Abstract
Background
Many hospitalized patients with heart failure and reduced ejection fraction (HFrEF) have a slow heart rate at discharge, and the effect of β‐blockers may be reduced in those patients. We sought to examine the variable effect of β‐blockers on clinical outcomes according to the discharge heart rate of hospitalized HFrEF patients.
Methods and Results
The KorAHF (Korean Acute Heart Failure) registry consecutively enrolled 5625 patients hospitalized for acute heart failure. In this analysis, we included patients with HFrEF (left ventricular ejection fraction ≤40%). Slow heart rate was defined as <70 beats per minute regardless of the use of β‐blockers. The primary outcome was 1‐year all‐cause postdischarge death according to heart rate. Among 2932 patients with HFrEF, 840 (29%) had a slow heart rate and 56% received β‐blockers at discharge. Patients with slow heart rates were older and had lower 1‐year mortality than those with high heart rates (P<0.001). A significant interaction between discharge heart rate and β‐blocker use was observed (P<0.001 for interaction). When stratified, only patients without a β‐blocker prescription and with a high heart rate showed higher 1‐year mortality. In a Cox‐proportional hazards regression analysis, β‐blocker prescription at discharge was associated with 24% reduced risk for 1‐year mortality in patients with high heart rates (hazard ratio: 0.76; 95% CI, 0.61–0.95) but not in those with slow heart rates (hazard ratio: 1.02; 95% CI, 0.68–1.55).
Conclusions
Many patients with acute heart failure have slow discharge heart rates, and β‐blockers may have a limited effect on HFrEF and slow discharge heart rate.
Clinical Trial Registration
URL: http://www.clinicaltrial.gov. Unique identifier: NCT01389843.
Keywords: β‐blocker, heart failure, heart rate, outcome
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
The favorable effects of β‐blockers have been attributed to their capacity to reduce heart rate, among others.
Because the magnitude of heart rate reduction by β‐blockers depends on the baseline heart rate, its effect may depend on the patient's heart rate.
In this study, β‐blocker use was associated with a reduced risk of 1‐year mortality in patients with high heart rate but not in those with slow heart rate, suggesting a differential effect of β‐blockers by heart rate.
What Are the Clinical Implications?
Not all patients benefit equally from β‐blocker therapy.
Introduction
The prognosis of patients with heart failure with reduced rejection fraction (HFrEF) has been steadily improving with the development of disease‐modifying drugs that target neurohumoral activation.1 Sympathetic tone blockage with β‐blockers has been one of the cornerstones of HFrEF treatment and has significantly improved outcomes.2 The favorable effect of β‐blockers has been attributed to its capacity to reduce heart rate, among others.3
Early initiation of β‐blockers, especially at discharge after acute heart failure, is beneficial4; however, a considerable number of patients with HFrEF have slow heart rate at discharge. Because the magnitude of heart rate reduction by the β‐blocker depends on the baseline heart rate and patients with low heart rate experience smaller reduction in heart rate,5 the effect of the β‐blocker may be reduced. In addition, slow heart rate may present a psychological barrier for the physicians in terms of prescribing β‐blockers.
In this study, we sought to examine, for the first time, the variable effect of β‐blockers on clinical outcomes according to the discharge heart rate in hospitalized patients with HFrEF in a large nationwide prospective cohort.
Methods
Patients
The KorAHF (Korean Acute Heart Failure) registry was a prospective multicenter cohort study that consecutively enrolled 5625 patients who were hospitalized for acute heart failure syndrome from 10 tertiary university hospitals throughout the country between March 2011 and December 2014. Detailed information on the study design and results has been previously reported elsewhere (ClinicalTrial.gov identifier NCT01389843).6, 7 Briefly, patients with signs or symptoms of heart failure and either lung congestion, objective findings of left ventricular systolic dysfunction, or structural heart disease were eligible for the study. The mortality data for patients who were lost to follow‐up were collected from the National Insurance data or National Death Records.
In this study, we included only patients with HFrEF. The study protocol was approved by the ethics committee or institutional review board at each hospital. Written informed consent was waived by the institutional review board. The study complied with the Declaration of Helsinki.
Study Variables and Definitions
HFrEF was defined as left ventricular ejection fraction of ≤40%. Patients who did not receive evidence‐based β‐blockers were excluded. Evidence‐based β‐blockers were defined as long‐acting metoprolol, bisoprolol, carvedilol, and nebivolol. The doses of prescribed β‐blockers were calculated into a percentage target dose of each β‐blocker, which was 200, 10, 50, and 10 mg for long‐acting metoprolol, bisoprolol, carvedilol, and nebivolol, respectively.
The use of a β‐blocker was evaluated at hospital discharge, and heart rate was measured clinically before discharge. In a restricted cubic‐spline model, there appeared to be a J‐curve relationship between the effect of β‐blockers on 1‐year all‐cause mortality and the heart rate; the effect of β‐blockers seemed to decrease as the heart rate decreased, and the effect of β‐blockers appeared to be neutral below a heart rate of 70 beats per minute (bpm). Using this cutoff, patients were categorized as having either slow (<70 bpm) or high (≥70 bpm) heart rate.8, 9
The primary outcome was postdischarge 1‐year all‐cause death.
Statistical Analysis
Data are presented as numbers and frequencies for categorical variables and as mean±SD for continuous variables. For comparisons among groups, the χ2 test (or Fisher exact test when any expected count was <5 for a 2×2 table) was used for categorical variables, and the unpaired Student t test or 1‐way ANOVA was used for continuous variables.
