Abstract
Background
Premenopausal women have a lower incidence of cardiovascular disease, which may partly be due to a protective effect of estrogen on endothelial function. Animal studies suggest that estrogen may also improve the relationship between shear rate (SR) and endothelial function. We aimed to explore the relationship between endothelial function (ie, flow‐mediated dilation [FMD]) and SR (ie, SR area under the curve [SRAUC]) in women versus men, and between pre‐ versus postmenopausal women.
Methods and Results
Brachial artery FMD and SRAUC were measured in accordance with expert‐consensus guidelines in 932 healthy participants who were stratified into young adults (18‐40 years, 389 men, 144 women) and older adults (>40 years, 260 men, 139 women). Second, we compared premenopausal (n=173) and postmenopausal women (n=110). There was evidence of a weak correlation between SRAUC and FMD in all groups but older men, although there was variation in strength of outcomes. Further exploration using interaction terms (age‐sex×SRAUC) in linear regression revealed differential relationships with FMD (young women versus young men [β=−5.8−4, P=0.017] and older women [β=−5.9−4, P=0.049]). The correlation between SRAUC and FMD in premenopausal women (r 2=0.097) was not statistically different from that in postmenopausal women (r 2=0.025; Fisher P=0.30). Subgroup analysis using stringent inclusion criteria for health markers (n=505) confirmed a stronger FMD‐SRAUC correlation in young women compared with young men and older women.
Conclusions
Evidence for a stronger relationship between endothelial function and the eliciting SR stimulus is present in young women compared with men. Estrogen may contribute to this finding, but larger healthy cohorts are required for conclusive outcomes.
Keywords: endothelial function, estrogen, sex‐specific, shear stress
Subject Categories: Ultrasound
Clinical Perspective
What Is New?
In a sample of 932 individuals we have shown that the correlation between brachial artery flow‐mediated dilation and its eliciting shear rate stimulus was not statistically different between sexes or age groups.
Systolic blood pressure was an important factor that influenced flow‐mediated dilation.
After repeated analysis using stringent inclusion criteria for blood pressure (n=505), sex‐ and age‐related differences were apparent in the relationship between flow‐mediated dilation and shear rate.
What Are the Clinical Implications?
Shear stress is a hemodynamic stimulus for acute artery vasodilation as well as chronic adaptation and promotes antiatherogenic properties for protection against the development and progression of atherosclerosis.
Premenopausal women benefit from the cardioprotective effects of estrogen, which may play a role in increasing sensitivity to a given shear stress stimulus.
A stronger relationship between shear stress and artery vasodilation may contribute to the lower incidence of cardiovascular disease observed in premenopausal women, compared with men of similar age and postmenopausal women.
Introduction
Cardiovascular diseases (CVD) remain the world's leading causes of morbidity and mortality in women. The vascular endothelium is responsive to hormonal and hemodynamic stimuli and plays a pivotal role in the development and progression of atherosclerosis.1 Consequently, endothelial dysfunction has been identified as an early biomarker of CVD2, 3 and predictor of future CVD.4, 5, 6 Although the incidence of CVD in women is lower compared with age‐matched men, an increase in CVD‐related mortality in women coincides with the onset of menopause.7 These sex‐related differences in CVD may at least partly relate to differences in endothelial function.8 Interestingly, premenopausal women exhibit enhanced endothelial function, assessed using the flow‐mediated dilation (FMD), compared with men.8, 9, 10, 11
An important physiological characteristic explaining sex differences in endothelial function relates to the sex hormone estrogen. FMD declines markedly in women after menopause,8, 12 and some studies show that FMD follows the fluctuating levels of estrogen across the menstrual cycle.13, 14, 15 The direct vasodilator effects of estrogen may contribute to the larger FMD in premenopausal women. An alternative explanation for sex differences in endothelial function relates to observations in animal studies, which suggest that estrogen improves the vascular responsiveness to changes in shear stress. For example, Huang and colleagues found that female and ovariectomized rats with estrogen replacement show significantly greater dilation in response to a given shear stress than male and ovariectomized rats.16 A stronger relationship between endothelial function and shear stress may therefore contribute to the enhanced endothelial function observed in premenopausal women compared with postmenopausal women and age‐matched men. To date, no study has examined this hypothesis in humans.
