Abstract
Background
Despite the epidemic of cardiovascular diseases in middle‐income countries, few trials are testing the benefits of cardiac rehabilitation (CR). This trial assessed (1) maintenance of functional capacity, risk factor control, knowledge, and heart‐health behaviors and (2) mortality and morbidity at 6 months following CR in a middle‐income country.
Methods and Results
Eligible Brazilian coronary patients were initially randomized (1:1:1 concealed) to 1 of 3 parallel arms (comprehensive CR [exercise plus education], exercise‐only CR, or wait‐list control). The CR programs were 6 months in duration, at which point follow‐up assessments were performed. Mortality and morbidity were ascertained from chart and patient or family report (blinded). Controls were then offered CR (crossover). Outcomes were again assessed 6 months later (blinded). ANCOVA was performed for each outcome at 12 months. Overall, 115 (88.5%) patients were randomized, and 62 (53.9%) were retained at 1 year. At 6 months, 23 (58.9%) of those 39 initially randomized to the wait‐list control elected to attend CR. Functional capacity, risk factors, knowledge, and heart‐health behaviors were maintained from 6 to 12 months in participants from both CR arms (all P>0.05). At 1 year, knowledge was significantly greater with comprehensive CR at either time point (P<0.001). There were 2 deaths. Hospitalizations (P=0.03), nonfatal myocardial infarctions (P=0.04), and percutaneous coronary interventions (P=0.03) were significantly fewer with CR than control at 6 months.
Conclusions
CR participation is associated with lower morbidity, long‐term maintenance of functional capacity, risk factors, and heart‐health behaviors, as well as with greater cardiovascular knowledge compared with no CR.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02575976.
Keywords: cardiac rehabilitation, coronary disease, morbidity/mortality, rehabilitation, risk factor
Subject Categories: Chronic Ischemic Heart Disease, Risk Factors, Secondary Prevention, Exercise, Mortality/Survival
Clinical Perspective
What Is New?
Cardiac rehabilitation participation was associated with lower morbidity and with maintenance of functional capacity, risk factors, and heart‐health behaviors at 6 months after the program compared with no cardiac rehabilitation.
Comprehensive cardiac rehabilitation was associated with significantly greater knowledge.
What Are the Clinical Implications?
Broader implementation of comprehensive cardiac rehabilitation services is needed.
Despite the epidemic of cardiovascular disease in middle‐income countries, the sustained impact of cardiac rehabilitation in mitigating this burden was unknown in these settings.
Cardiovascular diseases are among the leading burdens of disease globally and are the leading cause of death, with >80% of these deaths occurring in low‐ and middle‐income countries (LMICs)1 such as Brazil.2 Cardiac rehabilitation (CR) is an outpatient model of secondary prevention designed to mitigate this burden.3
CR comprises internationally agreed core components including structured exercise and patient education.3, 4 Meta‐analyses demonstrate that participation in CR is associated with 20% lower morbidity and cardiovascular mortality compared with usual care.5 However, included trials were all undertaken in high‐income countries, and there have been no trials with mortality or morbidity outcomes in an LMIC to our knowledge. This is a major knowledge gap, considering that resources in many LMICs are so limited that only the core component of exercise is offered; thus, a pragmatic trial is greatly needed. Moreover, recent meta‐analyses have questioned the impact of CR on all‐cause mortality in the current era.5, 6 The responses to the review by Powell et al6 suggested that such benefits would likely be observed in LMICs,3 given the lack of preventive care, risk factor management, and other differences with high‐income countries.7
Reduced mortality and morbidity in the long term are likely achieved through maintenance of heart‐health behavior changes initiated through CR, ensuring sustained risk factor control. Consequently, the objectives of this study were (1) to assess maintenance of functional capacity, risk factor control, knowledge, and heart‐health behaviors in the 6 months following CR (exercise‐only or comprehensive CR [CCR]) versus usual care, (2) to test the effects of CR on mortality and morbidity at 6 months after the program in a middle‐income country. It was hypothesized that patients who participate in CR, compared with patients who do not participate, maintain their gains and have lower mortality and morbidity.
Methods
Design
Research ethics approval was obtained from Federal University of Minas Gerais (898.235) and York University (e2015–172). The data that support the findings of this study may be made available in anonymized form by the corresponding author on reasonable request from qualified researchers trained in human subject confidentiality protocols, with appropriate research ethics approvals and secure data transfer agreements. This article presents secondary analysis of trial data, focusing on the mortality and morbidity outcomes that have not been previously reported, as well as primary, secondary, and tertiary outcomes of the trial reported not only from the prespecified 6‐month end point (corresponding with the end of CR for those in the intervention arms) but also longer term at 12 months from initial randomization.
A single‐blinded, single‐site, pragmatic, superiority randomized controlled trial was undertaken with 3 parallel arms: CCR (education and exercise), exercise‐only CR (no education), and wait‐list control (ie, no CR). Patient assessments were performed before randomization and 6 months later. A detailed protocol8 and primary and secondary9 outcomes at 6 months are reported elsewhere. Mortality and morbidity (the latter being added after trial commencement) were also assessed at 6 months.
After the 6‐month assessments, the wait‐list control group was offered CR (exercise only or CCR; ie, nonrandomized crossover; Figure 1) by a doctoral student (G.C.). Although not envisioned when the original protocol was designed, assessments of all outcomes, including mortality and morbidity, were undertaken again in all participants at 12 months from trial inception. Thus, the 5 conditions were exercise‐only CR, CCR (both of these conditions would be expected to have carryover effects, but no more CR sessions were offered after the 6‐month program), wait‐list control (and did not elect to participate in CR at posttest), exercise‐only CR after 6 months, and CCR after 6 months.
Figure 1.
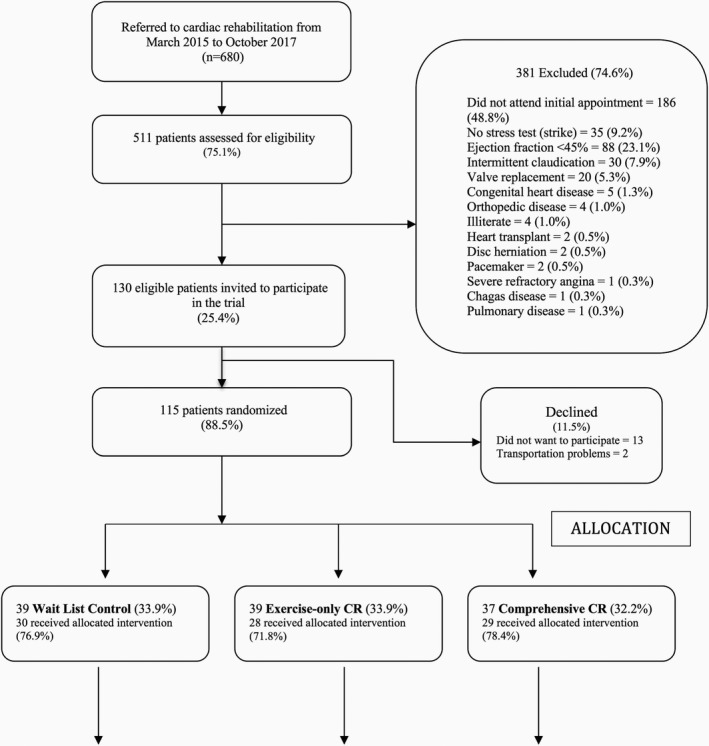
Study flow diagram. The threshold number of sessions for per‐protocol analysis was minimum of 24 of 36 exercise sessions (both CR arms) and an additional 16 of 24 education classes for comprehensive CR. CR indicates cardiac rehabilitation.
