Abstract
Background
Central arteriovenous fistula (AVF) creation is under investigation for treatment of severe hypertension. We evaluated the effects of AVF for initiation of hemodialysis on systolic, diastolic, and mean arterial blood pressure in patients with end‐stage renal disease.
Methods and Results
Data search included PubMed, Web of Science, and the Cochrane Library. A systematic review and meta‐analysis of peer‐reviewed studies reporting the effects of the creation/ligation of an AVF on blood pressure in patients with end‐stage renal disease was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analysis), PRISMA‐P (PRISMA for systematic review protocols), and ROBINS‐I (Risk of Bias in Non‐Randomized Studies) criteria by the Cochrane Bias Methods Group. All studies in which the results could have been biased by hemodialysis were excluded. A total of 14 trials including 412 patients with end‐stage renal disease (AVF creation, n=185; AVF ligation, n=227) fulfilled the criteria and were subsequently analyzed. Average blood pressure in patients with no/closed AVF was 140.5/77.6 mm Hg with a mean arterial blood pressure of 96.1 mm Hg. Following creation of AVF, systolic blood pressure significantly decreased by 8.7 mm Hg (P<0.001), diastolic blood pressure by 5.9 mm Hg (P<0.001), and mean arterial blood pressure by 6.6 mm Hg (P=0.02), whereas after ligation systolic blood pressure increased by 5.2 mm Hg (P=0.07), diastolic blood pressure by 3.8 mm Hg (P=0.02), and mean arterial blood pressure by 3.7 mm Hg (P=0.07) during short‐ to long‐term follow‐up.
Conclusions
Creation of AVF significantly decreases blood pressure in patients with end‐stage renal disease, whereas blood pressure tends to increase after ligation. These findings illustrate the hemodynamic consequences of AVF which are under investigation for severe hypertension.
Keywords: arteriovenous fistula, blood pressure, end‐stage renal disease, hypertension, shunt
Subject Categories: High Blood Pressure, Hypertension, Physiology, Treatment, Cardiovascular Surgery
Clinical Perspective
What Is New?
This is the first systematic review and meta‐analysis further specifying the effects of arteriovenous fistula creation and ligation on blood pressure.
This systematic meta‐analysis documented significant reductions in blood pressure following surgical arteriovenous fistula creation and, conversely, blood pressure increases after arteriovenous fistula ligation in patients with end‐stage renal disease.
What Are the Clinical Implications?
Owing to the large number of patients with end‐stage renal disease requiring hemodialysis treatment worldwide, the hemodynamic consequences following arteriovenous fistula are of importance.
These data enhance our understanding of the physiological changes in patients with end‐stage renal disease on hemodialysis and hypertension treatments using a stent‐coupler.
Central arteriovenous fistula (AVF) creation using a stent‐like device (ROX coupler) is under investigation for treatment of severe resistant hypertension and has been shown to significantly reduce office and ambulatory 24‐hour blood pressure (BP) when compared with drug treatment only.1 The principle is based on surgical observations in which BP variations were documented following surgical AVF creation, noted almost 100 years ago.2 Surgical creation of an AVF is often desirable for initiation of hemodialysis in patients with end‐stage renal disease (ESRD), which affects >1.5 million patients in Europe and the United States.3, 4 AVF exposes the circulation to significant hemodynamic changes as a consequence of fluid redistribution from the high pressure, low capacitance arterial to the low pressure, high capacitance venous system resulting in (1) reduced peripheral resistance, (2) increased right ventricular preload, and (3) left ventricular unloading. Although a large number of patients have undergone AVF creation, the focus so far has mainly been on adverse hemodynamic effects in ESRD.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Reports on other clinically relevant variables, particularly BP, are scarce. Consequently, in addition to a lowering of BP after AVF creation, an increase in BP may be observed following AVF ligation.12, 13, 14, 15, 16, 17, 18, 19 A systematic meta‐analysis on BP effects following AVF creation/closure has not been reported. Hence, we sought to identify and analyze eligible studies in which BP was recorded before and after creation and ligation of AVF.5, 6, 7, 8, 9, 10, 11, 12, 13, 16, 17, 18, 19, 20
Methods
The authors declare that all supporting data are available within the article. This systematic review and meta‐analysis were performed according to the PRISMA, PRISMA‐P, and ROBINS‐I (Risk of Bias in Non‐Randomized Studies) criteria by the Cochrane Bias Methods Group.21, 22, 23, 24
Study Protocol
A systematic literature search was completed for all peer‐reviewed and published studies which reported office BP (systolic blood pressure [SBP], diastolic blood pressure [DBP], and mean arterial blood pressure [MAP]) in patients prior and after creation/ligation of an AVF, respectively. Studies not providing valid information on if patients were on hemodialysis while BP was measured have been excluded such as trials in which the time to the last hemodialysis session remained unclear, as well as studies with insufficient BP data. Additionally, only original papers were included; systematic reviews and case reports were excluded.
Literature Research and Data Extraction
Two investigators (S.S.S., L.L.) searched PubMed, Web of Science, and the Cochrane Library independently for eligible studies published until June 2018. The search was performed using the terms: (((Graft fist*) or (cimin*) or (dialys* shunt*) or (arterio* fistul*)) and (blood pressure)). The same investigators screened the search results according to the title and abstract, reviewed the full‐text articles, considered the paper for inclusion, and extracted appropriate data from the publications. To ensure comparability of the measurements, we used only non‐invasive office BP measurements, with a clear relationship to hemodialysis. Measurements were taken before hemodialysis, on a non‐dialysis day, and in patients not requiring hemodialysis in general, as depicted in Tables 1 and 2. Further details on BP measurement modalities included automated,6, 9, 10, 11, 18, 19 sphygmomanometric,12, 13, 17, 20 and thoracic bioimpedance measurements.16 Details on attended/unattended measurements were not included. If not reported, corresponding authors were contacted to obtain missing data on BP values, thereafter effect‐size was calculated based on the available data. Information on BP (SBP, DBP, and MAP), type of intervention (AVF creation/ligation), heart rate, fistula flow, age, sex, medication, sample size, and chronic kidney disease stage were collected.
