Abstract
Background
Food deserts (FDs), defined as low‐income communities with limited access to healthy food, are a growing public health concern. We evaluated the impact of living in FDs on incident cardiovascular events.
Methods and Results
We recruited 4944 subjects (age 64±12, 64% male) undergoing cardiac catheterization into the Emory Cardiovascular Biobank. Using the US Department of Agriculture definition of FD, we determined whether their residential addresses had (1) poor access to healthy food, (2) low income, or (3) both (=FD). Subjects were prospectively followed for a median of 3.2 years for myocardial infarction (MI) and death. Fine and Gray's subdistribution hazard models for MI and Cox proportional hazard models for death/MI were used to examine the association between area characteristics (FD, poor access, and low income) and the rates of adverse events after adjusting for traditional risk factors. A total of 981 (20%) lived in FDs and had a higher adjusted risk of MI (subdistribution hazard ratio, 1.44 [95% CI, 1.06–1.95]) than those living in non‐FDs. In a multivariate analysis including both food access and area income, only living in a low‐income area was associated with a higher adjusted risk of MI (subdistribution hazard ratio, 1.40 [1.06–1.85]) and death/MI (hazard ratio, 1.18 [1.02–1.35]) while living in a poor‐access area was not significantly associated with either (subdistribution hazard ratio, 1.05 [0.80–1.38] and hazard ratio, 0.99 [0.87–1.14], respectively).
Conclusions
Living in an FD is associated with a higher risk of adverse cardiovascular events in those with coronary artery disease. Specifically, low area income of FDs, not poor access to food, was significantly associated with worse outcomes.
Keywords: cardiovascular disease, death, environment, food desert, myocardial infarction, socioeconomic position
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Epidemiology, Mortality/Survival
Clinical Perspective
What Is New?
Living in a food desert, defined as an area of low income and poor access to healthy foods, is associated with adverse cardiovascular outcomes in patients with coronary artery disease, independent of their traditional cardiovascular risk factor burden.
The association between food deserts and adverse outcomes is largely driven by low area income, not poor access to food.
What Are the Clinical Implications?
Living in low‐income neighborhoods is a risk factor for adverse outcomes in patients with coronary artery disease.
Whether community‐level or individual‐level interventions will improve outcomes needs to be investigated.
Introduction
First coined in the early 1990s, the term food deserts (FDs) identifies areas where residents have difficulty with access to affordable healthy food.1 While there is no consensus on the definition of FD in the literature, the US Department of Agriculture (USDA) currently defines FDs as areas with both poor food access and low area income,2 and an estimated 23.5 million people are living in such FDs across the United States.2, 3 With the growing interest in residential “place” as a determinant of health in the recent years,4 FDs have been increasingly recognized as important environmental contributors to individual health and as potential targets of community‐level interventions to improve health outcomes.5, 6 In the meantime, it is well established that cardiovascular risk and disease are not only associated with food choice and dietary intake patterns of individuals7, 8 but also influenced by their residential neighborhoods.9, 10, 11 Thus, a better understanding of FDs and their impact on cardiovascular risk factors and disease is imperative not only to improve our epidemiologic knowledge of cardiovascular disease, but more importantly to develop effective interventions to target vulnerable, disadvantaged areas of food resources to improve their cardiovascular outcomes.
The association between FDs and cardiovascular disease outcomes still remains understudied. A few studies have examined the cross‐sectional association between food environment and cardiovascular risk factors/disease. Recently, we demonstrated that living in an FD was associated with unfavorable cardiovascular risk profiles as well as subclinical vascular disease in a population without known clinical cardiovascular disease.12 Several other studies have also shown that food environment was associated with cardiovascular risk factors, such as obesity,13, 14, 15 diabetes mellitus,16 and hypertension.17, 18 However, despite these cross‐sectional findings, no study has yet directly examined the association between living in an FD and incident cardiovascular outcomes in a longitudinal study, which would be essential in establishing the causal relationship between FDs and cardiovascular disease. Furthermore, given the heterogeneity in the definitions of FD, an in‐depth examination of what aspects of FDs drive adverse cardiovascular outcomes will be also crucial to address and elucidate the mechanistic pathways from living in FDs to cardiovascular outcomes and to help design effective policies.
Herein, we examined the impact of living in FDs on adverse cardiovascular events using a cohort of patients with suspected or confirmed coronary artery disease (CAD). We investigated not only whether living in FDs predicted hard outcomes of cardiovascular disease, myocardial infarction (MI), and death, independent of the traditional demographic and clinical risk factors, but also investigated which component of FDs, poor access to food or low income, as defined by the USDA, is the driver of adverse outcomes. Given our prior work showing that area income drives the unfavorable cardiovascular disease risk profile and disease burden,12 we hypothesized that living in an FD would be an independent risk factor of adverse cardiovascular outcomes, independent of the traditional risk factors, and it would be largely driven by the low area income, not the level of access to food.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We studied 4944 adults aged ≥18 years with valid residential addresses from the Emory Cardiovascular Biobank,19 a prospective cohort of patients undergoing cardiac catheterization for suspected or confirmed CAD at 3 Emory Healthcare sites in Atlanta, Georgia, between 2003 and 2015. Subjects with congenital heart disease, heart transplantation, severe anemia, and cancer were excluded. Participants were interviewed to inquire about demographic characteristics, medical history, medication use, and behavioral habits as previously described at the time of cardiac catheterization upon enrollment.19 Collected variables of interest included age, sex, race, body mass index (BMI), level of education, and smoking history, as well as medical history including hypertension, diabetes mellitus, hyperlipidemia, CAD, heart failure (HF), ejection fraction, prior MI, prior coronary revascularization, and medications. Level of education and smoking history were obtained through a questionnaire completed by patients. History of hypertension, hyperlipidemia, and diabetes mellitus were reported by patients and confirmed by physician diagnosis. CAD was defined as angiographic evidence of luminal stenosis ≥20% of major epicardial vessels on the index coronary angiography upon enrollment. HF was defined by the presence of at least 1 of the following: self‐reported history of HF; physician diagnosis of HF noted in medical records; International Classification of Diseases, Ninth Revision (ICD‐9) discharge diagnosis of HF; or ejection fraction <50%. History of MI and coronary revascularization reported by the subjects were also verified by medical records and ICD‐9 diagnostic codes. Medication use was documented based on the medications being taken at enrollment as well as upon discharge from their index hospitalizations. Fasting lipid profile and serum creatinine levels were obtained from their hospital records at the time of enrollment, and estimated glomerular filtration rate was calculated on the basis of creatinine level using the Chronic Kidney Disease Epidemiology Collaboration equation.20 The study was approved by the institutional review board at Emory University (Atlanta, GA). All subjects provided written informed consent at the time of enrollment.
Environmental Characteristics
We utilized the FD data from the USDA Food Access Research Atlas.2 The data define FDs as areas based on the USDA classification at the census‐tract level, namely, those census tracts that meet the criteria of having both (1) low income and (2) poor access to food. According to the Atlas definitions, the criteria for identifying a census tract as low income are from the Department of Treasury's New Markets Tax Credit program. This program defines a low‐income census tract as any tract where the tract's poverty rate is 20% or greater, the tract's median family income is ≤80% of the statewide median family income, or the tract is in a metropolitan area and has a median family income ≤80% of the metropolitan area's median family income. Using established definitions used in the Atlas, poor access to healthy food at the census tract level is defined as having a significant number of individuals (at least 500 people) or share (at least 33%) of the population are >1 mile from a supermarket, supercenter, or large grocery store for an urban area or >10 miles for a rural area.
