Abstract
Background
The prognostic value of N‐terminal pro–brain natriuretic peptide (NT‐proBNP) in patients with hypertrophic cardiomyopathy who underwent septal myectomy has not been well studied.
Methods and Results
We retrospectively evaluated NT‐proBNP levels in 758 patients (46.1±13.8 years; median follow‐up, 936 days) who underwent septal myectomy in our center between March 2011 and April 2018. The median NT‐proBNP level was 1450.5 (interquartile range 682.6‐2649.5) pg/mL. Overall, 22 (2.9%) patients died during follow‐up; of these, 86.4% were cardiovascular deaths. The 3‐year survival free from all‐cause mortality by tertile was 95.2% (95% CI 91.1% to 97.4%; NT‐proBNP >2080 pg/mL), 98.3% (95% CI 94.6% to 99.5%; NT‐proBNP, 947‐2080 pg/mL), and 99.2% (95% CI, 94.4% to 99.9%; NT‐proBNP <947 pg/mL). The 3‐year survival rate free from cardiovascular mortality by tertiles was 95.2% in the highest tertile, 98.8% in the middle tertile, and 99.2% in the lowest tertile. Cox regression analysis indicated that Ln(NT‐proBNP) was a significantly independent predictor of all‐cause mortality (hazard ratio 2.380, 95% CI 1.356‐4.178, P=0.003) and cardiovascular mortality (hazard ratio 2.788, 95% CI 1.450‐5.362, P=0.002). In addition, concomitant coronary artery bypass grafting for coronary artery disease was also an independent predictor of cardiovascular mortality (hazard ratio 5.178, 95% CI 1.597‐16.789, P=0.006).
Conclusions
Increased preoperative NT‐proBNP level is a strong predictor of midterm mortality in patients undergoing septal myectomy.
Keywords: brain natriuretic peptide, hypertrophic cardiomyopathy, surgery, survival
Subject Categories: Cardiomyopathy, Heart Failure, Cardiovascular Surgery
Clinical Perspective
What Is New?
The N‐terminal pro–brain natriuretic peptide level is associated with many clinical parameters in patients with hypertrophic cardiomyopathy with left ventricular outflow tract obstruction, and increased preoperative N‐terminal pro–brain natriuretic peptide level is a strong predictor of midterm mortality in patients undergoing septal myectomy.
What Are the Clinical Implications?
Preoperative measurement of N‐terminal pro–brain natriuretic peptide may help in the preoperative risk stratification and management of patients with hypertrophic cardiomyopathy who desire to undergo septal myectomy.
Introduction
Hypertrophic cardiomyopathy (HCM) is characterized by left ventricular (LV) hypertrophy not solely explained by secondary loading conditions.1 According to previous reports, LV outflow tract (LVOT) obstruction is present in two thirds of patients with HCM.1 For those with medically refractory symptoms, septal myectomy is the standard of care to relieve LVOT obstruction and its associated symptoms.2, 3 In addition, those undergoing septal myectomy have excellent survival similar to that of an age‐ and sex‐matched general population.4, 5
Earlier studies reported several risk factors to predict postoperative outcomes of patients undergoing septal myectomy.2, 6, 7 A variety of preoperative clinical variables, including increasing age, left atrial enlargement, and extensive late gadolinium enhancement in cardiac magnetic resonance imaging, have been described to predict postoperative prognosis. Because no gold standard for preoperative risk stratification exists, it is valuable to investigate novel predictors and add them to the preoperative risk evaluation of the postoperative outcomes.
Pro–brain natriuretic peptide (proBNP) is a neurohormone synthesized and released primarily from cardiac myocytes as a response to wall stress.8 The proBNP is cleaved into brain natriuretic peptide (BNP) and N‐terminal proBNP (NT‐proBNP). NT‐proBNP is a useful diagnostic and prognostic marker in heart failure and is also used in risk stratification of several cardiovascular disorders.9, 10 Furthermore, it is considered to be more stable than BNP because of its relatively long half‐life.11 In HCM, NT‐proBNP is reported to be associated with echocardiographic features and long‐term outcomes.12, 13 However, there is a paucity of data on the prognostic impact of NT‐proBNP on survival in patients who underwent septal myectomy. Therefore, the purpose of this study was to determine the prognostic value of NT‐proBNP in these patients.
Methods
The authors will not make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedure.
Study Population
A total of 787 patients with HCM with LVOT obstruction and drug‐refractory symptoms who underwent septal myectomy in our center (by Dr Shuiyun Wang) were screened between March 2011 and April 2018. Medically refractory symptoms were defined as the persistence of symptoms despite maximally tolerated drug therapy. Data in our study showed that 582 patients received 1 drug, and 176 patients received more than 1 drug. Patients received maximal doses of drugs unless they could not tolerate the therapy. The diagnosis of HCM was based on the presence of myocardial hypertrophy (maximum wall thickness ≥15 mm) in the absence of any other cardiac or systemic cause that could lead to cardiac hypertrophy. Septal myectomy was performed in patients with HCM with medically refractory symptoms and maximum LVOT gradient or midventricular gradient ≥50 mm Hg at rest or with physiologic provocation.14 We reviewed the medical records of these patients. Finally, 758 eligible patients with preoperative NT‐proBNP were included in this study. Demographic, clinical, and drug use data of these enrolled patients were obtained. The New York Heart Association class was identified by cardiologists before operation. The study was approved by the institutional review committee of Fuwai Hospital, Chinese Academy of Medical Sciences. All subjects gave informed consent.