Table 1.
Study characteristics
| Author | Intervention | BPb (pre‐intervention) (mm Hg) | BPb (post‐intervention) (mm Hg) | HRb (bpm) | Shunt flowb (mL/min) | Medicationb | Time of Measurement (post‐intervention) |
|---|---|---|---|---|---|---|---|
| Casagrande5 |
Creation, n=20 (13 ♂/7 ♀) |
155/90/a±16/13/a | 141/84/a±15/8/a | 66±9 vs 66±9 | 360±160 | a | 10 d |
| Utescu6 |
Creation, n=31 (20♂/11♀) |
132/78/96±17/11/12 | 124/71/89±23/12/15 | 71±14 vs 70±14 | 1050±410 | a | 3 mo |
| Ori7 |
Creation, n=10 (5♂/5♀) |
154/90/113±32/14/21 | 147/87/107±22/11/15 | 83±18 vs 79±3 | 250 to 300 | 2.5±1.5 vs 2.2±1.4 | 13 d |
| Iwashima8 |
Creation, n=16 (11♂/5♀) |
159/83/a±16/12/a | 147/78/a±16/12/a | 66±8 vs 66±8 | a | No changes | 14 d |
| Korsheed9 |
Creation, n=30 (20♂/10♀) |
144/75/a±28/12/a | 134/66/a±21/11/a | 60±11 vs 60±11 | 735±600 | No changes | 2 wks |
| Vizinho10 |
Creation, n=44 (27♂/17♀) |
142/79/a±16/14/a | 132/77/a±31/19/a | 77±11 vs 78±12 | a | No changes | 2 mo |
| Saratzis11 |
Creation, n=10 (8♂/2♀) |
a/a/98±a/a/11 | a/a/90±a/a/12 | a | 457 to 1350 | a | 30 d |
| Kurita12 |
Ligation, n=33 (26♂/7♀) |
121/67/a±22/14/a | 126/71/a±21/10/a | 84±13 vs 77±13 | 2000±1300 | 1.2±1.4 vs 1.4±1.3 | 7 to 30 d |
| van Duijnhoven13 |
Ligation, n=20 (15♂/5♀) |
135/79/a±17/7 | 136/81/a±18/10/a | 72±10 vs 69±9 | 1790±648 | 1.8±1.6 vs 1.7±1.6 | 3 to 4 mo |
| Aitken16 |
Ligation, n=100 (51♂/49♀) |
a/a/90±a/a/17 | a/a/92±a/a/18 | 94±18 vs 86±22 | 965±235 | No changes | 15 min |
| Velez‐Roa17 |
Ligation, n=23 (13♂/10♀) |
a/a/98±a/a18 | a/a/112±a/a/19 | 71±14 vs 61±14 | a | a | 30 s |
| Unger18 |
Ligation, n=17 (7♂/10♀) |
131/78/96±19/15/16 | 138/85/103±14/10/11 | 73±10 vs 68±8 | 1371±727 | a | Within 21 mo |
| Vaes19 |
Ligation, n=23; HFS (10♂/13♀) |
111/57a±29/19/a | 123/63/a±29/24/a | Decrease by 3±1 | 3026±705 | No changes | 15 s |
|
Ligation, n=11; HAIDI (9♂/2♀) |
116/48/a±30/13/a | 122/50/a±30/13/a | Decrease by 3±1 | 1078±461 | |||
| Dundon20 |
Creation, n=24 (14♂/10♀) |
146±19 | 146±17 | 71±12 vs 76±11 | a | a | 6 mo |
♀ indicates female participants; ♂, male participants; antihypertensive medication (pre vs post); BP, blood pressure (mm Hg); DBP, diastolic blood pressure; HAIDI, hemodialysis access‐induced distal ischemia; HFS, high‐flow shunt; HR, heart rate (bpm); MAP, mean blood pressure, values expressed as SBP/DBP/MAP±SD, expressed as SDSBP/SDDBP/SDMAP; SBP, systolic blood pressure; shunt flow (mL/min).
Not available.
Values expressed as mean±SD.
Table 2.