One‐year outcomes were analyzed in the β‐blocker group in relation to heart rate at discharge. Kaplan–Meier curves were plotted and compared using the log‐rank test. A multivariable Cox proportional hazards regression model was used to determine the effect size of β‐blocker as an independent predictor of all‐cause death. Variables found to be statistically significant (P<0.1) in the univariable analysis were included in the multivariable model, except for variables with >10% missing values or variables that were closely related to other clinical variables. A 2‐sided probability value <0.05 was considered indicative of a statistically significant difference. Statistical tests were performed using SPSS v22 (IBM Corp).
Results
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patients
Among the 5625 patients enrolled in the KorAHF registry, left ventricular ejection fraction was available for 5103 (90.7%) patients. Among them, 3088 (60.5%) patients had HFrEF, and 156 patients died during hospital admission and 48 patients did not receive evidence‐based β‐blockers such as atenolol, leaving the data of 2884 patients available for the final analysis (Figure 1).
Figure 1.

The study patients. BB indicates β‐blocker; KorAHF, Korean acute heart failure; LVEF, left ventricular ejection fraction.
The mean heart rate decreased from 95.1±24.6 bpm at admission to 77.6±14.1 bpm at discharge (P<0.001). Overall, 840 (29.1%) patients had slow heart rate at discharge, and they were older and more likely to have ischemic heart disease and atrial fibrillation but less likely to have diabetes mellitus and malignancy than those with high heart rate (Table 1). Furthermore, they received more RAS (renin–angiotensin system) inhibitors, β‐blockers, and mineralocorticoid receptor antagonists than those with high heart rate.
Table 1.
Baseline Characteristics of the Study Population
| Slow Heart Rate | High Heart Rate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| All n=840 (29.1%) | β‐Blocker Yes n=524 (62.4%) | β‐Blocker No n=316 (37.6%) | P Valuea | All n=2044 (70.9%) | β‐Blocker Yes n=1108 (54.2%) | β‐Blocker No n=936 (45.8%) | P Valueb | P Valuec | |
| Discharge heart rate, bpm | 62.0±5.4 | 62.4±5.2 | 61.4±5.8 | 0.018 | 84.1±11.3 | 82.7±10.8 | 85.6±11.6 | <0.001 | <0.001 |
| Age, y | 67.3±13.8 | 66.3±13.8 | 68.8±13.9 | 0.009 | 65.5±15.1 | 64.3±15.0 | 66.9±15.1 | <0.001 | 0.002 |
| Men, % | 60.7 | 59.0 | 63.6 | 0.182 | 59.9 | 60.2 | 60.1 | 0.982 | 0.788 |
| De novo, % | 48.8 | 54.6 | 39.2 | <0.001 | 52.1 | 57.8 | 45.3 | <0.001 | 0.113 |
| Body mass index, kg/m2 | 23.2±3.5 | 23.5±3.7 | 22.8±3.2 | 0.003 | 23.1±3.9 | 23.5±3.9 | 22.7±3.8 | <0.001 | 0.455 |
| Past medical history | |||||||||
| Hypertension, % | 55.8 | 55.9 | 55.7 | 0.95 | 54.5 | 55.8 | 53.1 | 0.226 | 0.529 |
| Diabetes mellitus, % | 33.0 | 32.4 | 33.9 | 0.672 | 38.3 | 40.5 | 35.7 | 0.025 | 0.007 |
| GFR <60 mL/min/1.72 m2, % | 46.1 | 43.7 | 50.2 | 0.069 | 43.6 | 41.0 | 46.8 | 0.008 | 0.222 |
| Ischemic heart disease, % | 32.7 | 31.5 | 34.5 | 0.378 | 27.6 | 25.5 | 30.7 | 0.005 | 0.007 |
| Valvular heart disease, % | 8.7 | 6.3 | 12.7 | 0.002 | 8.8 | 6.1 | 12.0 | <0.001 | 0.951 |
| Atrial fibrillation, % | 27.4 | 22.3 | 35.8 | <0.001 | 21.8 | 21.0 | 22.6 | 0.376 | 0.001 |
| COPD, % | 10.6 | 8.8 | 13.6 | 0.028 | 10.4 | 8.0 | 13.1 | <0.001 | 0.858 |
| Cerebrovascular disease, % | 14.5 | 13.4 | 16.5 | 0.217 | 14.1 | 12.5 | 16.0 | 0.024 | 0.788 |
| Malignancy, % | 7.0 | 5.9 | 8.9 | 0.106 | 9.1 | 7.8 | 10.7 | 0.022 | 0.069 |
| Current smoking, % | 21.1 | 23.5 | 17.1 | 0.028 | 21.7 | 23.9 | 19.1 | 0.009 | 0.699 |
| ICD, % | 3.0 | 1.9 | 4.7 | 0.019 | 1.5 | 3.3 | 2.9 | 0.556 | 0.010 |
| CRT, % | 1.1 | 1.1 | 0.9 | 0.79 | 0.7 | 1.5 | 2.2 | 0.237 | 0.289 |
| NYHA functional class, % | 0.39 | 0.422 | 0.007 | ||||||
| II | 15.6 | 15.3 | 16.1 | 13.6 | 14.5 | 12.6 | |||