Using the USDA Food Access Research Atlas, we obtained 3 area characteristics for the residential address of each subject: whether its respective census tract is classified as (1) FD, (2) low income, or (3) poor access to food. A previous study had investigated the validation of the USDA FD measures; the USDA FDs were compared with secondary data sources of food outlets (Dun & Bradstreet and InfoUSA) across 169 census tracts in South Carolina, and the 2 sources had 94% concordance, 50% to 65% sensitivity, and 60% to 64% positive predictive value.21
In addition, we categorized the residential addresses of subjects as urban or rural using the Census Bureau's urbanized area definitions.2 Rural areas were defined as sparsely populated areas with fewer than 2500 people, whereas urban areas are areas with more than 2500 people.
Follow‐Up and Outcomes
The cohort was prospectively followed for adverse cardiovascular events. Follow‐up data were obtained by dedicated research personnel using phone contact, electronic medical record review, Georgia vital records, and the Social Security Death Index. The primary end point of the study was MI including fatal MI, defined as death within 28 days of MI onset, as well as nonfatal MI. The secondary outcome was the composite end point of death or MI. All death and MI events were adjudicated by the committee of physicians blinded to the data, by reviewing medical records as well as the aforementioned death certificates.
Statistical Methods
Baseline demographic and clinical characteristics of the cohort were summarized as means (SDs) or median (interquartile range) for continuous variables and frequency counts (%) for categorical variables. Patient characteristics were compared between those living in FDs and those not living in FDs using t tests or Mann–Whitney U tests for continuous variables and chi‐squared tests for categorical variables. The same comparison was also performed between those living in low‐income areas versus high‐income areas as well as between those living in areas with poor access versus adequate access to food.
We performed survival analysis to examine the risk of adverse events for subjects who lived in (1) FD versus non‐FD, (2) area with low income versus area with high income, and (3) area with poor access versus area with adequate access. Follow‐up time was defined as the time from enrollment until 1 of the following: death, MI, loss to follow‐up, or end of follow‐up. Initially, survival probability was compared between the 2 groups using Kaplan–Meier curves and log‐rank test. Subsequently, multivariable survival analysis was performed with adjustment of age, sex, race (black versus non‐black), BMI, level of education (high school or less, some college, or college graduate), smoking history, as well as medical history including hypertension, diabetes mellitus, hyperlipidemia, CAD, HF, ejection fraction, prior MI, prior coronary revascularization and medication use (aspirin, clopidogrel, statin, β‐blocker, and angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker) (Model 1). These covariates in the models were selected a priori to account for baseline differences of risk factor burden between subjects living in different areas of an FD, as we have recently demonstrated,12 and to examine the independent association between living in an FD and outcomes. Because individual household income was not collected in this cohort, we used education level as a surrogate for individual socioeconomic status. Previously, education had been shown to correlate with individual household income and cardiovascular risk factors and outcomes.22, 23, 24 For the primary end point of MI, Fine and Gray's subdistribution hazard model25 was used while treating death as a competing risk. For the secondary end point of death/MI, Cox proportional hazards models were used with the same aforementioned covariates used for the primary end point.
In addition, we examined the study end point by including both area income (high or low) and food access (poor or adequate) simultaneously as independent exposure variables in a single model (Model 2), in place of the FD variable. This was to examine which of the 2 components of FDs are driving the associations between the outcomes and FDs, if any. Additionally, we stratified the cohort into 4 groups, depending on the permutation of area income (high or low) and food access (poor or adequate) categories, and compared the differences in their Kaplan–Meier curves using pairwise log‐rank tests.
In the sensitivity analysis, we examined whether the association between an FD and adverse outcomes differed with respect to the presence of prespecified covariates by including the interaction term of FD×the covariate of interest (Model 1). The same sensitivity analysis was repeated with respect to the subcomponent of the FD (low area income or food access) that was identified as the primary driver in the main analysis on the association between the FD and outcomes.
Finally, we examined whether living in an FD, poor‐access, or low‐income area was associated with the risk of death following incident MI. Among those with incident MI, the rates of fatal MI, defined as death within 28 days of incident MI, were compared depending on the area characteristics (FD versus non‐FD; low income versus high income; poor access versus adequate access) using Cox proportional hazard models.
Throughout the analyses, there were no violations of the proportional hazards assumption, and P<0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Subjects Characteristics
Baseline demographic and clinical characteristics of the 4944 subjects are presented in Table 1. The cohort consisted of 64% male and 22% black individuals with a mean age of 64±12 years; 43% had a high school diploma or less, whereas 22% had some college education and 35% were college graduates. Individuals living in FDs (n=981; 20%) were more likely to be black (31% versus 20%) with less college education, were more likely to be living in urban areas (77% versus 73%) compared with those who were not living in FDs, and had a higher prevalence of hypertension and prior revascularization and higher BMI (Table 1).
Table 1.
Subject Characteristics by FD Status
| Total (N=4944) | Non–Food Desert (N=3963) | Food Desert (N=981) | P Value | |
|---|---|---|---|---|
| Age, y | 64±12 | 64±12 | 63±12 | 0.006a |
| Male | 3148 (64) | 2540 (64) | 608 (62) | 0.22 |
| Black race | 1087 (22) | 787 (20) | 300 (31) | <0.001a |
| Education | <0.001a | |||
| High school graduate or less | 2104 (43) | 1628 (41) | 476 (49) | |
| Some college | 1108 (22) | 883 (22) | 225 (23) | |
| College graduate | 1732 (35) | 1452 (37) | 280 (29) | |
| Urban | 2846 (74) | 2243 (73) | 603 (77) | 0.023a |
| Hypertension | 3913 (79) | 3110 (79) | 803 (82) | 0.028a |
| Diabetes mellitus | 1748 (36) | 1399 (36) | 349 (36) | 0.86 |
| Hyperlipidemia | 3537 (72) | 2848 (72) | 689 (71) | 0.32 |
| Smoking | 3393 (69) | 2723 (69) | 670 (68) | 0.82 |
| History of coronary artery disease | 4500 (91) | 3610 (91) | 890 (91) | 0.71 |
| History of myocardial infarction | 1178 (24) | 957 (25) | 221 (23) | 0.36 |
| History of revascularization | 2637 (53) | 2143 (54) | 494 (50) | 0.038a |
| History of heart failure | 956 (19) | 764 (19) | 192 (20) | 0.86 |
| BMI, kg/m2 | 30±6 | 29±6 | 30±7 | 0.002a |
| Total cholesterol, mg/dL | 166±45 | 166±44 | 166±47 | 0.65 |
| LDL, mg/dL | 94±38 | 94±37 | 96±39 | 0.11 |
| HDL, mg/dL | 43±16 | 43±14 | 43±23 | 0.9 |
| eGFR, mL/min per 1.73 m2 | 73±24 | 73±24 | 72±26 | 0.62 |
| Gensini angiographic score, median (IQR) | 6.5 (0–32) | 6.5 (0–33) | 7 (0–31) | 0.9 |
| Ejection fraction % | 53±13 | 53±13 | 52±13 | 0.24 |
| Medications | ||||
| ACE/ARB use | 2757 (56) | 2235 (56) | 522 (53) | 0.07 |
| Aspirin use | 3762 (76) | 3038 (77) | 724 (74) | 0.07 |
| Clopidogrel use | 2215 (45) | 1779 (45) | 436 (44) | 0.83 |
| Statin use | 3471 (70) | 2805 (71) | 666 (68) | 0.08 |
| β‐Blocker use | 3340 (68) | 2687 (68) | 653 (67) | 0.47 |
Values shown are mean±SDs or median (IQR) for continuous variables and number (percentage) for categorical variables. ACE indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; FD, food deserts; HDL, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL, low‐density lipoprotein cholesterol.