Additional Characteristics
| Author | Inclusion Criteria | Exclusion Criteria | Age (y) | BMI | Relationship to Hemodialysis | |
|---|---|---|---|---|---|---|
| Casagrande5 | ESRD, measurements performed on the day after midweek dialysis | No functional AVF 3 mo post‐intervention, extreme BP values (SBP >190 mm Hg or <80 mm Hg), severe congestive heart failure, previous AVF, acute heart failure, stroke, acute coronary syndrome in the preceding 3 months and during the study period | 60 | a | On the day after hemodialysis | |
| Utescu6 | ESRD, patients who were scheduled for an AVF creation | No functional AVF 3 mo post‐intervention, extreme BP values (SBP >190 mm Hg or <80 mm Hg), severe congestive heart failure, previous AVF, acute heart failure, stroke, acute coronary syndrome in the preceding 3 mo and during the study period, transfer to another institution | 58 | 29 | Before weekly hemodialysis | |
| Ori7 | ESRD, patients who were scheduled for an AVF creation | Valvular pathology or regional wall motion abnormalities on echocardiography | 60 | a | Before first hemodialysis | |
| Iwashima8 | ESRD, patients who were scheduled for an AVF creation | Wall motion abnormalities on echocardiography changes in medication, initiation of hemodialysis, with ischemic heart disease, including myocardial infarction, congestive heart failure, valvular heart disease, or atrial fibrillation | 68 | a | Before first hemodialysis | |
| Korsheed9 | ESRD (including CKD 4/5), >18 y, clinically stable, did not receive any hemodialysis modality before | Heart transplantation | 69 | 29 | Before first hemodialysis | |
| Vizinho10 | CKD patients not yet on dialysis and clinical indication for AVF creation, >18 y, AVF still functioning 2 months after surgery | Non‐eligibility for AVF construction, limb amputation and non‐consenting patients | 65 | a | Before first hemodialysis | |
| Saratzis11 | ESRD, patients who were scheduled for an AVF creation | Heart failure | 65 | a | Not after hemodialysis | |
| Kurita12 | Consecutive hemodialysis patients who were referred because of symptomatic and refractory heart failure and underwent AVF closure, AVF included both AVF and grafts, NYHA ≥2 | Pulmonary edema because of temporal over‐hydration | 68 | a | On a non‐hemodialysis day | |
| van Duijnhoven13 | Functioning kidney transplant with stable renal function Seventeen patients had a Cimino fistula, 2 patients a brachial fistula, and 1 patient a graft | Heart failure | 51 | a | Kidney transplant recipients | |
| Aitken16 | Patients with ESRD on hemodialysis, >18 y with an established radio (n=60)‐ or brachiocephalic (n=40) AVF | If the AVF was inadequately mature to sustain hemodialysis blood flows ≥250 mL/min or if it was under current investigation for possible stenosis | 57 | a | 15 min after digital compression immediately after hemodialysis | |
| Velez‐Roa17 |
Kidney transplant recipients with patent AVF All were in sinus rhythm |
46 | a | Kidney transplant recipients | ||
| Unger18 | Kidney transplant patients were referred to the cardiology department for cardiac assessment before closure of an AVF, all were in sinus rhythm | Wall motion abnormalities on echocardiography, congenital heart disease, heart transplantation, valvular heart disease | 48 | 24 | Kidney transplant recipients | |
| Vaes19 | HFS | Hemodialysis patients, only patients with an arm shunt, shunt flow ≥2 L/min, hand ischemia, objective signs of hypoperfusion | Language barrier, no consent, impaired mental capacity | 51 | a | 15 s after intra‐operative clamping |
| HAIDI | hemodialysis patients, only patients with an arm shunt, shunt flow ≥2 L/min, hand ischemia, objective signs of hypoperfusion | Language barrier, no consent, impaired mental capacity | 72 | a | 15 s after intra‐ operative clamping | |
| Dundon20 | ESRD, patients who were scheduled for an AVF creation | Significant preexisting valvular heart disease, contraindications to magnetic resonance imaging | 59 | a | Before first hemodialysis | |
AVF indicates arteriovenous fistula; BMI, body mass index; BP, blood pressure (mm Hg); CKD, chronic kidney disease; ESRD, end‐stage renal disease; HAIDI, hemodialysis access‐induced distal ischemia; HFS, high‐flow shunt; SBP, systolic blood pressure; NYHA: New york heart association.
Not available.
Assessment of Bias
The bias within and across the studies was further assessed (Table 3) by the investigators based on the ROBINS‐I criteria in Non‐Randomized Studies—of Interventions by the Cochrane Bias Methods Group (BMG). The overall bias was judged in reference to the criteria as moderate for all included studies. In case of disagreements a third investigator (F.M.) was consulted.
Table 3.
Risk of Bias in Non‐Randomized Studies
| Confounding | Selection of Participants | Classification | Deviations From Interventions | Missing Data | Measurement of Outcomes | Selective Reporting | Judgement of Overall Bias | |
|---|---|---|---|---|---|---|---|---|
| Casagrande5 | PN | U | PN | U | PN | U | N | Moderate |
| Utescu6 | PN | U | PN | U | PN | U | PN | Moderate |
| Ori7 | PN | U | PN | Y | PN | PN | PN | Moderate |
| Iwashima8 | PN | U | PN | N | PN | PN | PN | Moderate |
| Korsheed9 | PN | U | PN | Y | PY | PN | PN | Moderate |
| Vizinho10 | PN | U | PN | U | PY | U | PN | Moderate |
| Unger18 | PN | U | PN | Y | PY | PN | PN | Moderate |
| Kurita12 | PN | U | PN | PY | PN | PN | PN | Moderate |
| Duijnhoven13 | PN | U | PN | Y | PN | PN | PN | Moderate |
| Aitken16 | PN | U | PN | PY | PN | PN | PN | Moderate |
| Velez‐Roa17 | PN | U | PN | PY | PN | PN | PN | Moderate |
| Saratzis11 | PN | U | PN | PN | PN | PN | PN | Moderate |
| Vaes19 | PN | U | PN | U | PN | PN | PN | Moderate |
| Dundon20 | PN | U | PN | U | PN | PN | PN | Moderate |
N indicates low; PN, probably low; PY, probably high; U, unclear; Y, high.
Statistical Analysis
The effects of the intervention (AVF creation/ligation) on BP (SBP, DBP, and MAP) and heart rate were investigated by pooling the data (average BP value, standard deviation and sample size) from each study. Mean differences and pooled mean differences for SBP, DBP, and MAP were determined and presented using Forrest plots along with respective 95% CIs. This was done under the assumption that BP values before and after AVF creation originate from 2 unmatched samples. A fixed‐, or random‐effects model (DerSimonian‐Laird) was used to pool the data, where appropriate. Statistical heterogeneity between the trials was evaluated using Cochran Q Test and I2 statistic as a measure of variability. Relevant statistical heterogeneity was considered as Cochran Q‐Test P<0.05 and I2>50%, in which case a random‐effects was used to estimate the results. Eventual presence of publication bias was explored visually with Funnel plots and formally using the Egger regression asymmetry test. Presence of asymmetry in the Funnel plot was considered as the presence of publication bias. In studies not reporting standard deviation, it was calculated using standard error of the mean. If within one study more than one measurement was existing, the measurement with the highest number of patients and the longest observational period was selected for the meta‐analysis, respectively. Correlation of BP change (mm Hg) and shunt flow (mL/min) was assessed using Pearson correlation coefficient (r2). Shunt flow was measured using dilutional techniques,6, 12, 13, 19 ultrasound,5, 9, 12, 13 and decrease of CO after compression.18 Shunt flow of the ROX Coupler was assumed to be 1000 mL/min (800–1000 mL/min).25 Values are expressed as mean±SD. The statistical analysis was performed using comprehensive meta‐analysis software (CMA). A 2‐sided P≤0.05 was considered as statically significant.