| III | 41.0 | 42.7 | 38.0 | 36.5 | 36.5 | 36.5 | |||
| IV | 43.5 | 42.0 | 45.9 | 49.9 | 49.0 | 50.9 | |||
| Physical exam (at admission) | |||||||||
| Systolic BP, mm Hg | 130.4±28.5 | 132.8±28.0 | 126.4±28.8 | 0.002 | 128.4±29.2 | 130.9±29.6 | 125.4±28.4 | <0.001 | 0.095 |
| Diastolic BP, mm Hg | 79.9±18.2 | 81.7±18.4 | 76.8±17.4 | <0.001 | 79.7±18.8 | 81.9±19.3 | 77.0±17.8 | <0.001 | 0.785 |
| Heart rate, bpm | 88.7±25.6 | 89.3±25.1 | 87.8±26.3 | 0.44 | 97.7±23.7 | 98.3±23.5 | 96.9±23.9 | 0.164 | <0.001 |
| Laboratory findings | |||||||||
| Hemoglobin, mg/dL | 12.8±2.2 | 12.9±2.2 | 12.6±2.2 | 0.046 | 12.7±2.3 | 12.9±2.3 | 12.5±2.3 | <0.001 | 0.267 |
| Serum sodium, mmol/L | 137.9±4.7 | 138.1±4.5 | 137.6±5.0 | 0.15 | 137.4±4.5 | 137.8±4.1 | 136.9±4.9 | <0.001 | 0.005 |
| Serum potassium, mmol/L | 4.4±0.6 | 4.3±0.6 | 4.4±0.7 | 0.107 | 4.4±0.7 | 4.4±0.7 | 4.4±0.7 | 0.042 | 0.069 |
| BUN, mg/dL | 25.5±16.6 | 24.5±15.4 | 27.3±18.3 | 0.021 | 26.4±15.6 | 25.2±15.0 | 27.8±16.2 | <0.001 | 0.184 |
| Creatinine, mg/dL | 1.46±1.40 | 1.4±1.3 | 1.6±1.6 | 0.107 | 1.50±1.44 | 1.5±1.4 | 1.5±1.5 | 0.649 | 0.608 |
| BNP, pg/mL | 1479±1507 | 1407±1454 | 1609±1596 | 0.245 | 1624±1314 | 1535±1267 | 1738±1366 | 0.034 | 0.110 |
| NT‐proBNP, pg/mL | 9949±13 470 | 9921±14 210 | 9989±12 188 | 0.956 | 10 504±10 608 | 9681±10 339 | 11 455±10 843 | 0.005 | 0.378 |
| Troponin I, ng/mL | 2.5±26.9 | 2.7±32.8 | 2.3±10.8 | 0.837 | 2.2±11.9 | 2.2±10.8 | 2.4±13.2 | 0.738 | 0.717 |
| Troponin T, ng/mL | 0.16±0.69 | 0.2±0.9 | 0.1±0.3 | 0.416 | 0.24±0.84 | 0.3±1.1 | 0.2±0.6 | 0.332 | 0.405 |
| C‐reactive protein, mg/L | 1.7±3.3 | 1.6±2.9 | 2.1±3.9 | 0.04 | 2.3±4.1 | 2.2±4.1 | 2.4±4.1 | 0.425 | 0.001 |
| Echocardiographic parameters | |||||||||
| LVEF, % | 27.9±7.3 | 27.5±7.4 | 28.4±7.2 | 0.081 | 26.7±7.7 | 26.7±7.6 | 26.6±7.8 | 0.130 | <0.001 |
| Medications at discharge | |||||||||
| RAS inhibitor, % | 82.7 | 88.0 | 74.1 | <0.001 | 73.8 | 81.1 | 65.1 | <0.001 | <0.001 |
| β‐Blocker, % | 62.4 | 55.2 | <0.001 | ||||||
| MRA, % | 56.8 | 61.3 | 49.4 | 0.001 | 51.7 | 56.1 | 46.5 | <0.001 | 0.013 |
| Ivabradine, % | 0.1 | 0 | 0.3 | 0.198 | 0.1 | 0.2 | 0 | 0.193 | 0.873 |
| Digoxin, % | 31.3 | 31.3 | 31.3 | 0.890 | 29.8 | 30.1 | 29.5 | 0.242 | 0.675 |
BNP indicates B‐type natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; GFR, glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; RAS, renin–angiotensin system.
P value for β‐blocker yes vs no among patients with low heart rate.
P value for β‐blocker yes vs no among patients with high heart rate.
P value for low heart rate vs high heart.
Changes in Heart Rate and Blocker Prescription During Follow‐up
The β‐blocker prescription rate was 57% at discharge and 66.5% at 12 months, and the corresponding proportions of patients with slow heart rate were 29.1% and 30.3%, respectively (Figure 2A). During follow‐up, there was no difference in heart rate between patients with slow heart rate with or without β‐blocker use (Figure 2B).
Figure 2.
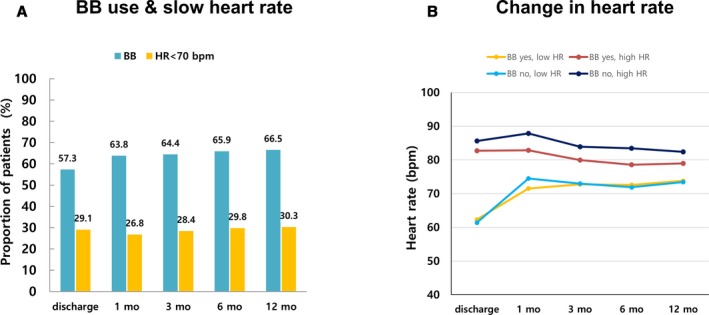
BB prescription rate and change in heart rate. A, BB prescription rate and proportion of patients with slow heart rate. B, Among patients with high heart rate, patients with BBs had lower heart rate than those without BBs (at discharge 82.7±10.8 vs 85.6±11.6 bpm, P<0.001; at 1 month: 82.8±16.7 vs 87.9±15.8 bpm, P<0.001; at 3 months: 78.0±15.5 vs 83.9±14.9 bpm, P<0.001; at 6 months: 75.6±13.9 vs 83.5±15.2 bpm, P<0.001; at 12 months: 79.0±14.4 vs 82.4±16.6 bpm, P=0.002). Among patients with slow heart rate, the heart rate did not differ after 3 months from discharge (at discharge: 62.3±5.2 vs 61.4±5.8 bpm P=0.015; at 1 month: 71.5±14.6 vs 74.5±16.6 bpm, P=0.036; at 3 months: 72.8±14.9 vs 73.0±16.9 bpm, P=0.649; at 12 months: 73.8±14.1 vs 73.5±15.4 bpm, P=0.806). BB indicates β‐blocker; bpm, beats per minute; HR, heart rate.
