Abstract
Background
The endothelial glycocalyx is a vasoprotective barrier between the blood and endothelium. We hypothesized that glycocalyx degradation is present in preeclampsia, a pregnancy‐specific hypertensive disorder characterized by endothelial dysfunction and activation.
Methods and Results
We examined the sublingual glycocalyx noninvasively using sidestream dark field imaging in the third trimester among women with normotensive pregnancies (n=73), early (n=14) or late (n=29) onset preeclampsia, or gestational diabetes mellitus (n=21). We calculated the width of the glycocalyx that was permeable to red blood cells (called the perfused boundary region, a measure of glycocalyx degradation) and the percentage of vessels that were filled with red blood cells ≥50% of the time (a measure of microvascular perfusion). In addition, we measured circulating levels of glycocalyx components, including heparan sulfate proteoglycans, hyaluronic acid, and SDC1 (syndecan 1), in a subset of participants by ELISA. Repeated‐measures ANOVA was performed to adjust for vessel diameter and caffeine intake. Women with early onset preeclampsia showed higher glycocalyx degradation, indicated by a larger perfused boundary region (mean: 2.14 [95% CI, 2.05–2.20]), than the remaining groups (mean: normotensive: 1.99 [95% CI, 1.95–2.02], P=0.002; late‐onset preeclampsia: 2.01 [95% CI, 1.96–2.07], P=0.024; gestational diabetes mellitus: 1.97 [95% CI, 1.91–2.04], P=0.004). The percentage of vessels that were filled with red blood cells was significantly lower in early onset preeclampsia. These structural glycocalyx changes were accompanied by elevated plasma concentrations of the glycocalyx components, heparan sulfate proteoglycans and hyaluronic acid, in early onset preeclampsia compared with normotensive pregnancy.
Conclusions
Glycocalyx degradation and reduced microvascular perfusion are associated with endothelial dysfunction and activation and vascular injury in early onset preeclampsia.
Keywords: gestational diabetes mellitus, microcirculation, preeclampsia/pregnancy, vascular glycocalyx
Subject Categories: Preeclampsia, Pathophysiology, Vascular Biology, Pregnancy
Clinical Perspective
What Is New?
Early onset preeclampsia is associated with structural and functional damage of the glycocalyx, a layer of proteoglycans and glycoproteins that lines the vascular endothelium and protects endothelial cells from endothelial activation.
What Are the Clinical Implications?
Endothelial activation, which is the result of adhesion of white blood cells and platelets, and proinflammatory and procoagulation effector molecules may contribute to vascular injury in preeclampsia.
Noninvasive glycocalyx measurements, using sidestream dark field imaging, offer a unique opportunity to assess endothelial activation in real time, which may provide insight into the pathophysiology of pregnancy complications.
Introduction
Preeclampsia is a leading cause of maternal and fetal morbidity and mortality that affects 2% to 7% of pregnancies.1, 2 This hypertensive pregnancy disorder is diagnosed in women presenting with new‐onset hypertension and often proteinuria after 20 weeks gestation.3 Preeclampsia can also be diagnosed in hypertensive pregnant women without proteinuria who have other signs of severe organ dysfunction.3 Delivery is the only known cure.
The role of endothelial dysfunction and endothelial cell activation in vascular disease has been increasingly recognized,4 and many of these postulates have been studied in preeclampsia. Endothelial dysfunction, which has been associated with impaired vasodilation resulting from a decrease in nitric oxide bioavailability or activity,4 has been documented in preeclampsia, using both circulating markers of endothelial injury and vascular reactivity studies.5, 6, 7 Endothelial activation is the result of endothelial expression of cell‐surface adhesion molecules that assist in recruitment and attachment of circulating leukocytes to the vessel wall, ultimately leading to vascular inflammation and atherosclerosis.4 Evidence of endothelial activation in preeclampsia includes upregulated levels of proinflammatory cytokines, such as TNF‐α (tumor necrosis factor α) and IL‐6 (interleukin 6),8 as well as upregulation of cell‐surface adhesion molecules.9 However, little is known about the glycocalyx, a layer of proteoglycans and glycoproteins that lines the vascular endothelium. The glycocalyx protects endothelial cells from endothelial activation, including adhesion of white blood cells and platelets, and proinflammatory and procoagulation effector molecules.10
Recent advances allow for measurements of glycocalyx degradation noninvasively using a hand‐held camera that captures video of red blood cells (RBCs) passing through small vessels under the tongue.11 Commercially available software estimates the depth to which RBCs can penetrate the glycocalyx.11 Healthy glycocalyx is relatively impermeable to RBCs and other circulating factors, whereas degradation creates gaps that allow RBCs to penetrate further into the glycocalyx.11 Glycocalyx damage, as measured by greater permeability of the glycocalyx by RBCs,11 is expressed as an increase in the perfused boundary region (PBR). Studies to date have shown that the PBR increases with diabetes mellitus12 and reduced renal function13 and in those patients with white matter lesions, which are indicative of small vessel disease.14 In HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count; a particularly severe form of preeclampsia), serum levels of SDC1 (syndecan 1), a protein core of proteoglycans that forms glycocalyx, are higher than in gestational age–matched normotensive pregnancies.15 However, these markers have not been studied in the context of different subtypes of preeclampsia. In addition, comparative in vivo measurements of glycocalyx in small sublingual vessels using sidestream dark field imaging have not been performed previously in preeclampsia.
The present study tests the hypothesis that glycocalyx degradation is enhanced in women with preeclampsia compared with normotensive pregnant women. In addition, we posited that glycocalyx degradation would be more pronounced in early preeclampsia (<34 gestational weeks) than in late preeclampsia (≥34 gestational weeks). Compositional and dimensional changes of glycocalyx were examined by using in vivo sidestream dark field imaging of the sublingual vasculature and by measuring circulating levels of glycocalyx components, including SDC1, heparan sulfate proteoglycans (HSPG), and hyaluronic acid. Microvascular perfusion was assessed by measuring the percentage of vessels that were filled with RBCs ≥50% of the time.
