Abstract
Background
Data are sparse on the association of cardiovascular health (CVH) in younger/middle age with the incidence of dementia later in life.
Methods and Results
We linked the CHA (Chicago Heart Association Detection Project in Industry) study data, assessed in 1967 to 1973, with 1991 to 2010 Medicare and National Death Index data. Favorable CVH was defined as untreated systolic blood pressure/diastolic blood pressure ≤120/≤80 mm Hg, untreated serum total cholesterol <5.18 mmol/L, not smoking, bone mass index <25 kg/m2, and no diabetes mellitus. International Classification of Diseases, Ninth Revision (ICD‐9) codes and claims dates were used to identify the first dementia diagnosis. Cox models were used to estimate hazard ratios of incident dementia after age 65 years by baseline CVH status. Among 10 119 participants baseline aged 23 to 47 years, 32.4% were women, 9.2% were black, and 7.3% had favorable baseline CVH. The incidence rate of dementia during follow‐up after age 65 was 13.9%. After adjustment, the hazard ratio for incident dementia was lowest in those with favorable baseline CVH and increased with higher risk factor burden (P‐trend<0.001). The hazards of dementia in those with baseline favorable, moderate, and 1‐only high‐risk factor were lower by 31%, 26%, and 20%, respectively, compared with those with ≥2 high‐risk factors. The association was attenuated but remained significant (P‐trend<0.01) when the model was further adjusted for competing risk of death. Patterns of associations were similar for men and women, and for those with a higher and lower baseline education level.
Conclusions
In this large population‐based study, a favorable CVH profile at younger age is associated with a lower risk of dementia in older age.
Keywords: aging, Alzheimer's disease, cardiovascular disease risk factors, dementia, epidemiology
Subject Categories: Epidemiology, Risk Factors, Aging
Clinical Perspective
What Is New?
This study reinforces previous findings on the association of favorable cardiovascular health in younger age and neuro‐cognitive disorder in older age with more comprehensive analyses using data from a large population‐based cohort of both men and women with decades‐long follow‐up.
It suggests that cardiovascular disease‐related health outcomes and brain health may share some common causes and may be responsive to the same preventive strategies.
What Are the Clinical Implications?
The finding allays a common fear that a longer lifespan may translate into a greater vulnerability for dementia, and thus a lower quality of life in later years, by suggesting delayed onset of dementia for those with a low risk profile.
The finding reinforces the argument for primordial prevention (ie, preventing the development of risk factors).
Introduction
Dementia, a major neurocognitive disorder commonly caused by Alzheimer disease and vascular disease, is highly prevalent in the elderly. In the United States, it is estimated that ≈11% of individuals aged ≥65 years, and 32% of people aged ≥85 years, suffered from dementia in 2016.1 Because of the decline in memory and other cognitive functions, dementia significantly diminishes an individual's quality of life and places a heavy burden on family and healthcare systems.2 Therefore, preventing dementia is critical to improving the health of the population.
Numerous epidemiological studies have focused on identifying individual risk factors for dementia. Previous research suggests that major cardiovascular disease (CVD) risk factors are associated with a higher risk of dementia, including hypertension,3, 4, 5, 6, 7 hypercholesterolemia,8, 9 diabetes mellitus,5, 7, 10 overweight/obesity,11, 12 and smoking.7, 13 However, the association of favorable cardiovascular health (CVH), a measure of overall risk factor burden defined by favorable levels of all major CVD risk factors, ie, low‐risk in young/middle ages, and the incidence of dementia later in life has not been thoroughly investigated. Moreover, previous studies examining the association between CVD risk profile and dementia did not account for the competing risk of death before dementia diagnosis, a critical issue in studies of the elderly whose death rate is high and affected by CVD risk factors.14, 15, 16
We linked baseline data from the CHA (Chicago Heart Association Detection Project in Industry) study assessed in 1967 to 1973 (for participants ages ≤65 years in 1991 and ≥67 years in 2010) with fee‐for‐service Medicare claims and National Death Index data from 1991 to 2010 to examine the association between favorable CVH profile in young/middle ages and the incidence of dementia ≥40 years of follow‐up.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Participants
The CHA Study is a prospective epidemiologic investigation of 39 665 men and women ages 18 to 74 at baseline in 1967 to 1973. The details of the CHA study have been published.17 Briefly, baseline examination involved collection of demographics, smoking history, medical history, and medical treatment data by questionnaires; measurement of height, weight, supine blood pressure, resting ECG; and blood collection for measurement of serum total cholesterol and plasma glucose.
The CHA cohort has been followed through personal contacts, National Death Index data, and linked Medicare data for all Medicare eligible participants starting from 1991 through 2010. The study has received periodic institutional review board approval and a waiver of consent.
To obtain data on incidence of dementia, we included newly Medicare‐eligible participants during 1991 to 2010 participants therefore had to be ≤65 years in 1991. To increase the likelihood that participants would have incurred Medicare claims so that we can approximate incident dementia, we included a 2‐year washout period; ie, they had to be aged ≥67 years in 2010.
There were 15 407 age‐eligible CHA participants. We excluded those who died before 1991 or before aged 65 years (n=1731), had missing data on baseline CVD risk factors (n=1093), were not found in the Medicare Denominator files (n=510), were unmatched by sex, year of birth, or social security number (n=1379), or were enrolled in Health Maintenance Organization plans after aged 65 (n=404). We also excluded those who had Medicare before age 65 (n=49), as they had a special disability or end‐stage renal disease to be eligible for Medicare. In addition, to decrease the likelihood that the participants would have been diagnosed with dementia before age 65 years, those who had any claims for dementia before age 67 were excluded (n=122). The final study sample included 10 119 participants (6839 men and 3280 women), representing 65.7% of the original age‐eligible CHA cohort (Figure S1).