Setting
This randomized controlled trial was conducted in the Brazilian city of Belo Horizonte, in the publicly funded academic center Hospital das Clínicas of the Universidade Federal de Minas Gerais. The wait‐list control group received usual care, which consists of patients’ follow‐up appointments with their physician as deemed medically appropriate. The standard of care for Brazilian adults with cardiovascular disease does not include access to CR, given the gross lack of capacity.10
Intervention Arms
The CR program is led by a physician and staffed by physiotherapists. Participants underwent an initial assessment including functional capacity and risk factors at intake.
The exercise program was 6 months in duration, consisting of 36 1‐hour supervised sessions offered in descending frequency.8 Each CR participant received an individualized exercise prescription based on a graded exercise stress test. Patients were requested to exercise in their communities on the days they were not on‐site so as to accumulate ≥30 minutes of physical activity at moderate to vigorous intensity on ≥5 days per week, as recommended in practice guidelines.3, 4, 11
In the CCR arm, 24 education sessions were also offered, each of 30‐minute duration. Sessions were delivered weekly, in a group setting, by a health educator, in Brazilian Portuguese.8 Participants received a validated education workbook to accompany the sessions (https://www.healtheuniversity.ca/pt/CardiacCollege/About/Pages/download-guide.aspx).12
Participants
Patients with coronary artery disease after myocardial infarction (MI) or those who had undergone percutaneous coronary intervention (PCI) or coronary artery bypass grafting and were referred to CR were eligible to participate in the trial. The inclusion criteria were patients aged >18 years and living in the Belo Horizonte area. The exclusion criteria were cardiac conditions associated with some risk during high‐intensity exercise (eg, heart failure with ejection fraction <45%, complex ventricular dysrhythmia), any comorbid physical condition (eg, leg amputation, advanced cancer, disabling stroke, Parkinson's disease) or serious mental illness that would interfere with the ability to exercise, according to CR clinical practice guidelines, or any visual or cognitive condition that would preclude the participant from completing the questionnaires.
Sample size calculations were performed for the primary outcome of functional capacity at 6 months, namely, distance walked (see Measures).8 Based on previous studies, we considered 70 m a clinically important difference and assumed a standard deviation of 139 m. In accordance, 62 participants were required per group to ensure 80% power at the 5% significance level to detect a statistically significant difference.
Procedures
A doctoral student (G.C.) approached consecutive patients during the first physician consultation after hospital discharge from March 2015 to April 2017. With informed written consent from the patient and CR clearance from the physician, eligible participants were scheduled to come on‐site to complete pretest assessments. This included completion of a survey and instruction on wearing the pedometer. Follow‐up assessments were performed between September 2015 and May 2018.
Randomization and Blinding
Eligible participants were initially randomized to 1 of the 3 arms: wait‐list control, exercise‐only CR, and CCR. The randomization sequence was generated using the Randomization.com website in random blocks of 4, with a 1:1:1 allocation ratio. To ensure allocation concealment, the local principal investigator (R.B.) has the allocation sequence in a password‐protected file and randomization information was provided to the doctoral student (G.C.) once the participant was confirmed to be eligible. Because of the nature of the intervention, the participants and the doctoral student could not be blind to treatment allocation.
Master's degree students blinded to random allocation undertook subsequent 6‐ and 12‐month assessments, outcome ascertainment, and data entry. To minimize loss to follow‐up, patients were called to remind them to come on‐site for these assessments.
Measures
Participants were asked to complete a sociodemographic questionnaire at baseline. Clinical characteristics were extracted from medical charts, including sex, age, risk factors, cardiac history, laboratory test results, comorbidities, and medications. CR session attendance (both exercise and education sessions) was extracted from program charts for participants at 6 months.
The primary outcome of the trial was functional capacity (incremental shuttle walk test; 7 metabolic equivalents of task was considered indicative of independent living and thus was the target).13 Secondary outcomes were risk factors (ie, blood pressure at rest; high blood pressure was considered at target if values were either <140 mm Hg systolic or <90 diastolic11), lipids (not assessed at 12 months), anthropometrics (those with body mass index <30 were considered at practice guideline target11; waist circumference of <102 cm in men and <88 cm in women were considered at guideline target for central obesity14), and depressive symptoms (Patient Health Questionnaire‐9; scores ≤10 considered subclinical and thus at target).15, 16 Tertiary outcomes were cardiovascular disease knowledge,17 heart‐health behaviors (ie, exercise, diet,18 and smoking [self‐report]), and mortality. Exercise was assessed via pedometer. Willing participants wore a Digi‐Walker SW200 (Yamax) 19, 20, 21, 22 for 7 days at each assessment point. Mean steps per week was computed, with ≥7500 steps/day considered commensurate with the guideline target for 150 minutes/week in populations with chronic disease.23
Mortality (all‐cause and cardiovascular) and morbidity were ascertained at 6 and 12 months from baseline. Morbidity included emergency department visits for cardiac causes, hospital admissions for cardiac causes, nonfatal MI, angina, revascularization (PCI or coronary artery bypass grafting), or heart failure. These data were obtained from the center's health system records and were supplemented by participant report (and/or family via phone if participants had died or did not come on‐site for the 12‐month assessment) to capture deaths, cardiac events, and procedures that may have occurred outside the hospital. Reason for death also was recorded when available. A form was developed to standardize the verbal interviewer‐administered assessment and was piloted.
Statistical Analysis
SPSS v24.0 (IBM Corp) was used, and the level of significance was set at P=0.05 for all tests. Before outcome analysis, differences in participants’ baseline sociodemographic and clinical characteristics were compared among the 5 conditions using Fisher exact tests and ANOVA, as appropriate. Moreover, the retention rate was computed, and differences in the sociodemographic and clinical characteristics of participants who were retained (ie, completed 12‐month assessment) versus lost to follow‐up were compared using Fisher exact tests and t tests, as appropriate.
Participants were included in the per‐protocol analyses if they met the threshold number of ≥24 exercise sessions in the exercise‐only arm and, additionally, ≥16 educational sessions in the CCR arm at the 6‐month assessment. Nevertheless, analyses were first performed on the basis of intention to treat (using last observation carried forward for all outcomes except morbidity; at the 12‐month assessment, wait‐listed participants who elected CR were analyzed as treated; see Figures 1 and 2).
Figure 2.