The most commonly prescribed β‐blocker was carvedilol (62.6%) followed by bisoprolol (33.5%), nebivolol (3.1%), and metoprolol (0.9%).
Clinical Outcomes
In total, 547 (19%) patients died during 1‐year follow‐up. As expected, they had unfavorable clinical characteristics with lower prescription rates of RAS inhibitors, β‐blockers and mineralocorticoid receptor antagonists.
Overall, patients with slow discharge heart rate (15.1% versus 20.5%, P=0.001) and those with β‐blocker prescription (15% versus 24.1%, P<0.001) had lower 1‐year all‐cause mortality. Under stratification according to heart rate and β‐blocker prescription at discharge, only patients with high heart rate and no β‐blocker prescription had higher 1‐year all‐cause mortality, whereas there was no significant difference among the other groups (Figure 3A). Regarding hospitalization for heart failure, patients with β‐blocker prescription and slow heart rate had the lowest hospitalization rate (Figure 3B).
Figure 3.
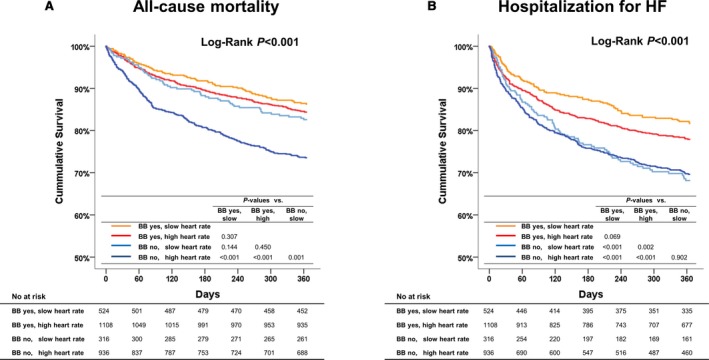
One‐year all‐cause death and hospitalization for HF according to heart rate and BB prescription at discharge. A, Only patients with high heart rate and no BB prescription at discharge had higher 1‐year all‐cause mortality, whereas there was no significant difference among the other groups. B, Patients without BB prescription had higher 1‐year hospitalization for HF regardless of heart rate, whereas those with BB prescription and slow heart rate had the lowest hospitalization. BB indicates β‐blocker; HF, heart failure.
In the Cox model, β‐blocker prescription at discharge was associated with a 24% reduced risk of 1‐year mortality in patients with high heart rate (hazard ratio [HR]: 0.76; 95% CI, 0.61–0.95, P=0.014) but not in those with slow heart rate (HR: 1.02; 95% CI, 0.68–1.55; P=0.909; Table 2).
Table 2.
Cox Proportional Hazards Regression Analysis for 1‐Year Mortality Stratified According to Heart Rate
| Heart Rate <70 bpm (n=840) | Heart Rate ≥70 bpm (n=2044) | |||||
|---|---|---|---|---|---|---|
| P Value | HR | 95% CI | P Value | HR | 95% CI | |
| Age | 0.002 | 1.03 | 1.01–1.05 | <0.001 | 1.03 | 1.03–1.05 |
| Body mass index | 0.087 | 0.95 | 0.89–1.01 | <0.001 | 0.93 | 0.90–0.96 |
| De novo heart failure | 0.101 | 0.68 | 0.43–1.08 | 0.546 | 0.93 | 0.73–1.18 |
| Hypertension | 0.577 | 1.13 | 0.73–1.75 | 0.504 | 1.08 | 0.86–1.37 |
| Diabetes mellitus | 0.800 | 1.06 | 0.69–1.63 | 0.099 | 1.21 | 0.97–1.52 |
| Ischemic heart disease | 0.849 | 0.96 | 0.62–1.47 | 0.391 | 1.11 | 0.88–1.40 |
| Valvular heart disease | 0.128 | 1.60 | 0.87–2.95 | 0.448 | 0.87 | 0.60–1.25 |
| Atrial fibrillation | 0.979 | 0.99 | 0.64–1.55 | 0.661 | 0.94 | 0.72–1.23 |
| COPD | 0.778 | 1.09 | 0.60–1.98 | 0.008 | 1.48 | 1.12–1.96 |
| Cerebrovascular disease | 0.041 | 1.66 | 1.02–2.70 | 0.362 | 1.13 | 0.87–1.48 |
| GFR <60 mL/min/1.72 m2 | 0.851 | 0.96 | 0.62–1.49 | 0.009 | 1.37 | 1.08–1.74 |
| Malignancy | 0.002 | 2.44 | 1.39–4.27 | 0.126 | 1.29 | 0.93–1.77 |
| Current smoking | 0.711 | 1.11 | 0.64–1.94 | 0.803 | 1.04 | 0.78–1.38 |
| ICD | 0.702 | 0.81 | 0.27–2.39 | 0.080 | 1.71 | 0.94–3.12 |
| CRT | 0.092 | 2.88 | 0.84–9.87 | 0.810 | 0.88 | 0.32–2.42 |
| NYHA III/IV | 0.678 | 1.13 | 0.64–1.98 | 0.013 | 1.59 | 1.10–2.30 |
| Systolic blood pressure | 0.258 | 1.00 | 0.99–1.00 | 0.010 | 0.96 | 0.99–1.00 |
| Hemoglobin | 0.001 | 0.84 | 0.77–0.93 | 0.028 | 0.94 | 0.89–0.99 |
| Serum sodium | 0.002 | 0.94 | 0.91–0.98 | <0.001 | 0.95 | 0.93–0.97 |
| Serum potassium | 0.791 | 0.96 | 0.72–1.29 | 0.659 | 1.03 | 0.90–1.19 |
| C‐reactive protein | 0.311 | 1.02 | 0.98–1.07 | 0.485 | 1.01 | 0.99–1.03 |
| RAS inhibitor at discharge | 0.567 | 0.87 | 0.54–1.40 | 0.001 | 0.68 | 0.55–0.86 |
| MRA at discharge | 0.784 | 0.94 | 0.63–1.42 | 0.706 | 0.96 | 0.78–1.19 |
| Β‐blocker at discharge | 0.909 | 1.02 | 0.68–1.55 | 0.014 | 0.76 | 0.61–0.95 |
COPD indicates chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; GFR, glomerular filtration rate; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; RAS, renin–angiotensin system.