Exposure and Covariates
CVH status was defined based on 5 established CVD risk factors assessed at baseline. The definition of the CVH profile is published.18, 19 Briefly, it included 4 categories: 1) Favorable, defined as favorable levels of all major CVD risk factors including untreated blood pressure, untreated serum cholesterol, bone mass index (BMI), diabetes mellitus, and smoking; 2) Elevated (moderate‐risk), defined as having 0 high‐risk factors but ≥1 unfavorable/borderline values for untreated blood pressure, untreated cholesterol, and BMI; 3) 1 high‐risk factor; or 4) ≥2 high‐risk factors (Table S1). Covariates included baseline age, race (black, non‐black), sex, educational attainment (years in school), and the annual number of medical care visits from age 65 years to the time dementia was first diagnosed or to the censored time.
Outcome Variables
The Medicare (fee‐for service) claims data contain International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes in all claim files from 1991 to 2010; they include the Medicare Provider Analysis and Review, outpatient, carrier, durable medical equipment, home health, and hospice files. As in prior studies, we used ICD‐9 codes of 290, 290.0–4, 290.8–9, 294.1, 331, 331.0–2, and 331.9 to define all types of dementia, then subtypes of dementia.20, 21 Our primary event of interest was the first diagnosis of dementia in any Medicare claim, regardless of whether it was the primary reason for the medical visit, and the outcome variable was years from aged 65 to the first event. It is worth noting that, in about 50% of the cases, dementia was the primary reason for the medical visit; and about 90% of dementia cases were established from ICD‐9 diagnoses codes in outpatient claims. Individuals who died without a dementia diagnosis were censored, or all‐cause death, instead of being censored, remained in the population at risk (ie, a competing event). Individuals who survived dementia‐free were censored on December 31, 2010. Information on deaths was obtained from National Death Index data.
Statistical Analysis
Baseline characteristics were compared across baseline CVD risk profile categories using F tests for continuous variables and Chi‐square tests for binary variables. The cumulative incidence of dementia with death treated as a competing risk was plotted using cumulative incidence function for each CVD risk category.
For the main analyses, we used the procedure regression analysis of survival data (PHREG) to fit a Cox proportional hazards model to estimate the association between CVH profile and the risk of developing dementia where individuals who died before dementia were censored (model 1). Because death may occur before the diagnosis of dementia in individuals who would have developed dementia (had they lived longer), death may be considered a competing event. Therefore, in model 2, we take into account the potential impact of death using Fine and Gray's extension of the Cox regression model.22 All hazard ratios with 95% CIs were compared between CVH groups with the high‐risk group as the reference. Models were adjusted for baseline age, race, sex, years of education, and the annual number of medical care visits before dementia diagnosis or until the censored time. We adjusted for the annual number of medical care visits to account for potential differences in the likelihood of dementia diagnosis because individuals with more visits may be more likely to be diagnosed. Linear trend across the CVH strata was also tested, with CVH status as an ordinal variable.
Proportional hazard assumptions were checked using Martingale residuals and the Kolmogorov‐type supremum test. Standard error estimates robust to misspecification of the Cox was also used. We also conducted sensitivity analyses, using interaction terms between covariates, such as CVH status and age, race, sex, or education; age and sex, race, or education; sex and race or education; and race and education; and splines effects of baseline age to inquire more flexible fits to the data for covariates.
Previous studies suggest that the prevalence and incidence of Alzheimer disease vary by sex.23 Therefore, analyses were further stratified by sex. Moreover, because the level of education is strongly associated with dementia,24 we stratified models by level of education at baseline (≤12 years at school versus otherwise). Furthermore, because stroke is considered an important cause of cognitive impairment,25 we used an exploratory analysis, adding into the models any interim stroke occurring from aged 65 years to the time dementia was first diagnosed (or to the censored time) to examine if having a stroke before dementia may be on the causal pathway of the association of interest.
Finally, models substituting individual risk factors for the combined CVH profile groups were used to examine the association of each risk factor separately with the incidence of dementia.
The analyses used SAS statistical software (version 9.4 with SAS/STAT 14.1, SAS Institute Inc, Cary, NC); a P<0.05 was considered statistically significant.
Results
Of 10 119 CHA participants aged 23 to 47 years at baseline, 32.4% were women, 9.2% were black, and 7.3% had a favorable baseline profile. Hypertension and smoking were the most prevalent risk factors, while diabetes mellitus was less common at baseline (41.3%, 40.7%, and 1.3%, respectively) (Table 1). People in the favorable group tended to be younger, female, better educated, and with the lowest average annual number of medical care visits from age 65 years to the first dementia diagnosis or the censored time.
Table 1.