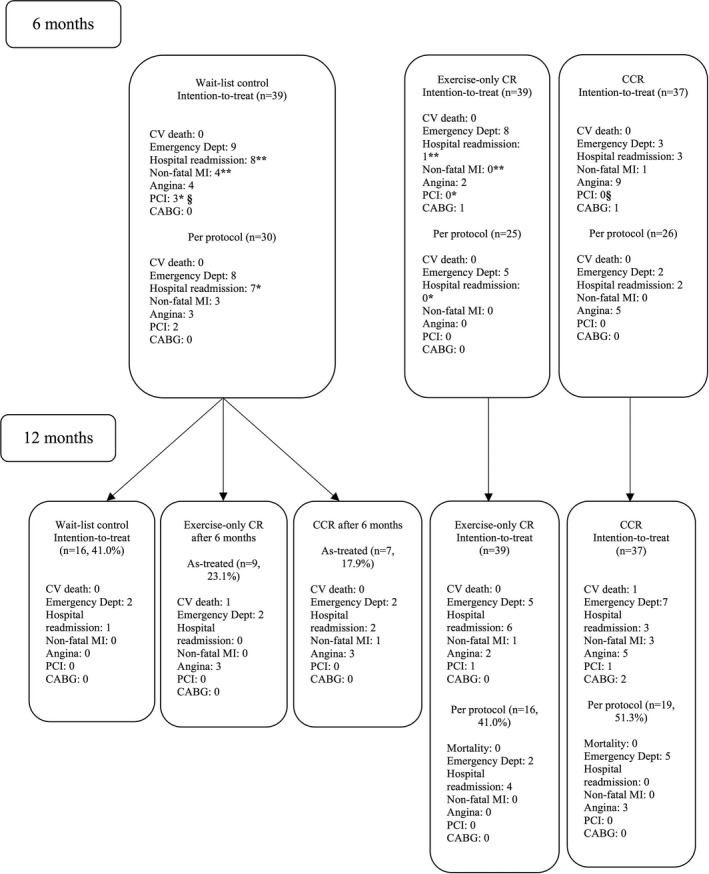
Mortality and morbidity by arm and time. The threshold number of sessions for per‐protocol analysis was minimum of 24of 36 exercise sessions (both CR arms) and an additional 16 of 24 education classes for CCR. Least significant difference post hoc: *§ P<0.05; ** P<0.01. CABG indicates coronary artery bypass grafting; CCR, comprehensive cardiac rehabilitation; CR, cardiac rehabilitation; CV, cardiovascular; Dept, department; MI, myocardial infarction; PCI, percutaneous coronary intervention.
To test the first objective, maintenance of functional capacity, risk factors, and heart‐health behavior from 6 to 12 months within condition was assessed using the paired t test (nonparametric test [Wilcoxon signed rank test]) for pedometer step counts, which were not normally distributed; outcomes were considered “maintained” if there was no significant difference in 6‐ and 12‐month scores (ie, P>0.05). To test the second objective, ANCOVA (adjusting for characteristics that differed by condition or retention, as assessed earlier) was performed for each of the outcomes at 12 months (except smoking, for which a Fisher exact test was performed), with condition (ie, 5) as the independent variable. Least significant difference post hoc tests were performed if significant differences were observed. Finally, to account for period effects, outcomes were described by condition regardless of time point (ie, outcomes for exercise only in those randomized to this arm at 6 months and in wait‐list controls electing exercise only at 12 months).
For mortality and morbidity, frequencies (and means for the latter) were calculated by arm and condition (ie, 3 arms at 6 months and 5 conditions at 12 months) for both follow‐up assessment points. Differences were tested using Fisher exact tests and ANOVA.
Results
Respondent Characteristics
A participant flow diagram is shown in Figure 1. The consent rate was very high, and 115 patients were randomized. Randomization was effective in ensuring equivalence across arms in most instances (only differences were in educational attainment and comorbid cancer).9 At 6 months, 93 (80.9%) were retained. There was no clinical bias and minimal sociodemographic bias in the retained sample, except that those retained were significantly older and less likely to be working than those lost to follow‐up.9
At 6 months, of those initially randomized to the wait‐list control, 12 (30.8%) elected to attend exercise‐only CR and 11 (28.2%) chose CCR (Figure 1). Table 1 presents baseline participant characteristics by condition. Only significant differences were noted for sex (more male participants chose exercise‐only CR than CCR after 6 months) and education (those retained in the exercise‐only CR arm initially were significantly more educated than retained participants both initially randomized to comprehensive CR as well as those on the wait‐list control who did not elect to participate in CR). No differences in clinical characteristics were observed.
Table 1.
Participants’ Baseline Sociodemographic and Clinical Characteristics by Condition
| Initial Randomization | Wait‐List Control (n=39) | Exercise Only (n=39) | Comprehensive (n=37) | Total (N=115) | ||
|---|---|---|---|---|---|---|
| Disposition at 6 mo | No CR (n=16) | Exercise Only (n=12) | Comprehensive (n=11) | No CR | No CR | |
| Sociodemographic | ||||||
| Sex (male) | 11 (68.8) | 12 (100.0)†† | 4 (36.4)†† | 28 (71.8) | 27 (73.0) | 82 (71.3)‡ |
| Age, y | 55.9±6.7 | 60.7±13.3 | 60.6±8.4 | 59.0±9.9 | 60.7±8.8 | 59.5±9.4 |
| Education (low)§ | 9 (56.3)* | 10 (83.3) | 9 (81.8) | 33 (84.6)*†† | 21 (56.8)†† | 82 (71.3)‡ |
| Marital status (married or equivalent)** | 9 (56.3) | 9 (75.0) | 9 (81.8) | 27 (69.2) | 20 (54.1) | 74 (64.3) |
| Work status (employed) | 11 (68.8) | 3 (25.0) | 3 (27.3) | 14 (35.9) | 15 (40.5) | 46 (40.0) |
| Monthly income (low)∥ | 13 (81.3) | 11 (91.7) | 11 (100.0) | 34 (87.2) | 31 (83.8) | 100 (87.0) |
| Clinical | ||||||
| CR indication (yes) | ||||||
| MI | 15 (93.8) | 11 (91.7) | 9 (81.8) | 37 (94.9) | 35 (94.6) | 107 (93.0) |
| Angina | 12 (75.0) | 8 (66.7) | 7 (63.6) | 21 (53.8) | 21 (56.8) | 69 (60.0) |
| PCI | 10 (62.5) | 9 (75.0) | 4 (36.4) | 23 (59.0) | 22 (59.5) | 68 (59.1) |
| Bypass surgery | 3 (18.8) | 4 (33.3) | 3 (27.3) | 7 (17.9) | 12 (32.4) | 29 (25.2) |
| First event (no) | 4 (25.0) | 2 (16.7) | 2 (18.2) | 8 (21.1) | 12 (32.4) | 28 (24.8) |
| Comorbidities (yes) | ||||||
| Depression | 3 (18.8) | 2 (16.7) | 2 (18.2) | 7 (17.9) | 6 (16.2) | 20 (17.4) |
| Kidney disease | 1 (6.3) | 2 (16.7) | 1 (9.1) | 3 (7.7) | 6 (16.2) | 13 (11.3) |
| Liver disease | 0 (0.0) | 1 (8.3) | 0 (0.0) | 2 (5.1) | 5 (13.5) | 8 (7.0) |
| Rheumatic disease | 1 (6.3) | 2 (16.7) | 1 (9.1) | 1 (2.6) | 2 (5.4) | 7 (6.1) |
| Cancer | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.6) | 5 (13.5) | 6 (5.2) |
| Stroke | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.6) | 2 (5.4) | 3 (2.6) |
| COPD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (7.7) | 0 (0.0) | 3 (2.6) |
| Functional capacity | ||||||
| Stress test (HR max, bpm) | 118.7±21.2 | 125.3±21.4 | 112.4±17.2 | 124.8±21.4 | 120.4±24.2 | 121.4±21.9 |
| Stress test (peak METs) | 7.2±2.7 | 7.9±2.3 | 6.6±1.9 | 7.7±2.6 | 7.8±2.6 | 7.6±2.5 |
| ISWT, m | 380.6±111.6 | 443.3±186.6 | 297.3±106.4 | 361.0±119.4 | 381.1±120.9 | 372.7±128.5 |
| Risk factors | ||||||
| BP systolic, mm Hg | 115.0±21.6 | 117.5±9.6 | 122.7±18.5 | 117.3±24.7 | 123.8±15.1 | 119.6±19.6 |
| BP diastolic, mm Hg | 75.0±17.5 | 71.7±11.1 | 77.3±19.0 | 77.7±13.0 | 77.0±11.0 | 76.4±13.5 |
| BMI, kg/m2 | 27.5±4.2 | 27.6±3.7 | 28.6±4.5 | 28.7±6.0 | 28.1±4.2 | 28.2±4.8 |
| WC, cm | 93.0±10.1 | 96.8±11.4 | 95.5±7.4 | 96.7±10.6 | 96.0±11.5 | 95.9±10.6 |
