Populations) clinical trial program.
Methods
Study Cohort
The study population was derived from the PROFICIO clinical trial program evaluating evolocumab. Data were analyzed from patients enrolled in 1 of 4 randomized, 12‐week, phase 3 evolocumab trials (Table S1).4, 5, 6, 17 All patients provided written informed consent, and the individual protocols were approved by the appropriate institutional review board. Qualified researchers may request data from Amgen clinical studies. Complete details are available online (http://www.amgen.com/datasharing).21
The evolocumab dosing regimens studied were either 140 mg subcutaneously every 2 weeks or 420 mg monthly (Table S1). A common central laboratory, which met applicable standards set forth by the Centers for Disease Control and Prevention and the National Heart, Lung, and Blood Institute,16 was used for lipid and apolipoprotein measurements. LDL‐C levels were determined by the Friedewald formula unless calculated LDL‐C was <40 mg/dL or triglyceride levels were >400 mg/dL, in which case LDL‐C levels were measured by preparative ultracentrifugation.4, 5, 6, 17 Lp(a) was measured using an immunoturbidometric assay with an Olympus AU5400 analyzer.12 The results were converted from nanomole per liter to milligram per deciliter using a conversion factor of 2.5.20
The following clinical trials registrations are related to this study: MENDEL‐2 (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Subjects Currently Not Receiving Drug Therapy for Easing Lipid Levels–2; NCT01763827); LAPLACE‐2 (LDL‐C Assessment With PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy–2; NCT01763866); GAUSS‐2 (Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin Intolerant Subjects–2; NCT01763905); RUTHERFORD‐2 (Reduction of LDL‐C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study–2; NCT01763918).
Efficacy and Safety End Points
The co–primary end points of each of the 4 trials were the percentage change from baseline in LDL‐C at week 12 and at the mean of weeks 10 and 12. Key safety end points were treatment‐emergent and serious adverse events, laboratory parameters, and antievolocumab antibodies.
There were 1558 patients who received evolocumab and adhered to the scheduled study treatment in the 4 phase 3 trials for this pooled analysis. To be eligible for the present analysis, patients needed to meet the following criteria: (1) no missing data at baseline and 12 weeks, (2) adhered to scheduled dose administration, and (3) baseline Lp(a) >10 mg/dL. A total of 895 patients met all inclusion criteria.
One set of criteria used to define response to therapy was set at LDL‐C reduction >35% and Lp(a) reduction >10%, as described in a prior study.13 This definition of discordance was developed to avoid spurious rejection or confirmation of the null hypothesis based on clinical trial data that demonstrate a 2:1 ratio between LDL‐C and Lp(a) lowering. The data from the large FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial recapitulated this observation with mean reductions (versus placebo) in LDL‐C and Lp(a) of 61% (95% CI, 60–61%) and 27% (95% CI, 26–29%), respectively.2 Consequently, we defined discordance as a ratio higher than 3.5:1, which should be a very rare event unless the effect of PCSK9 inhibition on Lp(a) is not exclusively or not always due to LDLR upregulation. Given that there is no widely accepted set of lipid cutoffs for such analyses, we also assessed the degree of concordance or discordance in response to PCSK9 inhibition by setting response to therapy as any LDL‐C reduction and any Lp(a) reduction.
Statistical Analysis
The analysis was performed for 3 groups: (1) baseline Lp(a) >10 mg/dL, (2) baseline Lp(a) >30 mg/dL, and (3) baseline Lp(a) >50 mg/dL. These cutoffs were selected based on recently published data relating the risk of coronary disease to Lp(a) levels.18, 19 Patients with missing data at baseline and/or week 12 were excluded from the analysis. Concordant response to therapy was defined as >35% reduction of LDL‐C and >10% reduction of Lp(a) from baseline to week 12.
Continuous variables were summarized with means and standard errors or medians and interquartile ranges (IQRs). Categorical variables were summarized with counts and percentages. The proportion of patients with a concordant response (proportion of patients with >35% reduction in LDL‐C and >10% reduction in Lp[a]) at week 12 was estimated, and a 95% CI (2‐sided) for this proportion was derived with the Wilson score interval. A 2‐sample t test with unequal variances using Satterthwaite degrees of freedom was performed to evaluate whether the percentage change in LDL‐C from baseline to week 12 was different between patients with ≤10% reduction and those with >10% reduction of Lp(a). These analyses were repeated for >40% reduction of Lp(a) at week 12. Correlations between Lp(a) and LDL‐C reductions for all patients were assessed using the Spearman correlation coefficient at week 12. The proportion of patients with any reduction of LDL‐C and Lp(a) (eg, LDL‐C reduction >0% and Lp[a] reduction >0%) at week 12 was also provided. All analyses were performed with SAS/STAT v9.4 software (SAS Institute).
Results
A total of 1558 patients enrolled in the 4 phase 3 clinical trials were included in this analysis. The final cohort meeting all eligibility criteria consisted of 895 patients (457 male; median age: 59.0 years [IQR: 51–66]). Patient characteristics are detailed in Table 1. Baseline mean LDL‐C level was 133.6 mg/dL (SE: 1.7) and median Lp(a) level was 46.4 mg/dL (IQR: 18.4–82.4). The estimated mean percentage reductions in LDL‐C and Lp(a) for evolocumab versus placebo were 63.3% (95% CI, 59.1–67.5%) and 29.6% (95% CI, 26.7–32.4%), respectively, confirming the expected ≈2:1 ratio. Consequently, the correlation between percentage of LDL‐C reduction and percentage of Lp(a) reduction was statistically significant (r=0.37, P<0.001; Figure 1).
