Abstract
Background
Heart failure is one of the most important late effects after treatment for cancer in childhood. The goals of this study were to evaluate the risk of heart failure, temporal changes by treatment periods, and the risk factors for heart failure in childhood cancer survivors (CCS).
Methods and Results
The DCOG‐LATER (Dutch Childhood Oncology Group–Long‐Term Effects After Childhood Cancer) cohort includes 6,165 5‐year CCS diagnosed between 1963 and 2002. Details on prior cancer diagnosis and treatment were collected for this nationwide cohort. Cause‐specific cumulative incidences and risk factors of heart failure were obtained. Cardiac follow‐up was complete for 5,845 CCS (94.8%). After a median follow‐up of 19.8 years and at a median attained age of 27.3 years, 116 survivors developed symptomatic heart failure. The cumulative incidence of developing heart failure 40 years after childhood cancer diagnosis was 4.4% (3.4%–5.5%) among all CCS. The cumulative incidence of heart failure grade ≥3 among survivors treated in the more recent treatment periods was higher compared with survivors treated earlier (Gray test, P=0.05). Mortality due to heart failure decreased in the more recent treatment periods (Gray test, P=0.02). In multivariable analysis, survivors treated with a higher dose of mitoxantrone or cyclophosphamide had a higher risk of heart failure than survivors who were exposed to lower doses.
Conclusions
CCS treated with mitoxantrone, cyclophosphamide, anthracyclines, or radiotherapy involving the heart are at a high risk for severe, life‐threatening or fatal heart failure at a young age. Although mortality decreased, the incidence of severe or life‐threatening heart failure increased with more recent treatment periods.
Keywords: childhood cancer survivors, heart failure
Subject Categories: Cardiovascular Disease, Risk Factors, Heart Failure
Clinical Perspective
What Is New?
In a nationwide cohort of 6165 5‐year childhood cancer survivors, we observed an increase in cumulative incidence of severe or life‐threatening heart failure in recent treatment periods (compared with earlier periods); a dose‐response relation of mitoxantrone and an association of cyclophosphamide with symptomatic heart failure were observed. These results should be replicated in a larger cohort.
What Are the Clinical Implications?
As the current cohort study demonstrates, childhood cancer survivors treated with cardiotoxic treatment have a high risk of developing heart failure at a relatively young age.
Therefore, it may be worthwhile to be vigilant of symptoms that suggest cardiac dysfunction/heart failure in adolescent and young adult childhood cancer survivors, even decades after initial treatment.
Other treatment possibilities, if available, should be considered in childhood cancer treatment protocols, and cardiotoxic doses should be limited because heart failure also develops after low doses of anthracyclines and/or mitoxantrone.
Introduction
Childhood cancer 5‐year survival rates have improved considerably, from 20% in the 1940s1 to 70% to 80% at present.2 It is well established that (childhood) cancer treatments can induce chronic health conditions in childhood cancer survivors (CCS).3, 4, 5 As a result of better survival over the years, the survivor population continues to grow in size. It is known that 75% of CCS will develop at least a chronic health condition,4 and among those aged 45 years, 80.5% of survivors will have a serious/disabling or life‐threatening health condition.5
Heart failure is one of the most frequent late effects in CCS, contributing to significant morbidity and mortality.3, 4, 5, 6, 7, 8, 9 Previous reports show that the cumulative incidence of symptomatic heart failure among CCS 30 years after diagnosis ranges between 2.7% and 4.1%.10, 11 The prevalence of asymptomatic heart failure, defined as a left ventricular shortening fraction <30%, has been found to be 27% in CCS treated with cardiotoxic treatment at a median of 15 years after diagnosis.12
A higher dose of anthracyclines and radiotherapy involving the heart region are associated with asymptomatic and symptomatic heart failure.10, 11, 13, 14, 15, 16, 17, 18 So far, the role of other types of chemotherapy such as mitoxantrone and cyclophosphamide on heart failure risk is not clear.11, 19
The risk of cardiotoxicity after childhood cancer treatment has already been known for several decades. Studies that investigate the temporal changes of heart failure by treatment periods are lacking. Recent studies did show a reduction in cardiac mortality of CCS in later periods of treatment compared with earlier treatment periods.8, 9
We aimed to determine the cumulative incidence, the temporal changes by treatment period, and factors that are associated with the cause‐specific incidence of systematically ascertained and validated symptomatic heart failure in CCS within a Dutch nationwide cohort. This knowledge will guide future treatment and surveillance protocols for children with cancer.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
We obtained our study population from the national DCOG‐LATER (Dutch Childhood Oncology Group—Long‐Term Effects After Childhood Cancer) nationwide cohort (n=6165), a collaborative effort of all Dutch pediatric oncology/hematology centers. Eligible survivors included 5‐year CCS diagnosed before the age of 18 years between January 1, 1963 and December 31, 2001 with a malignancy according to the third edition of the International Classification of Childhood Cancer.20 We only included CCS who were living in the Netherlands at the time of childhood cancer diagnosis and who were treated in 1 of the Dutch pediatric oncology/hematology centers (Academic Medical Center Amsterdam, VU University Medical Center, Leiden University Medical Center, Erasmus Medical Center, University Medical Center Groningen, Radboudumc, and University Medical Center Utrecht).