Methods
Deidentified data were deposited on the Open Science Framework website.16
Participants
A convenience sample of pregnant women in the third trimester were recruited through the Mayo Clinic Department of Obstetrics and Gynecology during prenatal appointments or following admission to the Family Birth Center. Normotensive women had no history of gestational hypertension or preeclampsia in prior pregnancies and did not develop these conditions during the index pregnancy. Preeclampsia was diagnosed based on established criteria3: (1) hypertension after 20 weeks of gestation, defined as blood pressure ≥140/90 mm Hg, and (2) proteinuria (defined as ≥300 mg of protein in a 24‐hour urine specimen and/or protein/creatinine ratio of 0.3 and/or 1+ (30 mg/L) dipstick urinalysis in the absence of urinary tract infection). In the absence of proteinuria, the diagnosis of preeclampsia was confirmed (1) if any of the following laboratory abnormalities are present: thrombocytopenia <100 000/μL, serum creatinine >1.1 mg/dL or its doubling, or elevated liver function tests (AST [aspartate aminotransferase] and ALT [alanine aminotransferase] >2 times the upper limit of normal); or (2) in the presence of pulmonary edema or cerebral or visual symptoms.3 Preeclampsia was categorized as early onset when diagnosed <34 weeks gestation or as late onset when diagnosed ≥34 weeks gestation. Heparin can alter the glycocalyx; therefore, preeclamptic women who were receiving heparin were excluded. A group of women with gestational diabetes mellitus (GDM) was also included because GDM is an important preeclampsia risk factor,17, 18 and endothelial dysfunction is hypothesized to contribute to the pathophysiology of GDM.19 GDM was diagnosed using the 2‐step approach outlined in the American Diabetes Association guidelines.20 Women completed a 3‐hour 100‐g oral glucose tolerance test if plasma glucose concentrations were ≥140 mg/dL 1 hour after a 50‐g glucose load test. GDM was diagnosed in women with at least 2 abnormal values (fasting: ≥95 mg/dL; 1 hour: ≥180 mg/dL; 2 hours: ≥155 mg/dL; 3 hours: ≥140 mg/dL).
The Mayo Clinic Institutional Review Board approved the study (no. 2104‐05). All participants provided written informed consent before participating. Participant characteristics, pregnancy outcomes, and medications administered to hospital inpatients for 24 hours before the glycocalyx test were abstracted from the medical record. Medical records were reviewed by 2 observers to confirm pregnancy diagnoses (V.D.G., T.L.W.).
Noninvasive Glycocalyx Measurements
Measurements were performed after a 10‐minute rest period. Images of RBCs flowing through sublingual vessels were obtained by sidestream dark field imaging (CapiScope Handheld Video Capillaroscopy System; KK Technologies). The camera is placed under the tongue and uses green light–emitting diodes to detect hemoglobin in RBCs (Figure 1A). Participants are asked not to apply pressure to the tongue when holding the camera to maintain normal blood flow. Automated software (GlycoCheck; GlycoCheck BV) records video clips when the image is stable and in focus. As described previously,11 the software identifies all measurable microvessels that are <30 μm in diameter based on contrast between the RBCs and the background. Measurements were made every 10 μm along each vessel. The participant held the camera stable while frames were recorded and then shifted the camera to a new location to view different vessels. This process was repeated until data on 3000 vascular segments were obtained (1–5 minutes). The software performs quality checks on each video clip to identify vascular segments that are in focus and have sufficient blood flow for analysis. For each valid vascular segment, the program calculates the vessel diameter (median width of the RBC column) and the width of the portion of the glycocalyx that is permeable to RBCs (PBR; Figure 1). Glycocalyx degradation allows RBCs to penetrate further into the glycocalyx, and this can be detected by a higher PBR value. The program also assesses perfusion by calculating the percentage of vessels in each size category that are filled with RBCs. PBR is calculated using validated methods.14, 21, 22, 23 Measurements are accurate to 0.05 μm.11 Previous studies in humans and animals have validated this technique.13, 14, 21, 22, 23, 24, 25, 26 Sublingual and retinal glycocalyx dimensions were both reduced in patients with type 2 diabetes mellitus, suggesting that sublingual glycocalyx degradation may reveal systemic effects.27 For each woman, glycocalyx measurements were obtained for 22 vessel‐size categories (median RBC column width of 3–25 μm). Each participant completed 3 trials separated by 5‐minute rest periods. We found no evidence of systematic differences in results across trials; therefore, the results of the 3 trials were averaged. Participants provided information on food and caffeine intake on the test day and medication use for 24 hours before the test.
Figure 1.
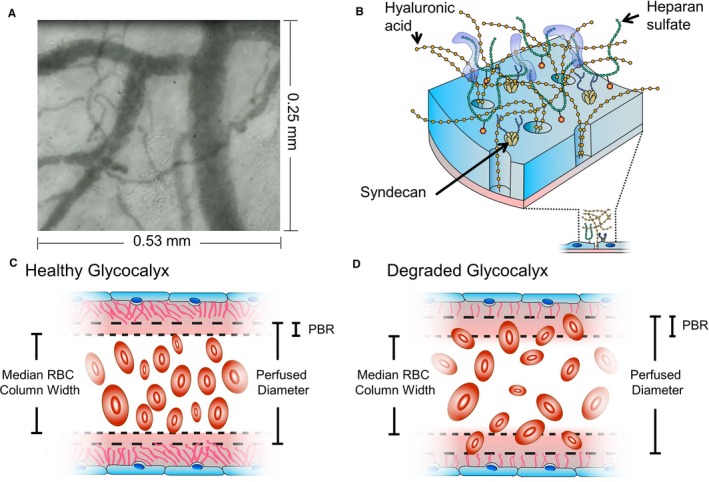
Glycocalyx measurements. A, The noninvasive camera records live video of RBCs moving through sublingual vessels. B, Schematic illustration of glycocalyx structure. C, The healthy glycocalyx is relatively impermeable to RBCs, resulting in a small PBR. D, Glycocalyx damage allows RBCs to penetrate further into the glycocalyx, increasing PBR width. PBR indicates perfused boundary region; RBC, red blood cell.
Reliability Study
A subset of women who were willing to return 24 hours later completed a second test, using identical procedures, 24 hours after the first test.
Blood Samples
Samples obtained by venipuncture were drawn into EDTA‐coated vacutainers and centrifuged. Plasma was stored at −80°C. Concentrations of 3 vascular glycocalyx components, SDC1, HSPG, and hyaluronic acid, were measured in duplicate using commercially available ELISAs. The average of duplicate values was used for analysis.
