Short abstract
See Article by Ma et al
Keywords: Editorials, autophagy, desmin‐related cardiomyopathy, heat shock protein B8, intermittent fasting, protein quality control mechanism, transcription factor EB
Subject Categories: Cell Signalling/Signal Transduction
Disruption of protein homeostasis has significant consequences in cells. In particular, accumulation of misfolded proteins is cytotoxic and leads to the development of numerous diseases.1 Cells, therefore, have protein quality control mechanisms to prevent or minimize proteotoxicity, including constant monitoring of protein structure, localization by chaperones, and degradation by the ubiquitin‐proteasome system and the autophagy‐lysosome system.2 Chaperones can function as key players in refolding of misfolded proteins and promote degradation of misfolded or damaged proteins not suitable for refolding via the ubiquitin‐proteasome system or autophagy.3 Although the ubiquitin‐proteasome system targets soluble misfolded proteins, large protein aggregates are primarily degraded by autophagy.4 Impairment of either chaperones or the protein degradation systems leads to accumulation of misfolded proteins or mislocalization of normal proteins and causes proteotoxicity; the diseases caused by proteotoxicity are collectively called proteinopathy. Proteinopathy has drawn the attention of many cardiovascular scientists recently because proteotoxicity has been identified in many cardiovascular conditions, including aging‐induced cardiac hypertrophy and heart failure.2
Perhaps one of the most well‐studied heart diseases caused by dysregulation of protein quality control mechanisms is desmin‐related cardiomyopathy (DRM). DRM is caused by a deficiency or mutation of desmin, a muscle‐specific type III intermediate filament that serves as a scaffold to link the sarcomere to other intracellular structures.5 αB‐crystallin (heat shock protein [HSP] B5) is a small HSP that binds to desmin and is abundant in cardiac and skeletal muscles. Mutations in αB‐crystallin cause a variety of muscular disorders, including hypertrophic and dilated cardiomyopathy and skeletal myopathy, characterized by the formation of insoluble protein aggregates consisting of αB‐crystallin, proteins, and mislocalized desmin, and consequent disruption of the sarcomere structure.6
In the current issue of the Journal of the American Heart Association (JAHA), Ma et al demonstrated that intermittent fasting (IF) rescues the advanced cardiomyopathy phenotype of transgenic mice with cardiac‐specific expression of human R120G αB‐crystallin (CryABR120G), a well‐established mouse model of DRM.7 Although several interventions that aim to improve protein quality control in the heart, including upregulation of chaperones and autophagy, have been shown to improve cardiac function in this mouse model,8 the study conducted by Ma et al7 provides useful additional insights into how treatment for DRM should be designed, which we would like to highlight below.
IF that involves alternating cycles of eating and fasting (Figure 1) has gained in popularity because of its potential beneficial effects on health.9 The authors have previously observed that IF transcriptionally upregulates genes encoding the autophagy‐lysosome machinery and stimulates autophagic flux to precondition the heart, reducing ischemia‐reperfusion injury and cell death.10 Ma et al7 extended this observation for the treatment of an advanced form of DRM and demonstrated that IF rescues the impaired autophagic flux, reduces protein aggregates, and improves cardiac function in CryABR120G mice. The fact that IF is able to improve the cardiac phenotype of this established and severe form of DRM is clinically promising.
Figure 1.
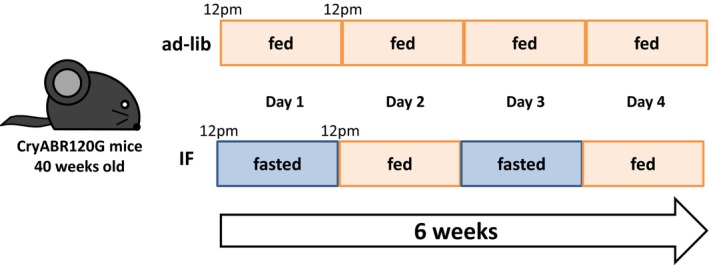
A feeding protocol of the intermittent fasting (IF) used in study by Ma et al.7 Adult mice are housed on cedar/one bedding and fed a chow (Lab Diet 5053). In the IF group, mice are fed every other day from 12 pm to 12 pm for 6 weeks. After 6 weeks, the body weight and the cumulative caloric intake were significantly less in the IF group than in the ad‐lib group. CryABR120G indicates R120G αB‐crystallin.