The purpose of this study was to explore the relationship between endothelial function (measured as FMD) and arterial shear rate (SR, ie, SR area under the curve [SRAUC]) between healthy men and women across the life span and also between pre‐ and postmenopausal women. We hypothesized that the relationship between FMD and its eliciting SR stimulus would be stronger in younger women than in men and that this relationship would be attenuated with older age and postmenopausal status.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Participants
This study utilized a retrospective design, including studies performed previously in our laboratories. From these studies, we identified 932 healthy individuals who were stratified into young adults (18‐40 years; 389 men, 144 women) and older adults (>40 years; 260 men, 139 women) (Table 1). The cut‐off level of 40 years was chosen on the basis of the increase in CVD incidence after this age17 and is in line with previous research.18 In addition, based on prescreening of menopausal status (postmenopause was defined as at least 1 year without a menstrual cycle/spotting12), subanalysis was performed between premenopausal (n=173) and postmenopausal women (n=110) (Table 2). All participants were nonsmokers, not taking any medication, and free of risk factors and signs or symptoms of cardiovascular or metabolic disease. Premenopausal women were not on any hormone‐based contraception, and postmenopausal women were not on hormone replacement therapy. All participants gave informed consent, and all studies were approved by the local ethics committees of Liverpool John Moores School of Sport and Exercise Science Research Ethics Committee, Radboud University Medical Center, or The University of Western Australia. All work adhered to the Declaration of Helsinki.
Table 1.
Subject Characteristics of Participants Divided on the Basis of Sex and Age Into Young Men and Women (18‐40 Years) and Older Men and Women (>40 Years)
| Young Adults (18‐40 y) | Older Adults (>40 y) | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Sex | Age | Sex×Age | |
| n | 144 | 389 | 139 | 260 | |||
| Age, y | 27±6 | 25±5 | 56±10 | 59±10 | 0.535 | <0.001 | <0.001 |
| Height, m | 1.69±0.08 | 1.80±0.07 | 1.63±0.07 | 1.77±0.06 | <0.001 | <0.001 | 0.003 |
| Body mass, kg | 69.6±14.0 | 76.3±10.3 | 69.7±14.0 | 82.9±14.1 | <0.001 | <0.001 | <0.001 |
| BMI, kg/m2 | 24.6±5.2 | 23.6±2.8 | 25.5±4.5 | 26.1±4.8 | 0.130 | <0.001 | 0.030 |
| SBP, mm Hg | 113±10 | 120±11 | 124±15 | 127±14 | <0.001 | <0.001 | 0.010 |
| DBP, mm Hg | 68±8 | 72±14 | 74±9 | 77±9 | <0.001 | <0.001 | 0.907 |
| MAP, mm Hg | 86±11 | 87±11 | 92±10 | 94±10 | <0.001 | <0.001 | 0.524 |
| Diameter, mm (rest) | 3.3±0.5 | 4.1±0.6 | 3.5±0.5 | 4.4±0.6 | <0.001 | <0.001 | 0.218 |
| Diameter, mm (peak) | 3.6±0.5 | 4.3±0.6 | 3.7±0.5 | 4.6±0.6 | <0.001 | <0.001 | 0.103 |
| FMD, % | 7.9±3.9 | 6.4±2.7 | 5.3±3.3 | 4.8±2.3 | <0.001 | <0.001 | 0.021 |
| SRAUC (s−1, ×103) | 23.0±12.0 | 20.4±10.7 | 21.6±11.0 | 19.7±9.0 | 0.003 | 0.175 | 0.662 |
| Time to peak, s | 51±25 | 59±30 | 64±30 | 58±28 | 0.575 | 0.006 | 0.002 |
Values are mean±SD. Comparisons between groups were made using a 2‐way ANOVA with sex and age as factors. BMI indicates body mass index; DBP, diastolic blood pressure; FMD, flow‐mediated dilation; MAP, mean arterial pressure; SBP, systolic blood pressure; SRAUC, shear rate area under the curve.
Table 2.
Subject Characteristics of Women Divided on the Basis of Menopausal Status
| Premenopause | Postmenopause | P Value | |
|---|---|---|---|
| n | 173 | 110 | |
| Age, y | 30±8 | 59±9 | <0.001 |
| Height, m | 1.69±0.08 | 1.62±0.07 | <0.001 |
| Body mass, kg | 69.6±13.7 | 70.0±15.1 | 0.938 |
| BMI, kg/m2 | 24.7±5.1 | 26.4±5.0 | 0.007 |
| SBP, mm Hg | 113±10 | 126±14 | <0.001 |
| DBP, mm Hg | 74±9 | 74±9 | <0.001 |
| MAP, mm Hg | 82±8 | 90±10 | <0.001 |
| Baseline diameter, mm | 3.3±0.5 | 3.6±0.5 | <0.001 |
| Peak diameter, mm | 3.6±0.5 | 3.8±0.6 | 0.018 |
| FMD, % | 7.8±3.9 | 4.9±3.1 | <0.001 |
| SRAUC (s−1, ×103) | 23.0±11.6 | 21.3±11.4 | 0.213 |
| Time to peak, s | 51±24 | 68±32 | <0.001 |
Values are mean±SD. P‐value refers to an independent t test. BMI indicates body mass index; DBP, diastolic blood pressure; FMD, flow‐mediated dilation; MAP, mean arterial pressure; SBP, systolic blood pressure; SRAUC, shear rate area under the curve.