Statistical Analysis
Detailed statistical methods are described in table and figure legends. Reliability of noninvasive glycocalyx measurements was assessed by calculating intraclass correlation coefficients to assess the trial‐to‐trial (trials 1–3) or day‐to‐day (days 1 and 2) reliability for individual vessel‐size categories, as well as composite measures. Participant demographics in the 4 groups of participants were compared using 1‐way ANOVA (least significance difference test for post hoc analyses) or the Kruskal–Wallis test (Mann–Whitney test for post hoc analyses). The effect of test conditions, including time of day, fasting for >4 hours, caffeine intake in the 6 hours before the test, patient‐reported intake of any medications in the previous 24 hours, and treatment with clinical medications to induce labor (misoprostol, oxytocin) or to treat signs of preeclampsia (antihypertensive medications, magnesium sulfate, betamethasone) were examined by repeated‐measures ANOVA with group as a between‐subjects factor and vessel‐size category as a within‐subjects factor (5–16 μm). Noninvasive glycocalyx measurements among vessels with a median RBC column width of 5 to 16 μm were compared using repeated‐measures ANOVA, with group as a between‐subjects factor, vessel size as a within‐subjects factor (5–16 μm), and caffeine intake as a covariate. Repeated‐measures ANOVAs did not include interaction terms. Composite PBR values were evaluated by ANOVA after adjusting for caffeine intake. Concentrations of SDC1, HSPG, and hyaluronic acid were compared using the Kruskal–Wallis test (Mann–Whitney for post hoc analyses). Correlations between gestational age and other variables were identified using Pearson or Spearman correlation coefficients, according to the data distribution. Interactive line graphs for noninvasive glycocalyx measurements were created using a free online tool.28 Analyses were performed using SPSS for Windows (v25.0; IBM SPSS). This was an exploratory study because published data using this technique in pregnant women were not available. There was no a priori power calculation.
Results
Reliability Study
Fifteen pregnant women were included in the reliability study at a median gestational age of 33 weeks (range: 27–39 weeks). Characteristics of the reliability study population are presented in Table S1. Two more women completed the reliability measurements but were excluded because of unstable preeclampsia requiring changes in medications during the study. To assess the reliability of glycocalyx measurements for the population of pregnant women, we examined trial‐to‐trial and day‐to‐day reliability for each of the 22 vessel‐size categories (based on the median RBC column width [3–25 μm]) and for composite PBR measurements for vessels in different size categories calculated by software (5–25, 5–9, 10–19, and 20–25 μm). Trial‐to‐trial reliability for PBR was poor for all 22 size categories on both days 1 and 2 (Table S2). When 3 trials were averaged, however, day‐to‐day reliability was moderate to excellent for vessels between 9 and 18 μm but poor for smaller and larger vessels. Larger vessels were uncommon in pregnant women, with many size categories having <5 vessel segments. Each size category >16 μm accounted for <0.5% of all vessel segments among participants in the reliability study (Figure S1), and similar results were observed in the larger cohort described below (n=137; Figure 2). Vessels with a median RBC column width of 9 μm showed poor reliability for measurements of the percentage of vessel segments that were filled with RBCs (data not shown). Based on these data, we developed a new composite PBR measure for pregnant women that included vessels with a median RBC column width of 10 to 16 μm; that measure showed good day‐to‐day reliability among pregnant women in the third trimester (Table S3). Day‐to‐day reliability for software composite measures, based on the average of 3 trials, was moderate to good for each composite measure (Table S3) and was also presented to facilitate comparisons with previously published studies in other populations.
Figure 2.
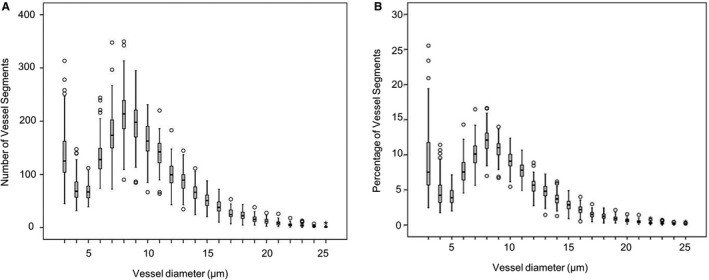
Vessel segments in each size category among pregnant women. Pooled data from 73 normotensive pregnant women, 21 women with gestational diabetes mellitus, 29 women with late‐onset preeclampsia, and 14 women with early onset preeclampsia. Values for the number (A) and percentage (B) of segments in each size category are the average of 3 trials.
Participants
In total, 139 pregnant women completed the study. The analysis included 73 women with normotensive pregnancies, 21 women with GDM, and 43 women with preeclampsia (early, n=14; late, n=29). Two women with preeclampsia were excluded from the analysis because of extreme trial‐to‐trial variability in noninvasive glycocalyx measurements. Three women in each preeclampsia group also had GDM.
Age, gravidity, parity, and fetal sex did not differ between women with normotensive pregnancies and those with GDM, early onset preeclampsia, or late‐onset preeclampsia (Table 1). The median gestational age at the time of the glycocalyx test was lower in women with early onset preeclampsia compared with the other groups. Women with early and late‐onset preeclampsia had higher blood pressures than women in the other 2 groups. Gestational age at delivery, delivery type, birth weight, and Apgar scores differed among groups because of earlier deliveries and worse outcomes among women with early onset preeclampsia.
Table 1.