Results
The initial literature search identified 1412 studies from various databases. After duplicates were removed (n=159), from the remaining 1253 studies, 21 were identified as potentially appropriate and eligible for full‐text review. Studies only providing a range of measurements (n=2), those providing insufficient BP data (n=1), and trials providing invalid information about the timing of the last hemodialysis session (n=4) were excluded. A total of 14 studies fulfilled the criteria (Figure 1) out of which, 11 provided data on SBP (n=279 patients), 10 on DBP (n=255), and 6 on MAP (n=191 patients), respectively (Figure 2). The final analysis included a total of 412 patients.
Figure 1.
PRISMA flow diagram showing search and selection strategies. BP indicates blood pressure (mm Hg).
Figure 2.
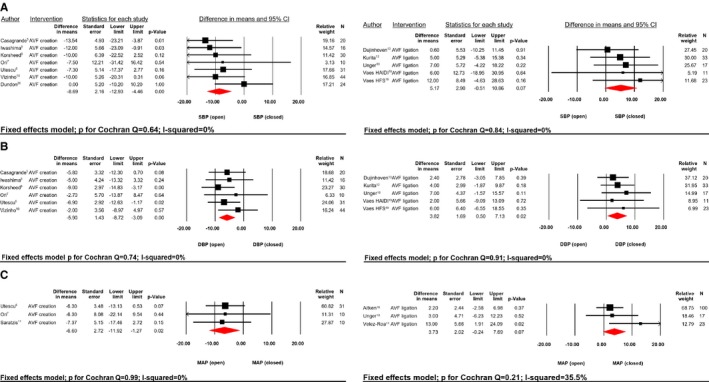
SBP (A), DBP (B), and MAP (C) difference in mean (mm Hg) AVF creation vs AVF ligation. AVF indicates arteriovenous fistula; relative weight (%); DBP, diastolic blood pressure; HAIDI, hemodialysis access‐induced distal ischemia; HFS, high‐flow shunt; MAP, mean blood pressure; SBP, systolic blood pressure.
All trials were unblinded, prospective, single‐center cohort‐studies, except for 1 retrospective analysis.12 The common inclusion criteria where age ≥18 years and scheduled for hemodialysis treatment or ligation of AVF. Common exclusion criteria were regional wall motion abnormalities, heart transplantation, congenital heart disease, extreme BP values (SBP >190 mm Hg, or <80 mm Hg), non‐functioning AVF (after 3 months), stroke, acute coronary syndrome within 3 months of procedure, heart failure and non‐sinus‐rhythm (Table 2).
An AVF ligation was performed when patients underwent kidney transplant, suffered from complications that were suspected to be caused by the AVF, or the hemodialysis treatment was terminated. Rarely, other complications (such as heart failure, swelling of the extremities, cosmetic reasons etc.) were observed and lead to the ligation of the AVF.
Patient characteristics were representative of ESRD patients and relatively homogeneous across all studies (Table 2). However, a lower average patient age was observed in 2 studies involving AVF ligation. Additionally, more male (60.4%) than female patients were included. Shunt flow varied considerably between the studies. The mean shunt flow rate was 725.9±474.7 mL/min (P<0.0001), which was provided by 8 studies (n=285, Table 1). The correlation of shunt flow in relationship to the observed BP changes are depicted in Figure 3. Shunt flow correlated with the SBP change (r2=0.59; P=0.03). Analysis of heart rate showed significant heterogeneity between the studies (P for Cochran Q=0.00; I2=99.9%). The extracted data items are summarized in Tables 1 and 2.
Figure 3.
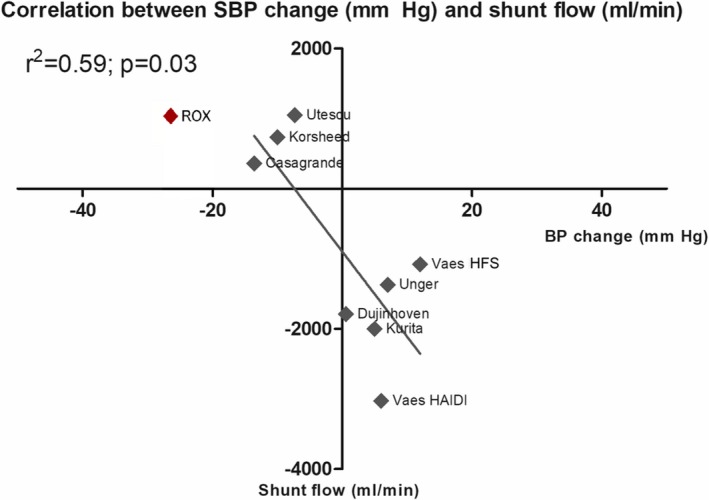
Correlation of mean SBP (mm Hg) and shunt flow (mL/min). Values are expressed as mean; r2: coefficient of determination; Shunt flow in ROX was visualized with 1000 mL/min (800–1000 mL/min)25 for comparison and not included in any calculations. BP indicates blood pressure; HAIDI, hemodialysis access‐induced distal ischemia; HFS, high‐flow shunt; SBP, systolic blood pressure; Rox: Rox medical coupler.
Average SBP, DBP, and MAP were higher in patients without/closed AVF when compared with open AVF (140.5/77.6/96.1±18.8/11.4/15.4 mm Hg versus 136.1/74.7/91.9±20/12.1/15.7 mm Hg; all P<0.0001), respectively. Creation of an AVF lead to a significant reduction in BP whereas AVF ligation, conversely, increased BP (Figure 2A‐C). After creation of an AVF, SBP was reduced by 8.7±28.5 mm Hg; P<0.0001 (P for Cochran Q=0.64; I2=0%), whereas BP tend to increase by 5.2±29.6 mm Hg; P=0.07 (P for Cochran Q=0.84; I2=0%) following closure. Pooling data of patients with closed versus open AVF lead to a significant reduction of mean SBP (Figure 4A) by 7.4±28.9 mm Hg; P<0.0001 (P for Cochran Q=0.83; I2=0%). No sign of significant publication bias (Figure 5) was observed (Funnel plot SBP, Egger test P=0.97).