Echocardiography
Transthoracic echocardiography including 2‐dimensional and Doppler type was performed in each patient using an E9 ultrasound system (GE Healthcare, Horten, Norway). Basal subaortic and midventricular gradients were measured with continuous‐wave Doppler in the apical 3‐chamber view. LV end‐diastolic diameter, LV ejection fraction, LV wall thickness, and left atrial diameter were quantified according to the recommendations of the American Society of Echocardiography.15 Rest LVOT obstruction was documented when a peak gradient ≥30 mm Hg in normal conditions was identified by Doppler.2 Mitral regurgitation was graded semiquantitatively and classified as mild, moderate, or severe.16 Pulmonary hypertension was defined as a pulmonary artery systolic pressure ≥35 mm Hg.
NT‐proBNP Measurement
Venous blood samples were obtained at the time of admission, which was within 10 days preceding myectomy. Blood samples were collected into tubes containing EDTA and immediately processed in the clinical lab in Fuwai Hospital. Plasma NT‐proBNP was measured using an electrochemiluminescence immunoassay (NT‐proBNP, Roche, Mannheim, Germany) by experienced operators. The sensitivity of the test is 5 pg/mL. Both intraassay and interassay coefficients of variance at 175 pg/mL are <5%.
Cardiac Surgery
As described previously, we performed an extended Morrow procedure.14 The hypertrophic ventricular septum resulting in systolic anterior motion of the mitral valve and LVOT obstruction was resected. The resection ranges were as follows: in the long axis, the myectomy started from ≈4 mm below the aortic ring to the apex of the LV; in the short axis, the myectomy started rightward to the nadir of the right aortic cusp and terminated near the mitral anterior commissure. Hypertrophy of the LV anterior free wall leading to LVOT narrowing might also require resection. Furthermore, the anomalous chordal attachments affecting the LVOT were also excised. If intraoperative transesophageal echocardiography detected a postoperative LVOT gradient >30 mm Hg or more than moderate mitral valve regurgitation after weaning from cardiopulmonary bypass, reoperation was required. Concomitant surgery was performed based on expert consensus among the experienced cardiac surgeons.
Follow‐Up
Clinical status was obtained through phone interview with patients or family members at least yearly after septal myectomy. Patients who died were censored the same day. The last follow‐up of survivors was conducted on June 2018. Survival analysis included all‐cause and cardiovascular mortality.
Statistical Analysis
Continuous values are presented as mean±SD. Categorical measures are presented as number (percentage). NT‐proBNP was transformed using ln(NT‐proBNP) to be treated as a normally distributed variable. Spearman rank correlation coefficients and Mann‐Whitney U test were appropriately used to test the correlations of NT‐proBNP levels and clinical variables. The study population was divided into 3 groups according to tertiles of NT‐proBNP. Survival free from the end points of this study (including all‐cause and cardiovascular mortality) was calculated by Kaplan‐Meier survival analysis, and the log‐rank test was used for comparison among the 3 groups. Univariable and multivariable Cox regression analyses were used to assess the association of individual variables with all‐cause and cardiovascular mortality. Age, sex, and variables with P<0.1 in the univariable analysis were included in the multivariable analysis. Of note, all subjects who died from other causes in the study period have been included in the cardiovascular mortality models. These subjects were treated as censored subjects, and the follow‐up time was defined as their dead time. Statistical tests were considered significant if a P‐value was <0.05 (2 sided). All analyses were performed using SPSS version 22.0 (IBM, Armonk, NY) and GraphPad 7.10 (GraphPad Software, La Jolla, CA).
Results
Baseline and Septal Myectomy Procedure Characteristics
A total of 758 consecutive patients with HCM were included in this study (456 men, 60.2%; mean age, 46.1±13.8 years). Of these patients, 618 (81.5%) were categorized as New York Heart Association class III or IV. Resting LVOT obstruction was identified in 691 patients (91.2%). The upper tertile contained more women; patients had lower body mass index (24.3±3.7 kg/m2), larger left atria (45.3±7.9 mm), larger maximum wall thickness (24.4±5.5 mm), and higher LVOT gradients (84.7±26.7 mm Hg). They were more likely to have atrial fibrillation and pulmonary hypertension. Detailed information is shown in Table 1.
Table 1.