Sinus Rhythm Versus Atrial Fibrillation
Overall, 2058 (60.3%) and 826 (28.6%) patients had sinus rhythm and atrial fibrillation, respectively.
Regarding the patients with sinus rhythm, those with β‐blockers had lower heart rates. Under stratification by heart rate, among patients with slow heart rate, the heart rate did not differ between those with or without β‐blockers after 3 months of follow‐up (at discharge: 62.8±5.0 versus 62.0±5.2 bpm, P=0.087; at 1 month: 70.8±12.3 versus 74.7±16.1 bpm, P=0.026; at 3 months: 72.6±13.5 versus 72.1±18.1 bpm, P=0.815; at 6 months: 71.6±14.3 versus 72.0±14.1 bpm, P=0.818; at 12 months: 73.6±13.2 versus 74.5±13.8 bpm, P=0.630). However, among patients with high heart rate, those with β‐blockers had lower heart rate throughout the follow‐up. Regarding the outcomes, only patients with high heart rate and no β‐blocker prescription had higher 1‐year all‐cause mortality, whereas there was no significant difference among the other groups (Figure 4A).
Figure 4.
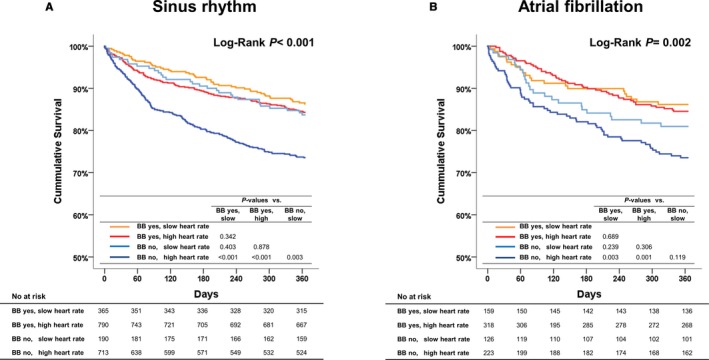
One‐year all‐cause deaths in patients with sinus rhythm and atrial fibrillation. A, Sinus rhythm. B, In the subgroup of patients with atrial fibrillation, patients taking a BB at discharge had better outcomes regardless of heart rate, whereas those without a BB and with high heart rate had the worst outcomes. BB indicates β‐blocker; HR, heart rate.
Among patients with atrial fibrillation, there was no difference in heart rate between patients with and without β‐blocker use (at discharge: 76±14 versus 76±15 bpm, P=0.652; at 12 months: 79±18 versus 28±17 bpm, P=0.891). Patients with a β‐blocker at discharge had better baseline characteristics (Table 3). In Kaplan–Meier survival analysis, patients with a β‐blocker at discharge had better outcomes regardless of heart rate. Patients with slow heart rate and without β‐blocker prescription had numerically higher 1‐year mortality than those with β‐blocker prescription (P=0.239). Nonetheless, patients without a β‐blocker and with high heart rate had the worst outcomes (Figure 4B). After adjustment for significant covariates, β‐blocker was not associated with improved 1‐year all‐cause mortality in all patients with atrial fibrillation; however, under stratification by heart rate, β‐blocker was associated with 37% reduced risk for mortality (HR: 0.63; 95% CI, 0.40–0.97; P=0.037) in patients with high heart rate but not in those with slow heart rate.
Table 3.