Denotes a statistically significant P‐value.
FD and Incident Cardiovascular Outcomes
During a median follow‐up period of 3.4 years, 812 patients (16%) died and 230 (5%) had incident MI (Table 2). In univariate analysis, patients living in an FD compared with those not living in an FD had a 46% higher risk of MI (subdistribution hazard ratio [sHR], 1.46 [95% CI, 1.08–1.96]; P=0.013), and a 22% higher risk of the composite outcome of death or MI (hazard ratio, 1.22 [1.05–1.43]; P=0.011) (Tables 2 and 3, Figures 1A and 2A). After adjustment for the baseline demographics and cardiovascular risk factors as described in the methods, patients living in FDs compared with those not living in FDs had a 44% higher risk of MI (sHR, 1.44 [1.04–1.91]; P=0.020), but the increase in risk for death/MI was no longer statistically significant hazard ratio, (hazard ratio, 1.16 [0.99–1.37]) (Table 3).
Table 2.
Rate of Adverse Events Stratified by Living in FD, Access to Food, and Area Income
| Overall | FD | Access to Food | Area Income | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FD | Non‐FD | P Value | Adequate | Poor | P Value | High | Low | P Value | ||
| MI | 230 (4.7) | 171 (4.3) | 59 (6) | 0.028a | 114 (4.9) | 116 (4.5) | 0.54 | 105 (3.8) | 125 (5.7) | 0.002a |
| Death | 812 (16.4) | 636 (16) | 176 (17.9) | 0.16 | 401 (17.1) | 411 (15.8) | 0.25 | 424 (15.4) | 388 (17.7) | 0.03a |
| Death or MI | 941 (19) | 734 (18.5) | 207 (21.1) | 0.07 | 471 (20.1) | 470 (18.1) | 0.09 | 480 (17.4) | 461 (21.1) | 0.001a |
Number of events (%) are shown. FD indicates food desert; MI, myocardial infarction.
Denotes a statistically significant P‐value.
Table 3.
Cox Regression Models for the Association Between Incident Cardiovascular Events and FD, Food Access, and Area Income
| MI | P Value | Death/MI | P Value | |
|---|---|---|---|---|
| sHR (95% CI) | HR (95% CI) | |||
| Univariate | ||||
| FD | 1.46 (1.08–1.96) | 0.013a | 1.22 (1.05–1.43) | 0.011a |
| Poor food access | 0.96 (0.74–1.24) | 0.75 | 0.95 (0.84–1.08) | 0.46 |
| Low area income | 1.54 (1.19–2.00) | 0.001a | 1.29 (1.13–1.46) | <0.001a |
| Multivariate model 1 | ||||
| FD | 1.44 (1.06–1.95) | 0.020a | 1.16 (0.99–1.37) | 0.068 |
| Poor food access | 1.00 (0.77–1.31) | 0.98 | 0.97 (0.85–1.11) | 0.67 |
| Low area income | 1.39 (1.06–1.83) | 0.019a | 1.18 (1.03–1.35) | 0.020a |
| Multivariate model 2 | ||||
| Poor food access | 1.05 (0.80–1.38) | 0.71 | 0.99 (0.87–1.14) | 0.91 |
| Low area income | 1.40 (1.06–1.85) | 0.018a | 1.18 (1.02–1.35) | 0.023a |
Model 1: adjusted for age, sex, race, diabetes mellitus, hypertension, hyperlipidemia, estimated glomerular filtration rate, body mass index, smoking history, heart failure, prior coronary revascularization, history of coronary artery disease, use of cardiovascular medications, previous MI, and education level. Model 2: poor food access and low area income were simultaneously treated as separate exposure variables with the same covariate adjustment for Model 1. FD indicates food desert; HR, hazard ratio; MI, myocardial infarction; sHR, subdistribution hazard ratio.
Denotes a statistically significant P‐value.
Figure 1.
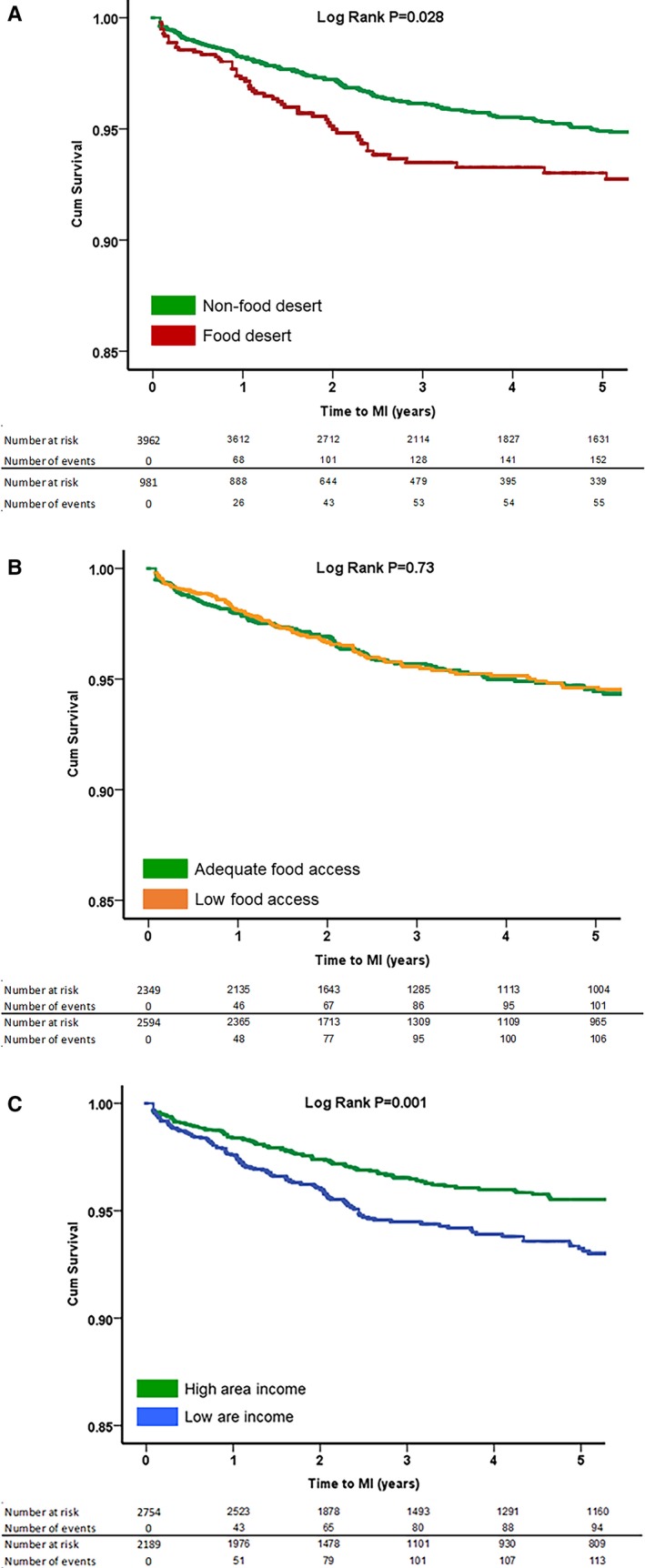
Kaplan–Meier curves for association between (A) food desert, (B) food access, and (C) area income with incident myocardial infarction (MI). P values were derived from log‐rank tests. A, Food desert and incident MI. B, Food access and incident MI. C, Area income and incident MI.