| TC, mg/dL | 137.3±29.0 | 154.2±40.3 | 165.4±30.2 | 148.7±39.4 | 165.0±61.9 | 155.7±46.9 |
| LDL, mg/dL | 68.8±21.2 | 84.1±38.9 | 93.3±24.3 | 80.4±23.7 | 86.4±29.7 | 83.1±27.9 |
| HDL, mg/dL | 42.6±6.5 | 39.3±7.5 | 44.2±6.8 | 40.4±14.3 | 39.5±7.9 | 40.7±10.1 |
| Triglycerides, mg/dL | 134.1±57.5 | 152.9±44.1 | 136.4±55.0 | 137.7±75.2 | 166.0±117.0 | 148.6±85.6 |
| Glucose (fasting), mg/dL | 106.3±25.6 | 110.8±34.8 | 112.2±52.5 | 107.2±35.3 | 104.6±20.2 | 107.3±32.0 |
| Sleep apnea (yes) | 2 (12.5) | 2 (16.7) | 1 (9.1) | 4 (10.3) | 4 (10.8) | 13 (11.3) |
| Smoking (current) | 1 (6.3) | 1 (8.3) | 0 (0.0) | 4 (10.3) | 3 (8.1) | 9 (7.8) |
| Medications (yes) | ||||||
| Statins | 16 (100.0) | 12 (100.0) | 10 (90.9) | 37 (94.9) | 36 (97.3) | 111 (98.2) |
| ASA | 16 (100.0) | 10 (83.3) | 10 (90.9) | 35 (92.1) | 35 (97.2) | 106 (94.6) |
| β‐Blockers | 16 (100.0) | 11 (91.7) | 10 (90.9) | 30 (78.9) | 33 (91.7) | 100 (89.3) |
| Antiplatelets | 13 (81.3) | 7 (58.3) | 8 (72.7) | 30 (78.9) | 23 (63.9) | 81 (72.3) |
| ACEIs | 6 (37.5) | 7 (58.3) | 7 (63.6) | 26 (68.4) | 27 (75.0) | 73 (65.2) |
| ARBs | 6 (37.5) | 5 (41.7) | 1 (9.1) | 8 (21.1) | 6 (16.7) | 26 (23.2) |
| Other | ||||||
| Cardiac knowledge¶ | 45.9±14.4 | 43.9±19.1 | 46.4±9.9 | 48.24±13.3 | 51.24±11.9 | 48.32±13.5 |
| Exercise (steps/wk) | 32 898.5±17 657.3 | 28 047.0±18 558.8 | 31 582.8±14 555.0 | 33 153.1±27 636.6 | 31 415.0±23 918.4 | 31 855.1±23 027.5 |
| Diet# | 10.1±5.8 | 9.2±7.7 | 3.4±5.7 | 5.92±7.4 | 4.65±7.7 | 6.18±7.4 |
Data are shown as n (%) or mean±SD. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; bpm, beats/minute; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; HDL, high‐density lipoprotein; HR, heart rate; ISWT, incremental shuttle walk test; LDL, low‐density lipoprotein; MET, metabolic equivalent of task; MI, myocardial infarction; PCI, percutaneous coronary intervention; TC, total cholesterol; WC, waist circumference.
Bonferroni post hoc tests: *†one symbol P<0.05; 2 symbols P<0.01.
ANOVA or Fisher exact test for characteristic by condition ‡ P<0.05.
ie, living together for many years, although not officially married.
Did not complete high school.
Less than 4 minimum wages per month.
Scores on the Coronary Artery Disease Questionnaire23 range from 0 to 93, with higher scores indicating better knowledge.
Scores on the Food Frequency Questionnaire range from −36 to +47, with higher scores indicating better diet.
Sixty (52.2%) participants completed the 12‐month assessment (Figure 1; however, hard outcomes were ascertained from medical records in 100% of the sample). As shown in Table 2, the only differences observed between the sociodemographic and clinical characteristics of those retained versus lost to follow‐up were that retained participants more often were married and had sleep apnea.
Table 2.
Participants’ Baseline Sociodemographic and Clinical Characteristics by Retention at 12‐Month Assessment
| Characteristic | Retained (n=62; 53.9%) | Lost to Follow‐up (n=53; 46.1%) | Total (n=115) |
|---|---|---|---|
| Sociodemographic | |||
| Sex (male) | 44 (71.0) | 38 (71.7) | 82 (71.3) |
| Age, y | 60.0±9.8 | 58.9±9.0 | 59.5±9.4 |
| Education (low)* | 48 (77.4) | 34 (64.2) | 82 (71.3) |
| Marital status (married or equivalent)** | 45 (72.6) | 29 (54.7) | 74 (64.3)† |
| Work status (employed) | 20 (32.3) | 26 (49.1) | 46 (40.0) |
| Monthly income (low)‡ | 56 (90.3) | 44 (83.0) | 100 (87.0) |
| Clinical | |||
| CR indication (yes) | |||
| MI | 57 (91.9) | 50 (94.3) | 107 (93.0) |
| Angina | 41 (66.1) | 28 (52.8) | 69 (60.0) |
| PCI | 40 (64.5) | 28 (52.8) | 68 (59.1) |
| Bypass surgery | 17 (27.4) | 12 (22.6) | 29 (25.2) |
| First event (no) | 15 (24.6) | 13 (25.0) | 28 (24.8) |
| Comorbidities (yes) | |||
| Depression | 8 (12.9) | 12 (22.6) | 20 (17.4) |
| Kidney disease | 9 (14.5) | 4 (7.5) | 13 (11.3) |
| Liver disease | 6 (9.7) | 2 (3.8) | 8 (7.0) |
| Rheumatic disease | 5 (8.1) | 2 (3.8) | 7 (6.1) |
| Cancer | 3 (4.8) | 3 (5.7) | 6 (5.2) |
| Stroke | 1 (1.6) | 2 (3.8) | 3 (2.6) |
| COPD | 2 (3.2) | 1 (1.9) | 3 (2.6) |
| Functional capacity | |||
| Stress test (HR max), bpm | 120.3±23.1 | 122.7±20.7 | 121.4±21.9 |
| Stress test (peak METs) | 7.7±2.5 | 7.4±2.5 | 7.6±2.5 |
| ISWT, m | 375.3±128.9 | 369.6±129.2 | 372.7±128.5 |
| Risk factors | |||
| BP systolic, mm Hg | 119.3±15.5 | 119.9±23.8 | 119.6±19.6 |
| BP diastolic, mm Hg | 74.7±12.4 | 78.5±14.6 | 76.4±13.5 |
| BMI, kg/m2 | 28.5±5.4 | 27.9±4.2 | 28.2±4.8 |
| WC, cm | 96.6±10.5 | 95.0±10.7 | 95.9±10.6 |
| TC, mg/dL | 159.0±40.3 | 151.2±54.8 | 155.7±46.9 |
| LDL, mg/dL | 88.1±32.1 | 76.7±20.0 | 83.1±27.9 |
| HDL, mg/dL | 41.1±11.5 | 40.1±8.1 | 40.7±10.1 |
| Triglycerides, mg/dL | 148.9±60.2 | 148.1±112.8 | 148.6±85.6 |
| Glucose (fasting), mg/dL | 112.6±37.3 | 100.9±23.1 | 107.3±32.0 |
| Sleep apnea | 11 (17.7) | 2 (3.8) | 13 (11.3)† |
| Smoking (current) | 6 (9.7) | 3 (5.7) | 9 (7.8) |
| Medications | |||
| Statins | 60 (96.8) | 51 (96.2) | 111 (98.2) |
| ASA | 57 (91.9) | 49 (92.4) | 106 (94.6) |
| β‐Blockers | 53 (85.5) | 47 (88.7) | 100 (89.3) |
| Antiplatelet | 43 (69.3) | 38 (71.7) | 81 (72.3) |
| ACEIs | 42 (67.7) | 31 (58.5) | 73 (65.2) |
| ARBs | 16 (25.8) | 10 (18.9) | 26 (23.2) |
| Other | |||
| Cardiac knowledge§ | 49.0±13.2 | 47.5±13.8 | 48.32±13.46 |
| Exercise (steps/d) | 29 781.3±20 435.2 | 34 287.9±25 729.9 | 4550.7±3289.6 |
| Diet∥ | 6.4±7.5 | 5.9±7.2 | 6.18±7.38 |
Data are shown as n (%) or mean±SD. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; bpm, beats per minute; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; HDL, high‐density lipoprotein; HR, heart rate; ISWT, incremental shuttle walk test; LDL, low‐density lipoprotein; MET, metabolic equivalent of task; MI, myocardial infarction; PCI, percutaneous coronary intervention; TC, total cholesterol; WC, waist circumference.