Baseline Characteristics of Patients With Atrial Fibrillation
| β‐Blocker Yes (n=477, 57.7%) | β‐Blocker No (n=349, 42.3%) | P Value | |
|---|---|---|---|
| Age, y | 67.5±12.9 | 68.5±12.8 | 0.123 |
| Men, % | 63.7 | 62.8 | 0.773 |
| De novo, % | 51.2 | 32.7 | <0.001 |
| Body mass index, kg/m2 | 23.7±3.8 | 23.0±3.6 | 0.011 |
| Past medical history | |||
| Hypertension, % | 56.6 | 51.0 | 0.110 |
| Diabetes mellitus, % | 29.8 | 27.8 | 0.536 |
| GFR <60 mL/min/1.72 m2, % | 42.1 | 48.0 | 0.095 |
| Ischemic heart disease, % | 26.8 | 31.1 | 0.222 |
| Valvular heart disease, % | 8.6 | 20.6 | <0.001 |
| COPD, % | 9.4 | 13.8 | 0.058 |
| Cerebrovascular disease, % | 18 | 17.5 | 0.838 |
| Malignancy, % | 7.8 | 8.9 | 0.561 |
| Current smoking, % | 23.1 | 13.5 | 0.001 |
| ICD, % | 1.5 | 2.6 | 0.252 |
| CRT, % | 1.0 | 0.9 | 0.785 |
| NYHA functional class, % | 0.529 | ||
| II | 12.2 | 13.5 | |
| III | 43.4 | 39.5 | |
| IV | 44.4 | 47.0 | |
| Physical exam (at admission) | |||
| Systolic BP, mm Hg | 127.3±25.6 | 124.3±26.9 | 0.109 |
| Diastolic BP, mm Hg | 82.1±18.4 | 78.4±17.9 | 0.004 |
| Heart rate, bpm | 99.3±29.1 | 98.2±30.2 | 0.601 |
| Laboratory findings | |||
| Hemoglobin, mg/dL | 13.5±2.1 | 13.0±2.2 | 0.001 |
| Serum sodium, mmol/L | 138.0±4.2 | 136.8±5.4 | <0.001 |
| Serum potassium, mmol/L | 4.4±0.6 | 4.5±0.6 | 0.006 |
| BUN, mg/dL | 25.3±15.1 | 29.1±17.1 | 0.001 |
| Creatinine, mg/dL | 1.3±1.0 | 1.5±1.2 | 0.124 |
| BNP, pg/mL | 1252±1053 | 1398±1344 | 0.261 |
| NT‐proBNP, pg/mL | 9031±9322 | 10 633±11 946 | 0.116 |
| Troponin I, ng/mL | 0.7±5.4 | 1.2±5.9 | 0.323 |
| Troponin T, ng/mL | 0.1±0.6 | 0.1±0.3 | 0.814 |
| C‐reactive protein, mg/L | 1.8±3.1 | 2.2±3.7 | 0.115 |
| Echocardiographic parameters | |||
| LVEF, % | 27.2±7.7 | 27.3±7.7 | 0.840 |
| Medications at discharge | |||
| RAS inhibitor, % | 80.7 | 64.2 | <0.001 |
| MRA, % | 57.0 | 47.0 | 0.004 |
| Digoxin, % | 58.5 | 51.6 | 0.019 |
BNP indicates B‐type natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; GFR, glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; RAS, renin–angiotensin system.
Β‐Blocker Dose
The median in percentage target dose of β‐blockers was 21%. Under stratification according to β‐blocker dose, there was no difference in all‐cause mortality between patients with β‐blocker doses greater or less than the median (Figure 5).
Figure 5.

One‐year all‐cause deaths according to BB dosage. In all patients and patients with high heart rate, patients without BB prescription at discharge had higher 1‐year all‐cause mortality than those with BB prescription; however, there was no difference in mortality between patients with BB dose above and below the median. Among patients with slow heart rate, 1‐year mortality did not differ between those with doses above and below the median and those without a BB. BB indicates β‐blocker; bpm, beats per minute; HR, heart rate.
Discussion
In this study we examined the effect of β‐blockers according to the discharge heart rate in a large cohort of patients with HFrEF. We demonstrated that patients with slow heart rate had favorable outcomes regardless of β‐blocker use and that the use of β‐blocker at discharge was associated with improved 1‐year mortality only among patients with high heart rate.
Β‐Blockers, Heart Rate, and Outcomes
Overall, more patients with slow heart rate received β‐blockers, which reflects the negative chronotropic effect of β‐blockers. Nevertheless, among patients without a β‐blocker prescription at discharge, 25% had slow heart rate; these patients may have low intrinsic heart rate, relatively low sympathetic tone, or increased sympathetic tone with sinoatrial node dysfunction.10 It is reported that heart failure patients typically have vagal attenuation and elevated sympathetic tone,11 as well as significantly impaired sinus node function with structural alterations.12
By blocking the adrenergic receptors in cardiomyocytes, β‐blockers exert negative chrono‐ and inotropic, antiarrhythmic, and antiproliferative effects, among others. Clinical trials of β‐blockers in patients with HFrEF showed a correlation between heart rate reduction and improved outcomes.5, 13, 14 Nonetheless, those studies could not separate the negative chronotropic effect from the other potentially beneficial effects of the β‐blockers. Notably, ivabradine, which blocks the If channel in the sinus node and reduces heart rate without the properties of a β‐blocker, has been shown to improve clinical outcomes, indicating that heart rate reduction per se improves outcomes.8 Heart rate reduction lowers energy expenditure,15 increases coronary perfusion,16 and unloads ventricular loading via alteration of vascular elastance.17 Thus, heart rate is not only a marker but also a mediator in patients with heart failure.
The interaction between heart rate and β‐blocker treatment effect has been controversial. Schleman et al reported that the efficacy of β‐blockers was greatest in patients with faster heart rates,18 whereas others rejected any association.5, 14 Cullington et al showed that the achieved heart rate was the main determinant of outcomes.19 In a recent meta‐analysis, β‐blockers could reduce mortality in patients with HFrEF in sinus rhythm regardless of pretreatment heart rate.20 High heart rate might reflect increased neurohumoral activity in favor of increased sympathetic tone. Consequently, the effect of β‐blockers may be more profound in those patients because it counteracts the deleterious effects of tachycardia in a failing heart. We showed that the effect of β‐blockers depends on the heart rate (Figure 6).
Figure 6.

Heart rate and outcomes. A, The risk of mortality increased as the heart rate elevated in patients both with and without BBs. B, Effect size of BBs on outcomes according to heart rate. The effect of BBs appeared to decrease as the heart rate declined. The solid line represents the estimated probability of 1‐year all‐cause mortality, the shaded area is 95% CI. Below is a density plot showing the distribution of observed heart rate. BB indicates β‐blocker.