Baseline Characteristics
| Variables | Whole Cohort | Lower Tertile (<947 pg/mL) | Middle Tertile (947‐2080 pg/mL) | Upper Tertile (>2080 pg/mL) | P Value |
|---|---|---|---|---|---|
| Number of patients | 758 | 253 | 252 | 253 | |
| Demographics | |||||
| Male | 456 (60.2%) | 182 (71.9%) | 163 (64.7%) | 111 (43.9%) | <0.001 |
| Age, y | 46.1±13.8 | 46.7±12.7 | 46.0±13.7 | 45.6±15.1 | 0.878 |
| BMI, kg/m2 | 25.2±6.8 | 26.6±10.4 | 24.6±3.4 | 24.3±3.7 | <0.001 |
| Family history of HCM | 113 (14.9%) | 28 (11.1%) | 42 (16.7%) | 43 (17.0%) | 0.109 |
| Hypertension | 165 (21.8%) | 68 (26.9%) | 48 (19.0%) | 49 (19.4%) | 0.054 |
| Diabetes mellitus | 27 (3.6%) | 10 (4.0%) | 7 (2.7%) | 10 (4.0%) | 0.713 |
| CAD | 67 (8.8%) | 29 (11.5%) | 19 (7.5%) | 19 (7.5%) | 0.198 |
| History of SRT | 27 (3.6%) | 6 (2.4%) | 11 (4.4%) | 10 (4.0%) | 0.443 |
| Atrial fibrillation | 103 (13.6%) | 17 (6.7%) | 34 (13.5%) | 52 (20.6%) | <0.001 |
| Biventricular pacemaker | 3 (0.4%) | 1 (0.4%) | 2 (0.8%) | 0 | 0.248 |
| Symptoms | |||||
| Chest distress | 698 (92.1%) | 233 (92.1%) | 233 (92.5%) | 232 (91.7%) | 0.951 |
| Chest pain | 227 (29.9%) | 77 (30.4%) | 67 (26.6%) | 83 (32.8%) | 0.306 |
| Syncope | 137 (18.1%) | 41 (16.2%) | 49 (19.4%) | 47 (18.6%) | 0.619 |
| Palpitations | 101 (13.3%) | 34 (13.4%) | 29 (11.5%) | 38 (15.0%) | 0.509 |
| NYHA class III or IV | 618 (81.5%) | 201 (79.4%) | 203 (80.6%) | 214 (84.6%) | 0.293 |
| Clinical variables | |||||
| Systolic blood pressure, mm Hg | 119.2±16.3 | 121.8±14.5 | 118.6±17.6 | 117.1±16.3 | 0.002 |
| Diastolic blood pressure, mm Hg | 71.6±10.1 | 72.7±11.0 | 71.8±9.3 | 70.4±9.8 | 0.016 |
| Heart rate, bpm | 72.5±9.3 | 72.4±8.6 | 72.2±9.4 | 72.8±9.8 | 0.608 |
| Creatinine, μmol/L | 76.2±17.4 | 76.2±15.4 | 76.2±15.7 | 76.3±20.7 | 0.659 |
| Echocardiography | |||||
| Maximum wall thickness, mm | 22.7±5.4 | 20.8±5.3 | 23.0±4.8 | 24.4±5.5 | <0.001 |
| Left atrial diameter, mm | 44.4±7.5 | 43.3±6.7 | 44.7±7.6 | 45.3±7.9 | 0.002 |
| Left atrial dimeter ≥45 mm | 364 (48.0%) | 103 (40.7%) | 130 (51.6%) | 131 (51.8%) | 0.017 |
| LVEDD, mm | 42.1±5.1 | 42.8±5.4 | 42.1±4.6 | 41.4±5.3 | 0.002 |
| LVEF, % | 71.4±6.2 | 71.4±6.1 | 71.1±6.0 | 71.7±6.3 | 0.769 |
| LVOT obstruction at rest | 619 (91.2%) | 213 (84.2%) | 241 (95.6%) | 237 (93.7%) | <0.001 |
| Maximum LVOT gradient, mm Hg | 81.8±26.5 | 78.6±24.2 | 82.2±28.2 | 84.7±26.7 | 0.038 |
| Moderate or severe MR | 419 (55.3%) | 130 (51.4%) | 141 (56.0%) | 148 (58.5%) | 0.265 |
| Pulmonary hypertension | 90 (11.9%) | 20 (7.9%) | 24 (9.5%) | 46 (18.2%) | 0.001 |
| Medications | |||||
| β‐Blocker | 701 (92.5%) | 239 (94.5%) | 230 (90.9%) | 232 (91.7%) | 0.335 |
| CCB | 79 (10.4%) | 35 (13.8%) | 21 (8.3%) | 23 (9.1%) | 0.090 |
| ACEI/ARB | 11 (1.5%) | 5 (2.0%) | 3 (1.2%) | 3 (1.2%) | 0.705 |
| Amiodarone | 83 (10.9) | 25 (9.9%) | 27 (10.7%) | 32 (12.6%) | 0.697 |
| Warfarin | 56 (7.4%) | 12 (4.7%) | 16 (6.3%) | 28 (11.1%) | 0.018 |
Values expressed as mean±SD or number of patients and percentage. ACEI/ARB indicates angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker; BMI, body weight index; bpm, beats/min; CAD, coronary artery disease; CCB, calcium channel blocker; HCM, hypertrophic cardiomyopathy; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; MR, mitral regurgitation; NYHA, New York Heart Association; SRT, septal reduction therapy.
Concomitant surgical procedures were performed in 295 patients (38.9%): mitral valve replacement/repair (96 [12.7%]), tricuspid valve repair (75 [9.9%]), aortic valve replacement/repair (7 [0.9%]), coronary artery bypass grafting (CABG) for myocardial bridging (59 [7.8%]), myocardial unroofing (55 [7.3%]), CABG for coronary artery disease (49 [6.5%]), and the maze procedure (44 [5.8%]). The mean residual LVOT gradient after surgery was 8.1±5.7 mm Hg. The median NT‐proBNP level of the entire cohort was 1450.5 (interquartile range 670‐2626.8) pg/mL. NT‐proBNP concentrations were higher in women (2590±2200 pg/mL; median 1948.0 pg/mL, interquartile range 1025.0‐3430.0 pg/mL) than in men (1635±1849 pg/mL; median 1152.4 pg/mL, interquartile range 590.7‐2026.8 pg/mL). The aortic clamp time, postoperative hospital stay, postoperative LVOT gradient, and maze procedure rate were significantly higher in the upper tertile (Table 2).
