Abstract
Background
Black individuals in the United States experience higher rates of ischemic stroke than other racial groups but have lower rates of clinically apparent atrial fibrillation (AF). It is unclear whether blacks truly have less AF or simply more undiagnosed AF.
Methods and Results
We performed a retrospective cohort study using inpatient and outpatient claims from 2009 to 2015 for a 5% nationally representative sample of Medicare beneficiaries. We included patients aged ≥66 years with at least 1 documented Current Procedural Terminology code for interrogation of an implantable pacemaker, cardioverter‐defibrillator, or loop recorder and no documented history of AF, atrial flutter, or stroke before their first device interrogation. Kaplan–Meier statistics and Cox proportional hazards models were used to examine the association between black race and the composite outcome of AF or atrial flutter while adjusting for age, sex, and vascular risk factors. Among 47 417 eligible patients, the annual incidence of AF/atrial flutter was 12.2 (95% CI, 11.5–13.1) per 100 person‐years among blacks and 17.6 (95% CI, 17.4–17.9) per 100 person‐years among non‐black beneficiaries. After adjustment for confounders, black beneficiaries faced a lower hazard of AF/atrial flutter than non‐black beneficiaries (hazard ratio, 0.75; 95% CI, 0.70–0.80). Despite the lower risk of AF, black patients faced a higher hazard of ischemic stroke (hazard ratio, 1.37; 95% CI, 1.22–1.53).
Conclusions
Among Medicare beneficiaries with implanted cardiac devices capable of detecting atrial rhythm, black patients had a lower incidence of AF despite a higher burden of vascular risk factors and a higher risk of stroke.
Keywords: atrial fibrillation, atrial flutter, health disparities, ischemic stroke, race and ethnicity
Subject Categories: Atrial Fibrillation, Ischemic Stroke, Race and Ethnicity
Clinical Perspective
What Is New?
Black Medicare beneficiaries with implantable cardiac devices capable of detecting atrial rhythm had lower incidence of atrial fibrillation than non‐black beneficiaries despite having a higher burden of vascular risk factors and a higher risk of ischemic stroke.
What Are the Clinical Implications?
Further investigation of this paradox may elucidate novel mechanisms of cardioembolic stroke and reveal targets for reducing racial disparities in stroke risk.
Introduction
Black individuals in the United States are significantly more likely to experience a stroke compared with other racial groups.1, 2 This disparity is partly attributable to known differences in established stroke risk factors.1 However, these known risk factors appear to account for only half of the observed stroke disparity.2 Atrial fibrillation (AF) is a major risk factor for ischemic stroke, but prior studies have suggested that AF may be less common among blacks in the United States.3, 4, 5, 6, 7, 8, 9
Prior investigations that found a lower incidence of AF in black individuals used self‐reported diagnosis,10 standard 12‐lead ECGs,5, 6, 7, 11, 12 and administrative claims data to ascertain AF.3 More recent trials evaluating the incidence of previously undiagnosed AF among the elderly demonstrated the superiority of long‐term implanted cardiac devices in detecting subclinical AF.13, 14 Few studies on racial differences in AF risk have taken advantage of the more thorough arrhythmia detection afforded by these implanted devices, and those that did included few black patients15 or limited their study to inpatient encounters.9 We therefore used inpatient and outpatient claims to evaluate the incidence of AF in black compared with non‐black patients with implanted cardiac devices capable of documenting atrial dysrhythmias.