Brachial Artery FMD
Participants reported to the temperature‐controlled (20°C to 22°C) laboratory on 1 occasion for FMD assessment. In preparation, participants abstained from strenuous exercise for 24 hours and alcohol for 8 hours as well from as any food/caffeine/stimulants for 6 hours before reporting to the laboratory.
Following 20 minutes of supine rest, brachial artery diameter was assessed via high‐resolution duplex ultrasound (Terason t3000/u‐smart 3300, Teratech, Burlington, MA; or Siemens Acuson Aspen, Mountain View, CA) with a 7.5‐ to 12‐MHz linear array probe. B‐mode images were obtained and optimized, and Doppler velocity was recorded simultaneously. Expert‐consensus protocol guidelines were followed for the performance of the FMD.19 Briefly, after 1 minute of baseline diameter and flow measurement, an occlusion cuff, connected to a rapid inflator (Hokanson, Bellevue, WA), placed distal to the olecranon process, was inflated to a suprasystolic pressure (>200 mm Hg) for 5 minutes. Brachial artery diameter and flow recordings were resumed 30 seconds before cuff deflation, and FMD was recorded for a further 3 minutes after cuff deflation.
All FMD data were analyzed using a specialized custom‐designed edge‐detection and wall‐tracking software, the reproducibility and validity of which have been previously reported.20 This software tracks the vessel walls and blood velocity trace in B‐mode frames via pixel density and frequency distribution algorithm. An optimal region of interest to be analyzed was selected by the sonographer, chosen on the basis of image quality, with a clear distinction between the artery walls and lumen. The FMD was defined as the percentage change in artery diameter from baseline to the peak captured during the 3 minutes following cuff release. The software automatically calculated the relative diameter change, time to peak (following cuff release), and SRAUC.21 SRAUC was calculated as the area under the SR curve between the points of cuff release (manually selected by the sonographer) to peak diameter (determined by the software).19 Despite the initial region of interest selection being operator determined, the remaining analysis was automated and independent of operator bias.
Statistical Analysis
Statistical analyses were performed using SPSS (Version 24, SPSS, Chicago, IL). Pearson correlation coefficient was used to calculate the correlation between FMD and SRAUC across age groups in men and women. This analysis was repeated using the allometrically scaled FMD to correct for baseline artery diameter.22 Fisher r‐to‐z transformation was used to compare the difference between 2 correlation coefficients in the independent groups (ie, sex, age, menopause status). Linear regression analysis was performed to examine the interaction between age‐sex group and SRAUC with FMD as the dependent outcome. Other variables (eg, age, sex, body mass index [BMI], blood pressure) that have been purported to influence SR and/or FMD were also considered in the model. Two‐way ANOVA was also used to examine the differences between sex and age. Independent t tests examined the differences between pre‐ and postmenopausal women. All data were presented as mean± SD unless stated otherwise. Statistical significance was assumed at P<0.05.
Results
Impact of Sex and Age
Older age was associated with lower FMD, and higher body mass, BMI, systolic, diastolic, and mean blood pressure, alongside higher baseline and peak brachial artery diameters (all P<0.05). There was a significant main effect for sex, with women demonstrating a lower height, body mass, systolic, diastolic, and mean blood pressure, baseline diameter, and peak diameter but a higher FMD response and SRAUC (P<0.05; Table 1). A significant interaction effect between age and sex was observed for height, body mass, BMI, systolic blood pressure, FMD response, and time to peak (P<0.05, Table 1).
A significant positive correlation between FMD response and SRAUC was evident in young men (r 2=0.042, P<0.001; Figure 1A). Young women also demonstrated a significant correlation between FMD and SRAUC (young women r 2=0.112, P<0.001), which did not significantly differ from that in young men (Fisher P=0.15). The correlation between FMD and SRAUC was nonsignificant in older men (r 2=0.011, P=0.098), whereas older women presented a very weak, but significant correlation (r 2=0.029, P=0.047; Figure 1B). Using the allometrically scaled FMD, we confirmed presence of a correlation in young women (r 2=0.108, P<0.001), and a lower correlation in older women (r 2=0.029, P=0.045), although this difference did not reach statistical significance (Fisher P=0.15). Young and older men did not demonstrate a significant correlation between the allometrically scaled FMD and SRAUC (r 2<0.001 and P=0.662, r 2<0.001 and P=0.779, respectively).
Figure 1.