Figure 4.
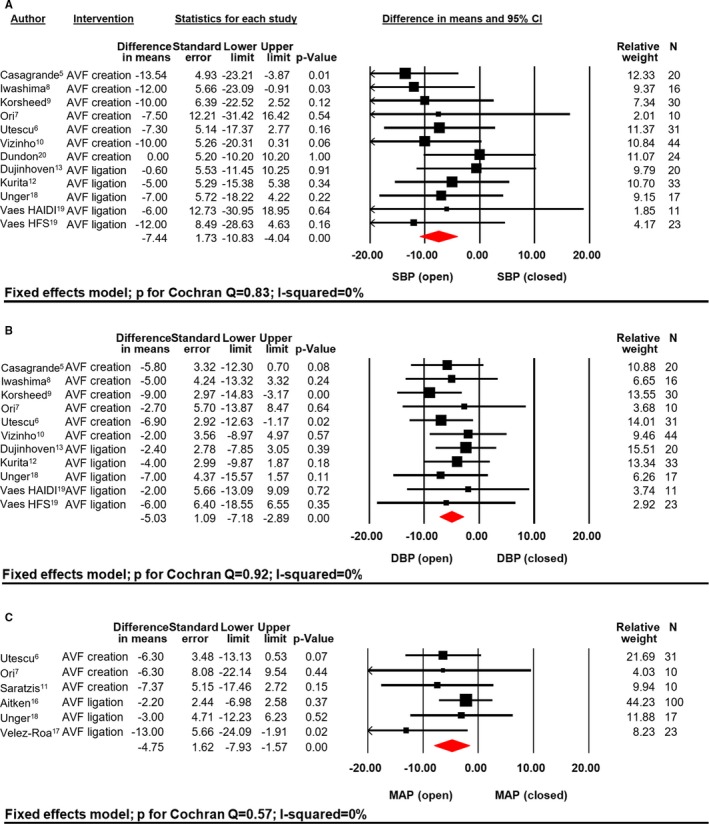
SBP (A), DBP (B), and MAP (C) difference in mean (mm Hg) open vs closed AVF. AVF indicates arteriovenous fistula; relative weight (%); HAIDI, hemodialysis access‐induced distal ischemia; HFS, high‐flow shunt; MAP, mean blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Figure 5.
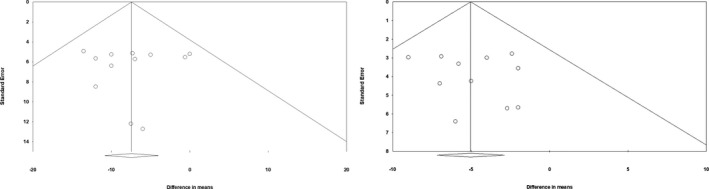
Funnel plot for SBP (left) and DBP (right). DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
After creation of an AVF, DBP was reduced by 5.9±17.6 mm Hg; P<0.0001 (P for Cochran Q=0.74; I2=0%) whereas ligation increased DBP by 3.8±17.23 mm Hg; P=0.02 (P for Cochran Q=0.91; I2=0%). Mean pooled difference in DBP (Figure 4B) between the groups closed versus open AVF resulted in a significant mean difference of 5.0±17.4 mm Hg; P<0.0001 (P for Cochran Q=0.91; I2=0%), showing no significant sign of publication bias, as depicted in Figure 5 (Funnel plot DBP, Egger test P=0.92).
Finally, creation of AVF led to a decrease of MAP (Figure 2C) by 6.6±19.4 mm Hg; P=0.02 (P for Cochran Q=0.99; I2=0%) and ligation to an increase by 3.7±23.9 mm Hg; P=0.07 (P for Cochran Q=0.21; I2=35.5%). Pooling the data for MAP resulted in a significant difference of 4.8±22.4 mm Hg; P=0.003 (P for Cochran Q=0.57; I2=0%, Figure 4C).
In addition, sensitivity analyses were conducted to assess potential biological heterogeneity and impact of time of follow‐up. Patient's age (<60 versus ≥60 years) had no significant impact on SBP (P for heterogeneity=0.12) or DBP (P for heterogeneity=0.88, Figure 6), respectively. Likewise, timing of BP measurement (ultrashort [<1 minute], short [<30 days] and mid‐ to long‐term [>30 days]) did not impact the BP‐lowering effects (P for heterogeneity of SBP=0.34, P for heterogeneity of DBP=0.76, Figure 7).
Figure 6.
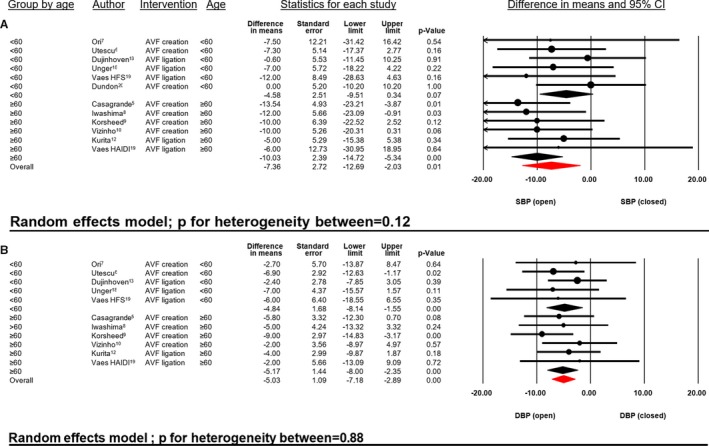
SBP (A) and DBP (B) difference in mean (mm Hg) open vs closed AVF in relationship to patient age (years). AVF indicates arteriovenous fistula; DBP, diastolic blood pressure; HAIDI, hemodialysis access‐induced distal ischemia; HFS, high‐flow shunt; SBP, systolic blood pressure.