Participant Characteristics
| Variable | Normotensive (n=73) | GDM (n=21) | Late‐Onset PE (n=29) | Early Onset PE (n=14) | P Value |
|---|---|---|---|---|---|
| Age, y, mean±SD | 29.8±4.5 | 30.3±4.2 | 28.9±4.8 | 29.6±5.7 | 0.740 |
| Race (% white) | 66 (93.0) | 20 (95.2) | 26 (92.9) | 14 (100.0) | 0.763 |
| Gravidity, median (IQR) | 2 (1–2) | 2 (1–4) | 2 (1–3) | 1 (0–1) | 0.589 |
| Parity, n (%) | |||||
| 0 | 43 (58.9) | 11 (52.4) | 19 (65.5) | 7 (50.0) | 0.722 |
| ≥1 | 30 (41.1) | 10 (47.6) | 10 (34.5) | 7 (50.0) | |
| At GlycoCheck test | |||||
| Gestational age, wk, median (IQR) | 39 (35–41)a | 39 (37–39) | 38 (37–39) | 31 (30–33) | <0.001 |
| Systolic BP, mm Hg, mean±SD | 111±12 | 118±17 | 141±13 | 141±13 | <0.001 |
| Diastolic BP, mm Hg, mean±SD | 67±9 | 73±14 | 83±11 | 88±13 | <0.001 |
| Proteinuria, median (IQR)b | NA | NA | 710 (460–1530) | 660 (544–1543) | |
| At delivery | |||||
| Gestational age, wk, median (IQR) | 41 (40–41) | 39 (38–40) | 38 (37–39) | 33 (31–34) | <0.001 |
| Preeclampsia with severe features, n (%) | NA | NA | 18 (62) | 11 (100) | <0.001 |
| Superimposed preeclampsia, n (%) | NA | NA | 5 (17.2) | 3 (21.4) | 0.741 |
| Delivery type | |||||
| Vaginal, n (%) | 61 (83.6) | 18 (85.7) | 18 (62.1) | 5 (35.7) | <0.001 |
| Cesarean section, n (%) | 12 (16.4) | 3 (14.3) | 11 (37.9) | 5 (64.3) | |
| Systolic BP, mm Hg, mean±SD | 121±10 | 125±12 | 145±17 | 147±15 | <0.001 |
| Diastolic BP, mm Hg, mean±SD | 76±9 | 77±12 | 93±8 | 84±12 | <0.001 |
| Fetal sex, n (% male) | 42 (57.5) | 10 (47.6) | 13 (44.8) | 5 (35.7) | 0.377 |
| Birth weight, g, mean±SD | 3643±474 | 3281±519 | 3083±618 | 1699±809 | <0.001 |
| Apgar, 1 min, median (IQR) | 8 (8–9) | 9 (8–9) | 7 (7–8) | 6 (4–7) | <0.001 |
| Apgar, 5 min, median (IQR) | 9 (9–9) | 9 (9–9) | 9 (8–9) | 8 (8–9) | <0.001 |
Normally distributed numerical data (mean±SD) were analyzed with 1‐way ANOVA and the least significant differences post hoc test. Numerical data that are not normally distributed (median [IQR]) were analyzed with a Kruskal–Wallis test and a Mann–Whitney post hoc test. Categorical data (n [%]) were analyzed with a χ2 test. BP indicates blood pressure; GDM, gestational diabetes mellitus; IQR, interquartile range; NA, not assessed; PE, preeclampsia.
Seventeen women completed glycocalyx testing between 27 and 34 weeks of gestation.
Twenty‐four–hour proteinuria was measured (early, n=8; late, n=9), or estimated from the protein creatinine ratio (early, n=5; late, n=20) or the protein osmolality ratio (early, n=1).
Test Conditions
Controlled test conditions are not possible in women with preeclampsia because of clinical instability. These patients often deliver soon after diagnosis to prevent worsening symptoms and adverse pregnancy outcomes. The potential impact of fasting, caffeine use, patient medication use, administration of clinical medications that are used to induce labor (misoprostol, oxytocin) or to manage symptoms of preeclampsia (eg, antihypertensive medications, magnesium sulfate), and time of day were examined (Table S4). None of these factors was significantly associated with changes in PBR. Caffeine intake was the only factor that significantly affected the percentage of vessel segments that were filled with RBCs. Among normotensive pregnant women, those who had consumed caffeine in the 6 hours before the glycocalyx measurement had a higher percentage of vessel segments that were filled with RBCs (P=0.011; Figure 3). PBR did not differ between women who had consumed caffeine and those who had not (P=0.118). There was no relationship between noninvasive glycocalyx measurements and gestational age in the third trimester (P>0.05 for all).
Figure 3.
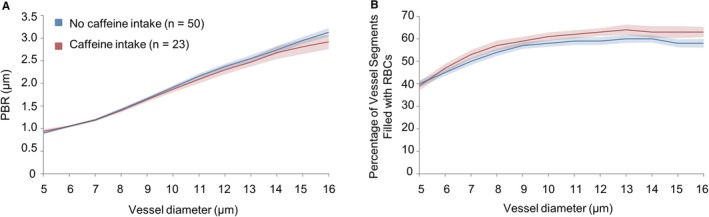
Effect of caffeine intake on noninvasive glycocalyx measurements in normotensive pregnant women. PBR (A) and the percentage of vessel segments that were filled with RBCs (B) were compared from 23 women who had caffeine in the 6 hours before the test and 50 women who did not have caffeine. Data were analyzed by repeated‐measures ANOVA with caffeine intake (yes vs no) as a between‐subjects factor and vessel size as a within‐subjects factor. Vessel size included 12 categories (5–16 μm), based on the median width of the RBC column. The main effect of caffeine intake was not statistically significant for PBR (mean: caffeine: 1.946 [95% CI, 1.888–2.005]; no caffeine: 2.003 [95% CI, 1.963–2.043]; F=2.501, df=1, P=0.118). There was a significant main effect of caffeine on the percentage of segments filled with RBCs (mean: caffeine: 0.579 [95% CI, 0.560–0.599]; no caffeine: 0.548 [95% CI, 0.535–0.562]; F=6.806, df=1, P=0.011). Post hoc analyses were performed with a least significant differences test. PBR indicates perfused boundary region; RBC, red blood cell.
Noninvasive Glycocalyx Measurements
We performed a repeated‐measures ANOVA to determine whether any relationship between pregnancy outcome and glycocalyx degradation was consistent across different size categories. Women with early onset preeclampsia had increased glycocalyx degradation, as indicated by a higher PBR, than normotensive pregnant women (P=0.002; Table S5, Figure 4) or women with GDM (P=0.004) or late onset preeclampsia (P=0.024). Microvascular perfusion, as indicated by the percentage of vessel segments filled with RBCs, was lower in women with early onset preeclampsia compared with normotensive pregnant women (P=0.045) or women with GDM (P=0.018) or late‐onset preeclampsia (P=0.024). Both analyses were adjusted for caffeine intake and included vessel‐size categories of 5 to 16 μm. Results were not different when analyses were restricted to vessel‐size categories with good reliability and high counts (10–16 μm; data not shown).
Figure 4.
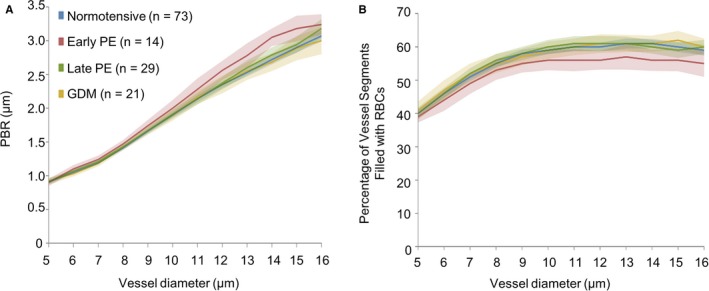
Relationship between pregnancy outcome and noninvasive glycocalyx measurements. PBR (A) and the percentage of vessel segments that were filled with RBCs (B) for vessel sizes 5 to 16 μm, among women with different pregnancy outcomes after adjusting for caffeine intake. See statistical results in Table S5. GDM indicates gestational diabetes mellitus; PBR indicates perfused boundary region; PE, preeclampsia; RBC, red blood cell.