Did not complete high school.
ie, living together for many years, although not officially married.
Unpaired t test: P<0.05.
Less than 4 minimum wages per month.
Scores on the Coronary Artery Disease Questionnaire range from 0 to 93, with higher scores indicating better knowledge.
Scores on the Food Frequency Questionnaire range from −36 to +47, with higher scores indicating better diet.
Outcomes
Mean scores for functional capacity, risk factor control (excluding lipids), knowledge, and heart‐health behaviors are shown in Table 3 by arm at 12 months. Table 4 shows the proportion of participants at outcome target at 12‐months by condition.
Table 3.
Functional Capacity, Risk Factors, Knowledge, and Heart‐Health Behaviors at 12 Months, by Condition, and Change From 6 Months (Per Protocol)*
| n | Estimated Marginal Mean† | 95% CI | P Value‡ | Change From 6 mo§ (MD±SD) | |
|---|---|---|---|---|---|
| Functional capacity (ISWT), m | |||||
| Wait‐list control only | 3 | 415.7 | 254.3–577.1 | 25.0±35.3 | |
| Exercise only after 6 mo | 8 | 463.9 | 360.2–567.6 | 121.2±108.1§ | |
| CCR after 6 mo | 7 | 508.4 | 401.1–615.8 | 50.0±72.3 | |
| Exercise only | 15 | 497.8 | 423.2–572.5 | −9.3±77.8 | |
| CCR | 19 | 444.4 | 377.6–511.2 | −15.8±93.1 | |
| Total (SD)∥ | 52 | 469.8 | (161.7) | 0.75 | 18.2±30.1 |
| Risk factors | |||||
| BP systolic, mm Hg | |||||
| Wait‐list control only | 3 | 113.6 | 88.6–138.7 | −5.0±7.0 | |
| Exercise only after 6 mo | 9 | 127.0 | 111.9–142.2 | 3.3±17.3 | |
| CCR after 6 mo | 7 | 124.2 | 107.5–140.8 | 15.7±25.0 | |
| Exercise only | 15 | 121.6 | 110.0–133.2 | 10.7±24.6 | |
| CCR | 19 | 120.1 | 109.8–130.5 | 4.8±22.8 | |
| Total (SD)∥ | 53 | 121.9 | (20.7) | 0.89 | 7.3±3.8 |
| BP diastolic, mm Hg | |||||
| Wait‐list control only | 3 | 77.3 | 62.6–92.0 | −5.0±7.0 | |
| Exercise only after 6 mo | 9 | 79.8 | 71.0–88.7 | 0.0±12.2 | |
| CCR after 6 mo | 7 | 84.0 | 74.2–93.7 | 14.2±16.1 | |
| Exercise only | 15 | 79.8 | 73.0–86.6 | 7.0±15.3 | |
| CCR | 19 | 78.6 | 72.5–84.6 | 3.1±14.9 | |
| Total (SD)∥ | 53 | 79.8 | (12.3) | 0.90 | 4.9±2.4 |
| BMI, kg/m2 | |||||
| Wait‐list control only | 3 | 26.4 | 21.8–31.0 | 0.3±0.8 | |
| Exercise only after 6 mo | 9 | 26.9 | 24.1–29.7 | 0.0±1.2 | |
| CCR after 6 mo | 7 | 26.6 | 23.5–29.6 | −0.6±3.2 | |
| Exercise only | 15 | 26.6 | 24.4–28.7 | −0.3±1.6 | |
| CCR | 19 | 28.9 | 27.0–30.9 | 0.1±1.5 | |
| Total (SD)∥ | 53 | 27.5 | (4.0) | 0.46 | −0.1±0.7 |
| WC, cm | |||||
| Wait‐list control only | 3 | 93.6 | 80.8–106.4 | 1.8±1.7 | |
| Exercise only after 6 mo | 9 | 95.1 | 87.4–102.8 | 1.5±2.8 | |
| CCR after 6 mo | 7 | 92.6 | 84.1–101.1 | −1.1±8.3 | |
| Exercise only | 15 | 92.9 | 87.0–98.8 | 0.07±3.3 | |
| CCR | 19 | 98.2 | 92.9–103.5 | 0.1±4.1 | |
| Total (SD)∥ | 53 | 95.2 | (11.2) | 0.70 | 0.3±2.1 |
| Depressive symptoms (PHQ‐9) | |||||
| Wait‐list control only | 3 | 6.2 | 0.8–11.6 | −3.6±3.0 | |
| Exercise only after 6 mo | 9 | 2.8 | −0.3 to 6.1 | −0.2±2.9 | |
| CCR after 6 mo | 7 | 2.7 | −0.8 to 6.3 | −0.7±4.2 | |
| Exercise only | 16 | 5.6 | 3.2–8.0 | 1.7±5.3 | |
| CCR | 19 | 3.5 | 1.2–5.7 | −0.4±3.9 | |
| Total (SD)∥ | 54 | 4.1 | (4.7) | 0.41 | 0.0±0.9 |
| Disease‐related knowledge¶ | |||||
| Wait‐list control only | 2 | 30.0¶¶∥∥* * | 14.6–45.3 | 8.5±6.3 | |
| Exercise only after 6 mo | 8 | 46.7††‡‡ | 39.0–54.4 | −2.0±8.2 | |
| CCR after 6 mo | 6 | 63.7¶¶‡‡ | 54.7–72.7 | 6.6±14.4 | |
| Exercise only | 16 | 54.1* *# | 48.7–59.6 | 1.4±10.2 | |
| CCR | 19 | 66.5∥∥††# | 57.4–67.6 | 0.1±7.2 | |
| Total (SD)∥ | 51 | 56.3 | (14.6) | <0.001 | 1.3±3.0 |
| Health behaviors | |||||
| Exercise, weekly mean steps | |||||
| Wait‐list control only | 3 | 27 572.7 | 3120.3–58 265.8 | −32 277.5±10 750.1 | |
| Exercise only after 6 mo | 7 | 28 849.8 | 8268.9–49 430.7 | 12 367.8±13 458.4§ | |
| CCR after 6 mo | 7 | 53 941.2 | 33 523.7–74 358.6 | 25 758.8±53 213.6 | |
| Exercise only | 16 | 49 920.5 | 36 285.8–63 555.2 | 5282.3±30 769.6 | |
| CCR | 18 | 36 844.6 | 23 867.1–49 822.0 | 5127.8±21 742.7 | |
| Total (SD)∥ | 51 | 41 650.7 | (28 913.8) | 0.25## | 7485.3±5421.3 |
| Diet*** | |||||
| Wait‐list control only | 3 | 4.4 | −4.2 to 13.1 | −3.0±5.5 | |