Figure 7.
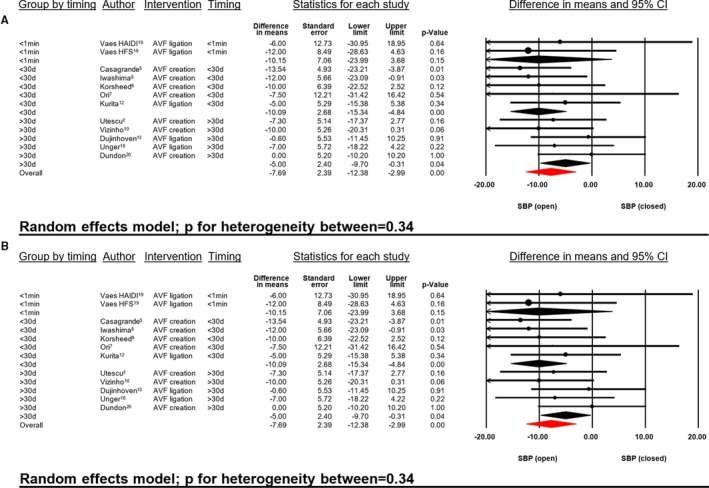
SBP (A) and DBP (B) difference in mean (mm Hg) open vs closed AVF in relationship to timing of BP measurements. AVF indicates arteriovenous fistula; DBP, diastolic blood pressure; HAIDI, hemodialysis access‐induced distal ischemia; HFS, high‐flow shunt; SBP, systolic blood pressure.
Discussion
Creation of an AVF in patients with ESRD requiring hemodialysis significantly lowered SBP, DBP, and MAP whereas ligation of an AVF was associated with an increase in BP. These findings provide novel insights into the hemodynamic consequences of AVF and support the principle of device‐based central iliac AVF creation using a dedicated coupler for treatment of hypertension.
To the best of our knowledge, comprehensive data on BP changes following AVF creation however, are lacking. Hence, this study provides the first systematic review and meta‐analysis, further specifying the effects of AVF creation and ligation on BP in ESRD. During short‐ to long‐term follow‐up after AVF creation, office SBP significantly decreased by 8.7 mm Hg (P<0.001), DBP by 5.9 mm Hg (P<0.001), and MAP by 6.6 mm Hg (P=0.02), respectively. Interestingly, shunt flow correlated with SBP changes during follow‐up. The hypothesis of a clinical meaningful BP reduction following AVF creation is furthermore supported by the documented BP increase following ligation, underlining the plausibility of observed effects (Figure 2).
The consequences of central AVF creation using a 4 mm stent device (shunt volume 800–1000 mL/min) have recently been investigated in ROX Control HTN study, an open‐label, multicenter, prospective, randomized, controlled trial. This study investigated the use of a novel implantable device (ROX Anastomotic Coupler; ROX Medical Inc, San Clemente) and documented a significant reduction of mean 24‐hour ambulatory BP by 13.5/13.5 mm Hg and office BP by 26.9/20.1 mm Hg at 6 months follow‐up in patients randomized to the active coupler‐treated group with no indication of secondary BP rise during 12‐month follow‐up.1, 25 These results indeed support the portrayed BP‐lowering effects after AVF creation in general, whether being surgically created or device‐based.1 The disparity in the magnitude of the observed BP changes may in part be related to differences in the underlying diseases (ESRD versus resistant hypertension) and the differences in baseline BP, which were significantly higher in the ROX coupler study compared with patients included in the present meta‐analysis (175/100 mm Hg versus 140.5/77.6 mm Hg).1 Although the creation of a central AVF using a coupler effectively lowers BP, one has to keep potential side effects in mind, i.e. venous stenosis, leg swelling, increased pulmonary pressure, and right heart dysfunction.1, 12, 13, 18, 19 A recently published case report, in which pressure‐volume loops were recorded before and immediately after central AV‐fistula creation, suggests increased cardiac output and stroke volume following AV‐coupling, though with reduced left ventricular end‐diastolic pressure.26
Average baseline BP of the patients included in this meta‐analysis was 140.5/77.6 mm Hg under treatment with antihypertensive drugs (Table 1), indicating that most of the patients were diagnosed with hypertension. Both systolic and diastolic BP decreased following AVF creation by 8.7/5.9 mm Hg and increased by 5.2/3.8 mm Hg following AVF ligation, substantiating the plausibility of the observed effects. It is important to interpret these findings in the context of cumulating evidence suggesting that even small changes in BP may correspond to significant improvements in cardiovascular morbidity and mortality in patients with hypertension and ESRD.27, 28 Owing to the large number of patients with ESRD requiring Hemodialysis treatment worldwide, the evaluation of the hemodynamic consequences following AVF is of importance. The connection of an artery and a vein typically increases cardiac output, ventricular work, and venous return to the heart. A recently published, retrospective study of 137 ESRD patients, documented at 2‐year follow‐up significant right heart dilatation and deterioration of right heart function, causing incident heart failure in >40% of the patients.14 These data suggest, that volume loading from surgically created shunts may place a major stress on the right heart, causing remodeling and dysfunction. Some studies indeed suggest that the incidence of heart failure and mortality are higher in AVF patients when compared with patients receiving peritoneal dialysis.29, 30, 31 On the other hand, it has been shown that creation of an AVF can modestly reduce left ventricular size, mass, and delay progression of chronic kidney disease in certain patients.14 However, 1‐year results from the ROX CONTROL Hypertension Trial showed no statistically significant change in mean eGFR (estimated glomerular filtration rate).32
Limitations
The results are limited by the observational nature of the published studies, which were qualitatively assessed by the ROBINS I criteria for observational studies. The overall quality of the included studies was judged as moderate. The analysis was not preregistered at PROSPERO. There may have been studies published, in which BP was documented but the word “blood pressure” was not provided in the title, topic, or key words. As a result, these studies may not be included in this meta‐analysis. As with all meta‐analysis, the risk of potential publication bias has to be considered when the results are evaluated. However, no indications for relevant publication bias could be determined using Funnel plot/Egger regression asymmetry test. As mentioned, timing of the hemodialysis and volume status can seriously impact subsequent BP readings, which caused us to exclude studies with unclear timing of hemodialysis sessions. The mean number of antihypertensive drugs remained unchanged over time, although rigorous assessment of adherence to medication was not reported, adherence to medication is typically dynamic and may have affected the BP results. None of the included studies focused on BP changes primarily, and the marked variability when follow‐up measurements were made limits our ability to make exact interferences about the timing of BP changes. To ensure comparability, only office BP measurements, with a clear relationship to hemodialysis, taken on a non‐dialysis day, and in patients not requiring hemodialysis in general, entered the analysis. Further details on BP measurement modalities (automated/semi‐automated) were not available, whereas no study included sufficient information on whether the measurements were attended/unattended.