Mechanistically, Ma et al showed that IF induces nuclear localization of transcription factor EB (TFEB), a key transcription factor that regulates autophagy and lysosomal machinery,7 and that TFEB directly induces transcriptional upregulation of HSPB8, a small HSP (Figure 2). TFEB and HSPB8 activate both general autophagy and chaperone‐assisted selective autophagy,11, 12 thereby removing protein aggregates and damaged organelles, including mitochondria. HSPB8 also promotes protein refolding as part of the BAG3‐HSPB8‐HSC70 (BAG, B‐cell lymphoma 2‐associated anthanogene; HSC, Heat shock cognate) complex13 and assists in restoring desmin to its physiological localization. More important, the study shows the first evidence that both TFEB and HSPB8 are indispensable for mediating the salutary effect of IF in CryABR120G mice.7
Figure 2.
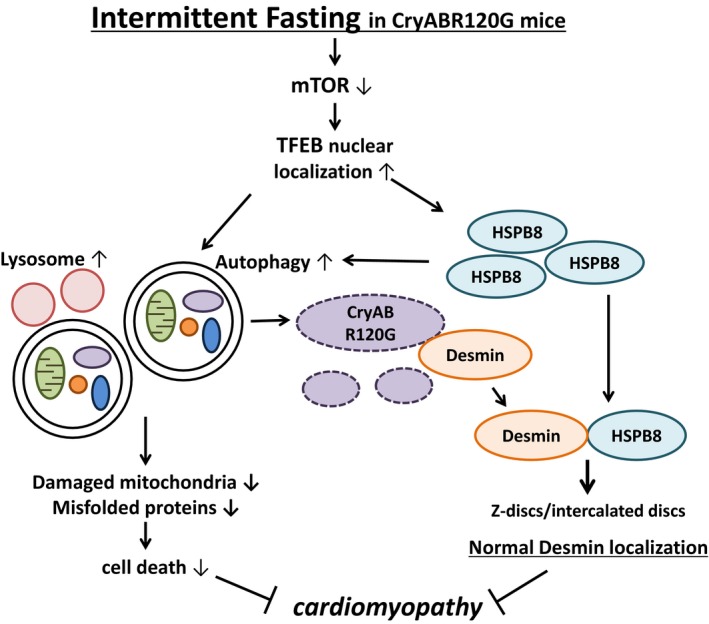
Molecular mechanisms through which intermittent fasting (IF) improves the cardiac phenotype in R120G αB‐crystallin (CryABR120G) mice. IF inactivates mammalian target of rapamycin (mTOR) and induces nuclear translocation of transcription factor EB (TFEB), a master regulator of lysosome biogenesis and autophagy. The salutary effect of IF is critically mediated by TFEB. TFEB not only activates autophagy, thereby eliminating protein aggregates and damaged mitochondria, but also transcriptionally upregulates heat shock protein (HSP) B8, which, in turn, normalizes localization of desmin. HSPB8 also stimulates autophagy through chaperone‐assisted selective autophagy and other unknown mechanisms. Autophagic degradation of protein aggregates frees desmin trapped inside, which, in turn, goes back to Z‐lines and intercalated discs with assistance of HSPB8 and allows restoration of the normal sarcomere structure. Although the study by Ma et al7 clearly showed that the salutary effects of TFEB in CryABR120G mice are critically mediated through HSPB8, whether stimulation of autophagy is indispensable remains to be clarified.