Characteristics of the Study Sample for All and by Baseline Cardiovascular Health Status
| Characteristicsa | All | Baseline Cardiovascular Health Status | P Valuee | |||
|---|---|---|---|---|---|---|
| Favorableb | Moderate‐Riskc | 1 High‐Risk Factord | ≥2 High‐Risk Factord | |||
| People, n | 10 119 | 742 | 2238 | 4220 | 2919 | |
| Baseline characteristics (1967–1973) | ||||||
| Age, y | 35.3 (5.5) | 33.9 (5.4) | 35.2 (5.4) | 35.2 (5.6) | 35.9 (5.5) | <0.001 |
| Female, % | 32.4 | 59.6 | 32.5 | 33.8 | 23.5 | <0.001 |
| Black, % | 9.2 | 8.4 | 7.2 | 9.6 | 10.4 | <0.001 |
| Education, y | 13.6 (2.6) | 14.1 (2.5) | 14.1 (2.7) | 13.6 (2.6) | 13.1 (2.6) | <0.001 |
| Smoking status, % | ··· | |||||
| Never smoker | 33.1 | 63.3 | 57.9 | 28.5 | 12.9 | |
| Former smoker | 25.7 | 36.7 | 42.1 | 23.6 | 13.9 | |
| Current smoker | 41.3 | 0.0 | 0.0 | 47.9 | 74.0 | |
| BMI, kg/m2 | 25.6 (3.9) | 22.1 (2.0) | 25.0 (2.7) | 25.0 (3.4) | 27.8 (4.5) | ··· |
| BMI ≥30 kg/m2, % | 11.4 | 0 | 0 | 5.5 | 31.5 | ··· |
| SBP, mm Hg | 131.8 (15.9) | 114.4 (6.2) | 124.0 (8.2) | 131.2 (15.3) | 142.9 (15.0) | ··· |
| DBP, mm Hg | 78.3 (10.6) | 69.8 (7.4) | 74.2 (7.4) | 77.8 (10.3) | 84.3 (10.6) | ··· |
| Hypertension,d % | 40.7 | 0 | 0 | 40.4 | 82.8 | ··· |
| Serum cholesterol, mmol/Lf | 196.7 (35.5) | 168.5 (19.9) | 190.6 (27.1) | 192.5 (31.5) | 214.6 (41.3) | ··· |
| Hypercholesterolemia,d % | 11.5 | 0 | 0 | 5.5 | 31.9 | ··· |
| Diabetes mellitus, % | 1.3 | 0 | 0 | 0.8 | 3.3 | ··· |
| Annual medical care visitsg | 13.2 (12.4) | 11.4 (10.9) | 11.9 (10.8) | 13.0 (12.1) | 15.0 (14.0) | <0.001 |
BMI indicates body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Numbers are mean (SD) unless otherwise indicated.
Favorable level of all major CVD risk factors (BP ≤120/≤80 mm Hg and no antihypertensive medication, serum cholesterol <5.18 mmol/L and no lipid‐lowering medication, not smoking, BMI <25 kg/m2, no diabetes mellitus).
One or more unfavorable/borderline levels of untreated systolic blood pressure 121 to 139 mm Hg or diastolic blood pressure 81 to 89 mm Hg, or untreated serum total cholesterol 5.18 to 6.21 mmol/L, or BMI 25.0 to 29.9 kg/m2, not smoking, no diabetes mellitus.
High‐risk factors: hypertension (systolic blood pressure/diastolic blood pressure (≥140/90) or using antihypertensive medication), hypercholesterolemia (serum total cholesterol ≥6.22 mmol/L or using lipid‐lowering medication), smoking, BMI ≥30.0 kg/m2, diabetes mellitus.
P values for overall group comparisons based on Chi‐square or F‐test except for cardiovascular health components.
Divide by 0.0259 to convert to mg/dL.
Annual number of medical care visits from age 65 years to the first time that dementia was diagnosed or to the censored time.
After up to 43 years of follow‐up since baseline examination (up to 19 years of follow‐up in Medicare after reaching age 65 years), 13.9% of the participants developed at least 1 type of dementia. The percent of those who survived without any dementia was highest in the favorable group (84.4%), and lowest in the ≥2 high‐risk factors group (60.3%). Conversely, the outcomes of dementia and all‐cause mortality were lowest in the favorable group (9.6% and 6.1%, respectively), and highest in the ≥2 high‐risk factors group (16.8% and 23.0%, respectively) (P<0.001) (Figure 1).
Figure 1.
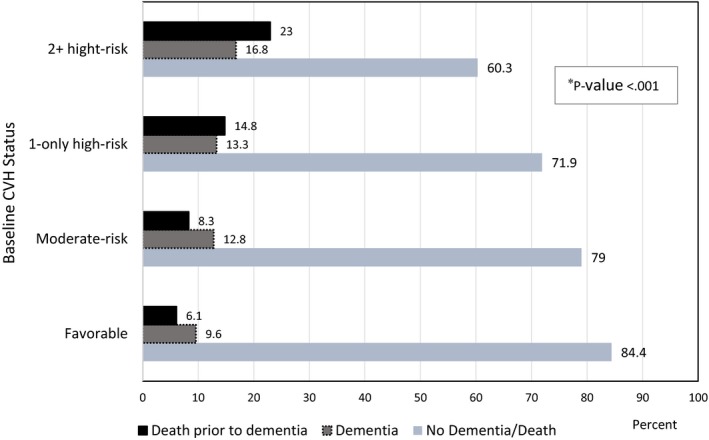
Dementia status and death before dementia during 1991 to 2010 by baseline cardiovascular health status. Death before dementia: all‐cause death before the first diagnosis of any type of dementia. Favorable level of all major cardiovascular disease risk factors (blood pressure ≤120/≤80 mm Hg and no antihypertensive medication, serum cholesterol <5.18 mmol/L and no lipid‐lowering medication, not smoking, bone mass index <25 kg/m2, no diabetes mellitus); Moderate‐risk: One or more unfavorable/borderline levels of untreated systolic blood pressure 121 to 139 mm Hg or diastolic blood pressure 81 to 89 mm Hg, or untreated serum total cholesterol 5.18 to 6.21 mmol/L, or BMI 25.0 to 29.9 kg/m2, not smoking, no diabetes mellitus; High‐Risk: High systolic blood pressure/diastolic blood pressure (≥140/90) or using antihypertensive medication, serum total cholesterol ≥6.22 mmol/L or using lipid‐lowering medication, smoking, bone mass index ≥30.0 kg/m2, diabetes mellitus. CVH indicates cardiovascular health. *P value for group comparison based on Chi‐square tests.