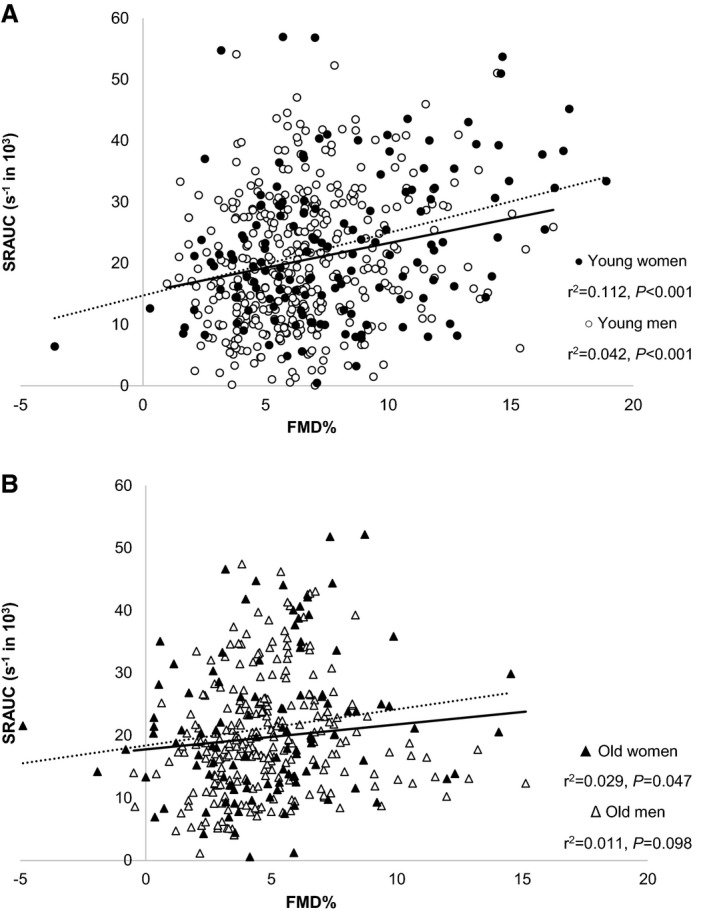
Brachial artery flow‐mediated dilation (FMD; percentage change from baseline) and the eliciting shear rate area under the curve (SRAUC) stimulus (in second−1) in healthy younger (A, total n=533) and older (B, total n=399) adults. In these figures data were presented and analyzed separately for younger men (open circles, n=389) and women (solid circles, n=144), but also for older men (open triangles, n=260) and women (solid triangles, n=139). Pearson's correlation coefficient was used to examine the relationship between the FMD and SRAUC in younger and older women (dotted line) and men (solid line).
The impact of age, sex, and SRAUC on FMD was investigated further using interaction terms in linear regression. This approach revealed evidence of a differential relationship between sex and age status and SRAUC on subsequent FMD outcomes. More specifically, young women demonstrated a significantly stronger relationship between SRAUC and FMD than young men (β=−5.8−4, P=0.017) and older women (β=−5.9−4, P=0.049). Age did not significantly alter the relationship between SRAUC and FMD in men (β=−2.5−4, P=0.30).
Other variables that might contribute to FMD response were also explored in the linear regression model. In addition to age‐sex–SRAUC interactions, FMD is influenced by systolic blood pressure (β=−0.035, P=0.001) but not by diastolic blood pressure (β=0.006, P=0.60) or BMI (β=0.033, P=0.26). Given the systolic blood pressure outcome, we repeated the bivariate correlations in a subset of n=505 who all fell within strict cut‐off values for normal blood pressure (systolic <130 mm Hg, diastolic <80 mm Hg), BMI (<25 kg/m2), and, when available, glucose (<5.6 mmol/L) and cholesterol levels (<4.9 mmol/L). Young men show evidence of a correlation between FMD response and SRAUC (r 2=0.02, P=0.024), but this response was significantly stronger in young women (r 2=0.124, P<0.001, Fisher P=0.05). Older men and women did not show a correlation between FMD and SRAUC (r 2=0.006 and 0.002, respectively, both P>0.05).
Impact of Menopausal Status
Compared with premenopausal women, postmenopausal women demonstrated a higher BMI and blood pressure but lower height and FMD (all P<0.05, Table 2). Premenopausal women demonstrated a significant correlation between FMD and SRAUC (r 2=0.097, P<0.001), although this correlation was not significant after menopause (r 2=0.025, P=0.100, Figure 2, Fisher P=0.19). Using the allometrically scaled FMD, we confirmed these findings in that the correlation with SRAUC in premenopausal women (r 2=0.095, P<0.001) disappeared after menopause (r 2=0.025, P=0.099, Fisher P=0.20). Reanalysis of the correlation coefficients within the subgroup of healthy participants (n=505) confirmed the presence of a correlation between FMD and SRAUC in premenopausal women (r 2=0.09, P=0.001) that was absent in postmenopausal women (r 2=0.006, P=0.73, Fisher P=0.30).