Figure 2.
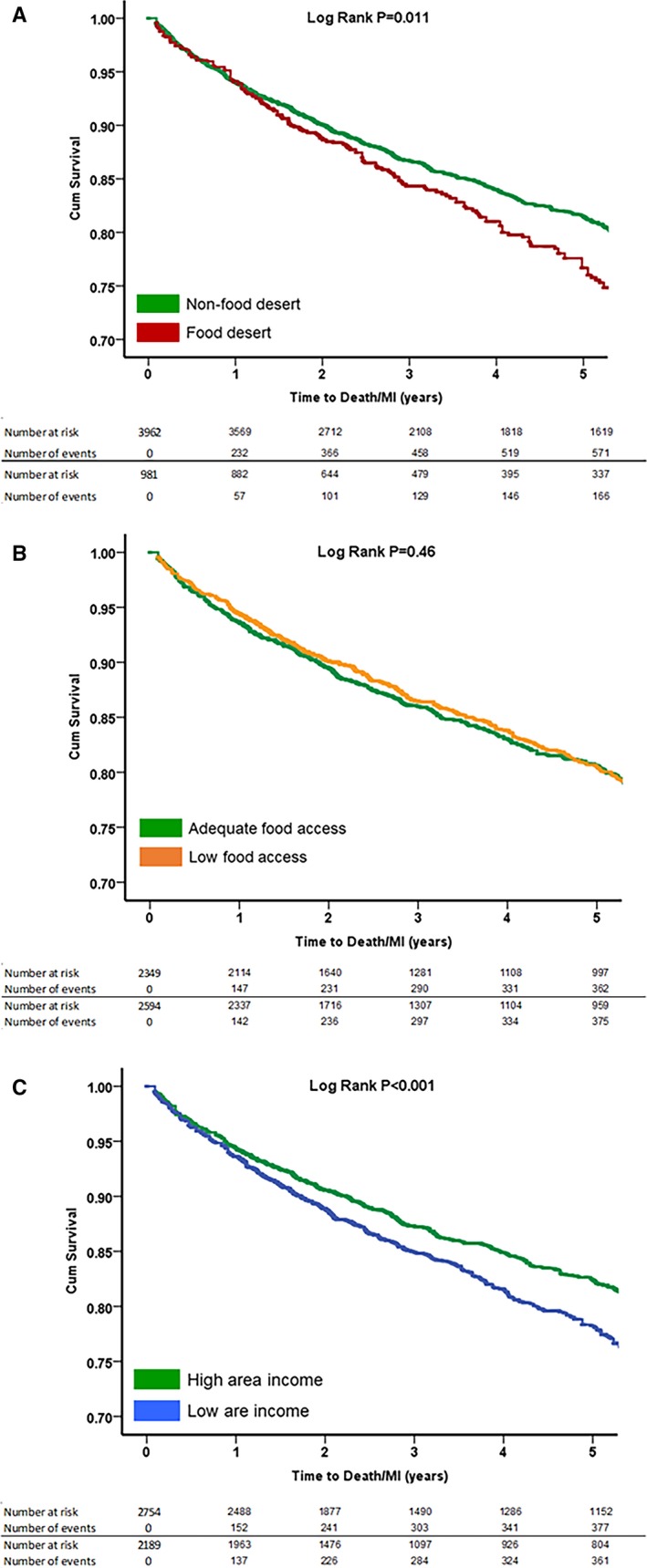
Kaplan–Meier curves for association between (A) food desert, (B) food access, and (C) area income with incident composite event rate of all‐cause death/myocardial infarction (MI). P values were derived from log‐rank tests. A, Food desert and incident death/MI. B, Food access and incident death/MI. C, Area income and incident death/MI.
Food Access and Incident Cardiovascular Outcomes
The rates of adverse outcomes were also compared between those living in areas with poor food access and those living in areas with adequate access (Table 2). Subjects living in areas with poor access to healthy foods were more likely to be younger and black, with higher education attainment than subjects living in favorable food access areas. Interestingly, subjects living in areas with poor food access had a lower prevalence of smoking and prior MI, higher high‐density lipoprotein cholesterol levels, and were more likely to live in urban areas (Table S1). However, there were no significant differences in the rates of adverse cardiovascular outcomes including MI or death/MI between patients living in areas with poor and adequate access to healthy foods in both univariate and multivariable analyses (Tables 2 and 3, Figures 1B and 2B).
Area Income and Incident Cardiovascular Outcomes
The cohort was also categorized on the basis of area income. Patients who lived in low‐income areas were more likely to be younger, female, and black, with lower educational attainment. They were also less likely to live in urban areas and had higher prevalence of hypertension, diabetes mellitus, smoking, and higher BMI as well as higher total cholesterol and low‐density lipoprotein cholesterol levels (Table S1). In univariate analysis, patients living in low‐income areas had a 54% higher risk of incident MI (sHR, 1.54 [1.19–2.00]; P=0.001) and 29% higher risk of death or MI (hazard ratio, 1.29 [1.13–1.46]; P<0.001) (Tables 2 and 3, Figures 1C and 2C). After adjustment for the baseline demographics and cardiovascular risk factors, patients living in low‐income compared with high‐income areas had a 39% higher risk of incident MI (sHR, 1.39 [1.06–1.83]; P=0.019]) and an 18% higher risk of death/MI (hazard ratio, 1.18 [1.03–1.35]; P=0.033) (Table 3).
Relationship Between Area Income and Food Access and Incident Cardiovascular Outcomes
When area income and food access were examined simultaneously in the same model with the aforementioned covariate adjustment, living in low‐income areas remained significantly associated with elevated risk of adverse outcomes, including MI (sHR, 1.40 [1.06–1.85]) and death/MI (1.18 [1.02–1.35]), independent of food access. On the other hand, living in areas with poor access to food continued to be unassociated with adverse outcomes (Table 3, Model 2).
Based on the combination of area income and food access, 1141 (23%) lived in a high‐income, adequate‐access area; 1614 (33%) in a high‐income, poor‐access area; 1208 (24%) in a low‐income, adequate‐access area; and the remainder (981; 20%) lived in an FD (Table S2). The prevalence of traditional cardiovascular risk factors, such as age, male sex, black race, hypertension, diabetes mellitus, and smoking habits, generally tended to be similar according to the income level of the area, not access to food (Table S2). Similarly, the incident rates of adverse outcomes were similar among those with similar level of area income, regardless of access to food (Figure 3, Table S3). For example, the incidence of MI was similar between subjects living in areas with low income/poor access (FD) and in areas with low income/adequate access (log‐rank P=0.49). Furthermore, subjects living in a high‐income/adequate‐access area had comparable risk of MI to those living in a high‐income/poor‐access area (log‐rank P=0.55) (Figure 3A). On the other hand, those living in an FD had a higher risk of incident MI compared with those living in both high‐income/poor‐access and high‐income/adequate‐access areas (log‐rank P=0.002 and 0.039, respectively) (Figure 3A). A similar pattern of relationship was seen with respect to the secondary end point of death/MI as well (Figure 3B).