| Exercise only after 6 mo | 8 | 5.3 | −0.2 to 10.9 | −2.3±5.5 | |
| CCR after 6 mo | 7 | 4.6 | −1.2 to 10.3 | 0.8±3.8 | |
| Exercise only | 14 | 7.5 | 3.4–11.6 | 0.2±7.2 | |
| CCR | 17 | 5.1 | 1.3–8.9 | −2.4±7.3 | |
| Total (SD)∥ | 49 | 5.8 | (7.3) | 0.88 | −1.2±1.4 |
| Smoking status (n, % current)††† | |||||
| Wait‐list control only | 6 | 1 | 16.7 | 0 | |
| Exercise only after 6 mo | 9 | 3 | 33.3 | 1 | |
| CCR after 6 mo | 7 | 0 | 0.0 | 0 | |
| Exercise only | 16 | 3 | 18.8 | −1 | |
| CCR | 19 | 1 | 5.3 | 1 | |
| Total (SD)∥ | 57 | 8 | (14.0) | 0.15 | 1 |
BMI indicates body mass index; BP, blood pressure; CCR, comprehensive cardiac rehabilitation; ISWT, incremental shuttle walk test; MD, mean difference; SD, standard deviation; PHQ‐9, Patient Health Questionnaire 9; WC, waist circumference.
Except the wait‐list control arms who elected either cardiac rehabilitation are shown as treated, because program adherence data were not available.
Adjusted for sex, education, marital status, and sleep apnea.
Differences by condition at 12 months. ANCOVA adjusted for sex, education, marital status, and sleep apnea.
A t test comparing 6 to 12 months, P<0.05 (note that for the pedometer, Wilcoxon tests were performed).
Unadjusted values.
Scores on the Coronary Artery Disease Questionnaire range from 0 to 93, with higher scores indicating better knowledge.
Least significant difference post hoc tests from ANCOVA: # P<0.05; ** ††‡‡ P<0.01; §§∥∥¶¶ P<0.001.
Kruskal–Wallis test also performed, as not normally distributed: P>0.05.
Scores on the Food Frequency Questionnaire range from −36 to +47, with higher scores indicating better diet.
Fisher exact test.
Table 4.
Proportion of Participants Meeting Outcome Targets and Total Morbidity at 12 Months by Condition
| Initial Randomization | Wait List Control (n=39) | Exercise Only (n=39) | Comprehensive (n=37) | Total (N=115) | ||
|---|---|---|---|---|---|---|
| Disposition at 6 mo | No CR (n=16) | Exercise Only (n=12) | Comprehensive (n=11) | No CR | No CR | |
| Functionally independent (≥7 METs from ISWT) | 0 (0.0) | 2 (16.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.7) |
| High BP (no) | 13 (81.2) | 9 (75.0) | 6 (54.5) | 28 (71.8) | 31 (83.7) | 87 (75.6) |
| Not obese (BMI <30) | 12 (75.0) | 9 (75.0) | 8 (72.7) | 27 (69.2) | 24 (64.9) | 80 (69.6) |
| Not centrally obese (WC)* | 10 (66.7) | 7 (58.3) | 4 (40.0) | 22 (57.9) | 18 (50.0) | 61 (53.0) |
| Subclinical depressive symptoms (PHQ‐9 <10) | 12 (75.0) | 11 (91.7) | 10 (91.0) | 29 (74.3) | 31 (83.9) | 93 (80.9) |
| Physically active (≥7500 steps/d) | 2 (12.5) | 1 (8.3) | 2 (18.2) | 10 (25.6) | 10 (27.0) | 25 (21.7) |
| Morbidities (n) | 1.5±0.7 | 2.0±1.1 | 2.2±0.9 | 3.0±2.3 | 3.5±2.2 | 2.4±0.7 |
| Morbidities (any) | 3 (18.7) | 5 (41.6) | 8 (72.7) | 6 (15.4) | 8 (21.6) | 30 (26.1) |
Data are shown as n (%) or mean±SD. BMI indicates body mass index; CR, cardiac rehabilitation; ISWT, incremental shuttle walk test; MET, metabolic equivalent of task; PHQ‐9, Patient Health Questionnaire 9; WC, waist circumference.
WC <102 cm in men and <88 cm in women.
Change from 6 to 12 months (ie, difference in outcome scores between these assessment points) is also shown in Table 3. A significant change was shown only for functional capacity in controls electing exercise‐only CR after 6 months; those post‐CR had no significant changes, supporting the hypothesis of maintenance or lack of decay after the program. Tests were not performed for wait‐list controls who did not elect CR, given the small retained sample size (n=3).
Differences by condition in all outcomes except morbidity and mortality at 12 months are also shown in Table 3. Significant differences are shown only in knowledge, with those who were randomized to CCR having significantly greater knowledge than those in exercise only (at either point) or wait‐list control, and those electing CCR after 6 months having greater knowledge than wait‐list controls and those electing exercise‐only CR. When considered regardless of time point (Table 5), functional capacity and exercise appear to have been lower in controls.
Table 5.