Figure 2.

Brachial artery flow‐mediated dilation (FMD; percentage change from baseline) and the eliciting shear rate area under the curve (SRAUC) stimulus (in second−1) in healthy premenopausal women (solid circles, n=173) and postmenopausal women (open circles, n=110). The Pearson correlation coefficient was used to examine the relationship between the FMD and SRAUC in pre‐ (solid line) and postmenopausal women (dotted line).
Discussion
Our initial analyses were suggestive of sex differences in conduit artery flow‐mediated dilation across the life span. However, given the impact of systolic blood pressure on FMD, we repeated our analysis on a subset of participants following the American Heart Association guidelines for blood pressure.23 This analysis revealed a significantly stronger relationship between FMD and SRAUC in young women compared with young men, and this was attenuated with advancing age. The sex‐related difference and the impact of menopausal status on the relationship between FMD and its eliciting shear stress stimulus suggest that estrogen may play a role in mediating the higher FMD in premenopausal women and, consequently, the reduced risk of CVD in comparison with young men.7
Our work in a large population of 932 healthy individuals confirms previous work on the association between FMD and SR in that a statistically significant correlation is present between endothelial function and the magnitude of the shear stress stimulus. This correlation remained present after the FMD had been corrected for individual differences in baseline diameter and when performed in a subset of healthy individuals (n=505). Given that SRAUC is the eliciting stimulus of the FMD response,24 we would expect to observe a moderate to strong correlation between FMD and SR. However, our data show a somewhat weaker correlation, in general, compared with previous work, especially in men.18 This finding could be attributed to a number of participant characteristics that may lead to a weaker or even absent relationship between FMD and SRAUC (eg, age, CVD risk factors).18, 25 Indeed, the subanalysis performed among individuals with no risk factors revealed a slightly higher r value. In addition, other factors that impact on the FMD response must be acknowledged, such as the response of the vascular smooth muscle cells to dilator signals (we did not assess endothelium‐independent dilation in our studies) and the structural properties of the artery (ie, wall thickness, stiffness, and diameter).26, 27, 28 Also, numerous studies have shown that baseline diameter is a stronger predictor of the FMD response than SRAUC,18, 24, 25, 29, 30 and our scaling of FMD responses to baseline diameter attempted to account for this.
In line with some previous observations, we observed sex‐related differences in the relationship between FMD and the eliciting SRAUC stimulus. More specifically, we found that young healthy women demonstrate a stronger correlation between FMD and SRAUC than their male peers, especially in the healthy subgroup. To examine the potential role of estrogen, we performed a subanalysis based on menopausal status and found that the relationship between FMD and SRAUC was absent in postmenopausal women. The potential cardioprotective properties of estrogen have been described before and may relate to upregulated endothelial nitric oxide (NO) synthase activity,31 vasodilator prostacyclin synthase, expression of vascular endothelial growth factor, inhibition of endothelial cell apoptosis, or vascular smooth muscle cell migration and/or proliferation.32, 33 These adaptations likely contribute to changes in vascular health, especially because some studies have shown that the cyclical estrogen levels across the menstrual cycle are mirrored by fluctuations in arterial stiffness34, 35 and endothelial function.13, 14, 15, 34 Some of this work used intrabrachial infusions to examine forearm blood flow responses, an endothelial assessment independent of SR, and confirmed that endothelial function per se fluctuates across the menstrual cycle.14 Studies that utilized FMD found that fluctuations in this variable across the menstrual cycle were independent of changes in the SR stimulus.13, 14, 15, 34 This suggests that these larger FMD responses are explained, at least partly, by enhanced sensitivity of the endothelium to SR.
Distinction between levels of estrogen receptors (ERα and ERβ, respectively) may contribute to the relationship between FMD and the SR stimulus in premenopausal women. Estrogen receptors are located within endothelial cells and play an important role in the vasodilator effects of estrogen.36 In animal models, abundance of ERα is linked to higher circulating estrogen levels,37, 38, 39 which are consequently linked to increased NO bioavailability.38, 40 In humans ERα expression was lower in the early follicular phase (ie, low estrogen) and also in postmenopausal women and was positively associated with (phosphorylated) endothelial NO synthase protein expression and brachial artery FMD.41 Indeed, the binding of estrogen to a receptor upregulates NO release, and because shear‐independent dilation also mirrors the menstrual cycle,14 this implies a greater release of NO with higher estrogen abundance. NO possesses a myriad of antiatherogenic properties to protect against the development of CVD42 and is negatively associated with traditional CVD risk factors.43 Given the above evidence, it could be suggested that estrogen receptors mediate the relationship between FMD and shear stress, resulting in greater dilator responses to a given shear stress stimulus. More research is required to explore the mechanisms underlying the FMD‐SRAUC relationships we observed.