Table 1.
Baseline Characteristics of the Study Population
| Baseline Characteristic | Evolocumab 140 mg SC Biweekly | Evolocumab 420 mg SC Monthly | Overall |
|---|---|---|---|
| n | 471 | 424 | 895 |
| Age, y, median (IQR) | 58.0 (51.0–66.0) | 60 (52.0–67.0) | 59.0 (51.0–66.0) |
| Female, n (%) | 228 (48.4) | 210 (49.5) | 438 (48.9) |
| LDL‐C, mg/dL, mean (SE) | 132.8 (2.3) | 134.5 (2.4) | 133.6 (1.7) |
| Lp(a), mg/dL, median (IQR) | 40.8 (18.0–82.2) | 48.6 (18.4–83.0) | 46.4 (18.4–82.4) |
| HDL‐C, mg/dL, mean (SE) | 53.6 (0.75) | 55.8 (0.80) | 54.6 (0.55) |
| Triglycerides, mg/dL, median (IQR) | 115.5 (86.5–166.0) | 114.0 (85.0–154.3) | 115.0 (86.0–160.0) |
| Non‐HDL‐C, mg/dL, mean (SE) | 159.5 (2.5) | 160.2 (2.6) | 159.8 (1.8) |
| apoB, mg/dL, mean (SE) | 102.9 (1.5) | 102.4 (1.4) | 102.7 (1.0) |
| PCSK9, ng/mL, mean (SE) | 363.7 (5.8) | 350.5 (6.2) | 357.5 (4.3) |
| hs‐CRP, mg/L, mean (SE) | 3.2 (0.3) | 3.6 (0.5) | 3.4 (0.3) |
| Coronary artery disease, n (%) | 114 (24.2) | 113 (26.7) | 227 (25.4) |
| Cerebrovascular or peripheral arterial disease, n (%) | 56 (11.9) | 59 (13.9) | 115 (12.8) |
| Tobacco use, n (%) | 69 (14.6) | 58 (13.7) | 127 (14.2) |
| Diabetes mellitus, n (%) | 58 (12.3) | 45 (10.6) | 103 (11.5) |
| Hypertension, n (%) | 231 (49.0) | 216 (50.9) | 447 (49.9) |
| Family history of premature coronary heart disease, n (%) | 123 (26.1) | 116 (27.4) | 239 (26.7) |
apoB indicates apolipoprotein B; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; Lp(a), lipoprotein(a); PCSK9, proprotein convertase subtilisin/kexin type 9; SC, subcutaneous.
Figure 1.
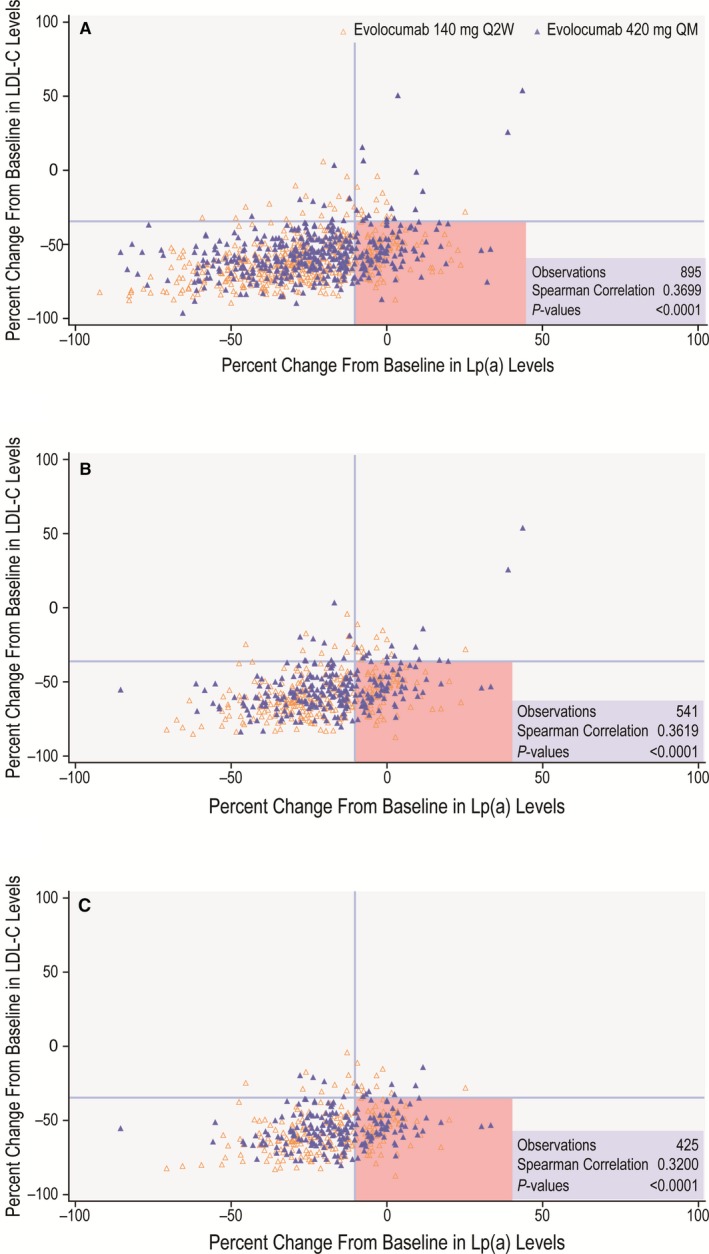
Relationship between percentage reduction in LDL‐C and Lp(a). Relationship between percentage reduction in LDL‐C and Lp(a) at 12 weeks of evolocumab therapy according to baseline Lp(a). A, Baseline Lp(a) >10 mg/dL. B, Baseline Lp(a) >30 mg/dL, C, Baseline Lp(a) >50 mg/dL. The quadrants shaded in pink represent patients with discordant LDL‐C and Lp(a) responses to evolocumab based on response to therapy defined as LDL‐C reduction >35% and Lp(a) reduction >10%. LDL‐C indicates low‐density lipoprotein cholesterol; Lp(a), lipoprotein(a); Q2W, every 2 weeks; QM, monthly.