IF improves insulin resistance in type 2 diabetes mellitus14 and reduces inflammation and high blood pressure.15, 16 IF has also been shown to increase the lifespan of rats and mice.17 Thus, although TFEB can protect cardiomyocytes with CryABR120G overexpression in a cell‐autonomous manner,7 the salutary effect of IF in CryABR120G mice may be mediated, in part, through extracardiac mechanisms as well.
The authors showed that downregulation of HSPB8 completely abrogates the cell‐protective effects of TFEB overexpression, despite the fact that removal of protein aggregates by autophagy is spared in this condition in cultured cardiomyocytes.7 This suggests that the chaperone activity of HSPB8 is essential for mediating the salutary effect of the IF‐TFEB pathway and that TFEB‐mediated activation of autophagy may not be sufficient. Although activation of autophagy by IF and TFEB may assist in the release of desmin from misfolded proteins and the removal of dysfunctional proteins and organelles, whether it is also essential for mediating the effect of IF or TFEB has yet to be determined. It would be interesting to test whether selective downregulation of autophagy, such as through loss of Atg7 (Autophagy) function, affects the salutary effect of IF or TFEB in CryABR120G mice. It is possible that autophagy and HSPB8 are both essential. Alternatively, upregulation of HSPB8 may be both necessary and sufficient to mediate the rescue of DRM in response to IF or TFEB, but induction of autophagy may not be. It has been suggested that protein aggregates themselves may not be directly correlated with the toxicity.18 Thus, determining the relative importance of HSPB8 versus autophagy in mediating the effect of IF and TFEB would provide an important clue to determine the key mechanism facilitating the cardiac pathological feature in CryABR120G mice.
Although mechanisms of protein degradation are supposed to be induced in the presence of misfolded proteins, the mechanisms that maintain the quality of proteins and organelles are obviously impaired or insufficient at advanced stages of dysfunction in CryABR120G mice. Impairment of the ubiquitin‐proteasome system is observed in CryABR120G transgenic mice hearts even before the mice develop cardiac dysfunction,19 and the autophagic activity is also reduced during the late stage of cardiac dysfunction.8 Elucidating the molecular mechanisms preventing activation of the quality control mechanisms is important. Ma et al7 propose that mammalian target of rapamycin activation, caused by amino acid release from the initial degradation of misfolded proteins, inhibits the nuclear import of TFEB. It is unclear, however, whether mammalian target of rapamycin activation is sustainable because autophagy is inhibited in the advanced stage of DRM. Further investigation is needed to elucidate the signaling mechanisms controlling protein quality control in DRM.
Increasing lines of evidence suggest that IF may improve other cardiovascular conditions, including hypertension and aging‐induced cardiac hypertrophy. The study by Ma et al7 suggests that it may also be possible to apply IF for the treatment of established heart disease, such as cardiomyopathy and heart failure. The study also suggests that it may be possible to develop an intervention to activate TFEB or upregulate HSPB8 in the heart for the treatment of DRM as an alternative to IF. Like caloric restriction mimetics, interventions that mimic the underlying mechanisms of IF may represent viable options for the treatment of DRM. Examples include trehalose, a disaccharide, and spermidine, a polyamine, which have been shown to upregulate TFEB. However, IF can exacerbate cardiac conditions if it is applied when autophagy and/or lysosomal function is irrecoverable, such as in some forms of lysosomal storage diseases. Thus, the application of IF should be carefully evaluated depending on the underlying mechanisms of cardiovascular conditions. Currently, clinical application of IF is still at a primitive stage and awaits careful evaluation of its safety and efficacy, as well as optimization of the feeding protocol.
Sources of Funding
This work was supported, in part, by US Public Health Service grants HL67724, HL91469, HL102738, HL112330, HL138720, and AG23039 (to Sadoshima). This work was also supported by the Leducq Foundation Transatlantic Network of Excellence 15CBD04 (to Sadoshima) and an American Heart Association Founders Affiliate Postdoctoral Fellowship 18POST34060247 (to Mukai).
Disclosures
None.