The new composite measure of vessels with a median RBC column width of 10 to 16 μm was also examined (PBR 10–16 μm; Table 2). Women with early onset preeclampsia had lower PBR values, indicating higher glycocalyx degradation, compared with women with late‐onset preeclampsia (P=0.023), GDM (P=0.004), or normotensive pregnancies (P=0.002) after adjusting for caffeine intake. The percentage of vessels segments that were filled with RBCs (RBC 10–16 μm) was also lower in women with early onset preeclampsia compared with the remaining groups after adjusting for caffeine intake (late‐onset preeclampsia: P=0.017; GDM: P=0.010; normotensive pregnancy: P=0.021). Gestational age did not correlate with PBR 10 to 16 μm (r=0.211, P=0.859) or RBC 10 to 16 μm (r=0.335, P=0.727), suggesting that differences in women with early onset preeclampsia were not due to gestational age. The 4 composite measures calculated by the software also showed differences between normotensive women and women with early onset preeclampsia after adjustment for caffeine intake (Table 2). Differences between women with early onset preeclampsia and those with late‐onset preeclampsia or GDM were observed for some composite measures calculated by the GlycoCheck software.
Table 2.
Composite Variables for Glycocalyx Assessment
| Variable | Normotensive (n=73) | GDM (n=21) | Late‐Onset PE (n=29) | Early Onset PE (n=14) |
|---|---|---|---|---|
| Suggested composite variables for pregnant women | ||||
| PBR 10–16 | 2.520 (2.469–2.572)* | 2.495 (2.399–2.591)* | 2.555 (2.473–2.636)* | 2.721 (2.604–2.838) |
| RBC 10–16 | 0.598 (0.587–0.610)* | 0.609 (0.587–0.631)* | 0.604 (0.585–0.622)* | 0.564 (0.537–0.591) |
| Composite variables calculated by the GlycoCheck software | ||||
| PBR 5–25 | 2.441 (2.392–2.490)* | 2.448 (2.356–2.539)* | 2.461 (2.384–2.539)* | 2.612 (2.500–2.723) |
| RBC 5–25 | 0.548 (0.537–0.558)* | 0.549 (0.529–0.568) | 0.549 (0.533–0.566)* | 0.518 (0.495–0.542) |
| PBR 5–9 | 1.243 (1.225–1.261) | 1.263 (1.230–1.296) | 1.261 (1.233–1.290) | 1.283 (1.242–1.323) |
| PBR 10–19 | 2.706 (2.651–2.762)* | 2.690 (2.587–2.793)* | 2.746 (2.658–2.834) | 2.872 (2.746–2.998) |
| PBR 20–25 | 3.092 (2.992–3.192)* | 3.101 (2.915–3.287)* | 3.086 (2.928–3.244)* | 3.426 (3.199–3.653) |
Data are presented as mean (95% CI). All variables were adjusted for caffeine intake. RBC composites denote composite values for the percentage of vessel segments that are filled with RBCs. Each composite variable was calculated as the median value of the averages of each vessel‐size category within the specified range (ie, 5–9 includes vessels with a median RBC column width of 5, 6, 7, 8, and 9 μm). Data were analyzed by univariate ANOVA (fixed factor: group; covariate: caffeine intake; post hoc test: least significant differences). GDM indicates gestational diabetes mellitus; PBR, perfused boundary region; PE, preeclampsia; RBC, red blood cell.
P<0.05 in comparison to early PE.
Composite measurements of the PBR and the percentage of vessel segments that were filled with RBCs were highly correlated (PBR 10–16 μm and RBC 10–16 μm: r=0.803, P<0.001; PBR 5–25 μm and RBC 5–25 μm: r=0.745, P<0.001).
Glycocalyx Components in Plasma
HSPG concentrations were higher in women with early onset preeclampsia compared with normotensive women (P=0.002; Table 3) and women with GDM (P=0.003). Women with early onset preeclampsia had also higher concentrations of hyaluronic acid than normotensive pregnant women (P=0.046). Gestational age was not correlated with HSPG (ρ=0.147, P=0.345) or hyaluronic acid (ρ=0.267, P=0.083), whereas SDC1 was correlated with gestational age (ρ=0.454, P=0.002; Figure 5). No differences in SDC1 were observed among the groups before or after the adjustment for gestational age.
Table 3.
Effect of Pregnancy Outcome on Plasma Glycocalyx Components
| Variable | Normotensive (n=43) | GDM (n=10) | Late‐Onset PE (n=11) | Early Onset PE (n=11) |
|---|---|---|---|---|
| Gestational age, wk | 39 (33–40)* | 37 (33–39)* | 38 (36–39)* | 33 (30–34) |
| HSPG, pg/mL | 1984 (1679–2618)* | 1566 (1398–1839)* | 3228 (2294–3697) | 2952 (2177–3572) |
| HA, ng/mL | 654 (585–719)* | 611 (550–667) | 754 (668–836) | 757 (642–991) |
| SDC1, ng/mL | 161 (70–1109) | 124 (86–450) | 125 (47–1872) | 189 (81–365) |
Data were skewed and are presented as median (interquartile range). Groups were compared with a Kruskal–Wallis test (Mann–Whitney post hoc test). One outlier was excluded. GDM indicates gestational diabetes mellitus; HA, hyaluronic acid; HSPG, heparan sulfate proteoglycans; PE, preeclampsia; SDC1, syndecan 1.
P<0.05 compared with early onset PE.
Figure 5.
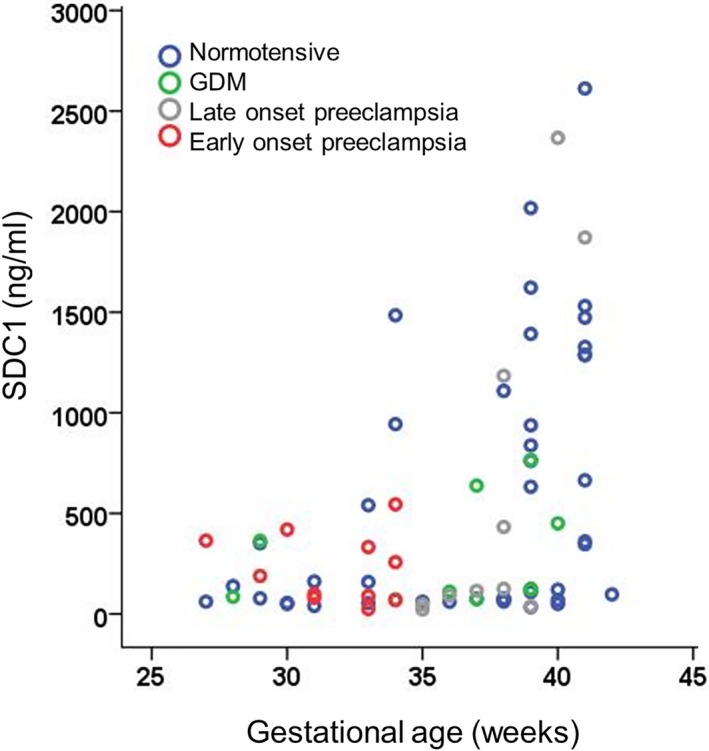
Plasma SDC1 (syndecan 1) concentrations in women with normotensive pregnancies, early and late‐onset preeclampsia, and GDM. SDC1 was correlated with gestational age (ρ=0.454, P=0.002, Spearman correlation coefficient; 1 outlier was excluded from this analysis). GDM indicates gestational diabetes mellitus.