In the overall study population (combined treatment groups with evolocumab 140 mg every 2 weeks and 420 mg monthly), the vast majority of patients achieved an LDL‐C reduction >35% (n=839; 93.7%) in response to PCSK9 inhibition. Achievement of Lp(a) reduction >10% was less common (n=699; 78.1%). For the remaining 196 patients, the Lp(a) response to evolocumab was either minimal or nonexistent. The prevalence of discordance was higher when baseline Lp(a) concentrations were >30 or >50 mg/dL. In patients with baseline Lp(a) levels either >30 or >50 mg/dL, appropriate LDL‐C reduction without Lp(a) lowering was observed in 133 of 502 (26.5%) and 112 of 392 (28.6%), respectively (Table 2). Importantly, the discordance was to some extent bidirectional, and some participants manifested significant Lp(a) reductions in the absence of the anticipated LDL‐C response to PCSK9 inhibition, although the absolute number of patients in this category was small because most achieved LDL‐C reduction >35%. Specifically, in patients with baseline Lp(a) levels >10, >30, or >50 mg/dL, 25 of 56, 15 of 39, and 13 of 33, respectively, achieved Lp(a) lowering without the anticipated LDL‐C reduction.
Table 2.
Percentage Reduction in LDL‐C and Lp(a), Based on Response to Therapy Defined as LDL‐C Reduction >35% and Lp(a) Reduction >10%, Stratified by Baseline Plasma Lp(a) Concentration
| Percentage Reduction at Week 12 | Evolocumab 140 mg SC Biweekly | Evolocumab 420 mg SC Monthly | Overall | |||
|---|---|---|---|---|---|---|
| LDL‐C ≤35% | LDL‐C >35% | LDL‐C ≤35% | LDL‐C >35% | LDL‐C ≤35% | LDL‐C >35% | |
| Baseline Lp(a) >10 mg/dL, n | 31 | 440 | 25 | 399 | 56 | 839 |
| Lp(a) ≤10% | 14 (45.2, 29.2–62.2) | 79 (18.0, 14.7–21.8) | 17 (68, 48.4–82.8) | 86 (21.6, 17.8–25.8) | 31 (55.4, 42.4–67.6) | 165 (19.7, 17.1–22.5) |
| Lp(a) >10% | 17 (54.8, 37.8–70.8) | 361 (82.0, 78.2–85.3) | 8 (32, 17.2–51.6) | 313 (78.4, 74.2–82.2) | 25 (44.6, 32.4–57.6) | 674 (80.3, 77.5–82.9) |
| Baseline Lp(a) >30 mg/dL, n | 21 | 263 | 18 | 239 | 39 | 502 |
| Lp(a) ≤10% | 12 (57.1, 36.5–75.5) | 65 (24.7, 19.9–30.3) | 12 (66.7, 43.7–83.7) | 68 (28.5, 23.1–34.5) | 24 (61.5, 45.9–75.1) | 133 (26.5, 22.8–30.5) |
| Lp(a) >10% | 9 (42.9, 24.5–63.5) | 198 (75.3, 69.7–80.1) | 6 (33.3, 16.3–56.3) | 171 (71.5, 65.5–76.9) | 15 (38.5, 24.9–54.1) | 369 (73.5, 69.5–77.2) |
| Baseline Lp(a) >50 mg/dL, n | 20 | 200 | 13 | 192 | 33 | 392 |
| Lp(a) ≤10% | 11 (55.0, 34.2–74.2) | 52 (26.0, 20.4–32.5) | 9 (69.2, 42.4–87.3) | 60 (31.3, 25.1–38.1) | 20 (60.6, 43.7–75.3) | 112 (28.6, 24.3–33.2) |
| Lp(a) >10% | 9 (45.0, 25.8–65.8) | 148 (74.0, 67.5–79.6) | 4 (30.8, 12.7–57.6) | 132 (68.8, 61.9–74.9) | 13 (39.4, 24.7–56.3) | 280 (71.4, 66.8–75.7) |
Data are shown as n (%, 95% CI) except as noted. LDL‐C indicates low‐density lipoprotein cholesterol; Lp(a), lipoprotein(a); SC, subcutaneous.