Data Collection on Chemotherapy Treatment
Data on childhood cancer diagnosis and treatment (including treatment for recurrences) were extracted from the medical records for all eligible CCS. The total cumulative anthracycline dose was calculated by summing the doxorubicin‐equivalent doses (daunorubicin [×0.45]21, epirubicin [×0.67], idarubicin [×3]) based on the previously published equivalence ratio.22 This cumulative anthracycline dose (the sum of all types of anthracyclines) was based directly on risk for heart failure and not on those doses that produce equivalent hematological toxicity with the assumption that hematological and cardiac toxicities are correlated.21
Data Collection on Radiotherapy Treatment
Detailed radiotherapy involving the heart was classified as follows: no radiotherapy involving the heart; radiotherapy potentially involving the heart (abdomen, para‐aortal, spleen, inverted Y, spine not otherwise specified, scapula, vertebrae, ribs/sternum/clavicle, kidney, liver, total abdominal radiation); radiotherapy involving the heart (total body radiation, thorax, mediastinum, total node, mantle field, spine [whole and thoracic], lung, parasternal). We used the maximum prescribed dose of the largest field involving the heart and added the dose of total body radiation. For the latter group, we used a cutoff point of 20 Gy, which is the median of the cumulative prescribed dose. Validation of radiotherapy data was performed by experts in radiotherapy.
Cardiac Outcome Data Collection and Cardiac Follow‐Up
The outcome of interest was heart failure, graded according to the Common Terminology Criteria for Adverse Events as grade 3 (severe), 4 (life‐threatening), or 5 (death).23 The incidence date of heart failure was defined as the date of an abnormal diagnostic test combined with symptoms.
Information on potential heart failure was collected from 3 different data sources: the DCOG‐LATER questionnaire, GP (primary physician) DCOG‐LATER questionnaire, and medical records (Figure S1). Cohort members resident in the Netherlands received a DCOG‐LATER questionnaire between 2012 and 2014. The DCOG‐LATER questionnaire is a general health and risk factor questionnaire including diseases of the circulatory system. To optimize response, CCS initially not willing to participate were offered the option of completing a brief telephone survey addressing sociodemographic characteristics and health status. CCS were considered nonresponders to the DCOG‐LATER questionnaire if they had not responded after a written invitation, a written reminder, and at least 2 telephone reminders. For nonresponders, we obtained data on heart failure from the GP DCOG‐LATER questionnaire, a short questionnaire on major health outcomes and risk factors sent to the GP of the CCS. The GP was considered a “GP nonresponder” if he or she had not responded after a written invitation, a written reminder, and at least 2 telephone reminders. For “GP nonresponders,” we extracted heart failure data from the DCOG‐LATER outpatient clinics where available. Finally, for known deceased CCS, the main cause of death and underlying diseases were extracted from the medical records. Potential heart failure was subsequently validated following a standardized method described previously (Figure S2 and Data S1 for more detailed information).24
For the DCOG‐LATER questionnaire data, written informed consent was obtained from the participating survivors. Informed consent was also sought from the parents of underage (<18 years of age) CCS. Data collection from the GP DCOG‐LATER questionnaire and medical records was exempt from institutional review board review.