Methods
The data used in this analysis include restricted Medicare claims data and therefore cannot be shared directly with other investigators because of the terms of the data use agreement. However, investigators can obtain access to these data by application to the Centers for Medicare and Medicaid Services.16
We performed a retrospective cohort study using inpatient and outpatient claims data from a 5% sample of Medicare beneficiaries between January 1, 2008 and September 30, 2015. The US federal government's Centers for Medicare and Medicaid Services provide health insurance to a large majority of US residents aged 65 years and up.16 Centers for Medicare and Medicaid Services makes data on claims, submitted by providers and hospitals, available to researchers. Hospital claims contain up to 25 International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis codes, 6 ICD‐9‐CM procedure codes, and hospitalization dates. Physician claims include ICD‐9‐CM diagnosis codes, Current Procedural Terminology (CPT) codes, and the date of service. Beneficiaries are assigned a unique, anonymous identifier code that allows linkage of multiple claims over the course of the patient's clinical care. This study was approved by the Weill Cornell Medical College institutional review board. The need for informed consent was waived because of the deidentified nature of our data. Our study abides by the Reporting of Studies Conducted using Observational Routinely‐Collected Health Data (RECORD) Statement.17
We limited our cohort to beneficiaries with continuous coverage in traditional fee‐for‐service Medicare (both parts A and B) for at least 1 year (or until death, if applicable). Additionally, only patients aged ≥66 years were included to ensure adequate time after Medicare eligibility at age 65 years for providers to document existing comorbidities. From among this sample, we identified beneficiaries with at least 1 documented interrogation of an implantable pacemaker, cardioverter‐defibrillator, or loop recorder. We used CPT codes 93285, 93291, 93298, and 93299 for interrogation of implantable loop recorders; 93288, 93294, and 93296 for interrogation of an implantable dual‐chamber pacemaker; and 93261, 93289, 93295, and 93296 for interrogation of an implantable cardioverter‐defibrillator. Patients without at least 1 year of Medicare coverage before their first device interrogation were excluded. We also excluded patients who at the time of their first device interrogation already had a diagnosis of AF, atrial flutter (AFL), or stroke, as defined below.
The primary predictor variable was black race. Race is documented in the Centers for Medicare and Medicaid Services denominator file as reported by beneficiaries or their surrogates. The primary outcome was AF/AFL diagnosed in an inpatient or outpatient setting, defined as ICD‐9‐CM codes 427.3, 427.31, or 427.32 in any diagnosis position. These codes have demonstrated good positive predictive value and sensitivity compared with expert review of medical records.18 AFL was included in the primary outcome because AFL frequently co‐occurs with AF19, 20, 21 and is an established stroke risk factor that results in similar patterns of care by physicians.22, 23 The inclusion of inpatient and outpatient claims, as done in our study, has been recommended for increased validity when identifying incident AF/AFL.18 Because racial differences in AFL may be paradoxically counter to those seen in AF,24 we conducted secondary analyses looking at AF (ICD‐9‐CM code 427.31) and AFL (427.32) separately. We additionally included ischemic stroke as a secondary outcome, defined by a validated ICD‐9‐CM code algorithm that uses codes 433.x1, 434.x1, or 436 in any hospital discharge diagnosis code position without a concurrent primary discharge code for rehabilitation (V57) or any codes for trauma (800–804 or 850–854), subarachnoid hemorrhage (430), or intracerebral hemorrhage (431).25
To adjust for potential confounders, we used the Medicare denominator file to ascertain age and sex, and standard ICD‐9‐CM codes to ascertain the following vascular risk factors for AF/AFL and/or stroke: hypertension, diabetes mellitus, coronary heart disease, heart failure, peripheral vascular disease, chronic kidney disease, valvular heart disease, chronic obstructive pulmonary disease, tobacco use, and alcohol abuse.4, 26, 27
Baseline characteristics were compared using the Chi‐square test and the t test or rank‐sum test, as appropriate. Survival analysis was used to calculate annual incidence rates, reported as cases per 100 person‐years along with exact CI. We additionally calculated cumulative incidence functions, stratified by race. Beneficiaries entered our analysis on the date of their first recorded device interrogation and were censored on the date of their last recorded device interrogation. Cox proportional hazards analysis was used to examine the association between black race and outcomes while adjusting for age, sex, and the vascular risk factors defined above. The proportional hazards assumption was verified by visual inspection of log‐log plots. The threshold of statistical significance was set at α=0.05. All analyses were performed using Stata/MP version 14 (College Station, TX).
Several sensitivity analyses were conducted. First, the frequency of device interrogation was included as an additional covariate in the Cox proportional hazards model. Frequency of device interrogation was defined as the number of interrogations divided by the time from the first interrogation until the censoring date, which was defined as the date of last interrogation. Second, we limited our sample to patients with at least 2 device interrogations at least 30 days apart; patients entered our analysis at the time of the second interrogation. Third, we limited our cohort to patients with a documented implantation of either a loop recorder or a dual‐chamber pacemaker or cardioverter‐defibrillator during the study period, since single‐chamber devices may not accurately capture atrial rhythm; we additionally analyzed only patients with implanted dual‐chamber devices since their AF/AFL‐detection algorithms may be more specific than those of implantable loop recorders.