Figure 2 shows the unadjusted cumulative incidence of dementia taking into account the competing risk of death. There was a significant difference in the dementia‐free survival rate (P<0.001) across baseline CVH groups. The cumulative incidence of dementia was lowest in the favorable group and increased with risk factor burden. Specifically, the hazards of dementia in those with baseline favorable, moderate, and 1 only high‐risk factor were lower by 28%, 22%, and 17%, respectively, compared with those with ≥2 high‐risk factors.
Figure 2.
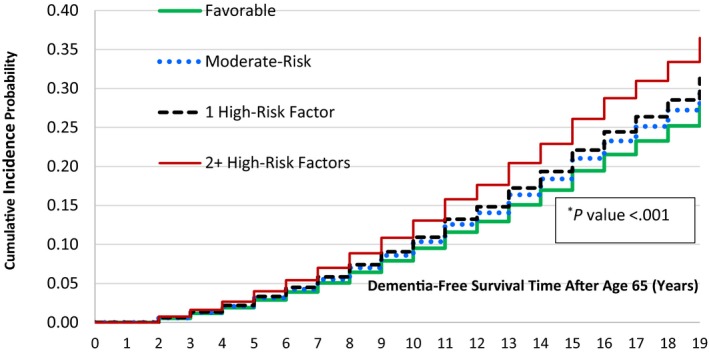
Cumulative incidence of dementia after aged 65 years during 1991 to 2010 by baseline cardiovascular health status. First diagnosis of any type of dementia. Favorable level of all major cardiovascular disease risk factors (blood pressure ≤120/≤80 mm Hg and no antihypertensive medication, serum cholesterol <5.18 mmol/L and no lipid‐lowering medication, not smoking, bone mass index <25 kg/m2, no diabetes mellitus); Moderate‐risk: One or more unfavorable/borderline levels of untreated systolic blood pressure 121 to 139 mm Hg or diastolic blood pressure 81 to 89 mm Hg, or untreated serum total cholesterol 5.18 to 6.21 mmol/L, or bone mass index 25.0 to 29.9 kg/m2, not smoking, no diabetes mellitus; High‐risk: high systolic blood pressure/diastolic blood pressure (≥140/90) or using antihypertensive medication, serum total cholesterol ≥6.22 mmol/L or using lipid‐lowering medication, smoking, bone mass index ≥30.0 kg/m2, diabetes mellitus. *P value for the Wald test.
Model 1 of Table 2 shows that a more favorable baseline risk profile was associated with a lower hazard of dementia after adjusting for covariates. Compared with the ≥2 high‐risk factors group, the hazards for dementia in the 1‐only high‐risk factor, moderate‐risk, and favorable groups were much lower—the hazards (95% CIs) of dementia were 0.80 (0.71–0.91), 0.74 (0.64–0.86), and 0.69 (0.54–0.89), respectively (P‐trend<0.001). The associations were attenuated when the competing risk of death was considered (model 2) but remained significant with P‐trends <0.01. There was no evidence that the proportional hazards assumption was violated as the Kolmogorov‐type supremum test results based on 1000 simulations for all the covariates were not significant at alpha level=0.05 (all P>0.05). The standard error estimates robust to misspecification of the Cox model were similar to the model‐based counterparts, ie, the main model—the ratios of the robust estimates of the standard error relative to the corresponding model‐based estimates for all variables ranged from 0.98 to 1.
Table 2.
Hazard Ratios (95% CIs) for Any Dementiaa After Age 65 Years During 1991 to 2010 by Baseline CVH Status (1967–1973)
| Baseline CVH Status | P‐Trende | ||||
|---|---|---|---|---|---|
| Favorableb (n=742) | Moderate Riskc (n=2238) | 1‐Only High‐Risk Factorc (n=4220) | ≥2 High‐Risk Factorsd (n=2919) | ||
| Model 1f | 0.69 (0.54–0.89) | 0.74 (0.64–0.86) | 0.80 (0.71–0.91) | 1.0 | <0.001 |
| Model 2g | 0.78 (0.61–0.99) | 0.85 (0.73–0.98) | 0.87 (0.77–0.98) | 1.0 | 0.009 |
CVH indicates cardiovascular health; ICD‐9, International Classification of Diseases, Ninth Revision.
Dementia was defined as any dementia‐related diagnoses with ICD‐9: 290, 290.0–4, 290.8–9, 294.1, 331, 331.0–2, 331.9. Medicare claims during 1991 to 2010 (including fee‐for‐service, for inpatient, outpatient [institutional and non‐institutional], skilled nursing, durable medical equipment, home health, and hospice claims).
Ideal levels of all major cardiovascular disease RFs (systolic blood pressure /diastolic blood pressure ≤120/≤80 mm Hg and no antihypertensive medication, serum total cholesterol <5.18 mmol/L and no cholesterol‐lowering medication, not smoking, bone mass index <25 kg/m2, and no diabetes mellitus).
Borderline levels of systolic blood pressure /diastolic blood pressure or serum total cholesterol, not smoking, bone mass index 25.0 to 29.9 kg/m2, and no diabetes mellitus.