Perspectives
This systematic meta‐analysis documented significant reductions in BP following AVF creation and conversely BP increases after AVF ligation in patients with ESRD. These data enhance our understanding of the physiological changes in patients with ESRD on hemodialysis and provide the principle of device‐based hypertension treatments using a stent‐coupler. Finally, further investigations are necessary to confirm the documented effects, preferably in randomized, masked, controlled studies, using more reliable measures of BP such as 24‐hour BP monitoring, providing homogeneous data on fistula flow, and long‐term outcomes.
Author Contributions
Scholz acquired the data, performed the analysis, and wrote the manuscript. Vukadinović extracted the data independently, contributed to the manuscript, and supported the statistical analysis. Lauder performed the data search and assessment of bias as a second independent. Ewen corrected the manuscript. Wagenpfeil provided statistical oversight and support. Böhm, Ukena, and Townsend provided critical revision for elemental intellectual features. Mahfoud supplemented key intellectual content, contributed to the manuscript, supported the statistical analysis, data search, assessment of bias, and designed the research. All authors approved the manuscript.
Disclosures
Drs Böhm and Mahfoud are supported by Deutsche Gesellschaft für Kardiologie, Deutsche Hochdruckliga, and Deutsche Forschungsgemeinschaft (SFB TRR 219), and have received grant support and personal fees from Medtronic and Recor Medical. Dr Townsend works with ROX as a consultant/Co‐Principal Investigator of the ROX Control HTN‐2 trial. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e011183 DOI: 10.1161/JAHA.118.011183.)
References
- 1. Lobo MD, Sobotka PA, Stanton A, Cockcroft JR, Sulke N, Dolan E, van der Giet M, Hoyer J, Furniss SS, Foran JP, Witkowski A, Januszewicz A, Schoors D, Tsioufis K, Rensing BJ, Scott B, Ng GA, Ott C, Schmieder RE. Central arteriovenous anastomosis for the treatment of patients with uncontrolled hypertension (the ROX CONTROL HTN study): a randomised controlled trial. Lancet. 2015;385:1634–1641. [DOI] [PubMed] [Google Scholar]
- 2. Holman E, Kolls AC. Experimental studies in arteriovenous fistulas: II. Pulse and blood pressure variations. Arch Surg. 1924;9:837. [Google Scholar]
- 3. United States Renal Data System . 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2017. Available at: https://www.usrds.org/2017/view/Default.aspx. Accessed September 8, 2018.
- 4. Kramer A, Stel V, Zoccali C, Heaf J, Ansell D, Gronhagen‐Riska C, Leivestad T, Simpson K, Palsson R, Postorino M, Jager K. An update on renal replacement therapy in Europe: ERA‐EDTA Registry data from 1997 to 2006. Nephrol Dial Transplant. 2009;24:3557–3566. [DOI] [PubMed] [Google Scholar]
- 5. Casagrande G, Lanzarone E, Miglietta F, Remuzzi A, Fumero R, Costantino ML. Determination of cardiovascular mechanics evolution in the presence of the arteriovenous fistula. ASAIO J. 2009;55:484–493. [DOI] [PubMed] [Google Scholar]
- 6. Utescu MS, LeBoeuf A, Chbinou N, Desmeules S, Lebel M, Agharazii M. The impact of arteriovenous fistulas on aortic stiffness in patients with chronic kidney disease. Nephrol Dial Transplant. 2009;24:3441–3446. [DOI] [PubMed] [Google Scholar]
- 7. Ori Y, Korzets A, Katz M, Perek Y, Zahavi I, Gafter U. Haemodialysis arteriovenous access—a prospective haemodynamic evaluation. Nephrol Dial Transplant. 1996;11:94–97. [PubMed] [Google Scholar]
- 8. Iwashima Y, Horio T, Takami Y, Inenaga T, Nishikimi T, Takishita S, Kawano Y. Effects of the creation of arteriovenous fistula for hemodialysis on cardiac function and natriuretic peptide levels in CRF. Am J Kidney Dis. 2002;40:974–982. [DOI] [PubMed] [Google Scholar]
- 9. Korsheed S, Eldehni MT, John SG, Fluck RJ, McIntyre CW. Effects of arteriovenous fistula formation on arterial stiffness and cardiovascular performance and function. Nephrol Dial Transplant. 2011;26:3296–3302. [DOI] [PubMed] [Google Scholar]
- 10. Vizinho RS, Santos C, Lucas C, Adragão T, Barata JD. Effect of the arteriovenous access for hemodialysis on subendocardial viability ratio, pulse pressure and hospitalizations. J Nephrol. 2014;27:563–570. [DOI] [PubMed] [Google Scholar]
- 11. Saratzis N, Saratzis A, Sarafidis PA, Melas N, Ktenidis K, Kiskinis D. Quantitative evaluation of the systemic effects of transposed basilic vein to brachial artery arteriovenous fistula: a prospective study. J Vasc Access. 2008;9:285–290. [PubMed] [Google Scholar]