Statistical Analyses
The following dates were assigned as the end of cardiac follow‐up: the date of death for decedents; the date of DCOG‐LATER (or GP DCOG‐LATER) questionnaire completion for responders; the date of the last recorded patient contact for nonresponders or for CCS lost to follow‐up. If a cohort member died from a cause other than heart failure, death was considered a competing event.25 Heart failure was evaluated if it started more than 5 years after childhood cancer diagnosis or if it started within 5 years of childhood cancer diagnosis and was still present after 5 years from diagnosis. The time at risk started 5 years after childhood cancer diagnosis, and it ended on the incidence date of heart failure diagnosis or at the end date of cardiac follow‐up, whichever occurred first.
Cumulative Incidence
We estimated the cumulative incidence overall and for different mutually exclusive treatment groups using the nonparametric Aalen‐Johansen estimator. Both follow‐up time since childhood cancer diagnosis and age at follow‐up were used as time scales. Additionally, we examined the cumulative incidence of heart failure (grades 3, 4, and 5) in relation to the period of treatment (1970–1979, 1980–1989, and 1990–2001). The differences between the groups were evaluated with Gray log‐rank tests.26 We also tested the differences between the period of treatment and the use of anthracyclines (yes versus no), anthracycline dose, mitoxantrone (yes versus no), mitoxantrone dose, and radiotherapy to the chest.
Risk Factor Analyses
We assessed risk factors for heart failure using a multivariable Cox proportional hazards model with attained age as the time scale. The model was adjusted for age at childhood cancer diagnosis, sex, and calendar year of childhood cancer diagnosis. We examined possible risk factors for heart failure based on the literature and clinical knowledge. We evaluated the effects of age at diagnosis, sex, and year of cancer diagnosis as well as the effect of treatment: anthracycline (per 1 mg/m2), mitoxantrone (per 1 mg/m2), cyclophosphamide (per 100 mg/m2), cisplatin (per 1 mg/m2), ifosfamide (per 1 mg/m2), vincristine (per 1 mg/m2), and radiotherapy involving the heart (no radiotherapy involving the heart; radiotherapy potentially involving the heart; radiotherapy involving the heart <20 Gy; radiotherapy involving the heart ≥20 Gy). We examined the dose‐response relationship between anthracyclines, mitoxantrone, and cyclophosphamide and the development of heart failure by modeling via restricted cubic splines using 3 knots (10%, 50%, 90% quantiles). In order to avoid overadjusting the model, we did not include treatment and diagnosis variables in the same model.
Two‐sided P‐values were reported, and those of less than 0.05 were considered statistically significant. Analyses were performed using R (version 3.1.1; R Foundation, Vienna, Austria) and SPSS (version 24; IBM SPSS Statistics, Armonk, NY).
Results
Study Population, Cardiac Follow‐Up, and Cardiac Events
The nationwide cohort included 6165 CCS. For 5845 CCS (94.8%), cardiac follow‐up information was retrieved. Of those 5845 CCS, cardiac follow‐up was complete for 84.7% in 2013. Data S2 and Figure S1 provide an extensive overview of the cardiac follow‐up. Table 1 presents demographic information, tumor characteristics, and follow‐up information on the cohort with cardiac follow‐up and CCS with heart failure. At the end of follow‐up 5278 (90.3%) CCS were alive, and 567 (9.7%) were deceased. Median follow‐up time since childhood cancer diagnosis was 19.9 years (range 5.0–50.4 years), and median attained age was 27.3 years (range 5.1–65.2 years).
Table 1.