In those with LDL‐C reduction >35% (n=839), Lp(a) reduction was >10% in 674 (80.3%) patients and ≤10% in 165 (19.7%) patients. Interestingly, the mean percentage of LDL‐C reduction was greater in 198 (23.6%) patients with Lp(a) reduction >40% than in those with Lp(a) reduction <10% (65.8% versus 50.1%; P<0.001).
Baseline mean LDL‐C and median Lp(a) for those with ≤35% reduction in LDL‐C were 136.2 mg/dL (SE: 9.2) and 70.8 mg/dL (IQR: 23.8–97.0), respectively. Although baseline mean LDL‐C was similar for patients with LDL‐C reduction ≤35% or >35%, baseline median Lp(a) was lower (43.2 versus 70.8 mg/dL) for those who achieved LDL‐C reduction >35% (Table 3). Baseline mean LDL‐C and median Lp(a) for those with ≤10% reduction in Lp(a) were 136.0 mg/dL (SE: 3.6) and 73.2 mg/dL (IQR: 38.8–86.8), respectively, whereas for patients with >10% reduction in Lp(a), they were 132.9 mg/dL (SE: 1.9) and 37.2 mg/dL (IQR: 17.2–77.6), respectively (Table 3). In patients with Lp(a) reduction of ≤10% in response to PCSK9 inhibition, the LDL‐C drop was 50.1% (SE: 1.4%), whereas in those with Lp(a) reduction of >10%, mean LDL‐C reduction was 60.7% (SE: 0.5%). In addition, we found a greater percentage of Lp(a) reduction in those with lower baseline Lp(a) concentration and a larger absolute Lp(a) reduction in those with higher baseline Lp(a) concentration.
Table 3.
Relationship of Baseline Lipid Concentrations on LDL‐C and Lp(a) Response to Treatment
| Baseline Lipid Concentration | Evolocumab 140 mg SC Biweekly | Evolocumab 420 mg SC Monthly | Overall | |||
|---|---|---|---|---|---|---|
| LDL‐C ≤35% n=31 | LDL‐C >35% n=440 | LDL‐C ≤35% n=25 | LDL‐C >35% n=399 | LDL‐C ≤35% n=56 | LDL‐C >35% n=839 | |
| Baseline LDL‐C, mg/dL, mean (SE) | 145.9 (13.5) | 131.9 (2.3) | 124.2 (11.8) | 135.1 (2.5) | 136.2 (9.2) | 133.4 (1.7) |
| Baseline Lp(a), mg/dL, median (IQR) | 76.4 (23.2–122.4) | 39.8 (17.8–80.8) | 63.6 (25.2–84.4) | 48.4 (18.0–82.8) | 70.8 (23.8–97.0) | 43.2 (18.0–81.6) |
|
Lp(a) ≤10% n=93 |
Lp(a) >10% n=378 |
Lp(a) ≤10% n=103 |
Lp(a) >10% n=321 |
Lp(a) ≤10% n=196 |
Lp(a) >10% n=699 |
|
|---|---|---|---|---|---|---|
| Baseline LDL‐C, mg/dL, mean (SE) | 140.8 (5.4) | 130.9 (2.6) | 131.7 (4.7) | 135.3 (2.9) | 136.0 (3.6) | 132.9 (1.9) |
| Baseline Lp(a), mg/dL, median (IQR) | 74.0 (39.4–91.2) | 34.8 (17.2–75.6) | 72.4 (32.4–85.6) | 38.6 (16.4–78.6) | 73.2 (38.8–86.8) | 37.2 (17.2–77.6) |
IQR indicates interquartile range; LDL‐C, low‐density lipoprotein cholesterol; Lp(a), lipoprotein(a); SC, subcutaneous.
Given that there is no widely accepted set of lipid cutoffs for such analyses and to provide a model for comparison, we performed the analyses with response to therapy defined as LDL‐C reduction >0% and Lp(a) reduction >0%. In this analysis, 807 (90.9%) patients had LDL‐C reduction >0% and Lp(a) reduction >0%.