Table 2.
Intraoperative and Postoperative Outcomes
| Variables | Whole Cohort | Lower Tertile (<947 pg/mL) | Middle Tertile (947‐2080 pg/mL) | Upper Tertile (>2080 pg/mL) | P Value |
|---|---|---|---|---|---|
| Aortic clamp time, min | 70.9±32.6 | 67.8±32.5 | 68.3±27.9 | 76.4±36.1 | 0.002 |
| Concomitant operative procedures | |||||
| Myocardial unroofing | 55 (7.3%) | 20 (7.9%) | 18 (7.1%) | 17 (6.7%) | 0.873 |
| CABG for myocardial bridge | 59 (7.8%) | 17 (6.7%) | 24 (9.5%) | 18 (7.1%) | 0.445 |
| CABG for CAD | 49 (6.5%) | 22 (8.7%) | 11 (4.4%) | 16 (6.3%) | 0.140 |
| Aortic valve procedure | 7 (0.9%) | 1 (0.4%) | 1 (0.4%) | 5 (2.0%) | 0.10 |
| Mitral valve procedure | 96 (12.7%) | 36 (14.2%) | 27 (10.7%) | 33 (13.0%) | 0.482 |
| Tricuspid valve procedure | 75 (9.9%) | 21 (8.3%) | 20 (7.9%) | 34 (13.4%) | 0.068 |
| Maze procedure | 44 (5.8%) | 7 (2.8%) | 12 (4.8%) | 25 (9.9%) | 0.002 |
| Perioperative pacemaker | 14 (1.8%) | 6 (2.4%) | 5 (2.0%) | 3 (1.2%) | 0.584 |
| Postoperative hospital stay, d | 8.4±4.7 | 8.0±2.9 | 7.8±2.9 | 9.5±6.8 | <0.001 |
| Postoperative LVOT gradient, mm Hg | 8.1±5.7 | 7.3±5.4 | 8.1±6.0 | 8.8±5.5 | 0.001 |
Values expressed as mean±SD, median and interquartile range, or number of patients and percentage. CABG indicates coronary artery bypass grafting; CAD, coronary artery disease; LVOT, left ventricular outflow tract.
The association of NT‐proBNP with demographic, clinical, and echocardiographic parameters is presented in Table 3. NT‐proBNP had a positive correlation with left atrial diameter and maximum wall thickness and a negative correlation with LV end‐diastolic diameter. Moreover, NT‐proBNP correlated with male sex, LVOT obstruction at rest, moderate or severe mitral regurgitation, pulmonary hypertension, and history of atrial fibrillation.
Table 3.
Relation of NT‐proBNP With Baseline Characteristics
| Variable | Spearman ρ | P Value |
|---|---|---|
| Age | −0.014 | 0.71 |
| Male | <0.001 | |
| Creatinine | −0.038 | 0.30 |
| Body mass index | −0.228 | <0.001 |
| NYHA class III or IV | 0.203 | |
| Left atrial diameter | 0.132 | <0.001 |
| Left atrial diameter ≥45 mm | 0.004 | |
| LV end‐diastolic dimension | −0.128 | <0.001 |
| LV ejection fraction | 0.000 | 0.995 |
| Maximum wall thickness | 0.315 | <0.001 |
| LVOT gradient | 0.098 | 0.007 |
| LVOT obstruction at rest | <0.001 | |
| Moderate or severe mitral regurgitation | 0.043 | |
| Pulmonary hypertension | <0.001 | |
| Atrial fibrillation | <0.001 |
Spearman rank correlation coefficients and Mann‐Whitney U test were appropriately used to test the correlations of NT‐proBNP levels and clinical variables. LV indicates left ventricular; LVOT, left ventricular outflow tract; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; NYHA, New York Heart Association.
Survival After Septal Myectomy
During a median follow‐up period of 936 (interquartile range 472‐1758) days, 22 patients (2.9%) died. Of these, 19 (86.4%) were cardiovascular deaths including 10 sudden cardiac deaths, 7 deaths related to heart failure, and 1 death each from myocardial infarction and infective endocarditis. The 3‐year survival of this study cohort was 97.5% (95% CI 94.0% to 98.3%). The 3‐year survival free from all‐cause mortality was 95.2% (95% CI 91.1% to 97.4%) in the upper tertile (NT‐proBNP >2080 pg/mL), 98.3% (95% CI 94.6% to 99.5%) in the middle tertile (NT‐proBNP, 947‐2080 pg/mL), and 99.2% (95% CI 94.4% to 99.9%) in the lowest tertile (NT‐proBNP <947 pg/mL). The 3‐year survival free from cardiovascular mortality was 95.2% (95% CI 91.1% to 97.4%) in the upper tertile, 98.8% (95% CI 95.0% to 99.7%) in the middle tertile, and 99.2% (95% CI 94.4% to 99.9%) in the lowest tertile. The Kaplan‐Meier lifetime analysis showed a significant difference in the survival free from either all‐cause mortality (overall log‐rank P=0.0014, Figure 1) or cardiovascular mortality (overall log‐rank P<0.001, Figure 2) among the 3 groups. Furthermore, the survival free from all‐cause mortality was significantly lower in the highest tertile (log‐rank P=0.0048 for highest and middle tertiles, and log‐rank P=0.0014 for highest and lowest tertiles, while log‐rank P=0.16 for lowest and middle tertiles). The survival free from cardiovascular mortality was significantly lower in the highest tertile (log‐rank P=0.0017 for highest and middle tertile, and log‐rank P=0.0014 for highest and lowest tertile, while log‐rank P=0.60 for lowest and middle tertile).