High‐risk factors: hypertension (systolic blood pressure /diastolic blood pressure [≥140/90] or using antihypertensive medication), hypercholesterolemia (serum total cholesterol ≥6.22 mmol/L or using lipid‐lowering medication), smoking, bone mass index ≥30.0 kg/m2, diabetes mellitus.
P value for graded association across 4 baseline risk factor groups with risk factor status as an ordinal variable.
Model 1: Adjusted for baseline age, sex, race, education attainment, and average number of claims per years before dementia diagnosis.
Model 2: Adjusted for baseline age, sex, race, education attainment, annual average number of claims before dementia diagnosis and death before dementia diagnoses.
In the sensitivity analyses, using interaction terms between covariates did not improve the fit of the model (P=0.916 for the likelihood ratio test). Moreover, the parameter estimates from the model with spline effects of baseline age were similar with the estimates from the model with baseline age as continuous variable and spline effects were not significant; and the Akaike information criterion of the model with spline effects (Akaike information criterion =23 123.627) was slightly bigger than the Akaike information criterion obtained in the model without spline function of age (Akaike information criterion =23 120.359).
In analyses of individual risk factors (Table S2), in model 1, baseline blood pressure and smoking status were important predictors of the hazards of dementia. For example, compared with current smokers, the hazards (95% CI) for dementia in those who were not smokers and former smokers were 0.88 (0.78–0.99) and 0.82 (0.71–0.93), respectively. When death before dementia was considered as a competing risk (model 2), the associations of baseline smoking status to dementia were no longer significant. The association of baseline blood pressure with dementia was slightly attenuated but remained significant; eg, in model 1, the hazards (95% CI) of dementia in those with a favorable blood pressure level was 0.85 (0.75, 0.98), compared with those with a high‐risk level, and it was 0.87 (0.76–0.99) in model 2.
In sex‐specific analyses, results in men and women were similar to the results in the main analyses, especially for model 1. For example, in men, compared with the ≥2 high‐risk factors group, the hazards (95% CIs) of dementia in the baseline low‐risk, moderate‐risk, and 1‐only high‐risk factor groups were 0.62 (0.40–0.97), 0.80 (0.67–0.96), and 0.83 (0.72–0.97), respectively; and, in women, they were 0.68 (0.49–0.94), 0.63 (0.49–0.82), and 0.74 (0.60–0.92), respectively (Table S3). The interaction terms for sex and CVH profile in both models, were not statistically significant (P>0.05).
In the analysis stratified by education level, results were also similar in both the low (≤12 years of schooling) and high (>12 years of schooling) baseline education groups, especially in model 1, in that the better the CVH status at younger age, the lower the likelihood of having dementia later in life with P‐trends <0.05 (Table S4). The interaction terms for the dichotomized education level and CVH profile in both models were not statistically significant (P>0.05).
In the analysis with an additional adjustment for any interim stroke before dementia, the association of CVH status and dementia remained strong, although the interim stroke slightly attenuated the association for vascular dementia. For example: in model 1 without adjustment for an interim stroke, the hazards for all types of dementia (95% CI) in the favorable group was 0.69 (0.54–0.89) versus the ≥2 high‐risk factors group, and with additional adjustment for interim stroke, it was 0.70 (0.54–0.90). The hazards for vascular dementia (95% CI) without adjustment for the interim stroke was 0.46 (0.31–0.69), and it was 0.51 (0.34–0.76) with adjustment for the interim stroke (Table S5).
Discussion
In this study of 6839 men and 3280 women aged 23 to 47 years at baseline, we found that after up to 43 years of follow‐up since baseline examination (with 19 years follow‐up in Medicare after reaching age 65 years), a favorable CVH profile at young/middle ages was associated with ≈30% lower risk of developing dementia later in life compared with those with ≥2 high‐risk factors. This lower risk was independent of baseline age, sex, race, education, and annual medical care visits before dementia diagnosis, and it increased in a graded fashion with burden of cardiovascular risk factors. With death free of dementia as a competing risk, the association was attenuated but remained significant. The associations were similar for men and women, and for those with a higher and lower baseline education level; they were mainly driven by baseline smoking and blood pressure levels.
Previous epidemiological studies have highlighted strong associations of the combined effects of favorable levels of all major CVD factors in young adulthood and middle age on subsequent health outcomes including CVD mortality and morbidity,26, 27, 28, 29 subclinical atherosclerosis and markers of inflammation.30, 31, 32 The benefits of favorable CVH have been seen for non‐CVD outcomes including lower risk of cancer33 and general functional disability,18 better quality of life,19, 34 and lower healthcare costs.29 Few longitudinal studies have addressed the association of the combined effect of CVD risk factors on cognitive function35, 36 and dementia;37, 38, 39, 40 our findings are consistent with results of these studies. For example, the Northern Manhattan Study found that among 1033 middle‐aged participants, those with a higher number of ideal CVH metrics at baseline (including the 5 factors considered here plus diet and physical activity) experienced smaller decline in cognitive performance during 6‐year follow‐up in older age (mean age at initial cognitive measurement 72 years).35 This study focused on a subgroup of survivors of the original study, hence the results may not be generalizable to the full study population.