Post‐Arm Outcomes, Regardless of Time Point (as Treated)
| Arm | Wait‐List Controls | Exercise Only | CCR |
|---|---|---|---|
| Assessment Point | 6 mo* (n=30) | 6 mo (n=25) and 12 mo (n=9) | 6 mo (n=26) and 12 mo (n=7) |
| Primary outcome | |||
| Functional capacity, (ISWT), m | 394.0±171.9 | 452.3±158.4 | 474.1±116.7 |
| Functionally independent (≥7 METs) | 1 (3.3) | 2 (5.8) | 0 (0.0) |
| Risk factors | |||
| BP systolic, mm Hg | 120.0±18.4 | 118.0±20.1 | 119.2±20.8 |
| BP diastolic, mm Hg | 77.0±15.3 | 76.6±12.9 | 77.1±13.2 |
| High BP (no) | 23 (76.6) | 29 (85.3) | 26 (78.8) |
| BMI, kg/m2 | 27.7±3.5 | 28.6±6.8 | 28.1±4.4 |
| Not obese (BMI <30) | 22 (56.4) | 27 (52.9) | 24 (50.0) |
| WC, cm | 95.1±9.2 | 95.8±11.1 | 95.5±12.0 |
| Not centrally obese (WC†) | 16 (41.0) | 23 (45.1) | 21 (43.8) |
| Depressive symptoms& | 4.3±4.6 | 4.8±5.9 | 4.0±4.8 |
| Subclinical depressive Symptoms (PHQ‐9 <10) | 28 (93.3) | 31 (91.2) | 30 (90.9) |
| Disease‐related knowledge‡ | 47.0±15.2 | 48.7±13.8 | 63.6±9.2 |
| Heart‐health behaviors | |||
| Exercise (steps/wk) | 25 967.6±18 525.3 | 31 687.7±25 149.6 | 41 506.3±35 107.3 |
| Physically active (≥7500 steps/d) | 4 (13.3) | 5 (14.7) | 6 (18.2) |
| Diet§ | 6.3±5.9 | 6.0±7.4 | 7.0±6.8 |
| Smoker | 3 (7.7) | 7 (13.7) | 2 (4.2) |
| Hard outcomes∥ | |||
| Mortality | 0 (0.0) | 1 (2.5) | 1 (2.5) |
| Morbidity, n | 5.6±2.7 | 2.5±2.4 | 2.9±2.5 |
Data are shown as n (%) or mean±SD. No inferential tests were performed. BMI indicates body mass index; BP, blood pressure; CCR, comprehensive cardiac rehabilitation; ISWT, incremental shuttle walk test; MET, metabolic equivalent of task; PHQ‐9, Patient Health Questionnaire 9; WC, waist circumference.
Because of potential carryover effects, the exercise‐only and CCR participant data from 12‐month assessment were not incorporated.
WC >102 cm for men and >88 cm for women.
Scores on the Coronary Artery Disease Questionnaire range from 0 to 93, with higher scores indicating better knowledge.
Scores on the Food Frequency Questionnaire range from −36 to +47, with higher scores indicating better diet.
Sample size larger because these outcomes were ascertained in all participants regardless of assessment completion.
Scores on the Patient Health Questionnaire‐9 range from 0 to 27, with higher scores indicating greater depressive symptoms.
There were no significant differences in the proportion of patients seeing a cardiac specialist by group at 6 months (n=100; 98.0%) or 12 months (n=72, 91.1%; both P>0.05). Mortality and morbidity are shown by time and condition in Figure 2 and by mean number of morbid events and proportion of participants having any morbidity in Table 4. There were no deaths at 6 months and 2 cardiovascular deaths at 12 months (1 male and 1 female). Mortality cell sizes were too small for inferential testing.
As shown, at 6 months, on the basis of intention to treat, there were significantly more hospital readmissions (P=0.03), nonfatal MIs (P=0.04), and PCIs (P=0.03) in the control group than among those receiving exercise‐only CR (and compared with those in CCR for PCI, P=0.03; Figure 2). Per protocol, there were also significantly more hospital readmissions in the wait‐list control group than among those receiving exercise‐only CR.
Cell sizes were too small to test for morbidity differences inferentially at 12 months (ie, only 7 participants elected CCR after wait listing). The mean number of morbidities was tested however, and there was no significant difference by condition (Table 4). As shown in Table 5, when examined regardless of time point, morbidity appeared to be greater in the no‐CR arm.
Discussion
In this one of few trials of CR outcomes in LMICs and the only trial with mortality and morbidity outcomes,24 long‐term follow‐up data generally corroborate the benefits achieved after the program.9 As previously reported, 6‐month “soft” outcomes from this trial demonstrated that CR participation results in significant increases in functional capacity and reductions in blood pressure from before to after the program and that CCR results in significantly greater functional capacity than no CR.9 Results also demonstrated that CCR participation results in significant increases in patients’ cardiovascular knowledge, exercise, and dietary behaviors, with greater knowledge, exercise, and diet than among those not receiving CR and/or participating in exercise‐only programs (G.L. Ghisi et al, unpublished data, 2019). As reported herein, maintenance of functional capacity, risk factors, knowledge, and heart‐health behaviors after CR was found at 1 year, as was greater knowledge with CCR than exercise only or control. Regarding mortality and morbidity, although there were too few deaths to consider group differences, morbidity was demonstrated to be significantly lower with any CR compared with none in the short term (long‐term outcomes are still unknown because of limited sample sizes).
In support of hypotheses, patients randomized to CR had significantly lower rates of hospital readmission, nonfatal MI, and PCI after the program compared with those who did not receive CR. The Cochrane review on the effects of CR showed 18% reductions in hospital admissions with CR5; however, there were no significant reductions in MI or PCI in that review. It is contended that CR may have greater benefits in LMICs (including for mortality); the benefits require further establishment, including magnitude of effect. These reductions hold meaningful implications for families (ie, costs for MI care and PCI are unaffordable for most, particularly given the gross lack of universal healthcare coverage) and health systems (ie, cost savings due to hospital avoidance).
A multisite trial powered for mortality is needed and appears highly feasible in the middle‐income country setting, considering the high consent rate and the degree of CR adherence in this trial. Nevertheless, these initial findings, corroborated by other studies (primarily nontrials),24, 25 suggest that CR is also beneficial for morbidity in LMICs. Results across all outcomes of this trial support provision of CCR over exercise‐only programs; however, firmer evidence is needed. Unfortunately, there is a gross lack of capacity in these settings.10 This trial bolsters calls for greater access26 so that patients can achieve these benefits.
Limitations
First, caution is warranted in interpreting the results of this study. Limits of generalizability, as well as selection and retention biases up to 6 months, are considered elsewhere.9 At 12 months, retention was only 50% (likely because this assessment was not planned until after trial accrual), but the only differences in those retained versus not were marital status and sleep apnea, suggesting considerable generalizability.
Second, the trial was not powered for mortality and morbidity outcomes, nor was it powered for 1‐year outcomes. Group sizes were small at 12 months, as was the number of events (a longer follow‐up period would be preferable). Although some differences in morbidity were observed, it cannot be ascertained from this trial whether CR results in reduced mortality in LMICs. Third, the morbidity outcomes were not prespecified.
Fourth, there are measurement issues. Mortality and morbidity that occurred out of hospital were self‐reported. Although lay language was used, ascertainment error is possible. Moreover, these outcomes were not adjudicated formally or blindly by a committee of independent clinicians. Fifth, there was no blood work at 12 months for ascertainment of dyslipidemia and dysglycemia, and no information was collected on program adherence in those control participants who elected to take CR.
Sixth, regarding design, we attempted to consider period and carryover effects in our analyses; however, there was not randomization of the wait‐list controls to arm at 6 months, and thus any differences observed could be related to differences in the nature of participants who elected CR versus those who did not (ie, sex and education). It was unfortunate that only 3 participants from the wait‐list control arm did not elect CR and were retained; this sample size was too small to examine differences, and thus comparison to a no‐CR control for the soft outcomes was not possible at the 12‐month assessment. Finally, because primary, secondary, and tertiary outcomes were considered in this article, multiple comparisons were performed, possibly increasing the chances of type I error.
Conclusion
CR participation is associated with lower morbidity and long‐term maintenance of functional capacity, risk factors, and heart‐health behaviors, as well as greater cardiovascular knowledge compared with no CR. These results, together with the primary and secondary outcomes of the trial demonstrating clinically significant improvements in functional capacity and risk factor management, confirm the need for greater implementation of CR in Brazil and other LMICs.