We did not specifically investigate the pathophysiologic mechanism for the ineffectiveness of β‐blockers in patients with slow heart. A possible explanation is that because the magnitude of heart rate reduction by β‐blockers depends on the baseline heart rate and patients with low heart rate experience smaller reduction in heart rate, the effect of the β‐blocker may be reduced. This explanation is consistent with the finding that there was no difference in heart rate between patients with or without β‐blockers among those with slow heart rate at discharge. Slow heart rate might reflect low sympathetic tone. Consequently, the effect of β‐blockers may be less prominent in patients with slow heart rate.
Atrial Fibrillation
The prognostic value of heart rate and the beneficial effect of β‐blockers in patients with heart failure and atrial fibrillation remain controversial. In patients with atrial fibrillation, slow heart rate may indicate a significant dysfunction in the conducting system, whereas high heart rate may represent compensatory response to the loss of atrial contraction. In some studies, high heart rate was associated with worse outcomes,21, 22 whereas in the post hoc analysis of the CHARM (Candesartan in Heart Failure‐Assessment of Reduction in Mortality and Morbidity) study,23 heart rate did not have any prognostic value. Similarly, the effect of β‐blockers was also inconsistent. In CIBIS‐II (The Cardiac Insufficiency Bisoprolol Study II), the morbidity and mortality rates were similar between the bisoprolol and placebo groups.24 In a meta‐analysis, Kotecha et al25 and Rienstra et al26 showed that β‐blockers did not lead to a reduction in death of patients with heart failure and atrial fibrillation. In contrast, Simpson et al27 showed improved survival with β‐blocker treatment despite the absence of heart rate reduction.
In this study, the β‐blocker prescription at discharge was not associated with a reduction in heart rate but was associated with better outcomes in patients with atrial fibrillation regardless of heart rate. Differences in the study populations might explain the varying results.
Clinical Implications
In patients with HFrEF, β‐blockers should be initiated as soon as possible, at best during hospitalization for acute heart failure.28 Concerns about worsening heart failure, bradycardia, and hypotension are psychological barriers to the prescribing of β‐blockers, especially in patients with slow heart rate. Regarding 1‐year mortality, our study showed that patients with slow heart rate seem to do equally well with or without a β‐blocker prescription, indicating that not all patients benefit equally from β blockade.
Nonetheless, β‐blockers had a beneficial effect on 1‐year mortality of patients with high heart rate and a favorable effect on hospitalization for heart failure regardless of heart rate. These results suggest that patients should receive a β‐blocker regardless of their heart rate, unless contraindicated, which is also in line with current practice guidelines.
Limitations
Because of the nature of the study design, albeit a large prospective cohort study, we were unable to exclude confounding factors that may have influenced the variable effect of the β‐blockers according to patients’ heart rates. The results remain to be proven in a randomized clinical trial of patients with low heart rate treated by β‐blockers or placebo. The proportion of β‐blocker usage and the prescribed doses were lower in this study compared with other clinical trials. Although these details may accurately reflect actual clinical practice in Asia, the study results cannot be extrapolated to other patients who receive higher dose of β‐blockers. Finally, it is unknown whether the same cutoff value for low heart rate can be applied for both atrial fibrillation and sinus rhythm. This cutoff should be tested in a larger cohort with atrial fibrillation patients.
Conclusions
In hospitalized patients with HFrEF, many patients have a slow heart rate at discharge and favorable 1‐year mortality regardless of β‐blocker prescription at discharge. β‐Blockers may have limited effect with HFrEF and slow heart rate at discharge. This study result is hypothesis generating, and whether β‐blockers may be deferred in hospitalized patients with HFrEF and slow heart rate at discharge must be confirmed in further clinical trials.
Sources of Funding
This work was supported by Research of Korea Centers for Disease Control and Prevention (2010‐E63003‐00, 2011‐E63002‐00, 2012‐E63005‐00, 2013‐E63003‐00, 2013‐E63003‐01, 2013‐E63003‐02, and 2016‐ER6303‐00) and by the Seoul National University Bundang Hospital Research Fund (grant no 14‐2015‐029, 16‐2017‐003).
Disclosures
None.
(J Am Heart Assoc. 2019;8:e011121. DOI: 10.1161/JAHA.118.011121.)