The Young Finns Study, which followed children aged 3 to 18 years at baseline for 31 years, found that the number of CVD risk factors measured in childhood was inversely associated with midlife cognitive performance.36 The Three‐City study in France, which focused on CVH at older age (mean age at baseline 74 years) among survivors who participated in the follow‐up test, found a lower hazard for dementia was associated with an increased number of optimal CVH factors.40 Other studies focused on dementia as the outcome but did not examine the combined effects of favorable levels of all risk factors earlier in life, examining instead only the effects of individual risk factors7 and/or using only standard Cox regression models.37, 38, 39 These other studies also focused only on men of the same age39 or measured risk factors at older age.38
To our knowledge, this study is the first American population‐based study to report that having a favorable CVH profile earlier in life is associated with lower incidence of dementia later in life, considering death before dementia as a competing risk in the analyses. Because of our large sample size and rich information on diagnoses from Medicare claims, we were able to examine the association not only for the whole cohort, but also in men and women, and for high and low education levels separately.
Our results on the associations of CVD risk factors assessed singly with the risk of dementia were also consistent with data previously published. We found baseline smoking status and blood pressure levels were all significantly associated with the risk of dementia.5, 6, 8, 9, 10, 12, 13 We also found an association between smoking status and dementia was affected by the competing risk of death;7 these are probably because of the high competing outcome of death in smokers and deaths (eg, by stroke) in hypertensive people is high.14, 41
Our study benefited from the use of data from a large population‐based cohort of >10 000 individuals, including both men and women. Also, all CVD risk factors were measured objectively >40 years ago when all the participants were at young/middle ages. Furthermore, the combination of CVD risk data with data from the National Death Index and 19 years of Medicare claims allowed us to assess the 4‐decade‐long association of baseline CVD risk profile and the cumulative incidence of dementia during older ages, considering the competing risk of death before dementia diagnosis.
Despite the strengths, the study has limitations. First, early and sustained drug therapy and changes in lifestyle may modify decades‐long CVD risks, thereby influencing the association of early CVD risk profile and dementia later in life—an issue we are unable to address in this study. Nonetheless, just a single ascertainment of the favorable CVD risk status at younger age highlights its important role not only for CVD but also non‐CVD related outcomes in this and other population‐based studies.18, 26, 27, 29, 42 Second, our analyses included only participants who enrolled in fee‐for‐service Medicare. However, CVH was not related to fee‐for‐service versus HMO enrollment. Third, the use of ICD‐9 diagnosis codes in Medicare claims only may lead to an imprecise estimate of actual dementia cases. However, administrative data generally are concordant with patient chart data in recording comorbidity (agreement >85%).43 Administrative data were found to be sensitive and specific for identifying older adults with Alzheimer and related dementias.44 Using Medicare claims to identify all types of dementia had a sensitivity of 85% and specificity of 89% compared with in‐home dementia assessment by clinicians.20 Medicare data have been used to estimate national prevalence of all types of dementia and the subtypes.45 Moreover, any possible misclassification on identifying dementia using Medicare data would be similar across CVD risk strata (non‐differential), ie, it would underestimate the association. Fifth, information on cognitive function at baseline was not available. However, CHA participants were all employed at baseline in 1967 to 1973, thus the proportion of poor cognitive performance at baseline was likely low. Finally, apart from modifiable risk factors, dementia also has genetic risk factors not assessed here.
Conclusion
Our findings highlight and extend the importance of having favorable levels of all major CVD heath factors in young adulthood and early middle age for preventing or delaying neuro‐cognitive disorder in older age. Our findings support an understanding that CVD‐related health outcomes and brain health share common underlying protective mechanisms.46 Our study therefore not only supports the goal of improving cardiovascular health,47 but also supports the national effort of attaining high‐quality, longer lives free of disability for individuals and reducing burdens on families and society.48
Findings from this study also have implications on the clinical perspective. In previous publications, we have shown that low risk or higher CVH profile is associated with longer lifespan26, 29 and delaying the onset of all‐cause and CVD morbidity.29 Yet there is a common fear that a longer lifespan may translate into a greater vulnerability for dementia, and thus a lower quality of life in later years. But our findings—that at any time during follow‐up, the hazard of dementia in the low risk people is lower than in the high risk people—together with our previous findings suggest delayed onset of dementia for those with a low risk profile, allaying some of the fear commonly associated with a longer lifespan and bolstering the argument for primordial prevention (ie, preventing the development of risk factors).
Sources of Funding
This research was supported by a grant from the National Heart, Lung, and Blood Institute (R01‐60036640 to Dr Allen).
Disclosures
None.
Supporting information
Table S1. Definitions of Favorable, Elevated and High Levels of Cardiovascular Health Factors
Table S2. Hazard Ratios (95% CIs) for Dementia* by Baseline Individual Risk Factors (1967–1973)
Table S3. Hazard Ratios (95% CIs) for Dementia# Age 65 During 1991–2010 by Baseline CVH Status (1967–1973), Stratified by Sex
Table S4. Hazard Ratios (95% CIs) for Dementia# After Age 65 During 1991–2010 by Baseline CVH Status (1967–1973), Stratified by Education Levels
Table S5. Hazard Ratios (95% CIs) for Dementia# After Age 65 During 1991–2010 by Baseline CVH Status (1967–1973), Further Adjusted for Interim Stroke
Figure S1. Flow chart depicting CHA‐MEDICARE* analysis sample.
Acknowledgments
The authors thank the other investigators, staff members, and volunteers involved in the CHA study. We also thank the participants for their valuable contributions.
(J Am Heart Assoc. 2019;8:e009730 DOI: 10.1161/JAHA.118.009730)
The abstract of this work was presented and selected for a videotaped interview that was covered at the EPI Lifestyle Scientific Sessions, March 10, 2017, in Portland, OR.