Because we sought to take advantage of the superior AF/AFL detection of implanted cardiac devices compared with routine clinical follow‐up, our selected cohort comprised only those with such devices. However, patients with these devices likely differ significantly from the general Medicare population, and there may also be racial differences in the likelihood of device implantation. To address these potential sources of bias, we conducted a confirmatory analysis of the incidence of AF/AFL among black versus non‐black patients in the general Medicare population. Aside from the broadened inclusion criteria, this analysis used the same definitions of AF/AFL and stroke as well as the same statistical approach described above. Details of similar analyses have been previously published.19
Results
We identified 47 417 eligible Medicare beneficiaries with at least 1 recorded interrogation of an implanted pacemaker, cardioverter‐defibrillator, or loop recorder during the study period (Figure 1). These patients’ mean age was 78.1 (±7.7) years, 20 183 (42.6%) were female, and 3192 (6.7%) were black. Among the 44 225 non‐black patients, 42 423 (95.9%) were white, 654 (1.5%) were Hispanic, 412 (0.9%) were Asian, 215 (0.5%) were American Native, 421 (1.0%) were of other races, and 100 (0.2%) had missing data on race. Black patients had a higher burden of vascular risk factors (Table 1). The number of device interrogations differed slightly between non‐black patients (median 5, interquartile range 2–9) and black patients (median 4, interquartile range 2–8). Devices were most often placed for sinoatrial node dysfunction (28.0%), syncope (17.7%), complete atrioventricular block (14.7%), and other atrioventricular block (6.5%); indications did not substantially differ between black and non‐black patients.
Figure 1.
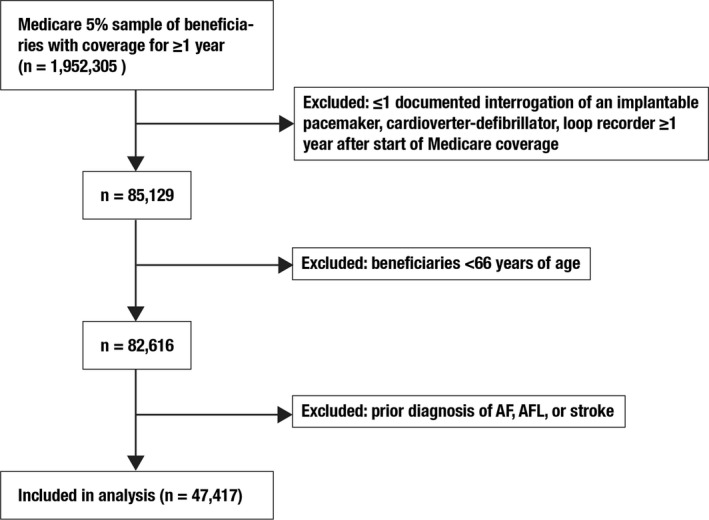
Patient population flow diagram. AF indicates atrial fibrillation; AFL, atrial flutter.
Table 1.
Characteristics of Medicare Beneficiaries With Implanted Cardiac Devices, Stratified by Race, 5% National Sample
| Characteristica | Black (N=3192) | All Other Races (N=44 225) |
|---|---|---|
| Age, mean (SD), y | 76.9 (7.8) | 78.3 (7.7) |
| Person‐years of follow‐up | 7590.3 | 100 045.9 |
| Female | 1675 (52.5) | 18 508 (41.9) |
| Hypertension | 2123 (66.5) | 22 871 (51.7) |
| Diabetes mellitus | 1185 (37.1) | 10 494 (23.7) |
| Coronary heart disease | 1278 (40.0) | 17 355 (39.2) |
| Heart failure | 996 (31.2) | 9141 (20.7) |
| Peripheral vascular disease | 372 (11.7) | 3830 (8.7) |
| Chronic kidney disease | 654 (20.5) | 4630 (10.5) |
| Valvular heart disease | 414 (13.0) | 5464 (12.4) |
| Chronic obstructive pulmonary disease | 374 (11.7) | 4930 (11.2) |
| Tobacco use | 130 (4.1) | 1477 (3.3) |
| Alcohol use | 117 (3.7) | 1070 (2.4) |
SD indicates standard deviation.
Data are presented as number (%) unless otherwise specified.