Discussion
The results of our study indicate greater glycocalyx degradation and lower microvascular perfusion among women with early onset preeclampsia compared with normotensive pregnant women. Glycocalyx degradation, as measured by higher PBR values, was consistently observed for both composite vessel measures and in analyses that included individual vessel‐size categories. Compared with normotensive pregnant women, women with early onset preeclampsia also had higher plasma concentrations of 2 glycocalyx components: HSPG and hyaluronic acid. This suggests that markers of glycocalyx degradation are present in systemic circulation at the time of disease in women with early onset preeclampsia. These findings appear to be specific to early onset preeclampsia, as glycocalyx degradation was not present in women with either late‐onset preeclampsia or GDM.
Our study provides new evidence of dimensional and compositional glycocalyx changes in preeclampsia. It has been increasingly recognized that preeclampsia is a heterogeneous disease, with the different clinical subtypes (eg, early versus late) reflecting distinct underlying pathological mechanisms.29 Women with early preeclampsia commonly have severe features, including thrombocytopenia, impaired liver function tests, new development of renal insufficiency, pulmonary edema, and new onset of cerebral or visual disturbances.3 Our data indicate that glycocalyx degradation is present in early but not late preeclampsia, suggesting that the resultant endothelial activation may contribute to differences in clinical presentation between these disease subtypes.
The mechanisms responsible for endothelial activation in preeclampsia are multifactorial and include upregulation of several potent mediators of endothelial cell dysfunction,30 and, most important, reduced nitric oxide bioavailability.4 Of note, nitric oxide prevents leukocyte recruitment and endothelial cell activation through inhibition of the transcription factor NF‐κB (nuclear factor κB), which mediates the induction of the cellular adhesion molecules.30, 31 Therefore, endothelial activation in preeclampsia may represent a missing link between endothelial dysfunction and vascular disease,4 the latter demonstrated by reduction in capillary density (ie, rarefaction) in skin32, 33 and by the presence of acute atherosis of the placental blood vessels in preeclampsia, which is similar to early stage atherosclerosis.34
Recent advances allow for the measurements of glycocalyx degradation noninvasively, using a hand‐held camera that captures videos of RBCs passing through small vessels under the tongue.11 Commercially available software estimates the depth to which RBCs can penetrate the glycocalyx.11 Although healthy glycocalyx is relatively impermeable to RBCs and other circulating factors, degradation creates gaps that allow RBCs to penetrate further into the glycocalyx.11
In the current study, dimensional glycocalyx changes were assessed noninvasively in the sublingual vasculature, whereas glycocalyx degradation was examined by measuring plasma concentrations of the glycocalyx components HSPG, hyaluronic acid, and SDC1. Proteoglycans form the backbone of the endothelial glycocalyx and are frequently classified by the types of glycosaminoglycan chains that are bound to them.10 Heparan sulfate is the most common type of glycosaminoglycan chain that binds proteoglycans in the endothelial glycocalyx.10 Hyaluronic acid is also common.35 Noncovalent interactions between hyaluronic acid and small proteoglycans are important for maintaining the structural stability and organization of the endothelial glycocalyx.35 SDC1 is a type I transmembrane HSPG. Whereas noninvasive sublingual measurements reflect maternal vascular glycocalyx degradation, circulating concentrations of glycocalyx components may reflect a combination of maternal vascular and placental glycocalyx sources. The placenta is hypothesized to be a major source of SDC1 in pregnancy36; however, placental‐staining studies suggest that the placenta does not secrete hyaluronic acid directly into the intervillous space.37 In contrast, hyaluronic acid released from infarcted areas or fibrin deposits in the placenta may contribute to elevated circulating concentrations in preeclampsia.37, 38 Plasma HSPG and hyaluronic acid concentrations were elevated in women with early onset preeclampsia in the present study compared with normotensive pregnant women; however, there were no differences in SDC1 concentrations after adjusting for gestational age. Although serum SDC1 concentrations were elevated in women with HELLP syndrome,15 lower concentrations of plasma SDC1 have been reported before the onset of preeclampsia (20 weeks) and at the time of preeclampsia compared with normotensive pregnant women.36 These divergent results may be explained by the small sample sizes of existing studies. Alternatively, differences between women with and without preeclampsia may depend on preeclampsia subtype, and the proportion of women with different subtypes may vary among studies. Larger studies examining circulating glycocalyx components in women with different types of preeclampsia are needed.
Previous studies suggest that glycocalyx degradation may occur in other tissues among women with preeclampsia. A study of black South African women reported higher urinary concentrations of HSPG and chondroitin sulfate proteoglycans among preeclamptic women, compared with normotensive pregnant women.39 Urinary HSPG excretion was correlated with 24‐hour urinary protein excretion.39 The structure and composition of the syncytiotrophoblast glycocalyx is abnormal in severe preeclampsia.40 Taken together, these studies and our data suggest that glycocalyx dysregulation is present in multiple vessel beds; this suggestion is consistent with a systemic nature of preeclampsia.
Implications for Noninvasive Glycocalyx Measurements
The reliability study suggests that investigators should pool multiple trials when performing noninvasive glycocalyx measurements, to reduce measurement error and to improve reliability. Although the automated analysis program offers several composite measures (ie, PBR 5–25, 5–9, 10–19, and 20–25 μm), the size categories included in these measures may need to be adjusted for different populations. Compared with previous studies in men,41 the vessel‐size distribution in pregnant women appears to shift downward. Although counts in young men peaked at 10 μm,41 counts in pregnant women peaked at 8 μm (Figure 2). Studies should control for caffeine intake, which significantly influences the percentage of vessels filled with RBCs. Previous reports examining the effects of oral caffeine on microvascular perfusion in different tissue beds have yielded conflicting results. No significant differences in microvascular perfusion in the hand were observed following a small dose or oral caffeine42; however, small differences that the study was not powered to detect cannot be ruled out.43 Oral caffeine may reduce microvascular blood flow in the ocular fundus.44 Although gestational age did not affect noninvasive glycocalyx measurements among women in the third trimester in the present study, studies characterizing longitudinal changes in glycocalyx degradation throughout pregnancy are needed.