References
- 1. Alzheimer's Association . 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. [DOI] [PubMed] [Google Scholar]
- 2. Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Razay G, Williams J, King E, Smith AD, Wilcock G. Blood pressure, dementia and Alzheimer's disease: the OPTIMA longitudinal study. Dement Geriatr Cogn Disord. 2009;28:70–74. [DOI] [PubMed] [Google Scholar]
- 4. Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and dementia—a comprehensive review. Ther Adv Neurol Disord. 2009;2:241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haring B, Leng X, Robinson J, Johnson KC, Jackson RD, Beyth R, Wactawski‐Wende J, von Ballmoos MW, Goveas JS, Kuller LH, Wassertheil‐Smoller S. Cardiovascular disease and cognitive decline in postmenopausal women: results from the Women's Health Initiative Memory Study. J Am Heart Assoc. 2013;2:e000369 DOI: 10.1161/JAHA.113.000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS. Associations between midlife vascular risk factors and 25‐year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon A, Kareholt I, Ngandu T, Winblad B, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M. Serum cholesterol changes after midlife and late‐life cognition: twenty‐one‐year follow‐up study. Neurology. 2007;68:751–756. [DOI] [PubMed] [Google Scholar]
- 9. Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late‐life Alzheimer disease. Ann Intern Med. 2002;137:149–155. [DOI] [PubMed] [Google Scholar]
- 10. Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: a population‐based neuropathologic study. Neurology. 2010;75:1195–1202. [DOI] [PubMed] [Google Scholar]
- 11. Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late‐life as a risk factor for dementia: a meta‐analysis of prospective studies. Obes Rev. 2011;12:e426–e437. [DOI] [PubMed] [Google Scholar]
- 12. Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–326. [DOI] [PubMed] [Google Scholar]
- 13. Rusanen M, Kivipelto M, Quesenberry CP Jr, Zhou J, Whitmer RA. Heavy smoking in midlife and long‐term risk of Alzheimer disease and vascular dementia. Arch Intern Med. 2011;171:333–339. [DOI] [PubMed] [Google Scholar]
- 14. Hernan MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19:448–450. [DOI] [PubMed] [Google Scholar]
- 15. Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marzona I, Baviera M, Vannini T, Tettamanti M, Cortesi L, Riva E, Nobili A, Marcon G, Fortino I, Bortolotti A, Merlino L, Roncaglioni MC. Risk of dementia and death in patients with atrial fibrillation: a competing risk analysis of a population‐based cohort. Int J Cardiol. 2016;220:440–444. [DOI] [PubMed] [Google Scholar]
- 17. Greenland P, Xie X, Liu K, Colangelo L, Liao Y, Daviglus ML, Agulnek AN, Stamler J. Impact of minor electrocardiographic ST‐segment and/or T‐wave abnormalities on cardiovascular mortality during long‐term follow‐up. Am J Cardiol. 2003;91:1068–1074. [DOI] [PubMed] [Google Scholar]
- 18. Vu TH, Lloyd‐Jones DM, Liu K, Stamler J, Garside DB, Daviglus ML. Optimal levels of all major cardiovascular risk factors in younger age and functional disability in older age: the Chicago Heart Association Detection Project in Industry 32‐year follow‐up health survey. Circ Cardiovasc Qual Outcomes. 2016;9:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allen NB, Badon S, Greenlund KJ, Huffman M, Hong Y, Lloyd‐Jones DM. The association between cardiovascular health and health‐related quality of life and health status measures among U.S. adults: a cross‐sectional study of the National Health and Nutrition Examination Surveys, 2001–2010. Health Qual Life Outcomes. 2015;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor DH Jr, Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guerra C, Linde‐Zwirble WT, Wunsch H. Risk factors for dementia after critical illness in elderly Medicare beneficiaries. Crit Care. 2012;16:R233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:13. [Google Scholar]
- 23. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crimmins EM, Saito Y, Kim JK, Zhang YS, Sasson I, Hayward MD. Educational differences in the prevalence of dementia and life expectancy with dementia: changes from 2000 to 2010. J Gerontol B Psychol Sci Soc Sci. 2018;73:S20–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. 2016;1862:915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, Dyer AR, Liu K, Greenland P. Low risk‐factor profile and long‐term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle‐aged men and women. JAMA. 1999;282:2012–2018. [DOI] [PubMed] [Google Scholar]
- 27. Lloyd‐Jones DM, Dyer AR, Wang R, Daviglus ML, Greenland P. Risk factor burden in middle age and lifetime risks for cardiovascular and non‐cardiovascular death (Chicago Heart Association Detection Project in Industry). Am J Cardiol. 2007;99:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daviglus ML, Stamler J, Pirzada A, Yan LL, Garside DB, Liu K, Wang R, Dyer AR, Lloyd‐Jones DM, Greenland P. Favorable cardiovascular risk profile in young women and long‐term risk of cardiovascular and all‐cause mortality. JAMA. 2004;292:1588–1592. [DOI] [PubMed] [Google Scholar]