During a mean follow‐up time of 2.3 (±2.1) years between the first and last documented device interrogation, there were 18 572 documented cases of incident AF/AFL. The annual incidence of AF/AFL was 12.2 (95% CI, 11.5–13.1) per 100 black beneficiaries per year and 17.6 (95% CI, 17.4–17.9) per 100 non‐black beneficiaries per year. By 5 years after the first documented device interrogation, the cumulative incidence of AF/AFL was lower among black beneficiaries (33.7%; 95% CI, 31.8–35.6%) compared with non‐black beneficiaries (44.4%; 95% CI, 43.9–45.0%) (Figure 2). After adjustment for demographic variables and vascular risk factors, black patients faced a lower hazard of AF/AFL than non‐black patients (hazard ratio [HR], 0.75; 95% CI, 0.70–0.80) (Table 2).
Figure 2.
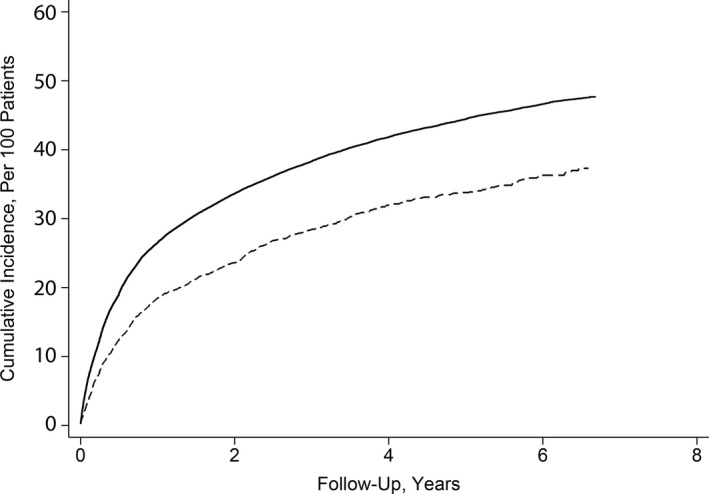
Cumulative incidence function of atrial fibrillation/flutter in black vs non‐black Medicare beneficiaries with implanted cardiac devices.
Table 2.
Hazard Ratios for the Association Between Black Race and AF or AFL in Medicare Beneficiaries With Implanted Cardiac Devices, 5% National Sample
| Outcome | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| AF/AFL | 0.70 (0.65–0.74) | 0.72 (0.67–0.77) | 0.74 (0.70–0.80) |
| AF | 0.68 (0.64–0.73) | 0.70 (0.66–0.75) | 0.73 (0.69–0.79) |
| AFL | 1.01 (0.82–1.25) | 1.09 (0.88–1.35) | 1.10 (0.89–1.36) |
AF indicates atrial fibrillation; AFL, atrial flutter.
Data represent hazard ratios (95% CI) from unadjusted Cox proportional hazards models.
Data represent hazard ratios (95% CI) from Cox proportional hazards models adjusted for age and sex.
Data represent hazard ratios (95% CI) from Cox proportional hazards models adjusted for age, sex, coronary heart disease, hypertension, diabetes mellitus, heart failure, peripheral vascular disease, chronic kidney disease, chronic obstructive pulmonary disease, valvular heart disease, tobacco use, and alcohol abuse.
After adjustment for potential confounding factors, the hazard of AF remained lower in black patients (HR, 0.73; 95% CI, 0.68–0.79) (Table 2). The adjusted hazard of AFL in black patients was not significantly different from that of non‐black patients (HR, 1.10; 95% CI, 0.89–1.36) (Table 2).
During the study period, there were 3937 documented ischemic strokes. The annual incidence of ischemic stroke was 3.6 (95% CI, 3.2–4.0) per 100 black patients per year versus 2.6 (95% CI, 2.5–2.7) per 100 non‐black patients per year. The higher hazard persisted following adjustment for demographic variables and vascular risk factors (HR, 1.37; 95% CI, 1.22–1.53).
Our results were unchanged in sensitivity analyses. First, although the number of device interrogations differed slightly between black and non‐black patients, the likelihood of AF/AFL remained lower in black patients after we adjusted for interrogation frequency (HR, 0.76; 95% CI, 0.72–0.82). Second, black race was associated with a lower risk of AF/AFL when we limited our cohort to patients with at least 2 device interrogations at least 30 days apart (HR, 0.83; 95% CI, 0.76–0.91). Third, we found a similarly lower risk of AF/AFL in black patients relative to non‐black patients when limiting our cohort to patients with a documented implantation of a loop recorder or dual‐chamber device (HR, 0.67; 95% CI, 0.56–0.79), or when limiting our cohort to only those with a dual‐chamber device (HR, 0.65; 95% CI, 0.54–0.78).