Figure 1.
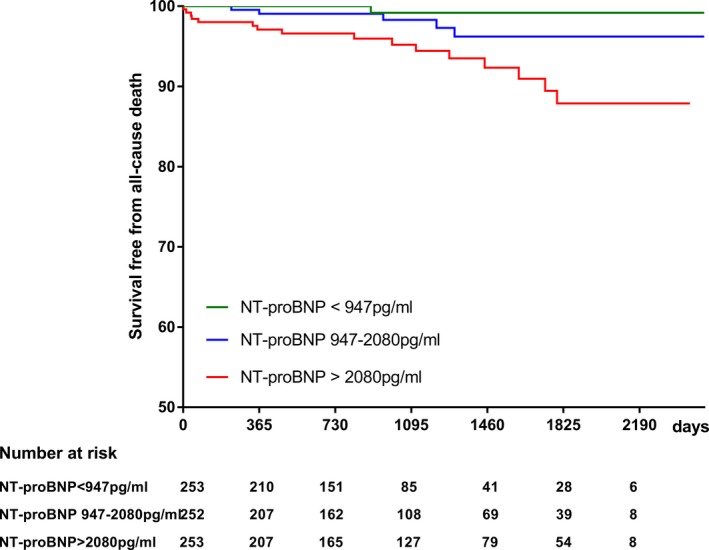
Kaplan‐Meier analysis showing the relation of N‐terminal pro–brain natriuretic peptide level with survival free from all‐cause mortality (overall log‐rank=0.0014). NT‐proBNP indicates N‐terminal pro–brain natriuretic peptide.
Figure 2.
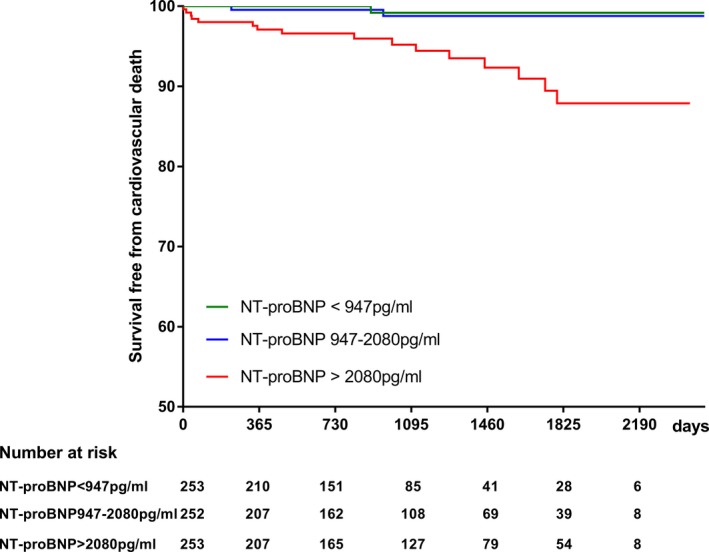
Kaplan‐Meier analysis showing the relation of N‐terminal pro–brain natriuretic peptide level with survival free from cardiovascular mortality (overall log‐rank <0.001). NT‐proBNP indicates N‐terminal pro–brain natriuretic peptide.
Predictors of All‐Cause and Cardiovascular Mortality
Univariable and multivariable Cox regression analyses were performed to investigate the predictors of all‐cause and cardiovascular mortality in the midterm follow‐up. Ln(NT‐proBNP) was a significantly independent predictor of all‐cause mortality (hazard ratio 2.380, 95% CI 1.356‐4.178, P=0.003) and cardiovascular mortality (hazard ratio 2.788, 95% CI 1.450‐5.362, P=0.002). In addition, concomitant CABG for coronary artery disease was also an independent predictor of cardiovascular mortality (hazard ratio 5.178, 95% CI 1.597‐16.789, P=0.006) (Table 4).
Table 4.