J Am Heart Assoc. 2019;8:e011863 DOI: 10.1161/JAHA.118.011863.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction—Alzheimer's disease of the heart? N Engl J Med. 2013;368:455–464. [DOI] [PubMed] [Google Scholar]
- 2. Sandri M, Robbins J. Proteotoxicity: an underappreciated pathology in cardiac disease. J Mol Cell Cardiol. 2014;71:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McLendon PM, Robbins J. Proteotoxicity and cardiac dysfunction. Circ Res. 2015;116:1863–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knaevelsrud H, Simonsen A. Fighting disease by selective autophagy of aggregate‐prone proteins. FEBS Lett. 2010;584:2635–2645. [DOI] [PubMed] [Google Scholar]
- 5. Dalakas MC, Dagvadorj A, Goudeau B, Park KY, Takeda K, Simon‐Casteras M, Vasconcelos O, Sambuughin N, Shatunov A, Nagle JW, Sivakumar K, Vicart P, Goldfarb LG. Progressive skeletal myopathy, a phenotypic variant of desmin myopathy associated with desmin mutations. Neuromuscul Disord. 2003;13:252–258. [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Osinska H, Klevitsky R, Gerdes AM, Nieman M, Lorenz J, Hewett T, Robbins J. Expression of R120G‐alphaB‐crystallin causes aberrant desmin and alphaB‐crystallin aggregation and cardiomyopathy in mice. Circ Res. 2001;89:84–91. [DOI] [PubMed] [Google Scholar]
- 7. Ma X, Mani K, Liu H, Kovacs A, Murphy JT, Foroughi L, French BA, Weinheimer CJ, Kraja A, Benjamin IJ, Hill JA, Javaheri A, Diwan A. Transcription Factor EB activation rescues advanced αB‐crystallin mutation‐induced cardiomyopathy by normalizing desmin localization. J Am Heart Assoc. 2019;8:e010866 DOI: 10.1161/JAHA.118.010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pattison JS, Osinska H, Robbins J. Atg7 induces basal autophagy and rescues autophagic deficiency in CryABR120G cardiomyocytes. Circ Res. 2011;109:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Godar RJ, Ma X, Liu H, Murphy JT, Weinheimer CJ, Kovacs A, Crosby SD, Saftig P, Diwan A. Repetitive stimulation of autophagy‐lysosome machinery by intermittent fasting preconditions the myocardium to ischemia‐reperfusion injury. Autophagy. 2015;11:1537–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Furst DO, Saftig P, Saint R, Fleischmann BK, Hoch M, Hohfeld J. Chaperone‐assisted selective autophagy is essential for muscle maintenance. Curr Biol. 2010;20:143–148. [DOI] [PubMed] [Google Scholar]
- 13. Carra S, Seguin SJ, Landry J. HspB8 and Bag3: a new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy. 2008;4:237–239. [DOI] [PubMed] [Google Scholar]
- 14. Liu H, Javaheri A, Godar RJ, Murphy J, Ma X, Rohatgi N, Mahadevan J, Hyrc K, Saftig P, Marshall C, McDaniel ML, Remedi MS, Razani B, Urano F, Diwan A. Intermittent fasting preserves beta‐cell mass in obesity‐induced diabetes via the autophagy‐lysosome pathway. Autophagy. 2017;13:1952–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varady KA, Bhutani S, Church EC, Klempel MC. Short‐term modified alternate‐day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–1143. [DOI] [PubMed] [Google Scholar]
- 16. Aksungar FB, Topkaya AE, Akyildiz M. Interleukin‐6, C‐reactive protein and biochemical parameters during prolonged intermittent fasting. Ann Nutr Metab. 2007;51:88–95. [DOI] [PubMed] [Google Scholar]
- 17. Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–260. [DOI] [PubMed] [Google Scholar]
- 18. Chen B, Retzlaff M, Roos T, Frydman J. Cellular strategies of protein quality control. Cold Spring Harb Perspect Biol. 2011;3:a004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, Robbins J. Desmin‐related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci USA. 2004;101:10132–10136. [DOI] [PMC free article] [PubMed] [Google Scholar]