We additionally conducted a number of post‐hoc sensitivity analyses. First, we narrowed our definition of AF to include only instances where beneficiaries had ≥2 outpatient or ≥1 inpatient claim; we found a similarly lower risk of AF/AFL in black patients versus non‐black patients (HR, 0.75; 95% CI, 0.70–0.81). Second, because race could potentially influence the frequency of device interrogation, we conducted a sensitivity analysis in which we did not censor patients at time of last interrogation, instead censoring them only at the time of death or end of Medicare coverage. Black patients continued to have a lower risk of AF/AFL (HR, 0.85; 95% CI, 0.80–0.91) than those of other races. Third, our results were unchanged when we conducted a sensitivity analysis adjusting for obesity (identified through ICD‐9‐CM codes) based on prior literature associating increased BMI with AF risk among black patients (AF/AFL [HR, 0.75; 95% CI, 0.70–0.80], AF [HR, 0.73; 95% CI, 0.69–0.79], and AFL [HR, 1.10; 95% CI, 0.89–1.36]).28 Fourth, our findings were similar in a competing‐risks regression model accounting for the competing risk of death in a 10% random subset of the cohort (HR, 0.68; 95% CI, 0.55–0.85).
In our overall sample of Medicare beneficiaries with and without implanted cardiac devices, black patients again had a lower adjusted likelihood of AF/AFL than patients of other races (HR, 0.64; 95% CI, 0.63–0.64) despite a higher adjusted risk of ischemic stroke (HR, 1.62; 95% CI, 1.58–1.66).
Discussion
In a large cohort of elderly Medicare beneficiaries with implanted cardiac devices, black patients had a higher burden of vascular risk factors but a lower incidence of AF/AFL than patients of other races, most of whom were white. The lower incidence of the composite end point of AF/AFL reflected a lower risk of AF, while the risk of AFL was similar in both groups. Despite the lower incidence of AF, a common and potent stroke risk factor, black patients had a significantly higher risk of stroke than non‐black patients. These findings were robust across multiple sensitivity analyses.
Our findings build on prior studies that have reported lower rates of AF among black individuals.3, 4, 5, 6, 7, 9, 10, 11, 15 Because AF can be paroxysmal, prior studies using records of single ECGs or diagnosis codes among the general population may not have captured the true burden of AF. Our cohort of patients with implanted cardiac devices and our access to diagnosis data from both inpatient and outpatient device interrogations likely allowed for better detection of AF, since data from clinical trials indicate that most cases of incident AF cannot be identified without continuous heart‐rhythm monitoring.13, 14 In general, these trials enrolled few black subjects and did not report data on racial differences in AF incidence. It has been posited that the black‐white disparity in stroke risk may be partly attributable to relative under‐detection of AF in black patients compared with white patients.10, 29 In this context, our study helps to advance our understanding of AF and stroke by indicating that the true underlying risk of AF in blacks is likely lower than in whites, and that unexplained racial disparities in stroke risk between blacks and whites involve factors other than differences in AF diagnosis.
Given that black patients have a higher prevalence of risk factors for AF, their lower rate of AF may indicate that atrial disease in black individuals follows a distinct pathophysiology as compared with individuals of other races.30 The variation among our studied population in racial disparities between AF and AFL may align with this hypothesis; the divergent racial patterns of AF versus AFL may reflect subtle but potentially significant differences between the pathologies of the 2 arrhythmias.31 Blacks have lower rates of left atrial dilatation5, 32, 33 and lower levels of plasma natriuretic peptides,34 both of which are associated with AF,35, 36 but exhibit higher rates of electrocardiographic left atrial abnormalities.11 While both black and white individuals may develop abnormal atrial substrate in response to aging and vascular risk factors, these abnormalities may predispose them to AF to varying degrees, and/or perhaps white individuals are more susceptible to developing arrhythmias in the setting of the same abnormal tissue substrate.6 Excess stroke risk in blacks may also result from a greater prevalence of other types of cardiac pathology or non‐cardiac pathologies such as intracranial atherosclerosis.37