- 29. Allen NB, Zhao L, Liu L, Daviglus M, Liu K, Fries J, Shih YT, Garside D, Vu TH, Stamler J, Lloyd‐Jones DM. Favorable cardiovascular health, compression of morbidity, and healthcare costs: forty‐year follow‐up of the CHA Study (Chicago Heart Association Detection Project in Industry). Circulation. 2017;135:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vu TH, Stamler J, Liu K, McDermott MM, Lloyd‐Jones DM, Pirzada A, Garside DB, Daviglus ML. Prospective relationship of low cardiovascular risk factor profile at younger ages to ankle‐brachial index: 39‐year follow‐up–the Chicago Healthy Aging Study. J Am Heart Assoc. 2012;1:e001545 DOI: 10.1161/JAHA.112.001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vu TH, Liu K, Lloyd‐Jones DM, Stamler J, Pirzada A, Shah S, Garside DB, Daviglus ML. Favorable levels of all major cardiovascular risk factors at younger ages and high‐sensitivity C‐reactive protein 39 years later—The Chicago Healthy Aging Study. Prev Med Rep. 2015;2:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vu TT, Daviglus ML, Liu K, Allen NB, Garside DB, Lloyd‐Jones DM. Long‐term favorable cardiovascular risk profile and 39‐year development of major and minor electrocardiographic abnormalities—The Chicago Healthy Aging Study (CHAS). J Electrocardiol. 2018;51:863–869. [DOI] [PubMed] [Google Scholar]
- 33. Rasmussen‐Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk in Communities study. Circulation. 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, Feinglass J, Guralnik JM, Greenland P, Stamler J. Favorable cardiovascular risk profile in middle age and health‐related quality of life in older age. Arch Intern Med. 2003;163:2460–2468. [DOI] [PubMed] [Google Scholar]
- 35. Gardener H, Wright CB, Dong C, Cheung K, DeRosa J, Nannery M, Stern Y, Elkind MS, Sacco RL. Ideal cardiovascular health and cognitive aging in the Northern Manhattan Study. J Am Heart Assoc. 2016;5:e002731 DOI: 10.1161/JAHA.115.002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rovio SP, Pahkala K, Nevalainen J, Juonala M, Salo P, Kahonen M, Hutri‐Kahonen N, Lehtimaki T, Jokinen E, Laitinen T, Taittonen L, Tossavainen P, Viikari JSA, Rinne JO, Raitakari OT. Cardiovascular risk factors from childhood and midlife cognitive performance: the Young Finns Study. J Am Coll Cardiol. 2017;69:2279–2289. [DOI] [PubMed] [Google Scholar]
- 37. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. [DOI] [PubMed] [Google Scholar]
- 38. Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ronnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk factors and dementia: 40‐year follow‐up of a population‐based cohort. Dement Geriatr Cogn Disord. 2011;31:460–466. [DOI] [PubMed] [Google Scholar]
- 40. Samieri C, Perier MC, Gaye B, Proust‐Lima C, Helmer C, Dartigues JF, Berr C, Tzourio C, Empana JP. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peng M, Chen G, Tang KL, Quan H, Smith EE, Faris P, Hachinski V, Campbell NRC. Blood pressure at age 60‐65 versus age 70‐75 and vascular dementia: a population based observational study. BMC Geriatr. 2017;17:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stokes J III, Kannel WB, Wolf PA, Cupples LA, D'Agostino RB. The relative importance of selected risk factors for various manifestations of cardiovascular disease among men and women from 35 to 64 years old: 30 years of follow‐up in the Framingham Study. Circulation. 1987;75:V65–V73. [PubMed] [Google Scholar]
- 43. Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived rom ICD‐9‐CCM administrative data. Med Care. 2002;40:675–685. [DOI] [PubMed] [Google Scholar]
- 44. Jaakkimainen RL, Bronskill SE, Tierney MC, Herrmann N, Green D, Young J, Ivers N, Butt D, Widdifield J, Tu K. Identification of physician‐diagnosed Alzheimer's disease and related dementias in population‐based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimers Dis. 2016;54:337–349. [DOI] [PubMed] [Google Scholar]
- 45. Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM. Prevalence of dementia subtypes in United States Medicare fee‐for‐service beneficiaries, 2011–2013. Alzheimers Dement. 2017;13:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gorelick PB, Furie KL, Iadecola C, Smith EE, Waddy SP, Lloyd‐Jones DM, Bae HJ, Bauman MA, Dichgans M, Duncan PW, Girgus M, Howard VJ, Lazar RM, Seshadri S, Testai FD, van Gaal S, Yaffe K, Wasiak H, Zerna C; American Heart Association/American Stroke A . Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lloyd‐Jones D, Hong Y, Labarthe D, Mozaffarian D, Appel L, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli G, Arnett D, Fonarow G, Ho P, Lauer M, Masoudi F, Robertson R, Roger V, Schwamm L, Sorlie P, Yancy C, Rosamond W; Committee obotAHASPTFaS . AHA special report. Defining and setting national goals for cardiovascular health promotion and disease reduction. The American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 48. ODPHP . The Vision, Mission, and Goals of Healthy People 2020. Office of Disease Prevention and Health Promotion. U.S. Department of Health and Human Services; Available at: https://www.healthypeople.gov/sites/default/files/HP2020Framework.pdf. Accessed November 28, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definitions of Favorable, Elevated and High Levels of Cardiovascular Health Factors
Table S2. Hazard Ratios (95% CIs) for Dementia* by Baseline Individual Risk Factors (1967–1973)
Table S3. Hazard Ratios (95% CIs) for Dementia# Age 65 During 1991–2010 by Baseline CVH Status (1967–1973), Stratified by Sex
Table S4. Hazard Ratios (95% CIs) for Dementia# After Age 65 During 1991–2010 by Baseline CVH Status (1967–1973), Stratified by Education Levels
Table S5. Hazard Ratios (95% CIs) for Dementia# After Age 65 During 1991–2010 by Baseline CVH Status (1967–1973), Further Adjusted for Interim Stroke
Figure S1. Flow chart depicting CHA‐MEDICARE* analysis sample.