Figure 3.
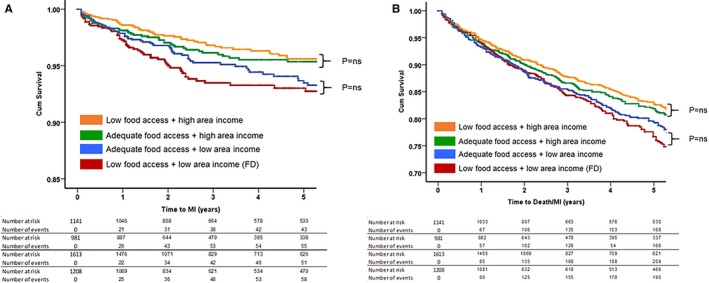
Kaplan–Meier curves for association between food access and area income with (A) incident myocardial infarction (MI) and (B) incident composite event rate of all‐cause death/MI. P values were derived from log‐rank tests. A, Incident MI. B, Incident death/MI. FD indicates food desert; ns, not significant.
Sensitivity Analysis
The association between living in an FD and the increased risk of adverse outcomes was similar across the subgroups stratified by prespecified covariates (Table 4). We also performed sensitivity analyses with respect to low‐income areas, as they were identified as the primary driver of the association between FD and adverse outcomes in the main analysis (Model 2). Similar to FD, the increase in risk of incident MI or death/MI for those living in low‐income areas did not differ across most subgroups stratified by the same covariates. However, we found a trend for a greater increase in risk of MI associated with low‐income areas among those with diabetes mellitus (P‐interaction=0.065) and those living in urban areas (P‐interaction=0.084) compared with their counterparts. For the composite end point of death/MI, there was a trend for greater increase in risk with living in low‐income areas among those without HF than in those with HF (P‐interaction=0.062) (Table 5).
Table 4.
Interaction of Clinical Covariates With Adverse Outcomes Associated With Living in FD
| Subgroups | MI | Death/MI | ||||
|---|---|---|---|---|---|---|
| sHR (95% CI) | P Value | Interaction P Value | HR (95% CI) | P Value | Interaction P Value | |
| Age ≤65 y | 1.48 (0.98–2.24) | 0.06 | 0.61 | 1.23 (0.96–1.58) | 0.10 | 0.36 |
| Age >65 y | 1.36 (0.85–2.18) | 0.19 | 1.12 (0.90–1.39) | 0.31 | ||
| Female | 1.55 (0.93–2.61) | 0.096 | 0.72 | 1.30 (1.00–1.70) | 0.050 | 0.36 |
| Male | 1.41 (0.96–2.08) | 0.078 | 1.11 (0.90–1.37) | 0.32 | ||
| Nonblack | 1.24 (0.84–1.84) | 0.28 | 0.20 | 1.18 (0.98–1.42) | 0.08 | 0.60 |
| Black | 1.88 (1.10–3.22) | 0.022 | 1.05 (0.75–1.48) | 0.78 | ||
| No smoking | 1.73 (0.92–3.23) | 0.087 | 0.47 | 1.17 (0.85–1.61) | 0.35 | 0.93 |
| Smoking | 1.34 (0.94–1.91) | 0.10 | 1.17 (0.97–1.41) | 0.11 | ||
| No heart failure | 1.39 (0.97–1.98) | 0.072 | 0.83 | 1.23 (1.01–1.49) | 0.038 | 0.36 |
| Heart failure | 1.80 (0.95–3.42) | 0.071 | 1.06 (0.78–1.44) | 0.72 | ||
| No CAD | 1.96 (0.61–6.33) | 0.26 | 0.49 | 1.42 (0.96–2.11) | 0.079 | 0.24 |
| CAD | 1.41 (1.03–1.94) | 0.033 | 1.12 (0.94–1.34) | 0.22 | ||
| No diabetes mellitus | 1.16 (0.73–1.85) | 0.53 | 0.14 | 1.22 (0.98–1.51) | 0.072 | 0.81 |
| Diabetes mellitus | 1.78 (1.16–2.72) | 0.008 | 1.12 (0.87–1.45) | 0.38 | ||
| No hypertension | 1.43 (0.52–3.90) | 0.49 | 0.87 | 1.06 (0.69–1.63) | 0.79 | 0.63 |
| Hypertension | 1.45 (1.05–2.00) | 0.025 | 1.19 (1.00–1.42) | 0.057 | ||
| No hyperlipidemia | 1.71 (0.89–3.30) | 0.11 | 0.38 | 1.23 (0.91–1.66) | 0.18 | 0.70 |
| Hyperlipidemia | 1.36 (0.96–1.92) | 0.085 | 1.14 (0.93–1.38) | 0.20 | ||
| Urban | 1.51 (1.03–2.22) | 0.035 | 0.65 | 1.14 (0.92–1.42) | 0.22 | 0.72 |
| Rural | 1.29 (0.58–2.87) | 0.53 | 1.30 (0.92–1.84) | 0.14 | ||
| No college education | 1.57 (1.06–2.35) | 0.026 | 0.59 | 1.14 (0.91–1.43) | 0.24 | 0.57 |
| College education | 1.26 (0.77–2.06) | 0.36 | 1.20 (0.94–1.52) | 0.14 | ||
CAD indicates coronary artery disease; FD, food desert; HR, hazard ratio; MI, myocardial infarction; sHR, subdistribution hazard ratio.
Table 5.