Discussion
Studies have demonstrated that therapy with PCSK9 inhibitors not only potently reduces LDL‐C by 50% to 60% but also lowers plasma concentrations of Lp(a) by 25% to 30%.7, 8, 14 Lp(a) is an atherogenic particle consisting of a molecule of apo(a) (apolipoprotein[a]), a nonfunctional mimic of plasminogen, covalently bound to apoB on the LDL particle.10 Epidemiologic and genetic data have suggested that Lp(a) is an independent risk factor for atherosclerotic cardiovascular disease.11, 15, 22, 23, 24, 25 The atherogenicity of Lp(a) is likely multifactorial and related to both its LDL and apo(a) moieties and to its enrichment in oxidized phospholipids.23 Furthermore, given its similarity with a portion of plasminogen, Lp(a) may interfere with the fibrinolytic system, thereby facilitating atherothrombosis.26 However, no effective therapies that target Lp(a) exist currently, leaving this potential major need unmet.27 The mechanisms underpinning the effects of PCSK9 inhibition on Lp(a) levels are unknown and remain an area of interest for further research. It has been suggested that the Lp(a) reduction achieved with PCSK9 inhibition is secondary to the profound increase in LDLR expression, although a decrease in Lp(a) is not observed in patients taking statins, which also upregulate LDLR.9 Although LDLR may mediate plasma Lp(a) removal, additional candidate clearance receptors for Lp(a) may exist, including LRP1 (LDL receptor–related protein 1), CD36 (CD36 molecule), TLR2 (toll‐like receptor 2), SR‐B1 (Scavenger receptor‐B1), and plasminogen receptors.28 It is also conceivable that PCSK9 inhibition reduces Lp(a), at least to some extent, by disrupting its synthesis, secretion, or assembly. The FLOREY (Effects on Lipoprotein Metabolism From PCSK9 Inhibition Utilizing a Monoclonal Antibody) study suggests that evolocumab reduces Lp(a) by inhibition of apo(a) production and upregulation of the LDLR activity on Lp(a) clearance.29 Although therapeutic antibodies do not enter the hepatocyte, they can still have an effect on Lp(a) production because the assembly of this particle is likely pericellular and relies on availability of preformed LDL, which is drastically reduced by PCSK9 inhibition.30 Experimental data demonstrate that PCSK9 augments the secretion of apo(a) from hepatocytes, an effect dampened with a PCSK9 antibody inhibitor.31 Others have suggested that when plasma LDL‐C levels are very low, Lp(a) can compete more favorably for the LDLR,9 perhaps via apoE‐mediated processes.32 Indeed, we observed greater Lp(a) reduction in patients who achieved lower on‐treatment LDL‐C levels, regardless of baseline LDL‐C (Figure 2A and 2B). Nevertheless, our finding of discordance between LDL‐C and Lp(a) reduction with evolocumab, for which the cutoffs for response to therapy were LDL‐C reduction >35% and Lp(a) reduction >10%, suggests that additional mechanisms might be at play. If PCSK9 inhibition does in fact lower Lp(a) simply and exclusively through LDLR‐mediated clearance, then the Lp(a) response would likely be proportional to the LDL‐C response. However, we observed differential patterns of LDL‐C and Lp(a) reductions as a function of baseline Lp(a) plasma levels.
Figure 2.
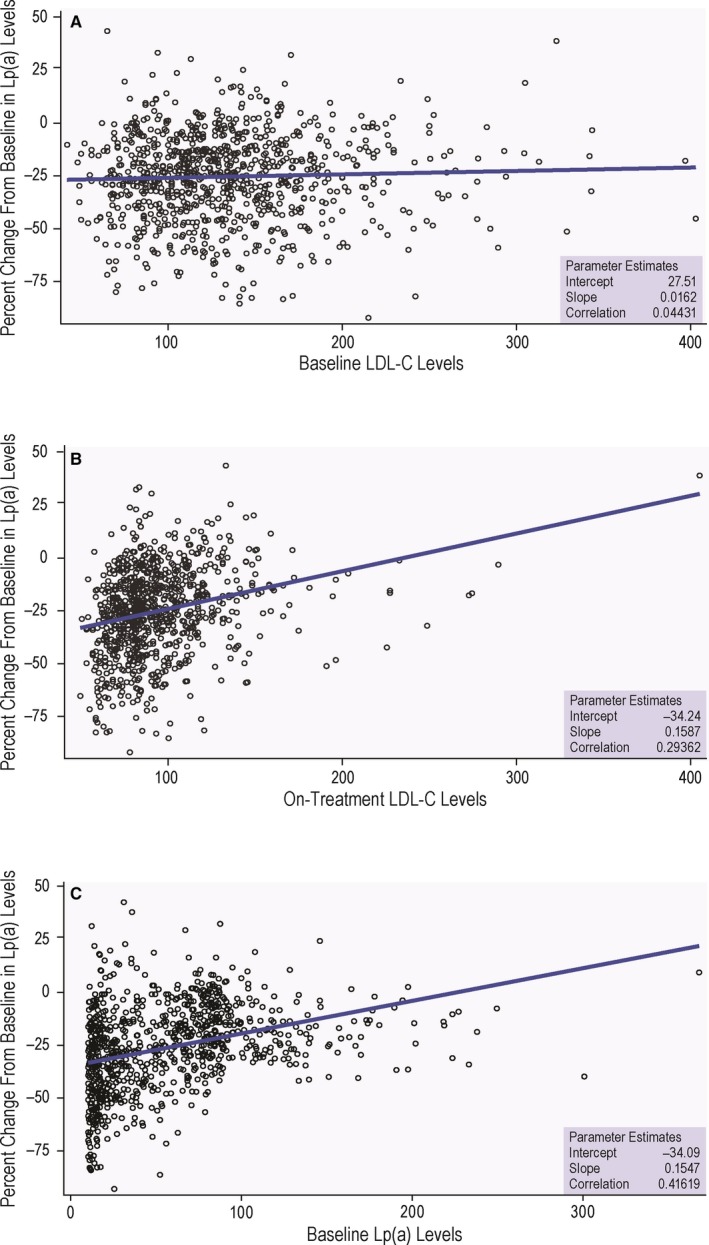
Relationship between percentage reduction in Lp(a) and selected variables. Relationship between percentage reduction in Lp(a) and baseline LDL‐C (A), on‐treatment LDL‐C (B), and baseline Lp(a) concentration (C) for the overall cohort at 12 weeks of evolocumab therapy. LDL‐C indicates low‐density lipoprotein cholesterol; Lp(a), lipoprotein(a).