Future studies should examine whether glycocalyx degradation in women with early onset preeclampsia is present before pregnancy or precedes the onset of disease. If so, the vascular glycocalyx may be a new preeclampsia‐prevention target. Follow‐up studies should also determine whether vascular glycocalyx degradation in early onset preeclampsia is correlated with glycocalyx degradation in other tissues, such as placental trophoblast cells and the renal glomerulus. Finally, future studies should examine whether glycocalyx degradation in women with early onset preeclampsia persists for months or years after delivery. A recent meta‐analysis concluded that vascular dysfunction, as measured by flow‐mediated dilation, persists for at least 3 years following a preeclamptic pregnancy.5 If these changes are accompanied by glycocalyx degradation and reduced microvascular perfusion, treatments that target the vascular glycocalyx may aid in preventing cardiovascular disease in women who have had preeclampsia.
Conclusions
Early onset preeclampsia is associated with signs of structural and functional glycocalyx damage. We propose that noninvasive glycocalyx measurements, using sidestream dark field imaging, offer a unique opportunity to assess endothelial activation in real time. The potential role of this methodology in identifying women with increased cardiovascular risks based on their histories of pregnancy complications needs to be determined.
Sources of Funding
This research was supported by American Heart Association grant 16GRNT30950002 (Weissgerber). Additional support was provided by a Robert W. Fulk Career Development Award (Mayo Clinic Division of Nephrology and Hypertension, Weissgerber), R01HL 136348 (Garovic) from the National Heart, Lung, and Blood Institute and R01 DK47060 (Nath) from the National Institute of Diabetes and Digestive and Kidney Diseases. Weissgerber was supported by the Office of Research on Women's Health (Building Interdisciplinary Research Careers in Women's Health, K12HD065987). The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health. The writing of the article and the decision to submit it for publication were solely the authors’ responsibilities.
Disclosures
None.
Supporting information
Table S1. Participant Characteristics and Pregnancy Outcomes for Reliability Study
Table S2. Trial‐to‐Trial and Day‐to‐Day Reliability for Perfused Boundary Region Among Vessels in Each Size Category
Table S3. Day‐to‐Day Reliability for Perfused Boundary Region Composite Measures
Table S4. Test Conditions
Table S5. Effect of Gestational Diabetes Mellitus and Preeclampsia on Noninvasive Glycocalyx Measurements
Figure S1. Number of vessel segments for each size category among women in the reliability study.
(J Am Heart Assoc. 2019;8:e010647 DOI: 10.1161/JAHA.118.010647.)
References
- 1. Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Curet LB, Catalano PM, Morris CD. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–28. [DOI] [PubMed] [Google Scholar]
- 2. Knuist M, Bonsel GJ, Zondervan HA, Treffers PE. Intensification of fetal and maternal surveillance in pregnant women with hypertensive disorders. Int J Gynaecol Obstet. 1998;61:127–133. [DOI] [PubMed] [Google Scholar]
- 3. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 4. Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013;123:540–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weissgerber TL, Milic NM, Milin‐Lazovic JS, Garovic VD. Impaired flow‐mediated dilation before, during, and after preeclampsia: a systematic review and meta‐analysis. Hypertension. 2016;67:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cockell AP, Poston L. Flow‐mediated vasodilatation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension. 1997;30:247–251. [DOI] [PubMed] [Google Scholar]
- 7. Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7:375–384. [DOI] [PubMed] [Google Scholar]
- 8. Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW. Tumor necrosis factor‐alpha, interleukin‐6, and interleukin‐10 levels are altered in preeclampsia: a systematic review and meta‐analysis. Am J Reprod Immunol. 2013;70:412–427. [DOI] [PubMed] [Google Scholar]
- 9. Molvarec A, Szarka A, Walentin S, Beko G, Karadi I, Prohaszka Z, Rigo J Jr. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol. 2011;9:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, Oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee DH, Dane MJ, van den Berg BM, Boels MG, van Teeffelen JW, de Mutsert R, den Heijer M, Rosendaal FR, van der Vlag J, van Zonneveld AJ, Vink H, Rabelink TJ; NEO Study Group . Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS One. 2014;9:e96477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M, Heine RJ, Hoekstra JB, Kastelein JJ, Stroes ES, Vink H. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. [DOI] [PubMed] [Google Scholar]
- 13. Dane MJ, Khairoun M, Lee DH, van den Berg BM, Eskens BJ, Boels MG, van Teeffelen JW, Rops AL, van der Vlag J, van Zonneveld AJ, Reinders ME, Vink H, Rabelink TJ. Association of kidney function with changes in the endothelial surface layer. Clin J Am Soc Nephrol. 2014;9:698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martens RJH, Vink H, van Oostenbrugge RJ, Staals J. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis. 2013;35:451–454. [DOI] [PubMed] [Google Scholar]
- 15. Hofmann‐Kiefer KF, Knabl J, Martinoff N, Schiessl B, Conzen P, Rehm M, Becker BF, Chappell D. Increased serum concentrations of circulating glycocalyx components in hellp syndrome compared to healthy pregnancy: an observational study. Reprod Sci. 2013;20:318–325. [DOI] [PubMed] [Google Scholar]
- 16. Weissgerber T, Garcia‐Valencia O, Milic N, Codsi E, Cubro H, Nath M, White WM, Nath K, Garovic V. Early onset preeclampsia is associated with glycocalyx degradation and reduced microvascular perfusion. Open Science Framework. Published December 14, 2018. Available at: https://osf.io/wfcv9/. Accessed December 14, 2018. [DOI] [PMC free article] [PubMed]
- 17. Schneider S, Freerksen N, Rohrig S, Hoeft B, Maul H. Gestational diabetes and preeclampsia–similar risk factor profiles? Early Hum Dev. 2012;88:179–184. [DOI] [PubMed] [Google Scholar]