The results reported here must be considered within the context of several limitations. First, our analysis was isolated to patients with implanted cardiac devices and may not be generalizable to the greater population. However, our findings were similar in a confirmatory analysis of our overall Medicare sample. Second, analyses of administrative claims data are subject to misclassification error. To mitigate such error, we limited our study to individuals with documented interrogations of implanted cardiac devices and used validated diagnosis codes for AF/AFL and stroke. In a sensitivity analysis narrowing the coding scheme for AF to ≥2 outpatient or ≥1 inpatient claim, our results were unchanged as well. However, the use of diagnosis codes precluded a nuanced evaluation of arrhythmias. Because we lacked access to patients’ actual device interrogation results, we may have missed some atrial tachyarrhythmias that were detected but not documented because of a perceived lack of clinical significance. This would be relevant if such non‐coding occurred in a systemically different way in black patients versus white patients. This seems unlikely given that we found a similar incidence of AFL between the 2 groups, suggesting an absence of systemic undercoding of atrial arrhythmias in black patients. Similarly, other institutionalized differences in care, particularly with regards to indications for device implantation and the frequency of device interrogation, may explain disparate rates of AF between racial groups. However, we found a minimal difference in the number of documented interrogations between black patients and patients of other races, and our results were unchanged in a sensitivity analysis adjusting for the number of device interrogations. Additionally, our findings persisted in a sensitivity analysis censoring patients only at time of death or end of Medicare coverage as opposed to at time of last interrogation. Furthermore, our results were similar in a confirmatory analysis of all Medicare beneficiaries, not just those with implanted devices. We additionally found comparable rates of AFL in both groups, arguing against institutionalized differences in device placement or interrogation as a major explanation of our findings. Third, the CPT codes we used to identify device interrogations in our primary analysis apply to both single‐ and dual‐lead cardioverter‐defibrillators and pacemakers, and thus, not all interrogations were derived from devices capable of detecting atrial rhythm. However, our results were unchanged in a sensitivity analysis limited to patients with implanted loop recorders or dual‐chamber cardioverter‐defibrillators or pacemakers.
Conclusion
In summary, we found that among Medicare beneficiaries with implanted cardiac devices capable of detecting atrial rhythm, black patients had a lower incidence of AF despite a higher incidence of ischemic stroke. Further investigation of this paradox may elucidate novel mechanisms of cardioembolic stroke and reveal targets for reducing racial disparities in stroke risk.
Sources of Funding
This study was funded by National Institutes of Health grant R01NS097443. Dr Merkler is supported by American Heart Association grant 18CDA34110419 and the Leon Levy Fellowship in Neuroscience. Dr Kamel is supported by National Institutes of Health grants K23NS082367, R01NS097443, and U01NS095869 and the Michael Goldberg Research Fund.
Disclosures
Dr Levitan has received research funding from Amgen, served on Amgen advisory boards, and consulted on a research project funded by Novartis, all related to the treatment of heart failure. Dr Kamel serves on the steering committee for the Medtronic Stroke AF study and on the advisory board for Roivant Sciences in relation to a Factor XIa inhibitor. Dr Kamel additionally receives non‐financial support from Bristol‐Myers Squibb and Roche as Principal Investigator for the ARCADIA trial. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e010661 DOI: 10.1161/JAHA.118.010661.)
This study was presented as a poster presentation at the International Stroke Conference, held February 6–8, 2019 in Honolulu, HI, USA.
References
- 1. Kleindorfer D. Sociodemographic groups at risk: race/ethnicity. Stroke. 2009;40:S75–S78. [DOI] [PubMed] [Google Scholar]
- 2. Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE. Traditional risk factors as the underlying cause of racial disparities in stroke. Stroke. 2011;42:3369–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128:2470–2477. [DOI] [PubMed] [Google Scholar]