Sources of Funding
Professor Britto was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq no. 305786/2014‐8), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG no. PPM‐00869‐15 and BCS00290‐16) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Disclosures
None.
Acknowledgments
We are grateful for the help of the undergraduate and master's degree students at Federal University of Minas Gerais who helped in recruiting and evaluating all patients in this study. We also acknowledge Maureen Pakosh, MISt, for undertaking the search of the literature for cardiac rehabilitation trials in low‐ or middle‐income countries with mortality or morbidity as an outcome. Finally, we acknowledge Dr Bruno Passos and Dr Debra Pantuzzo who helped to referral the patients to the cardiac rehabilitation program.
(J Am Heart Assoc. 2019;8:e011228 DOI: 10.1161/JAHA.118.011228.)
References
- 1. World Health Organization . Global Status Report on Noncommunicable Diseases. Geneva: WHO; 2014. Available at: http://www.who.int/nmh/publications/ncd-status-report-2014/en/. Accessed October 07, 2018. [Google Scholar]
- 2. Brasil Ministério da Saúde . Sistema de Informações de Mortalidade SIM e IBGM. 2014. Available at: http://tabnet.datasus.gov.br/CGI/idb2006/matriz.htm. Accessed October 06, 2018.
- 3. Grace SL, Turk‐Adawi KI, Contractor A, Atrey A, Campbell N, Derman W, Melo Ghisi GL, Oldridge N, Sarkar BK, Yeo TJ, Lopez‐Jimenez F, Mendis S, Oh P, Hu D, Sarrafzadegan N. Cardiac rehabilitation delivery model for low‐resource settings. Heart. 2016;102:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. British Association for Cardiovascular Prevention and Rehabilitation . The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2017. 3rd ed London: British Cardiovascular Society; 2017. Available at: http://www.bacpr.com/resources/BACPR_Standards_and_Core_Components_2017.pdf. Accessed December 14, 2018. [Google Scholar]
- 5. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;1:CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powell R, McGregor G, Ennis S, Kimani PK, Underwood M. Is exercise‐based cardiac rehabilitation effective? A systematic review and meta‐analysis to re‐examine the evidence. BMJ Open. 2018;8:e019656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S; PURE Investigators‐Writing Group . The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low‐, middle‐, and high‐income countries. Am Heart J. 2009;158:1–7.e1. [DOI] [PubMed] [Google Scholar]
- 8. Chaves GSS, Ghisi GLM, Grace SL, Oh P, Ribeiro AL, Britto RR. Effects of comprehensive cardiac rehabilitation on functional capacity and cardiovascular risk factors in Brazilians assisted by public health care: protocol for a randomized controlled trial. Braz J Phys Ther. 2016;20:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaves GSS, Ghisi GLM, Grace SL, Oh P, Ribeiro AL, Britto RR. Comprehensive cardiac rehabilitation in a middle‐income setting: a randomized trial. Heart. 2018. Available at: https://heart.bmj.com/content/early/2018/10/03/heartjnl-2018-313632. Accessed February 8, 2019. [Google Scholar]
- 10. Turk‐Adawi K, Supervia Pola M, Lopez Jimenez F, Pesah E, Rongjing D, Britto R, Bjarnason‐Wehrens B, Derman W, Abreu A, Grace SL; on behalf of ICCPR Working Group . Cardiac rehabilitation availability and density around the globe. World Congress of Cardiology & Cardiovascular Health Dubai, United Arab Emirates. December. (moderated poster). Glob Heart. 2018;13:381. [Google Scholar]
- 11. Herdy AH, Lopez‐Jimenez F, Terzic CP, Milani M, Stein R, Carvalho T, Serra S, Araujo CG, Zeballos PC, Anchique CV, Burdiat G, Gonzalez K, Fernandez R, Santibanez C, Rodriguez‐Escudero JP, Ilarraza‐Lomeli H. South American guidelines for cardiovascular disease prevention and rehabilitation. Arq Bras Cardiol. 2014;103:1–31. [DOI] [PubMed] [Google Scholar]
- 12. Ghisi GLM, Scane K, Sandison N, Maksymiu S, Skeffington V, Oh P. Development of an educational curriculum for cardiac rehabilitation patients and their families. J Clin Exp Cardiolog. 2015;6:373. [Google Scholar]
- 13. Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47:1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Institutes of Health . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6:51–209. [PubMed] [Google Scholar]
- 15. Santos IS, Tavares BF, Munhoz TN, Almeida LSP, Silva NTB, Tams BD, Patella AM, Matijasevich A. Sensitivity and specificity of the Patient Health Questionnaire‐9 (PHQ‐9) among adults from the general population. Cad Saude Publica. 2013;29:1533–1543. [DOI] [PubMed] [Google Scholar]
- 16. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghisi GL, Grace SL, Thomas S, Evans M, Oh P. Development and psychometric validation of the second version of the Coronary Artery Disease Education Questionnaire (CADE‐Q II). Patient Educ Couns. 2015;98:378–383. [DOI] [PubMed] [Google Scholar]
- 18. Laviolle B, Froger‐Bompas C, Guillo P, Sevestre A, Letellier C, Pouchard M, Daubert JC, Paillard F. Relative validity and reproducibility of a 14‐item semi‐quantitative food frequency questionnaire for cardiovascular prevention. Eur J Cardiovasc Prev Rehabil. 2005;12:587–595. [DOI] [PubMed] [Google Scholar]
- 19. Bassett DR, John D. Use of pedometers and accelerometers in clinical populations: validity and reliability issues. Phys Ther Rev. 2010;15:135–142. [Google Scholar]
- 20. Schneider PL, Crouter SE, Lukajic O, Bassett DR. Accuracy and reliability of 10 pedometers for measuring steps over a 400‐m walk. Med Sci Sports Exerc. 2003;35:1779–1784. [DOI] [PubMed] [Google Scholar]
- 21. Crouter SE, Schneider PL, Karabulut M, Bassett DR. Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35:1455–1460. [DOI] [PubMed] [Google Scholar]
- 22. Grace SL, Midence L, Oh P, Brister S, Chessex C, Stewart DE, Arthur HM. Cardiac rehabilitation program adherence and functional capacity among women: a randomized controlled trial. Mayo Clin Proc. 2016;91:140–148. [DOI] [PubMed] [Google Scholar]
- 23. Tudor‐locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, Ewald B, Gardner AW, Hatano Y, Lutes LD, Matsudo SM, Ramirez‐Marrero FA, Rogers LQ, Rowe DA, Schmidt MD, Tully MA, Schmidt MD. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ragupathi L, Stribling J, Yakunina Y, Fuster V, McLaughlin MA, Vedanthan R. Availability, use, and barriers to cardiac rehabilitation in LMIC. Glob Heart. 2017;12:323–334. [DOI] [PubMed] [Google Scholar]
- 25. Shanmugasegaram S, Perez‐Terzic C, Jiang X, Grace SL. Cardiac rehabilitation services in low‐and middle‐income countries: a scoping review. J Cardiovasc Nurs. 2014;29:454–463. [DOI] [PubMed] [Google Scholar]
- 26. Babu AS, Lopez‐Jimenez F, Thomas RJ, Isaranuwatchai W, Herdy AH, Hoch JS, Grace SL. Advocacy for outpatient cardiac rehabilitation globally. BMC Health Serv Res. 2016;16:471. [DOI] [PMC free article] [PubMed] [Google Scholar]