Univariable and Multivariable Cox Regression Analyses to Predict Mortality
| All‐Cause Mortality | Cardiovascular Mortality | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Univariable | ||||
| Age | 1.003 (0.970‐1.037) | 0.874 | 0.994 (0.960‐1.029) | 0.737 |
| Male | 0.994 (0.425‐2.327) | 0.989 | 0.760 (0.309‐1.872) | 0.551 |
| Previous atrial fibrillation | 1.730 (0.638‐4.694) | 0.281 | 2.133 (0.768‐5.927) | 0.146 |
| Previous syncope | 1.776 (0.723‐4.367) | 0.211 | 1.779 (0.675‐4.690) | 0.244 |
| NYHA class III or IV | 6.274 (0.842‐46.756) | 0.073 | 30.343 (0.297‐3097.2) | 0.148 |
| Left atrial dimeter ≥45 mm | 2.232 (0.935‐5.326) | 0.070 | 2.750 (1.044‐7.244) | 0.041 |
| LV end‐diastolic dimension | 1.021 (0.938‐1.1112) | 0.631 | 0.998 (0.910‐1.094) | 0.969 |
| LV ejection fraction | 0.981 (0.918‐1.049) | 0.572 | 0.987 (0.919‐1.060) | 0.716 |
| Moderate or severe MR | 2.146 (0.888‐5.187) | 0.090 | 1.652 (0.654‐4.173) | 0.288 |
| LVOT obstruction at rest | 0.720 (0.167‐3.102) | 0.659 | 1.310 (0.174‐9.873) | 0.793 |
| Maximal LV wall thickness ≥30 mm | 1.296 (0.437‐3.844) | 0.640 | 1.563 (0.516‐4.729) | 0.430 |
| Myocardial unroofing | 2.721 (0.613‐12.076) | 0.188 | 3.076 (0.682‐13.866) | 0.144 |
| CABG for myocardial bridge | 0.038 (0.000‐8.158) | 0.233 | 0.038 (0. 000‐12.852) | 0.271 |
| CABG for CAD | 2.494 (0.902‐6.898) | 0.078 | 3.104 (1.091‐8.831) | 0.034 |
| Aortic valve procedure | 5.412 (0.727‐40.281) | 0.099 | 6.427 (0.857‐48.212) | 0.070 |
| Mitral valve procedure | 0.846 (0.197‐3.637) | 0.822 | 0.986 (0.226‐4.293) | 0.985 |
| Tricuspid valve procedure | 1.812 (0.525‐6.257) | 0.347 | 2.171 (0.615‐7.672) | 0.229 |
| Maze procedure | 2.758 (0.814‐9.345) | 0.103 | 3.327 (0.966‐11.461) | 0.057 |
| Residual LVOT gradient | 0.989 (0.920‐1.064) | 0.772 | 0.979 (0.903‐1.063) | 0.617 |
| Pulmonary hypertension | 1.170 (0.346‐3.955) | 0.801 | 1.385 (0.403‐4.756) | 0.605 |
| Ln(NT‐proBNP) | 2.667 (1.538‐4.622) | <0.001 | 3.274 (1.781‐6.019) | <0.001 |
| Multivariablea | ||||
| Age | 0.994 (0.960‐1.029) | 0.713 | 0.980 (0.944‐1.017) | 0.278 |
| Male | 1.319 (0.519‐3.351) | 0.561 | 0.902 (0.329‐2.472) | 0.841 |
| NYHA class III or IV | 4.603 (0.609‐34.764) | 0.139 | ··· | ··· |
| Left atrial diameter ≥45 mm | 1.559 (0.585‐4.149) | 0.374 | 2.368 (0.796‐7.046) | 0.121 |
| Moderate or severe MR | 1.636 (0.647‐4.140) | 0.298 | ··· | ··· |
| Aortic valve procedure | 3.416 (0.400‐29.147) | 0.261 | 3.908 (0.406‐37.624) | 0.238 |
| Maze procedure | ··· | ··· | 2.117 (0.554‐8.093) | 0.273 |
| Ln(NT‐proBNP) | 2.380 (1.356‐4.178) | 0.003 | 2.788 (1.450‐5.362) | 0.002 |
| CABG for CAD | 2.902 (0.973‐8.656) | 0.056 | 5.178 (1.597‐16.789) | 0.006 |
CABG indicates coronary artery bypass grafting; CAD, coronary artery disease; HR, hazard ratio; LV, left ventricular; LVOT, left ventricular outflow tract; MR, mitral regurgitation; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; NYHA, New York Heart Association.
Age, male, NYHA class III or IV, left atrial diameter ≥45 mm, moderate or severe MR, CABG for CAD, aortic valve procedure, and Ln(NT‐proBNP) were included in the multivariable Cox regression analysis of all‐cause mortality. Age, male, left atrial diameter ≥45 mm, CABG for CAD, aortic valve procedure, maze procedure, and Ln(NT‐proBNP) were included in the multivariable Cox regression analysis of cardiovascular mortality.
Discussion
The present study demonstrates that an increased preoperative NT‐proBNP level is associated with midterm all‐cause and cardiovascular mortality. These data add valuable information in the risk stratification of patients who need to undergo septal myectomy. Moreover, we demonstrated that concomitant surgical operation is the independent predictor of cardiovascular death in the midterm follow‐up.
Elevated ventricular wall stress stimulates the secretion of proBNP, and then proBNP is cleaved into BNP and NT‐proBNP.8 A previous study has shown that NT‐proBNP level increases in patients with HCM relative to that in the general population.13 Further analysis of NT‐proBNP in HCM demonstrated its association with the severity of symptoms and echocardiographic patterns.17, 18 In 2013, 2 large studies focusing on the predictive role of BNP and NT‐proBNP in HCM identified that either BNP or NT‐proBNP was an independent predictor of survival regardless of the presence or absence of LVOT obstruction.13, 19 These findings reflect the prognostic utility of NT‐proBNP in patients with HCM.