- 4. Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR, Alonso A. Racial differences in atrial fibrillation‐related cardiovascular disease and mortality: the Atherosclerosis Risk in Communities (ARIC) study. JAMA Cardiol. 2016;1:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, Hulley SB, Schiller NB. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375.e371–375. e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, Fox ER, Levitzky YS, Mehra R, Kerr KF. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 2013;61:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lipworth L, Okafor H, Mumma MT, Edwards TL, Roden DM, Blot WJ, Darbar D. Race‐specific impact of atrial fibrillation risk factors in blacks and whites in the Southern Community Cohort Study. Am J Cardiol. 2012;110:1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamel H, Kleindorfer DO, Bhave PD, Cushman M, Levitan EB, Howard G, Soliman EZ. Rates of atrial fibrillation in black versus white patients with pacemakers. J Am Heart Assoc. 2016;5:e002492 DOI: 10.1161/JAHA.115.002492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prineas RJ, Soliman EZ, Howard G, Howard VJ, Cushman M, Zhang Z‐M, Moy CS. The sensitivity of the method used to detect atrial fibrillation in population studies affects group‐specific prevalence estimates: ethnic and regional distribution of atrial fibrillation in the REGARDS study. J Epidemiol. 2009;19:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soliman EZ, Prineas RJ, Case LD, Zhang Z‐M, Goff DC. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Wachter R, Pouliot E, Ziegler PD. Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high‐risk population: the REVEAL AF study. JAMA Cardiol. 2017;2:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Healey JS, Alings M, Ha A, Leong‐Sit P, Birnie DH, de Graaf JJ, Freericks M, Verma A, Wang J, Leong D, Dokainish H, Philippon F, Barake W, McIntyre WF, Simek K, Hill MD, Mehta SR, Carlson M, Smeele F, Pandey AS, Connolly SJ. Subclinical atrial fibrillation in older patients. Circulation. 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 15. Lau C‐P, Gbadebo TD, Connolly SJ, Van Gelder IC, Capucci A, Gold MR, Israel CW, Morillo CA, Siu C‐W, Abe H. Ethnic differences in atrial fibrillation identified using implanted cardiac devices. J Cardiovasc Electrophysiol. 2013;24:381–387. [DOI] [PubMed] [Google Scholar]
- 16. Centers for Medicare and Medicaid Services . Medicare limited dataset files. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/. Accessed January 10, 2018.
- 17. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sørensen HT, von Elm E, Langan SM; RECORD Working Committee . The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al‐Kawaz M, Omran SS, Parikh NS, Elkind MS, Soliman EZ, Kamel H. Comparative risks of ischemic stroke in atrial flutter versus atrial fibrillation. J Stroke Cerebrovasc Dis. 2018;27:839–844. [DOI] [PubMed] [Google Scholar]
- 20. Halligan SC, Gersh BJ, Brown RD, Rosales AG, Munger TM, Shen W‐K, Hammill SC, Friedman PA. The natural history of lone atrial flutter. Ann Intern Med. 2004;140:265–268. [DOI] [PubMed] [Google Scholar]
- 21. Seidl K, Hauer B, Schwick NG, Zellner D, Zahn R, Senges J. Risk of thromboembolic events in patients with atrial flutter. Am J Cardiol. 1998;82:580–583. [DOI] [PubMed] [Google Scholar]
- 22. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 23. January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soliman EZ, Alonso A, Goff DC Jr. Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5:547–556. [DOI] [PubMed] [Google Scholar]
- 25. Tirschwell DL, Longstreth W. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 26. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation. Circ Res. 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 27. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Efird JT, Gudimella P, O'Neal WT, Griffin WF, Landrine H, Kindell LC, Davies SW, Sarpong DF, O'Neal JB, Crane P, Nelson MA, Ferguson TB, Chitwood WR, Kypson AP, Anderson EJ. Comparison of risk of atrial fibrillation in black versus white patients after coronary artery bypass grafting. Am J Cardiol. 2016;117:1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;41:581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soliman EZ. Race and atrial flutter: a needed update to understand the atrial fibrillation race paradox. Future Cardiol. 2017;13:423–427. [DOI] [PubMed] [Google Scholar]
- 32. Manolio TA, Gottdiener JS, Tsang TS, Gardin JM. Left atrial dimensions determined by M‐mode echocardiography in black and white older (≥ 65 years) adults (The Cardiovascular Health Study). Am J Cardiol. 2002;90:983–987. [DOI] [PubMed] [Google Scholar]
- 33. Yaghi S, Moon YP, Mora‐McLaughlin C, Willey JZ, Cheung K, Di Tullio MR, Homma S, Kamel H, Sacco RL, Elkind MS. Left atrial enlargement and stroke recurrence. Stroke. 2015;46:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bajaj NS, Gutierrez OM, Arora G, Judd SE, Patel N, Bennett A, Prabhu SD, Howard G, Howard VJ, Cushman M, Arora P. Racial differences in plasma levels of N‐terminal pro‐B‐type natriuretic peptide and outcomes: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. JAMA Cardiol. 2018;3:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. [DOI] [PubMed] [Google Scholar]
- 37. Qiao Y, Suri FK, Zhang Y, Liu L, Gottesman R, Alonso A, Guallar E, Wasserman BA. Racial differences in prevalence and risk for intracranial atherosclerosis in a US community‐based population. JAMA Cardiol. 2017;2:1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]