- 18. Nerenberg KA, Johnson JA, Leung B, Savu A, Ryan EA, Chik CL, Kaul P. Risks of gestational diabetes and preeclampsia over the last decade in a cohort of Alberta women. J Obstet Gynaecol Can. 2013;35:986–994. [DOI] [PubMed] [Google Scholar]
- 19. de Resende Guimaraes MF, Brandao AH, de Lima Rezende CA, Cabral AC, Brum AP, Leite HV, Capuruco CA. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow‐mediated dilation of brachial artery. Arch Gynecol Obstet. 2014;290:441–447. [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes A . Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mulders TA, Nieuwdorp M, Stroes ES, Vink H, Pinto‐Sietsma SJ. Non‐invasive assessment of microvascular dysfunction in families with premature coronary artery disease. Int J Cardiol. 2013;168:5026–5028. [DOI] [PubMed] [Google Scholar]
- 22. Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23:1900–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Donati A, Damiani E, Domizi R, Romano R, Adrario E, Pelaia P, Ince C, Singer M. Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvasc Res. 2013;90:86–89. [DOI] [PubMed] [Google Scholar]
- 24. Dane MJ, van den Berg BM, Lee DH, Boels MG, Tiemeier GL, Avramut MC, van Zonneveld AJ, van der Vlag J, Vink H, Rabelink TJ. A microscopic view on the renal endothelial glycocalyx. Am J Physiol Renal Physiol. 2015;308:F956–F966. [DOI] [PubMed] [Google Scholar]
- 25. Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eskens BJ, Mooij HL, Cleutjens JP, Roos JM, Cobelens JE, Vink H, Vanteeffelen JW. Rapid insulin‐mediated increase in microvascular glycocalyx accessibility in skeletal muscle may contribute to insulin‐mediated glucose disposal in rats. PLoS One. 2013;8:e55399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weissgerber TL, Garovic VD, Savic M, Winham SJ, Milic NM. From static to interactive: transforming data visualization to improve transparency. PLoS Biol. 2016;14:e1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Powers RW, Roberts JM, Plymire DA, Pucci D, Datwyler SA, Laird DM, Sogin DC, Jeyabalan A, Hubel CA, Gandley RE. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertension. 2012;60:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garovic VD, Hayman SR. Hypertension in pregnancy: an emerging risk factor for cardiovascular disease. Nat Clin Pract Nephrol. 2007;3:613–622. [DOI] [PubMed] [Google Scholar]
- 31. Liao JK, Shin WS, Lee WY, Clark SL. Oxidized low‐density lipoprotein decreases the expression of endothelial nitric oxide synthase. J Biol Chem. 1995;270:319–324. [DOI] [PubMed] [Google Scholar]
- 32. Nama V, Manyonda IT, Onwude J, Antonios TF. Structural capillary rarefaction and the onset of preeclampsia. Obstet Gynecol. 2012;119:967–974. [DOI] [PubMed] [Google Scholar]
- 33. Hasan KM, Manyonda IT, Ng FS, Singer DR, Antonios TF. Skin capillary density changes in normal pregnancy and pre‐eclampsia. J Hypertens. 2002;20:2439–2443. [DOI] [PubMed] [Google Scholar]
- 34. Kim JY, Kim YM. Acute atherosis of the uterine spiral arteries: clinicopathologic implications. J Pathol Transl Med. 2015;49:462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ziganshina MM, Pavlovich SV, Bovin NV, Sukhikh GT. Hyaluronic acid in vascular and immune homeostasis during normal pregnancy and preeclampsia. Acta Naturae. 2016;8:59–71. [PMC free article] [PubMed] [Google Scholar]
- 36. Gandley RE, Althouse A, Jeyabalan A, Bregand‐White JM, McGonigal S, Myerski AC, Gallaher M, Powers RW, Hubel CA. Low soluble syndecan‐1 precedes preeclampsia. PLoS One. 2016;11:e0157608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matejevic D, Neudeck H, Graf R, Muller T, Dietl J. Localization of hyaluronan with a hyaluronan‐specific hyaluronic acid binding protein in the placenta in pre‐eclampsia. Gynecol Obstet Invest. 2001;52:257–259. [DOI] [PubMed] [Google Scholar]
- 38. Uzun H, Konukoglu D, Albayrak M, Benian A, Madazli R, Aydin S, Gelisgen R, Uludag S. Increased maternal serum and cord blood fibronectin concentrations in preeclampsia are associated with higher placental hyaluronic acid and hydroxyproline content. Hypertens Pregnancy. 2010;29:153–162. [DOI] [PubMed] [Google Scholar]
- 39. Khedun SM, Naicker T, Moodley J, Gathiram P. Urinary heparan sulfate proteoglycan excretion in black African women with pre‐eclampsia. Acta Obstet Gynecol Scand. 2002;81:308–312. [DOI] [PubMed] [Google Scholar]
- 40. Sukhikh GT, Ziganshina MM, Nizyaeva NV, Kulikova GV, Volkova JS, Yarotskaya EL, Kan NE, Shchyogolev AI, Tyutyunnik VL. Differences of glycocalyx composition in the structural elements of placenta in preeclampsia. Placenta. 2016;43:69–76. [DOI] [PubMed] [Google Scholar]
- 41. Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, van Loon LJ. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (1985). 2014;116:998–1005. [DOI] [PubMed] [Google Scholar]
- 42. Knight R, Pagkalos J, Timmons C, Jose R. Caffeine consumption does not have an effect on digital microvascular perfusion assessed by laser Doppler imaging on healthy volunteers: a pilot study. J Hand Surg Eur Vol. 2015;40:412–415. [DOI] [PubMed] [Google Scholar]
- 43. Knight R, Pagkalos J, Timmons C, Jose R. Reply. Re: Knight R, Pagkalos J, Timmons C et al. Caffeine consumption does not have an effect on digital microvascular perfusion assessed by laser Doppler imaging on healthy volunteers: a pilot study. J Hand Surg Eur Vol. 2016;41:235. [DOI] [PubMed] [Google Scholar]
- 44. Okuno T, Sugiyama T, Tominaga M, Kojima S, Ikeda T. Effects of caffeine on microcirculation of the human ocular fundus. Jpn J Ophthalmol. 2002;46:170–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Participant Characteristics and Pregnancy Outcomes for Reliability Study
Table S2. Trial‐to‐Trial and Day‐to‐Day Reliability for Perfused Boundary Region Among Vessels in Each Size Category
Table S3. Day‐to‐Day Reliability for Perfused Boundary Region Composite Measures
Table S4. Test Conditions
Table S5. Effect of Gestational Diabetes Mellitus and Preeclampsia on Noninvasive Glycocalyx Measurements
Figure S1. Number of vessel segments for each size category among women in the reliability study.