- 12. Kurita N, Mise N, Tanaka S, Tanaka M, Sai K, Nishi T, Miura S, Kigawa I, Miyairi T, Sugimoto T. Arteriovenous access closure in hemodialysis patients with refractory heart failure: a single center experience. Ther Apher Dial. 2011;15:195–202. [DOI] [PubMed] [Google Scholar]
- 13. van Duijnhoven ECM, Cheriex ECM, Tordoir JHM, Kooman JP, van Hooff JP. Effect of closure of the arteriovenous fistula on left ventricular dimensions in renal transplant patients. Nephrol Dial Transplant. 2001;16:368–372. [DOI] [PubMed] [Google Scholar]
- 14. Reddy YNV, Obokata M, Dean PG, Melenovsky V, Nath KA, Borlaug BA. Long‐term cardiovascular changes following creation of arteriovenous fistula in patients with end stage renal disease. Eur Heart J. 2017;38:1913–1923. [DOI] [PubMed] [Google Scholar]
- 15. Nakano J, Schryver CD. Effects of arteriovenous fistula on systemic and pulmonary circulations. Am J Physiol. 1964;207:1319–1324. [DOI] [PubMed] [Google Scholar]
- 16. Aitken E, Kerr D, Geddes C, Berry C, Kingsmore D. Cardiovascular changes occurring with occlusion of a mature arteriovenous fistula. J Vasc Access. 2015;16:459–466. [DOI] [PubMed] [Google Scholar]
- 17. Velez‐Roa S, Neubauer J, Wissing M, Porta A, Somers VK, Unger P, van de Borne P. Acute arterio‐venous fistula occlusion decreases sympathetic activity and improves baroreflex control in kidney transplanted patients. Nephrol Dial Transplant. 2004;19:1606–1612. [DOI] [PubMed] [Google Scholar]
- 18. Unger P, Velez‐Roa S, Wissing KM, Hoang AD, van de Borne P. Regression of left ventricular hypertrophy after arteriovenous fistula closure in renal transplant recipients: a long‐term follow‐up. Am J Transplant. 2004;4:2038–2044. [DOI] [PubMed] [Google Scholar]
- 19. Vaes RH, Tordoir JH, Scheltinga MR. Systemic effects of a high‐flow arteriovenous fistula for hemodialysis. J Vasc Access. 2014;15:163–168. [DOI] [PubMed] [Google Scholar]
- 20. Dundon BK, Torpey K, Nelson AJ, Wong DTL, Duncan RF, Meredith IT, Faull RJ, Worthley SG, Worthley MI. The deleterious effects of arteriovenous fistula‐creation on the cardiovascular system: a longitudinal magnetic resonance imaging study. Int J Nephrol Renovasc Dis. 2014;7:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. PRISMA‐P Group , Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Systematic Reviews. Available at: http://systematicreviewsjournal.biomedcentral.com/articles/10.1186/2046-4053-4-1. Accessed May 9, 2018. [DOI] [PMC free article] [PubMed]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A‐W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: https://handbook-5-1.cochrane.org/. Accessed June 12, 2018.
- 25. Foran JP, Jain AK, Casserly IP, Kandzari DE, Rocha‐Singh KJ, Witkowski A, Katzen BT, Deaton D, Balmforth P, Sobotka PA. The ROX coupler: creation of a fixed iliac arteriovenous anastomosis for the treatment of uncontrolled systemic arterial hypertension, exploiting the physical properties of the arterial vasculature. Catheter Cardiovasc Interv. 2015;85:880–886. [DOI] [PubMed] [Google Scholar]
- 26. Ewen S, Lauder L, Böhm M, Mahfoud F. Real‐time left ventricular pressure–volume loops during percutaneous central arteriovenous anastomosis. Eur Heart J. 2018;39:2330–2331. [DOI] [PubMed] [Google Scholar]
- 27. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 28. Coentrão L, Santos‐Araújo C, Dias C, Neto R, Pestana M. Effects of starting hemodialysis with an arteriovenous fistula or central venous catheter compared with peritoneal dialysis: a retrospective cohort study. BMC Nephrol. 2012;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trespalacios FC, Taylor AJ, Agodoa LY, Bakris GL, Abbott KC. Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis. 2003;41:1267–1277. [DOI] [PubMed] [Google Scholar]
- 30. van de Luijtgaarden MWM, Jager KJ, Segelmark M, Pascual J, Collart F, Hemke AC, Remón C, Metcalfe W, Miguel A, Kramar R, Aasarød K, Abu Hanna A, Krediet RT, Schön S, Ravani P, Caskey FJ, Couchoud C, Palsson R, Wanner C, Finne P, Noordzij M. Trends in dialysis modality choice and related patient survival in the ERA‐EDTA Registry over a 20‐year period. Nephrol Dial Transplant. 2016;31:120–128. [DOI] [PubMed] [Google Scholar]
- 31. Rao NN, Dundon BK, Worthley MI, Faull RJ. The impact of arteriovenous fistulae for hemodialysis on the cardiovascular system. Semin Dial. 2016;29:214–221. [DOI] [PubMed] [Google Scholar]
- 32. Lobo MD, Ott C, Sobotka PA, Saxena M, Stanton A, Cockcroft JR, Sulke N, Dolan E, van der Giet M, Hoyer J, Furniss SS, Foran JP, Witkowski A, Januszewicz A, Schoors D, Tsioufis K, Rensing BJ, Scott B, Ng GA, Schmieder RE. Central iliac arteriovenous anastomosis for uncontrolled hypertension: one‐year results from the ROX CONTROL HTN trial. Hypertension. 2017;70:1099–1105. [DOI] [PubMed] [Google Scholar]