Interaction of Clinical Covariates With Adverse Outcomes Associated With Living in Low‐Income Areas
| Subgroups | MI | Death/MI | ||||
|---|---|---|---|---|---|---|
| sHR (95% CI) | P Value | Interaction P Value | HR (95% CI) | P Value | Interaction P Value | |
| Age ≤65 y | 1.38 (0.92–2.05) | 0.12 | 0.65 | 1.12 (0.90–1.39) | 0.32 | 0.78 |
| Age >65 y | 1.37 (0.93–2.02) | 0.11 | 1.17 (0.98–1.40) | 0.080 | ||
| Female | 1.63 (1.00–2.66) | 0.051 | 0.46 | 1.19 (0.95–1.49) | 0.14 | 0.89 |
| Male | 1.33 (0.95–1.87) | 0.10 | 1.16 (0.98–1.38) | 0.085 | ||
| Nonblack | 1.36 (0.99–1.89) | 0.061 | 0.79 | 1.16 (1.00–1.35) | 0.055 | 0.63 |
| Black | 1.44 (0.84–2.47) | 0.19 | 1.11 (0.81–1.51) | 0.51 | ||
| No smoking | 1.49 (0.85–2.63) | 0.16 | 0.69 | 1.09 (0.84–1.43) | 0.51 | 0.65 |
| Smoking | 1.34 (0.97–1.84) | 0.074 | 1.19 (1.01–1.40) | 0.034 | ||
| No heart failure | 1.53 (1.11–2.11) | 0.009 | 0.13 | 1.26 (1.07–1.49) | 0.006 | 0.062 |
| Heart failure | 1.10 (0.61–1.97) | 0.75 | 0.97 (0.76–1.24) | 0.79 | ||
| No CAD | 1.49 (0.48–4.67) | 0.49 | 0.50 | 1.00 (0.70–1.42) | 0.99 | 0.55 |
| CAD | 1.39 (1.05–1.84) | 0.023 | 1.19 (1.02–1.38) | 0.026 | ||
| No diabetes mellitus | 1.10 (0.74–1.62) | 0.65 | 0.065 | 1.20 (1.00–1.44) | 0.047 | 0.91 |
| Diabetes mellitus | 1.77 (1.19–2.65) | 0.005 | 1.13 (0.91–1.40) | 0.27 | ||
| No hypertension | 1.50 (0.67–3.35) | 0.32 | 0.94 | 1.11 (0.79–1.55) | 0.55 | 0.75 |
| Hypertension | 1.37 (1.02–1.83) | 0.038 | 1.17 (1.00–1.36) | 0.045 | ||
| No hyperlipidemia | 1.41 (0.74–2.68) | 0.29 | 0.54 | 1.11 (0.86–1.44) | 0.42 | 0.63 |
| Hyperlipidemia | 1.37 (1.01–1.86) | 0.047 | 1.18 (1.00–1.38) | 0.052 | ||
| Urban | 1.34 (0.93–1.94) | 0.12 | 0.084 | 1.21 (1.00–1.46) | 0.054 | 0.94 |
| Rural | 2.75 (1.29–5.86) | 0.009 | 1.25 (0.93–1.68) | 0.15 | ||
| No college education | 1.75 (1.16–2.63) | 0.007 | 0.12 | 1.11 (0.92–1.35) | 0.29 | 0.43 |
| College education | 1.10 (0.73–1.66) | 0.64 | 1.21 (1.00–1.47) | 0.052 | ||
CAD indicates coronary artery disease; HR, hazard ratio; MI, myocardial infarction; sHR, subdistribution hazard ratio.
We also examined whether living in FD, poor‐access, or low‐income areas was associated with a higher risk of death following incident MI. Of 230 patients who had MI during the follow‐up, 70 (30.4%) died within 28 days of MI (fatal MI). Nevertheless, living in an FD, poor‐access area, or low‐income area was not significantly associated with the risk of death within 28 days of the index MI (hazard ratio, 1.07 [0.63–1.82], 1.18 [0.73–1.88], and 1.12 [0.70–1.80], respectively).
Discussion
In this large cohort of patients with suspected or confirmed CAD, we demonstrate the clinical impact of living in an FD and its components, area income and food access, on adverse cardiovascular outcomes. We found that subjects living in FDs not only had an unfavorable cardiovascular risk profile but also a higher risk of incident MI independent of cardiovascular risk factors, cardiovascular disease, and education level. Further analyses of the components of FDs revealed that their adverse health impact is mainly driven by area income rather than access to healthy food. To the best of our knowledge, our study is the first to demonstrate the risk of incident adverse cardiovascular outcomes associated with living in an FD, as currently defined by the USDA, using a well‐characterized clinical cohort of patients with cardiovascular disease.
Our findings substantially augment the current understanding of the food environment and its role in cardiovascular disease, by directly demonstrating an independent association between living in an FD and worse outcomes. Over the past 2 decades, a growing body of literature has demonstrated that disadvantaged neighborhoods that are deprived socioeconomically and/or in their physical/social resources are associated with an elevated risk of unfavorable cardiovascular risk profiles10, 26, 27 as well as incident adverse outcomes.28, 29, 30 Similarly, studies also examined factors of food environment in relation to dietary intake patterns31 as well as cardiovascular risk factors, such as obesity,13, 14, 15, 32 diabetes mellitus,16 and hypertension.17, 18 Yet whether living in areas with poor food environment, such as FDs, would lead to adverse health outcomes remains understudied. Recently, we have demonstrated among those without known cardiovascular disease that living in an FD is independently associated with a higher prevalence of cardiovascular risk factors, inflammation, oxidative stress, and arterial stiffness in a cross‐sectional analysis.12 Now, by demonstrating that living in an FD also predicts adverse cardiovascular outcomes including MI and death, independent of the traditional cardiovascular risk factors and demographic differences, we highlight that the relationship between FDs and cardiovascular disease is more likely causal, likely mediated via the unfavorable cardiovascular risk and disease burden associated with FDs, and our finding calls for due attention from a public policy standpoint to develop strategies to intervene and improve cardiovascular outcomes in FDs.
Another unique contribution of our analysis is that we simultaneously examined the 2 crucial components of FDs, low area income and poor access to healthy food, as defined by the USDA, to demonstrate that the association between FDs and adverse outcomes were largely driven by low area income rather than access to healthy foods. This finding indicates that area income and hence the ability to afford healthy food was a greater predictor of cardiovascular events rather than access to healthy foods. Overall, our results suggest that neighborhood characteristics should be considered in risk assessment of patients, while it can also help to further explore and tailor appropriate interventions and public resources to underserved areas.
These results have 3 main implications. First, access to healthy food may be overridden by income and the ability to actually purchase healthy food regardless of whether there is local access to healthy food options. Among those with poor access, living in an area with high income was clearly associated with lower incidence of adverse events. On the other hand, among those living in high‐income areas, whether living in areas with poor or adequate access did not result in any significant difference in the risk of adverse outcomes. Therefore, access to healthy food by itself may not significantly contribute to improved cardiovascular outcomes, and the relative cost of higher‐quality food rather than access may be a major barrier to healthy lifestyle and choices.33, 34 As neighborhood poverty increases, there are fewer supermarkets and more grocery stores, convenience stores, and fast food restaurants, and prices for healthy foods tend to be higher.35, 36, 37, 38 Other characteristics of low‐income areas such as social deprivation, lack of cohesion, decreased access to recreational resources, lack of public space, and safety were not measured in this study but may play an important role in determining outcomes in these area residents.39 It appears that these neighborhood attributes are correlated with area income, which can be the driver for adverse health outcomes, as demonstrated in our analysis.40, 41 Thus, simply providing interventions and resources focused on providing access to healthy food alone may not be enough to overcome disease risk, especially as it relates to cardiovascular outcomes.