When exploring the effects of age, we found an attenuated FMD‐SRAUC relationship with advancing age in both men and women, which confirms previous findings.18 Notably, we observed a weak but significant correlation in older women. However, this observation may be attributable to the inclusion of 29 (21%) premenopausal women in the older (over 40 years) group. Our findings therefore provide further evidence that older age impairs the FMD‐SRAUC relationship. Various components of vascular aging, including alterations in blood vessel structure,44, 45 shear patterns,46, 47, 48, 49 and attenuated NO bioavailability,50, 51 may potentially contribute to the age‐related attenuation in the FMD‐SRAUC relationship. Because these processes are also present in women, we may question the relative importance of age (versus estrogen) in the loss of the relationship between FMD and SRAUC in postmenopausal women. Given the more gradual impact of age on these factors compared with the relatively rapid alterations in estrogen, we may hypothesize that the loss of estrogen may represent a stronger factor than age in explaining the loss of the relationship between FMD and SRAUC. Future studies are required to untangle the effects of age and sex on this relationship.
A potential lifestyle factor underlying the age‐ and sex‐related differences in the FMD‐SRAUC relationship relates to fitness and/or physical activity levels. It has been well established that physical activity and subsequent fitness are associated with enhanced endothelial function52, 53, 54 among a myriad of other health markers, mediated by the activity‐induced exposure to increases in cyclical shear stress.55 Because studies highlight a trend for declining physical activity levels with advancing age,56, 57 age‐related differences in physical activity may represent a confounding variable in the relationship between FMD and SRAUC. Future studies are warranted to better understand this potential link.
The clinical relevance of our findings relates to the importance of changes in shear stress as an important hemodynamic stimulus for acute58, 59 and chronic60, 61 adaptation in vascular function and structure.62 High levels of shear stress have also been linked to the upregulation of antiatherogenic proteins and to the downregulation of proatherogenic substances62, 63, 64 to provide further protection against the development/progression of atherosclerosis. Accordingly, enhanced sensitivity of the endothelium to increases in shear stress (eg, those induced by physical activity) in younger women may contribute to the relatively lower risk for CVD events in this cohort. In addition, such changes may also contribute to impaired ability for remodeling of arteries in response to prolonged periods of changes in shear stress in older women. Importantly, shear stress–mediated changes in endothelial function, for example by exercise training, lead to clinically important improvements in vascular health. Notably, meta‐analyses have concluded that a 1% increase in brachial FMD is associated with 8% to 13% reduction in CVD risk.4, 6, 65
Limitations
First, we do not have data available on estrogen levels, which makes it difficult to directly link our observations to menstrual status and/or to estrogen. Furthermore, we must acknowledge that the timing and duration of menopause may also play a role in mediating the FMD‐SRAUC relationship. However, vigorous eligibility screening for the respective study established menopause status. Furthermore, markers of endothelial activation and/or damage were not available, which may have helped to better explain the age‐related changes in endothelial function and/or the role of shear stress. Another limitation is that data were collected from different laboratories, which may have contributed to some variation. Nonetheless, all laboratories strictly followed expert‐consensus guidelines19 and utilized identical data collection and validated software analysis procedures, which resulted in high reproducibility of FMD.66
In conclusion, a stronger relationship between endothelial function and the eliciting SR stimulus was found in women, compared with men, with this sex difference being attenuated with advancing age in the healthy subgroup. We suggest that endogenous estrogen may play a role in mediating the relationship between SRAUC and FMD. Therefore, the stronger relationship between endothelial function and shear stress (compared with that in men) may contribute to the cardioprotection of young women and subsequent lower prevalence of CVD.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e010994 DOI: 10.1161/JAHA.118.010994.)