The presence of LVOT obstruction increases the hemodynamic burden on the LV, which could result in the elevation of the NT‐proBNP level. In this study we confirmed the relationship of NT‐proBNP and LVOT obstruction and markers of increased LV filling pressures including left atrial diameter and pulmonary hypertension. Furthermore, we also found that NT‐proBNP was related to moderate or severe mitral regurgitation. Additionally, long‐standing LVOT obstruction could also to some extent account for the elevation of NT‐proBNP.
Septal myectomy is a reliable and safe approach to relieve LVOT obstruction and gives most patients a lifespan similar to that in an age‐matched population.5, 20 The midterm mortality in the current study is low, with a 3‐year survival rate of 97.5%. Previously, we reported the significant decrease in BNP after septal myectomy.21 Thus, NT‐proBNP could serve as a useful biomarker to reveal the change in LVOT gradient and LV filling pressure and predict the clinical outcome in those undergoing septal myectomy.
Several studies have been designed to investigate the predictors of postoperative survival in patients undergoing septal myectomy.2, 6, 7 Increasing age, preoperative atrial fibrillation, left atrial enlargement, concomitant CABG, and extensive late gadolinium enhancement help predict postoperative survival of patients undergoing septal myectomy.3, 7 So far, few studies have evaluated the ability of preoperative NT‐proBNP to predict postoperative survival after septal myectomy in patients with LVOT obstruction–related symptoms. Data from this study show that postoperative survival was significantly lower in those patients with preoperative NT‐proBNP ≥2080 pg/mL. Furthermore, NT‐proBNP is an independent predictor of midterm all‐cause mortality in patients who underwent septal myectomy. The predictive ability is much higher for cardiovascular death (mostly heart failure deaths and sudden cardiac death). In the context of the known association of NT‐proBNP with adverse cardiac remodeling17, 18 and late gadolinium enhancement described by cardiac magnetic resonance imaging,22 these results could be reasonable.
Concomitant CABG for coronary artery disease is also independently associated with midterm cardiovascular mortality in this cohort. This finding is consistent with previous reports. Woo and colleagues3 demonstrated that concomitant CABG at the time of myectomy was associated with long‐term mortality. Coronary atherosclerosis in HCM is likely to be associated with myocardial ischemia, which has been reported to adversely affect the prognosis.23, 24 Contrary to a previous report,3 increasing age, preoperative atrial fibrillation, and left atrial enlargement were not related to postoperative survival in this study. However, preoperative left atrial diameter ≥45 mm was related to midterm cardiovascular mortality in a univariable Cox regression analysis. The discrepancy could be partly explained by the low mortality rate during a relatively short follow‐up period.
Preoperative NT‐proBNP might not be reliable as a “stand‐alone” risk factor for outcomes in clinical practice because of the presence of other reported factors.3, 7 However, based on our study, we believe that preoperative measurement of NT‐proBNP is useful in clinical practice. Preoperative measurement of NT‐proBNP might help clinicians in making therapeutic decisions about adopting the appropriate operative strategies and conducting longer postoperative intensive care unit observation, which might improve the outcome. Moreover, low levels of NT‐proBNP might predict a good outcome, but closer clinical, echocardiographic, and electrocardiographic follow‐up after septal myectomy is necessary in patients with high levels of NT‐proBNP.
Study Limitations
This is a retrospective study with some inherent limitations. The number of patients who died was small, resulting in wide CIs. The present study was an observational, single‐center study, and the current results may not be entirely generalizable. As a retrospective study, we had to exclude those patients without records of preoperative NT‐proBNP, which could affect the results to some extent. We could not obtain enough postoperative information to evaluate the prognostic value of the change in NT‐proBNP from preoperation to follow‐up.
Conclusions
The present study demonstrates that preoperative NT‐proBNP level is an independent predictor of midterm survival in patients who underwent septal myectomy. Our findings suggest that measurement of NT‐proBNP may help in the preoperative risk stratification and management of patients with HCM who are desired to undergo septal myectomy.
Sources of Funding
This work was supported by the National Key Research and Development Program of China (grant number 2016YFC1300901).
Disclosures
None.
(J Am Heart Assoc. 2019;8:e011075 DOI: 10.1161/JAHA.118.011075.)
Contributor Information
Shuiyun Wang, Email: wsymd@sina.com.
Xiaohong Huang, Email: huangxiaohong@fuwaihospital.org.