Second, access to healthy food options does not necessarily encourage people to purchase and engage in healthy eating behaviors that may decrease cardiovascular disease risk. Studies on the association of food resources, specifically proximity to food establishments, and cardiovascular risk have been inconclusive and limited.42, 43 A study of the Framingham offspring cohort did not find a consistent relationship between BMI and food access.42 Although prior data suggest that FDs may negatively affect risk factors and health behaviors, more research is needed to determine how access to healthy foods influences healthy behaviors and the types of foods. While public health advocates would hope that increased access to affordable and nutritious foods would increase the intake of those foods, studies have shown that even after healthier food options are made more widely available in FDs, consumers often continue to make unhealthy choices based on personal preferences.44 Thus, the role of local food environment interventions to improve healthy food choices coupled with providing food access in communities should be discussed. Evidence‐based interventions to improve healthy food choices and access to food are also needed to inform public health efforts focused on the food environment.45
Third, the fact that our results show that the definition of FD as a composite measure of area income and food access have different associations with cardiovascular outcomes when analyzed individually raise important issues and challenges when measuring the neighborhood environment that should not be overlooked. Importantly, we must consider not only what we measure in the neighborhood environment but how it is measured. Low access to healthy food was measured at the census tract level and defined as having a significant number of individuals (at least 500 people) or share (at least 33%) of the population are >1 mile from a supermarket, supercenter, or large grocery store or supermarket for an urban area or >10 miles for a rural area. One possibility for the null associations found between food access and cardiovascular outcomes in this study may be due to measurement bias or inability to accurately capture food access. A previous study that investigated the validation of the USDA FD measures using secondary data sources of food outlets (Dun & Bradstreet and InfoUSA) to compare with the USDA FD found that across 169 census tracts in South Carolina, the 2 sources had 94% concordance, 50% to 65% sensitivity, and 60% to 64% positive predictive value.21 However, other definitions of food access may be more consistent with how people navigate within their neighborhood and how they define their neighborhood rather than larger areas such as census tracts, which are delineated and based on administrative geographic areas. These differences in associations and considerations suggest that the USDA definition of FD might need further research, validation, and revision if needed on area definitions and measures of FDs and its individual components.
Finally, while there was no statistically significant interaction with selected covariates examined, we noted an interesting trend demonstrating that the impact of living in low‐income areas for adverse outcomes may differ depending whether subjects lived in rural or urban communities. Subjects residing in rural low‐income areas had substantially higher risk of incident MI compared with their rural high‐income area counterparts, whereas this difference was less apparent in urban communities. A study from the National Center for Health Statistics showed similar findings of regional disparity where cardiovascular mortality remained higher in rural compared with urban areas and also in the southern United States.46, 47, 48 Residents in rural areas tend to be older, have higher rates of smoking, hypertension, obesity, and decreased levels of physical activity, with these differences being more prominent in the South.49, 50 Furthermore, residents of rural areas have higher rates of poverty and less access to health care and are less likely to have health insurance.50 The implications of our results are certainly limited given its statistically insignificant interaction term, the number of subgroup comparisons, and the imbalance of the sample size (74% in urban areas). Thus, further examination of the urban/rural differences in relation to the role of food environment and individual health outcomes would be necessary and important to pursue in the future research.
Strengths and Limitations
Our study has several strengths. It investigates the association between components of FDs, both area income and food access, together and separately on cardiovascular outcomes in a region of the United States where there is significant racial and regional disparity in the incidence of cardiovascular disease. Unlike a number of previously published population‐based studies in non–cardiovascular disease populations, our study investigated a population with a high prevalence of CAD. Limitations include a single‐center study with 3 sites from an urban, southeastern US population that may not represent other regions in the United States. Dietary intake data were not available in our cohort, and to demonstrate whether differences in diet mediate the association between living in an FD and adverse outcomes would be important to examine in future research. Furthermore, geographic locations and features can alter over time, and some patients may have changed neighborhoods before and during the course of the study. A recent analysis by the USDA showed a relatively small change in low‐income and poor‐access areas between 2010 and 2015 data.51 Moreover, previous reports suggest that individuals usually move to areas with similar socioeconomic status across their life course.52, 53
Conclusion
Living in an FD was associated with higher risk of incident MI and mortality among patients with CAD, and this association was largely driven by area income rather than access to healthy food. The reasons for the risks posed by low‐income areas in patients with CAD need further exploration. This realization may help refine and better navigate governmental and nongovernmental resources to low‐income areas.
Sources of Funding
Dr Quyyumi is supported by NIH grants 5P01HL101398‐02, 1P20HL113451‐01, 1R56HL126558‐01, 1RF1AG051633‐01, R01 NS064162‐01, R01 HL89650‐01, HL095479‐01, 1U10HL110302‐01, 1DP3DK094346‐01, 2P01HL086773, and the American Heart Association Grant no. 0000031288. Drs Kelli, Kim, Samman Tahhan, and Sandesara are supported by the Abraham J. and Phyllis Katz Foundation. Additional funding sources include NHLBI T32 THL130025A for Drs Kelli and Sullivan, the American Heart Association Grant no. 0000031288 for Dr Kim, and the National Institutes of Health/National Institute on Aging grant AG051633 for Dr Samman Tahhan, and NIH K12HD085850 for Dr. Sullivan. The sponsors of this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
None.
Supporting information
Table S1. Subject Characteristics Stratified by Neighborhood Food Access and by Area Income
Table S2. Subject Characteristics Stratified by Both Neighborhood Food Access and Area Income
Table S3. Rate of Adverse Events According to Area Income and Access to Food
Acknowledgments
The authors appreciate the patients who volunteered to enroll in the Emory Cardiovascular Biobank.
(J Am Heart Assoc. 2019;8:e010694 DOI: 10.1161/JAHA.118.010694.)
References
- 1. Cummins S, Macintyre S. “Food deserts”—evidence and assumption in health policy making. BMJ. 2002;325:436–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Economic Research Service . Food Access Research Atlas 2015. United States Department of Agriculture. https://www.ers.usda.gov/data-products/food-access-research-atlas.
- 3. Economic Research Service . Access to affordable and nutritious food ‐ measuring and understanding food deserts and their consequences: Report to Congress. United States Department of Agriculture. https://www.ers.usda.gov/publications/pub-details/?pubid=42729. Published June 25, 2009. Accessed July 29, 2015.