In our overall study population with baseline Lp(a) >10 mg/dL, we noted discordance between reductions in LDL‐C and Lp(a) for which the cutoffs for response to therapy were LDL‐C reduction >35% and Lp(a) reduction >10% (Table 2). Most cases of discordance were found in patients who achieved the anticipated LDL‐C (>35%) reduction but had little to no (≤10%) reduction in Lp(a) (165 of 839; 19.7%). Interestingly, few patients (25 of 56) demonstrated discordance in the other direction, achieving Lp(a) reductions >10% despite LDL‐C reductions ≤35%. This finding suggests that alternative mechanisms exist beyond LDLR clearance of Lp(a). To examine the impact of baseline Lp(a) on the prevalence of discordant behavior between LDL and Lp(a), we repeated the analysis with stratification to baseline Lp(a) values of >30 and >50 mg/dL. Increasing baseline Lp(a) was associated with increased prevalence of discordance, with 112 of 392 (28.6%) patients with baseline Lp(a) >50 mg/dL manifesting the anticipated LDL‐C reduction without a corresponding reduction in Lp(a). Similar trends were observed in the individual treatment groups (evolocumab 140 mg every 2 weeks and 420 mg monthly). Although we highlighted the prevalence of discordance in our study population, our results demonstrate that concordant reductions in LDL‐C and Lp(a) are indeed the most common responses to evolocumab.
In patients who achieved >35% reduction in LDL‐C with evolocumab, baseline Lp(a) levels were lower than in those who failed to achieve robust LDL‐C reduction (43.2 versus 70.8 mg/dL; Table 3). There was a moderate correlation between baseline Lp(a) levels and percentage of lowering in Lp(a) with evolocumab, such that patients with lower baseline Lp(a) showed greater Lp(a) reduction (37.2 versus 73.2 mg/dL; Figure 2C), an effect that was not related to baseline LDL‐C concentration (Table 3; Figure 2). The fact that the evolocumab‐induced Lp(a) lowering was related to baseline Lp(a) concentration suggests that Lp(a) clearance may be dependent on apo(a) isoform size. We postulate that although Lp(a) clearance is partly regulated by the LDLR pathway, it is also likely to be modulated by apo(a) isoform size, with kringle 4 type 2 chain length being a major determinant of its ability to bind to the LDLR versus alternative receptors. It is well established that plasma levels of Lp(a) are inversely correlated with apo(a) isoform size, with patients carrying small isoforms exhibiting high plasma Lp(a) levels.23 To the extent that Lp(a) mass is inversely related to apo(a) isoform size, our data suggest that patients with larger apo(a) isoforms may respond with more robust Lp(a) reductions after treatment with evolocumab.
Large gaps remain in our understanding of PCSK9's physiologic function and, specifically, how antagonism of PCSK9 relates to LDL‐C and Lp(a) reduction. We found some degree of discordant responses between these 2 parameters when the cutoffs for response to therapy were LDL‐C reduction >35% and Lp(a) reduction >10%; this may provide clues to the fundamental biology of Lp(a) catabolism, an issue that has been unresolved since the discovery of this unique lipoprotein in 1963.33 These findings may also suggest that PCSK9 inhibition activates alternative mechanisms and/or additional factors beyond the simple LDLR that ultimately determine the degree to which Lp(a) levels are reduced. Indeed, our study shows that although Lp(a) does act like an LDL subtype in many patients, it acts very differently in a substantial proportion of participants, suggesting that the LDLR route is not used by all Lp(a) isoforms. Future work will need to uncover the pathways by which PCSK9 inhibition enhances Lp(a) clearance and/or decreases its production. Our results have potential translational value in that they suggest that apo(a) isoforms may determine whether Lp(a) uses the LDLR pathway for its plasma clearance, an easily testable hypothesis that will serve as a pretest to identify patients likely to respond to PCSK9 inhibitor therapy with significant Lp(a) lowering.