References
- 1. Authors/Task Force Members , Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Seggewiss H, Schaff HV. Hypertrophic obstructive cardiomyopathy: surgical myectomy and septal ablation. Circ Res. 2017;121:771–783. [DOI] [PubMed] [Google Scholar]
- 3. Woo A, Williams WG, Choi R, Wigle ED, Rozenblyum E, Fedwick K, Siu S, Ralph‐Edwards A, Rakowski H. Clinical and echocardiographic determinants of long‐term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111:2033–2041. [DOI] [PubMed] [Google Scholar]
- 4. Smedira NG, Lytle BW, Lever HM, Rajeswaran J, Krishnaswamy G, Kaple RK, Dolney DO, Blackstone EH. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2008;85:127–133. [DOI] [PubMed] [Google Scholar]
- 5. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, Schaff HV, Danielson GK, Tajik AJ, Nishimura RA. Long‐term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. [DOI] [PubMed] [Google Scholar]
- 6. Desai MY, Bhonsale A, Smedira NG, Naji P, Thamilarasan M, Lytle BW, Lever HM. Predictors of long‐term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation. 2013;128:209–216. [DOI] [PubMed] [Google Scholar]
- 7. Tang B, Song Y, Cui H, Ji K, Zhu C, Zhao S, Huang X, Yu Q, Hu S, Wang S. Prediction of mid‐term outcomes in adult obstructive hypertrophic cardiomyopathy after surgical ventricular septum myectomy. J Am Coll Cardiol. 2017;70:2092–2094. [DOI] [PubMed] [Google Scholar]
- 8. Hall C. Essential biochemistry and physiology of (NT‐pro)BNP. Eur J Heart Fail. 2004;6:257–260. [DOI] [PubMed] [Google Scholar]
- 9. Sodeck G, Domanovits H, Schillinger M, Janata K, Thalmann M, Ehrlich MP, Endler G, Laggner A. Pre‐operative N‐terminal pro‐brain natriuretic peptide predicts outcome in type A aortic dissection. J Am Coll Cardiol. 2008;51:1092–1097. [DOI] [PubMed] [Google Scholar]
- 10. Morrow DA, de Lemos JA, Sabatine MS, Murphy SA, Demopoulos LA, DiBattiste PM, McCabe CH, Gibson CM, Cannon CP, Braunwald E. Evaluation of B‐type natriuretic peptide for risk assessment in unstable angina/non‐ST‐elevation myocardial infarction: B‐type natriuretic peptide and prognosis in TACTICS‐TIMI 18. J Am Coll Cardiol. 2003;41:1264–1272. [DOI] [PubMed] [Google Scholar]
- 11. Weber M, Hamm C. Role of B‐type natriuretic peptide (BNP) and NT‐proBNP in clinical routine. Heart. 2006;92:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mutlu B, Bayrak F, Kahveci G, Degertekin M, Eroglu E, Basaran Y. Usefulness of N‐terminal pro‐B‐type natriuretic peptide to predict clinical course in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2006;98:1504–1506. [DOI] [PubMed] [Google Scholar]
- 13. Coats CJ, Gallagher MJ, Foley M, O'Mahony C, Critoph C, Gimeno J, Dawnay A, McKenna WJ, Elliott PM. Relation between serum N‐terminal pro‐brain natriuretic peptide and prognosis in patients with hypertrophic cardiomyopathy. Eur Heart J. 2013;34:2529–2537. [DOI] [PubMed] [Google Scholar]
- 14. Wang S, Cui H, Yu Q, Chen H, Zhu C, Wang J, Xiao M, Zhang Y, Wu R, Hu S. Excision of anomalous muscle bundles as an important addition to extended septal myectomy for treatment of left ventricular outflow tract obstruction. J Thorac Cardiovasc Surg. 2016;152:461–468. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 16. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ; American Society of Echocardiography . Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 17. D'Amato R, Tomberli B, Castelli G, Spoladore R, Girolami F, Fornaro A, Caldini A, Torricelli F, Camici P, Gensini GF, Cecchi F, Olivotto I. Prognostic value of N‐terminal pro‐brain natriuretic peptide in outpatients with hypertrophic cardiomyopathy. Am J Cardiol. 2013;112:1190–1196. [DOI] [PubMed] [Google Scholar]
- 18. Maron BJ, Tholakanahalli VN, Zenovich AG, Casey SA, Duprez D, Aeppli DM, Cohn JN. Usefulness of B‐type natriuretic peptide assay in the assessment of symptomatic state in hypertrophic cardiomyopathy. Circulation. 2004;109:984–989. [DOI] [PubMed] [Google Scholar]
- 19. Geske JB, McKie PM, Ommen SR, Sorajja P. B‐type natriuretic peptide and survival in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;61:2456–2460. [DOI] [PubMed] [Google Scholar]
- 20. Schaff HV, Dearani JA, Ommen SR, Sorajja P, Nishimura RA. Expanding the indications for septal myectomy in patients with hypertrophic cardiomyopathy: results of operation in patients with latent obstruction. J Thorac Cardiovasc Surg. 2012;143:303–309. [DOI] [PubMed] [Google Scholar]
- 21. Cui H, Wu X, Wang S, Tang B, Zhu C, Chen H, Zhou X, Wu R, Yu Q, Huang X. Time and age dependent decrease of NT‐proBNP after septal myectomy in hypertrophic obstructive cardiomyopathy. Clin Chem Lab Med. 2017;55:1614–1620. [DOI] [PubMed] [Google Scholar]
- 22. Payá E, Marín F, González J, Gimeno JR, Feliu E, Romero A, Ruiz‐Espejo F, Roldán V, Climent V, de la Morena G, Valdés M. Variables associated with contrast‐enhanced cardiovascular magnetic resonance in hypertrophic cardiomyopathy: clinical implications. J Card Fail. 2008;14:414–419. [DOI] [PubMed] [Google Scholar]
- 23. Elliott PM, Kaski JC, Prasad K, Seo H, Slade AK, Goldman JH, McKenna WJ. Chest pain during daily life in patients with hypertrophic cardiomyopathy: an ambulatory electrocardiographic study. Eur Heart J. 1996;17:1056–1064. [DOI] [PubMed] [Google Scholar]
- 24. Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–1035. [DOI] [PubMed] [Google Scholar]