- 4. Diez Roux AV, Christina M. Neighborhoods and health. Ann NY Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 5. Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: a review of food deserts literature. Health Place. 2010;16:876–884. [DOI] [PubMed] [Google Scholar]
- 6. Beaulac J, Kristjansson E, Cummins S. A systematic review of food deserts, 1966–2007. Prev Chronic Dis. 2009;6:A105. [PMC free article] [PubMed] [Google Scholar]
- 7. Anand SS, Hawkes C, de Souza RJ, Mente A, Dehghan M, Nugent R, Zulyniak MA, Weis T, Bernstein AM, Krauss R, Kromhout D, Jenkins DJA, Malik V, Martinez‐Gonzalez MA, Mozafarrian D, Yusuf S, Willett WC, Popkin BM. Food consumption and its impact on cardiovascular disease: importance of Solutions focused on the globalized food system: a report from the workshop convened by the World Heart Federation. J Am Coll Cardiol. 2015;66:1590–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diez Roux AV, Mujahid MS, Hirsch JA, Moore K, Moore LV. The impact of neighborhoods on CV risk. Glob Heart. 2016;11:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Unger E, Diez‐Roux AV, Lloyd‐Jones DM, Mujahid MS, Nettleton JA, Bertoni A, Badon SE, Ning H, Allen NB. Association of neighborhood characteristics with cardiovascular health in the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calling S, Li X, Kawakami N, Hamano T; Sundquist K. Impact of neighborhood resources on cardiovascular disease: a nationwide six‐year follow‐up. BMC Public Health. 2016;16:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelli HM, Hammadah M, Ahmed H, Ko YA, Topel M, Samman‐Tahhan A, Awad M, Patel K, Mohammed K, Sperling LS, Pemu P, Vaccarino V, Lewis T, Taylor H, Martin G, Gibbons GH, Quyyumi AA. Association between living in food deserts and cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2017;10:e003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gibson DM. The neighborhood food environment and adult weight status: estimates from longitudinal data. Am J Public Health. 2011;101:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghosh‐Dastidar B, Cohen D, Hunter G, Zenk SN, Huang C, Beckman R, Dubowitz T. Distance to store, food prices, and obesity in urban food deserts. Am J Prev Med. 2014;47:587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morland KB, Evenson KR. Obesity prevalence and the local food environment. Health Place. 2009;15:491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Auchincloss AH, Roux A, Mujahid MS, Shen M, Bertoni AG, Carnethon MR. Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: the Multi‐Ethnic Study of Atherosclerosis. Arch Intern Med. 2009;169:1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suarez JJ, Isakova T, Anderson CAM, Boulware LE, Wolf M, Scialla JJ. Food access, chronic kidney disease, and hypertension in the U.S. Am J Prev Med. 2015;49:912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubowitz T, Ghosh‐Dastidar M, Eibner C, Slaughter ME, Fernandes M, Whitsel EA, Bird CE, Jewell A, Margolis KL, Li W, Michael YL, Shih RA, Manson JE, Escarce JJ. The Women's Health Initiative: the food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity (Silver Spring). 2012;20:862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ko Y‐A, Hayek S, Sandesara P, Samman Tahhan A, Quyyumi A. Cohort profile: the Emory Cardiovascular Biobank (EmCAB). BMJ Open. 2017;7:e018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma X, Battersby SE, Bell BA, Hibbert JD, Barnes TL, Liese AD. Variation in low food access areas due to data source inaccuracies. Appl Geogr. 2013;45:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–2888. [DOI] [PubMed] [Google Scholar]
- 23. Sylwester K. Income inequality, education expenditures, and growth. J Dev Econ. 2000;63:379–398. [Google Scholar]
- 24. Rogot E, Sorlie PD, Johnson NJ. Life expectancy by employment status, income, and education in the National Longitudinal Mortality Study. Public Health Rep. 1992;107:457. [PMC free article] [PubMed] [Google Scholar]
- 25. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26. Diez Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, Cooper LS, Watson RL, Szklo M. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol. 1997;146:48–63. [DOI] [PubMed] [Google Scholar]
- 27. Laraia BA, Karter AJ, Warton EM, Schillinger D, Moffet HH, Adler N. Place matters: neighborhood deprivation and cardiometabolic risk factors in the Diabetes Study of Northern California (DISTANCE). Soc Sci Med. 2012;74:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diez Roux AV, Borrell LN, Haan M, Jackson SA, Schultz R. Neighbourhood environments and mortality in an elderly cohort: results from the Cardiovascular Health Study. J Epidemiol Community Health. 2004;58:917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borrell LN, Diez Roux AV, Rose K, Catellier D, Clark BL. Neighbourhood characteristics and mortality in the Atherosclerosis Risk in Communities Study. Int J Epidemiol. 2004;33:398–407. [DOI] [PubMed] [Google Scholar]
- 30. Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 31. Morland K, Wing S, Roux AD. The contextual effect of the local food environment on residents’ diets: the Atherosclerosis Risk in Communities Study. Am J Public Health. 2002;92:1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morland K, Diez Roux AV, Wing S. Supermarkets, other food stores, and obesity: the Atherosclerosis Risk in Communities Study. Am J Prev Med. 2006;30:333–339. [DOI] [PubMed] [Google Scholar]
- 33. Holsten JE. Obesity and the community food environment: a systematic review. Public Health Nutr. 2009;12:397–405. [DOI] [PubMed] [Google Scholar]
- 34. Morland K, Wing S, Diez Roux A, Poole C. Neighborhood characteristics associated with the location of food stores and food service places. Am J Prev Med. 2002;22:23–29. [DOI] [PubMed] [Google Scholar]
- 35. Alwitt LF, Donley TD. Retail stores in poor urban neighborhoods. J Consum Aff. 1997;31:139–164. [Google Scholar]
- 36. Giang T, Karpyn A, Laurison HB, Hillier A, Perry RD. Closing the grocery gap in underserved communities: the creation of the Pennsylvania Fresh Food Financing Initiative. J Public Health Manag Pract. 2008;14:272–279. [DOI] [PubMed] [Google Scholar]
- 37. Lewis LB, Sloane DC, Nascimento LM, Diamant AL, Guinyard JJ, Yancey AK, Flynn G. African Americans’ access to healthy food options in South Los Angeles restaurants. Am J Public Health. 2005;95:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung C, Myers SL Jr. Do the poor pay more for food? An analysis of grocery store availability and food price disparities. J Consum Aff. 1999;33:276–296. [Google Scholar]
- 39. Diez Roux AV. Residential environments and cardiovascular risk. J Urban Health. 2003;80:569–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mode NA, Evans MK, Zonderman AB. Race, neighborhood economic status, income inequality and mortality. PLoS One. 2016;11:e0154535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Block JP, Christakis NA, O'Malley AJ, Subramanian S. Proximity to food establishments and body mass index in the Framingham Heart Study offspring cohort over 30 years. Am J Epidemiol. 2011;174:kwr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the US. Am J Prev Med. 2009;36:74–81.e10. [DOI] [PubMed] [Google Scholar]
- 44. Dubowitz T, Ghosh‐Dastidar M, Cohen DA, Beckman R, Steiner ED, Hunter GP, Flórez KR, Huang C, Vaughan CA, Sloan JC, Zenk SN, Cummins S, Collins RL. Diet and perceptions change with supermarket introduction in a food desert, but not because of supermarket use. Health Aff. 2015;34:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Penney TL, Brown HE, Maguire ER, Kuhn I, Monsivais P. Local food environment interventions to improve healthy food choice in adults: a systematic review and realist synthesis protocol. BMJ Open. 2015;5:e007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gillum RF, Mehari A, Curry B, Obisesan TO. Racial and geographic variation in coronary heart disease mortality trends. BMC Public Health. 2012;12:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Enterline PE, Rikli AE, Sauer HI, Hyman M. Death rates for coronary heart disease in metropolitan and other areas. Public Health Rep. 1960;75:759. [PMC free article] [PubMed] [Google Scholar]
- 48. Kulshreshtha A, Goyal A, Dabhadkar K, Veledar E, Vaccarino V. Urban‐rural differences in coronary heart disease mortality in the United States: 1999–2009. Public Health Rep. 2014;129:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martin SL, Kirkner GJ, Mayo K, Matthews CE, Larry J, Hebert JR. Urban, rural, and regional variations in physical activity. J Rural Health. 2005;21:239–244. [DOI] [PubMed] [Google Scholar]
- 50. Moy E. Leading causes of death in nonmetropolitan and metropolitan areas—United States, 1999–2014. MMWR Surveill Summ. 2017;66:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rhone A, Ver Ploeg M, Dicken C, Williams R, Breneman V. Low‐income and low‐supermarket‐access census tracts, 2010–2015. United States Department of Agriculture, Economic Research Service; January 2017 Economic Information Bulletin‐165.
- 52. Sampson RJ, Sharkey P. Neighborhood selection and the social reproduction of concentrated racial inequality. Demography. 2008;45:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Glass TA, Bilal U. Are neighborhoods causal? Complications arising from the “stickiness” of ZNA. Soc Sci Med. 2016;166:244–253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Subject Characteristics Stratified by Neighborhood Food Access and by Area Income
Table S2. Subject Characteristics Stratified by Both Neighborhood Food Access and Area Income
Table S3. Rate of Adverse Events According to Area Income and Access to Food