References
- 1. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 2. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 3. Kjekshus J, Gullestad L. Heart rate as a therapeutic target in heart failure. Eur Heart J Suppl. 1999;1:64–69. [Google Scholar]
- 4. Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta‐blockers in heart failure: findings from the OPTIMIZE‐HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gullestad L, Wikstrand J, Deedwania P, Hjalmarson A, Egstrup K, Elkayam U, Gottlieb S, Rashkow A, Wedel H, Bermann G, Kjekshus J; MERIT‐HF Study Group . What resting heart rate should one aim for when treating patients with heart failure with a beta‐blocker? Experiences from the Metoprolol Controlled Release/Extended Release Randomized Intervention Trial in Chronic Heart Failure (MERIT‐HF). J Am Coll Cardiol. 2005;45:252–259. [DOI] [PubMed] [Google Scholar]
- 6. Lee SE, Cho HJ, Lee HY, Yang HM, Choi JO, Jeon ES, Kim MS, Kim JJ, Hwang KK, Chae SC, Seo SM, Baek SH, Kang SM, Oh IY, Choi DJ, Yoo BS, Ahn Y, Park HY, Cho MC, Oh BH. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16:700–708. [DOI] [PubMed] [Google Scholar]
- 7. Lee SE, Lee HY, Cho HJ, Choe WS, Kim H, Choi JO, Jeon ES, Kim MS, Kim JJ, Hwang KK, Chae SC, Baek SH, Kang SM, Choi DJ, Yoo BS, Kim KH, Park HY, Cho MC, Oh BH. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF). Korean Circ J. 2017;47:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L; SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet. 2010;376:875–885. [DOI] [PubMed] [Google Scholar]
- 9. Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R; BEAUTIFUL investigators . Heart rate as a prognostic risk factor in patients with coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. [DOI] [PubMed] [Google Scholar]
- 10. Monfredi O, Boyett MR. Sick sinus syndrome and atrial fibrillation in older persons—a view from the sinoatrial nodal myocyte. J Mol Cell Cardiol. 2015;83:88–100. [DOI] [PubMed] [Google Scholar]
- 11. Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J. 2015;36:1974–1982b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanders P, Kistler PM, Morton JB, Spence SJ, Kalman JM. Remodeling of sinus node function in patients with congestive heart failure: reduction in sinus node reserve. Circulation. 2004;110:897–903. [DOI] [PubMed] [Google Scholar]
- 13. Lechat P, Hulot JS, Escolano S, Mallet A, Leizorovicz A, Werhlen‐Grandjean M, Pochmalicki G, Dargie H. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation. 2001;103:1428–1433. [DOI] [PubMed] [Google Scholar]
- 14. Metra M, Torp‐Pedersen C, Swedberg K, Cleland JG, Di Lenarda A, Komajda M, Remme WJ, Lutiger B, Scherhag A, Lukas MA, Charlesworth A, Poole‐Wilson PA. Influence of heart rate, blood pressure, and beta‐blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J. 2005;26:2259–2268. [DOI] [PubMed] [Google Scholar]
- 15. Mulder P, Barbier S, Chagraoui A, Richard V, Henry JP, Lallemand F, Renet S, Lerebours G, Mahlberg‐Gaudin F, Thuillez C. Long‐term heart rate reduction induced by the selective I(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674–1679. [DOI] [PubMed] [Google Scholar]
- 16. Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther. 2004;308:236–240. [DOI] [PubMed] [Google Scholar]
- 17. Reil JC, Reil GH, Bohm M. Heart rate reduction by I(f)‐channel inhibition and its potential role in heart failure with reduced and preserved ejection fraction. Trends Cardiovasc Med. 2009;19:152–157. [DOI] [PubMed] [Google Scholar]
- 18. Schleman KA, Lindenfeld JA, Lowes BD, Bristow MR, Ferguson D, Wolfel EE, Abraham WT, Zisman LS. Predicting response to carvedilol for the treatment of heart failure: a multivariate retrospective analysis. J Card Fail. 2001;7:4–12. [DOI] [PubMed] [Google Scholar]
- 19. Cullington D, Goode KM, Clark AL, Cleland JG. Heart rate achieved or beta‐blocker dose in patients with chronic heart failure: which is the better target? Eur J Heart Fail. 2012;14:737–747. [DOI] [PubMed] [Google Scholar]
- 20. Kotecha D, Flather MD, Altman DG, Holmes J, Rosano G, Wikstrand J, Packer M, Coats AJS, Manzano L, Bohm M, van Veldhuisen DJ, Andersson B, Wedel H, von Lueder TG, Rigby AS, Hjalmarson A, Kjekshus J, Cleland JGF; Beta‐Blockers in Heart Failure Collaborative Group . Heart rate and rhythm and the benefit of beta‐blockers in patients with heart failure. J Am Coll Cardiol. 2017;69:2885–2896. [DOI] [PubMed] [Google Scholar]
- 21. Bertomeu‐Gonzalez V, Nunez J, Nunez E, Cordero A, Facila L, Ruiz‐Granell R, Quiles J, Sanchis J, Bodi V, Minana G, Bertomeu V, Llacer A. Heart rate in acute heart failure, lower is not always better. Int J Cardiol. 2010;145:592–593. [DOI] [PubMed] [Google Scholar]
- 22. Rienstra M, Van Gelder IC, Van den Berg MP, Boomsma F, Hillege HL, Van Veldhuisen DJ. A comparison of low versus high heart rate in patients with atrial fibrillation and advanced chronic heart failure: effects on clinical profile, neurohormones and survival. Int J Cardiol. 2006;109:95–100. [DOI] [PubMed] [Google Scholar]
- 23. Castagno D, Skali H, Takeuchi M, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pfeffer MA, McMurray JJ, Solomon SD. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) program. J Am Coll Cardiol. 2012;59:1785–1795. [DOI] [PubMed] [Google Scholar]
- 24. Lechat PP. Beta‐blocker efficacy according to heart rate and rhythm in patients with heart failure. Commentary on the Cardiac Insufficiency Bisoprolol Study II analysis. Card Electrophysiol Rev. 2003;7:233–235. [DOI] [PubMed] [Google Scholar]
- 25. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, von Lueder TG, Wedel H, Rosano G, Shibata MC, Rigby A, Flather MD; Beta‐Blockers in Heart Failure Collaborative Group . Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual‐patient data meta‐analysis. Lancet. 2014;384:2235–2243. [DOI] [PubMed] [Google Scholar]
- 26. Rienstra M, Damman K, Mulder BA, Van Gelder IC, McMurray JJ, Van Veldhuisen DJ. Beta‐blockers and outcome in heart failure and atrial fibrillation: a meta‐analysis. JACC Heart Fail. 2013;1:21–28. [DOI] [PubMed] [Google Scholar]
- 27. Simpson J, Castagno D, Doughty RN, Poppe KK, Earle N, Squire I, Richards M, Andersson B, Ezekowitz JA, Komajda M, Petrie MC, McAlister FA, Gamble GD, Whalley GA, McMurray JJ; Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) . Is heart rate a risk marker in patients with chronic heart failure and concomitant atrial fibrillation? Results from the MAGGIC meta‐analysis. Eur J Heart Fail. 2015;17:1182–1191. [DOI] [PubMed] [Google Scholar]
- 28. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]