References
- 1. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. [DOI] [PubMed] [Google Scholar]
- 2. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 3. Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium‐dependent flow‐mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82:1535–1539. [DOI] [PubMed] [Google Scholar]
- 4. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: a meta‐analysis. Int J Cardiovasc Imaging. 2010;26:631–640. [DOI] [PubMed] [Google Scholar]
- 5. Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow‐mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57:363–369. [DOI] [PubMed] [Google Scholar]
- 6. Ras RT, Streppel MT, Draijer R, Zock PL. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 7. Townsend N, Williams J, Bhatnagar P, Wickramasinghe K, Rayner M. Cardiovascular Disease Statistics 2015. London, UK: British Heart Foundation; 2015:13. [Google Scholar]
- 8. Green DJ, Hopkins ND, Jones H, Thijssen DH, Eijsvogels TM, Yeap BB. Sex differences in vascular endothelial function and health in humans: impacts of exercise. Exp Physiol. 2016;101:230–242. [DOI] [PubMed] [Google Scholar]
- 9. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age‐related decline in women. J Am Coll Cardiol. 1994;24:471–476. [DOI] [PubMed] [Google Scholar]
- 10. Juonala M, Kahonen M, Laitinen T, Hutri‐Kahonen N, Jokinen E, Taittonen L, Pietikainen M, Helenius H, Viikari JS, Raitakari OT. Effect of age and sex on carotid intima‐media thickness, elasticity and brachial endothelial function in healthy adults: the Cardiovascular Risk in Young Finns Study. Eur Heart J. 2008;29:1198–1206. [DOI] [PubMed] [Google Scholar]
- 11. Yao F, Liu Y, Liu D, Wu S, Lin H, Fan R, Li C. Sex differences between vascular endothelial function and carotid intima‐media thickness by Framingham Risk Score. J Ultrasound Med. 2014;33:281–286. [DOI] [PubMed] [Google Scholar]
- 12. Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97:4692–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium‐dependent flow‐mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. [DOI] [PubMed] [Google Scholar]
- 14. Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:5389–5395. [DOI] [PubMed] [Google Scholar]
- 15. Brandão AHF, Serra PJ, Zanolla K, Cabral ACV, Geber S. Variation of endothelial function during the menstrual cycle evaluated by flow‐mediated dilatation of brachial artery. JBRA Assist Reprod. 2014;18:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang A, Sun D, Koller A, Kaley G. Gender difference in flow‐induced dilation and regulation of shear stress: role of estrogen and nitric oxide. Am J Physiol. 1998;275:R1571–R1577. [DOI] [PubMed] [Google Scholar]
- 17. Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–1678. [DOI] [PubMed] [Google Scholar]
- 18. Thijssen DH, Bullens LM, van Bemmel MM, Dawson EA, Hopkins N, Tinken TM, Black MA, Hopman MT, Cable NT, Green DJ. Does arterial shear explain the magnitude of flow‐mediated dilation? A comparison between young and older humans. Am J Physiol Heart Circ Physiol. 2009;296:H57–H64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow‐mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge‐detection software system. J Appl Physiol. 2001;91:929–937. [DOI] [PubMed] [Google Scholar]
- 21. Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol. 2002;93:361–368. [DOI] [PubMed] [Google Scholar]
- 22. Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow‐mediated dilation protocol. Atherosclerosis. 2013;226:425–427. [DOI] [PubMed] [Google Scholar]
- 23. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 24. Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow‐mediated dilation? J Appl Physiol. 2007;102:1510–1519. [DOI] [PubMed] [Google Scholar]
- 25. Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow‐mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. [DOI] [PubMed] [Google Scholar]
- 26. Koller A, Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. Am J Physiol. 1991;260:H862–H868. [DOI] [PubMed] [Google Scholar]
- 27. Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–392. [DOI] [PubMed] [Google Scholar]
- 28. Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol. 2008;295:H1927–H1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silber HA, Ouyang P, Bluemke DA, Gupta SN, Foo TK, Lima JA. Why is flow‐mediated dilation dependent on arterial size? Assessment of the shear stimulus using phase‐contrast magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2005;288:H822–H828. [DOI] [PubMed] [Google Scholar]
- 30. Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow‐mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004;97:499–508. [DOI] [PubMed] [Google Scholar]
- 31. Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, Hidaka H, Iguchi A. Estrogen increases endothelial nitric oxide by a receptor‐mediated system. Biochem Biophys Res Commun. 1995;214:847–855. [DOI] [PubMed] [Google Scholar]
- 32. Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium‐dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci USA. 1994;91:5212–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Majmudar NG, Robson SC, Ford GA. Effects of the menopause, gender, and estrogen replacement therapy on vascular nitric oxide activity. J Clin Endocrinol Metab. 2000;85:1577–1583. [DOI] [PubMed] [Google Scholar]
- 34. Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood). 2010;235:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robb AO, Mills NL, Din JN, Smith IB, Paterson F, Newby DE, Denison FC. Influence of the menstrual cycle, pregnancy, and preeclampsia on arterial stiffness. Hypertension. 2009;53:952–958. [DOI] [PubMed] [Google Scholar]