Although our study is derived from prospective randomized data, our analysis is retrospective in nature and should be considered hypothesis generating. The study population is larger than a previous report and the effect size is similar, but a more precise estimate of the prevalence of discordance would require an even larger number of patients. The interval between baseline and postevolocumab LDL‐C and Lp(a) measurements was relatively short (12 weeks). It is conceivable that longer exposure to evolocumab would yield different results, with either a lower or higher prevalence of discordance. Most important, although our data suggest that the Lp(a) lowering associated with evolocumab may be contingent on apo(a) isoform size, mechanistic studies will need to be carried out to fully understand the basis for this observation. In addition, because discordance analyses of the LDL‐C– and Lp(a)‐lowering effect of PCSK9 inhibitors are rare, we selected our previously published LDL‐C and Lp(a) percentage reduction thresholds to avoid spurious rejection or confirmation of the null hypothesis. In building our definition of discordance, we wanted to identify significant variation from the apparent concordance seen in clinical trials, in which the average reduction in Lp(a) levels is about half of the reduction in LDL levels (a 1:2 ratio). In our cohort, the same ratio was well above 1:4 in ≈20% of our participants. Our approach was designed to identify only clear discordance among patients with clinically relevant Lp(a) levels, thus avoiding a vast gray zone of uncertain significance. Finally, although it is well known that the cholesterol in Lp(a) is included in the calculated LDL‐C, the commonly used methods to assay Lp(a) (mass and molar concentration) do not allow for a reliable way to reconcile the absolute quantities of cholesterol carried by true LDL versus Lp(a). We have used the most accurate method for isoform‐independent determination of Lp(a) levels.34 Nonetheless, the study has strengths. We used prospectively gathered data from randomized controlled clinical trials. In aggregating clinical trial data, the article extends important recent results from a single center in a sample of larger size under controlled trial conditions.13
Conclusions
The purpose of this study was to evaluate the relationship between LDL‐C– and Lp(a)‐lowering responses to a therapeutic monoclonal antibody targeting PCSK9 in a large population. We demonstrate a high prevalence of discordance in the degree to which these 2 lipoprotein fractions respond to evolocumab when the cutoffs for response to therapy were LDL‐C reduction >35% and Lp(a) reduction >10%. The results suggest that Lp(a) may not always or exclusively utilize the LDLR as a clearance receptor. Alternative pathways of Lp(a) reduction beyond LDLR‐mediated clearance may be affected by PCSK9 blockade.
Author Contributions
Shapiro and Fazio had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Shapiro, Fazio, Kassahun, Somaratne. Acquisition, analysis, or interpretation of data: All authors. Drafting of the article: Shapiro. Critical revision of the article for important intellectual content: All authors. Statistical analysis: Minnier, Flower.
Source of Funding
This study was approved by Amgen Inc, but no direct or indirect funding was provided to either Oregon Health and Science University (OHSU) or the coauthors from OHSU. The sponsor provided funding for the parent studies and via employee coauthors provided input into the design and conduct of the study and collection, management, and analysis of the data review. The sponsor reviewed the article before submission, but the preparation, approval, and decision to submit the article for publication were done by the coauthors.
Disclosures
Dr Shapiro is supported by NIH K12HD043488 and has received compensation for advisory activities from Kastle, Novartis, and Regeneron (modest). Fazio has received compensation for advisory activities from Amarin, Akcea, Aegerion, Amgen Inc, and Kowa (modest). Minnier and Tavori have no disclosures. Kassahun is an employee of Amgen Inc and holds Amgen stock/stock options (significant). Somaratne is a former employee of Amgen Inc, holds Amgen stock, and is an inventor on at least 1 pending patent application owned by Amgen Inc relating to evolocumab (significant). Flower is a former employee of Amgen Inc.
Supporting information
Table S1. Phase 3 Trials With Evolocumab Included in the Study Cohort
Acknowledgments
The authors thank Mahta Nili, PhD, of Amgen for editorial support.
(J Am Heart Assoc. 2019;8:e010932 DOI: 10.1161/JAHA.118.010932.)
References
- 1. Maxwell KN, Breslow JL. Adenoviral‐mediated expression of Pcsk9 in mice results in a low‐density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci USA. 2004;101:7100–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; Committees OO, Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 4. Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM, Bays H; MENDEL‐2 Investigators . Anti‐PCSK9 monotherapy for hypercholesterolemia: the MENDEL‐2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. [DOI] [PubMed] [Google Scholar]
- 5. Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni‐Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D; RUTHERFORD‐2 Investigators . PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:331–340. [DOI] [PubMed] [Google Scholar]
- 6. Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, Xue A, Scott R, Wasserman SM, Rocco M; GAUSS‐2 Investigators . Anti‐PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS‐2 randomized, placebo‐controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–2548. [DOI] [PubMed] [Google Scholar]
- 7. Gaudet D, Kereiakes DJ, McKenney JM, Roth EM, Hanotin C, Gipe D, Du Y, Ferrand AC, Ginsberg HN, Stein EA. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am J Cardiol. 2014;114:711–715. [DOI] [PubMed] [Google Scholar]