- 36. Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor‐α in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284:E184–E192. [DOI] [PubMed] [Google Scholar]
- 38. Pinna C, Cignarella A, Sanvito P, Pelosi V, Bolego C. Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor α agonists. Hypertension. 2008;51:1210–1217. [DOI] [PubMed] [Google Scholar]
- 39. Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res. 2002;91:814–820. [DOI] [PubMed] [Google Scholar]
- 40. Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium‐derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997;99:2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. 2009;94:3513–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bloodsworth A, O'Donnell VB, Freeman BA. Nitric oxide regulation of free radical‐ and enzyme‐mediated lipid and lipoprotein oxidation. Arterioscler Thromb Vasc Biol. 2000;20:1707–1715. [DOI] [PubMed] [Google Scholar]
- 43. Yetik‐Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol. 2006;45:268–276. [DOI] [PubMed] [Google Scholar]
- 44. Dinenno FA, Jones PP, Seals DR, Tanaka H. Age‐associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278:H1205–H1210. [DOI] [PubMed] [Google Scholar]
- 45. Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S; Reference Values for Arterial Measurements Collaboration . Reference intervals for common carotid intima‐media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013;34:2368–2380. [DOI] [PubMed] [Google Scholar]
- 46. Credeur DP, Dobrosielski DA, Arce‐Esquivel AA, Welsch MA. Brachial artery retrograde flow increases with age: relationship to physical function. Eur J Appl Physiol. 2009;107:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Pro‐atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis. 2010;211:390–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: role of nitric oxide. Hypertension. 2011;57:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Casey DP, Padilla J, Joyner MJ. α‐Adrenergic vasoconstriction contributes to the age‐related increase in conduit artery retrograde and oscillatory shear. Hypertension. 2012;60:1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age‐related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. [DOI] [PubMed] [Google Scholar]
- 51. Al‐Shaer MH, Choueiri NE, Correia ML, Sinkey CA, Barenz TA, Haynes WG. Effects of aging and atherosclerosis on endothelial and vascular smooth muscle function in humans. Int J Cardiol. 2006;109:201–206. [DOI] [PubMed] [Google Scholar]
- 52. Hagg U, Wandt B, Bergstrom G, Volkmann R, Gan LM. Physical exercise capacity is associated with coronary and peripheral vascular function in healthy young adults. Am J Physiol Heart Circ Physiol. 2005;289:H1627–H1634. [DOI] [PubMed] [Google Scholar]
- 53. Siasos G, Chrysohoou C, Tousoulis D, Oikonomou E, Panagiotakos D, Zaromitidou M, Zisimos K, Marinos G, Mazaris S, Kampaksis M, Papavassiliou AG, Pitsavos C, Stefanadis C. The impact of physical activity on endothelial function in middle‐aged and elderly subjects: the Ikaria study. Hellenic J Cardiol. 2013;54:94–101. [PubMed] [Google Scholar]
- 54. Davison K, Bircher S, Hill A, Coates AM, Howe PR, Buckley JD. Relationships between obesity, cardiorespiratory fitness, and cardiovascular function. J Obes. 2010;2010:191253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thosar SS, Johnson BD, Johnston JD, Wallace JP. Sitting and endothelial dysfunction: the role of shear stress. Med Sci Monit. 2012;18:RA173–RA180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Milanovic Z, Pantelic S, Trajkovic N, Sporis G, Kostic R, James N. Age‐related decrease in physical activity and functional fitness among elderly men and women. Clin Interv Aging. 2013;8:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Townsend N, Wickramasinghe K, Williams J, Bhatnagar P, Rayner M. Physical Activity Statistics 2015. London, UK: British Heart Foundation; 2015:18. [Google Scholar]
- 58. Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension. 2009;54:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Greyling A, Schreuder TH, Landman T, Draijer R, Verheggen RJ, Hopman MT, Thijssen DH. Elevation in blood flow and shear rate prevents hyperglycemia‐induced endothelial dysfunction in healthy subjects and those with type 2 diabetes. J Appl Physiol. 2015;118:579–585. [DOI] [PubMed] [Google Scholar]
- 60. Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–318. [DOI] [PubMed] [Google Scholar]
- 61. Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol. 2011;300:H664–H669. [DOI] [PubMed] [Google Scholar]
- 62. Green DJ, Hopman MT, Padilla J, Laughlin H, Thijssen DH. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev. 2017;97:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Newcomer SC, Thijssen DH, Green DJ. Effects of exercise on endothelium and endothelium/smooth muscle cross talk: role of exercise‐induced hemodynamics. J Appl Physiol. 2011;111:311–320. [DOI] [PubMed] [Google Scholar]
- 64. Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand. 2000;168:81–88. [DOI] [PubMed] [Google Scholar]
- 65. Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow‐mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta‐analysis. J Am Heart Assoc. 2015;4:e002270 DOI: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Greyling A, van Mil AC, Zock PL, Green DJ, Ghiadoni L, Thijssen DH; TIFN International Working Group on Flow Mediated Dilation . Adherence to guidelines strongly improves reproducibility of brachial artery flow‐mediated dilation. Atherosclerosis. 2016;248:196–202. [DOI] [PubMed] [Google Scholar]