- 8. Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, Albizem M, Scott R, Stein EA. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol. 2014;63:1278–1288. [DOI] [PubMed] [Google Scholar]
- 9. Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Blom D, Seidah NG, Honarpour N, Lira A, Xue A, Chiruvolu P, Jackson S, Di M, Peach M, Somaratne R, Wasserman SM, Scott R, Stein EA. PCSK9 inhibition‐mediated reduction in Lp(a) with evolocumab: an analysis of 10 clinical trials and the LDL receptor's role. J Lipid Res. 2016;57:1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albers JJ, Kennedy H, Marcovina SM. Evidence that Lp[a] contains one molecule of apo[a] and one molecule of apoB: evaluation of amino acid analysis data. J Lipid Res. 1996;37:192–196. [PubMed] [Google Scholar]
- 11. Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M; PROCARDIS Consortium . Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. [DOI] [PubMed] [Google Scholar]
- 12. Dati F, Tate JR, Marcovina SM, Steinmetz A; International Federation of Clinical Chemistry and Laboratory Medicine, IFCC Working Group for Lipoprotein(a) Assay Standardization . First WHO/IFCC international reference reagent for lipoprotein(a) for immunoassay—Lp(a) SRM 2B. Clin Chem Lab Med. 2004;42:670–676. [DOI] [PubMed] [Google Scholar]
- 13. Edmiston JB, Brooks N, Tavori H, Minnier J, Duell B, Purnell JQ, Kaufman T, Wojcik C, Voros S, Fazio S, Shapiro MD. Discordant response of low‐density lipoprotein cholesterol and lipoprotein(a) levels to monoclonal antibodies targeting proprotein convertase subtilisin/kexin type 9. J Clin Lipidol. 2017;11:667–673. [DOI] [PubMed] [Google Scholar]
- 14. Gaudet D, Watts GF, Robinson JG, Minini P, Sasiela WJ, Edelberg J, Louie MJ, Raal FJ. Effect of alirocumab on lipoprotein(a) over ≥1.5 years (from the Phase 3 ODYSSEY Program). Am J Cardiol. 2017;119:40–46. [DOI] [PubMed] [Google Scholar]
- 15. Kamstrup PR, Tybjaerg‐Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. [DOI] [PubMed] [Google Scholar]
- 16. Myers GL, Cooper GR, Winn CL, Smith SJ. The Centers for Disease Control‐National Heart, Lung And Blood Institute Lipid Standardization Program. An approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9:105–135. [PubMed] [Google Scholar]
- 17. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R; LAPLACE‐2 Investigators . Effect of evolocumab or ezetimibe added to moderate‐ or high‐intensity statin therapy on LDL‐C lowering in patients with hypercholesterolemia: the LAPLACE‐2 randomized clinical trial. JAMA. 2014;311:1870–1882. [DOI] [PubMed] [Google Scholar]
- 18. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 19. Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36:2239–2245. [DOI] [PubMed] [Google Scholar]
- 20. Yeang C, Witztum JL, Tsimikas S. ‘LDL‐C’ = LDL‐C + Lp(a)‐C: implications of achieved ultra‐low LDL‐C levels in the proprotein convertase subtilisin/kexin type 9 era of potent LDL‐C lowering. Curr Opin Lipidol. 2015;26:169–178. [DOI] [PubMed] [Google Scholar]
- 21. Welcome to AmgenTrials. Available at: http://www.amgen.com/datasharing. Accessed: December 24, 2018.
- 22. Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta‐analysis of prospective studies. Circulation. 2000;102:1082–1085. [DOI] [PubMed] [Google Scholar]
- 23. Lamon‐Fava S, Marcovina SM, Albers JJ, Kennedy H, Deluca C, White CC, Cupples LA, McNamara JR, Seman LJ, Bongard V, Schaefer EJ. Lipoprotein(a) levels, apo(a) isoform size, and coronary heart disease risk in the Framingham Offspring Study. J Lipid Res. 2011;52:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Luke MM, Shiffman D, Devlin JJ. Genetic variants in the apolipoprotein(a) gene and coronary heart disease. Circ Cardiovasc Genet. 2011;4:565–573. [DOI] [PubMed] [Google Scholar]
- 25. Gencer B, Kronenberg F, Stroes ES, Mach F. Lipoprotein(a): the revenant. Eur Heart J. 2017;38:1553–1560. [DOI] [PubMed] [Google Scholar]
- 26. Eaton DL, Fless GM, Kohr WJ, McLean JW, Xu QT, Miller CG, Lawn RM, Scanu AM. Partial amino acid sequence of apolipoprotein(a) shows that it is homologous to plasminogen. Proc Natl Acad Sci USA. 1987;84:3224–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgozoglu L, Tybjaerg‐Hansen A; European Atherosclerosis Society Consensus Panel . Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoover‐Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watts GF, Chan DC, Somaratne R, Wasserman SM, Scott R, Marcovina SM, Barrett PHR. Controlled study of the effect of proprotein convertase subtilisin‐kexin type 9 inhibition with evolocumab on lipoprotein(a) particle kinetics. Eur Heart J. 2018;39:2577–2585. [DOI] [PubMed] [Google Scholar]
- 30. Kostner KM, Kostner GM. Lipoprotein (a): a historical appraisal. J Lipid Res. 2017;58:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villard EF, Thedrez A, Blankenstein J, Croyal M, Tran TT, Poirier B, Le Bail JC, Illiano S, Nobecourt E, Krempf M, Blom DJ, Marais AD, Janiak P, Muslin AJ, Guillot E, Lambert G. PCSK9 modulates the secretion but not the cellular uptake of lipoprotein(a) ex vivo: an effect blunted by alirocumab. JACC Basic Transl Sci. 2016;1:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moriarty PM, Varvel SA, Gordts PL, McConnell JP, Tsimikas S. Lipoprotein(a) mass levels increase significantly according to APOE genotype: an analysis of 431 239 patients. Arterioscler Thromb Vasc Biol. 2017;37:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berg K. A new serum type system in man—the LP system. Acta Pathol Microbiol Scand. 1963;59:369–382. [DOI] [PubMed] [Google Scholar]
- 34. Tsimikas S, Fazio S, Viney NJ, Xia S, Witztum JL, Marcovina SM. Relationship of lipoprotein(a) molar concentrations and mass according to lipoprotein(a) thresholds and apolipoprotein(a) isoform size. J Clin Lipidol. 2018;12:1313–1323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Phase 3 Trials With Evolocumab Included in the Study Cohort