Patient, Cancer, and Treatment Characteristics of the 5‐Year Survivors of the DCOG‐LATER Cohort With Complete Cardiac Follow‐Up
| Characteristics | Cohort With Cardiac Follow‐Up (n=5845) (94.8%)* | CCS With Heart Failure (n=116) |
|---|---|---|
| n (%) | n (%) | |
| Patient characteristics | ||
| Sex | ||
| Female | 2588 (44.3) | 44 (37.9) |
| Male | 3257 (55.7) | 72 (62.1) |
| Cancer characteristics | ||
| Primary childhood cancer (ICCC) | ||
| Leukemias, myeloproliferative diseases, and myelodysplastic diseases | 2034 (34.8) | 30 (25.9) |
| Lymphomas and reticuloendothelial neoplasms | 1019 (17.4) | 26 (22.4) |
| CNS and miscellaneous intracranial and intraspinal neoplasms | 749 (12.8) | 3 (2.6) |
| Neuroblastoma and other peripheral nervous cell tumors | 306 (5.2) | 2 (1.7) |
| Retinoblastoma | 29 (0.5) | 0 (0) |
| Renal tumors | 571 (9.8) | 11 (9.5) |
| Hepatic tumors | 46 (0.8) | 0 (0) |
| Bone tumors | 355 (6.1) | 25 (21.6) |
| Soft tissue and other extraosseous sarcomas | 422 (7.2) | 18 (15.5) |
| Germ cell tumors, trophoblastic tumors, and neoplasms of gonads | 219 (3.7) | 0 (0) |
| Other malignant epithelial neoplasms and malignant melanomas | 88 (1.5) | 1 (0.9) |
| Other and unspecified malignant neoplasms | 7 (0.1) | 0 (0) |
| Age at diagnosis (y), median (IQR) | 5.5 (2.8–10.5) | 6.1 (2.8–10.5) |
| 0 to 4 | 2692 (46.1) | 49 (42.2) |
| 5 to 9 | 1567 (26.8) | 35 (30.2) |
| 10 to 14 | 1233 (21.1) | 28 (24.1) |
| 15 to 17 | 353 (6.0) | 4 (3.4) |
| Treatment period | ||
| 1963 to 1979 | 990 (16.9) | 22 (19.0) |
| 1980 to 1989 | 1853 (31.7) | 58 (50.0) |
| 1990 to 2001 | 3002 (51.4) | 36 (31.0) |
| Overall treatment modality | ||
| Surgery only | 587 (10.0) | 0 (0) |
| Chemotherapy±Surgery | 2882 (49.3) | 60 (51.7) |
| Radiotherapy±Surgery | 445 (7.6) | 0 (0) |
| Chemotherapy and Radiotherapy±Surgery | 1854 (31.7) | 55 (47.4) |
| No therapy/unknown | 77 (1.3) | 1 (0.9) |
| Cardiotoxic treatment | ||
| No cardiotoxic treatment | 2845 (48.7) | 3 (2.6) |
| Cardiotoxic CT only | 2304 (39.4) | 83 (71.6) |
| RT involving the heart only | 211 (3.6) | 4 (3.4) |
| Cardiotoxic CT and chest RT | 434 (7.4) | 25 (21.6) |
| Unknown | 51 (0.9) | 1 (0.9) |
| Anthracyclines median dose (IQR) | 175 (114–272) | 360 (201–450) |
| No anthracyclines | 3099 (53.0) | 13 (11.2) |
| 1 to 100 mg/m2 | 491 (8.4) | 21 (18.1) |
| 100 to 250 mg/m2 | 1402 (24.0) | 33 (28.4) |
| >250 mg/m2 | 720 (12.3) | 42 (36.2) |
| Missing | 133 (2.3)† | 7 (6.0) |
| Mitoxantrone median dose (IQR) | 40 (20–60) | 45 (20–120) |
| No mitoxantrone | 5660 (96.8) | 103 (88.8) |
| <40 mg/m2 | 81 (1.4) | 6 (5.2) |
| >40 mg/m2 | 58 (1.0) | 6 (5.2) |
| Missing | 46 (0.7)‡ | 1 (0.9) |
| Cyclophosphamide (intravenous) | ||
| None | 3674 (62.8) | 34 (29.3) |
| Any | 2132 (36.5) | 81 (69.8) |
| Unknown | 39 (0.7) | 1 (0.9) |
| Cisplatin | ||
| None | 5363 (91.8) | 103 (88.8) |
| Any | 443 (7.6) | 12 (10.3) |
| Unknown | 39 (0.7) | 1 (0.9) |
| Ifosfamide | ||
| None | 5107 (87.4) | 98 (84.5) |
| Any | 699 (12.0) | 17 (14.7) |
| Unknown | 39 (0.7) | 1 (0.9) |
| Vincristine | ||
| None | 1642 (28.1) | 16 (13.8) |
| Any | 4164 (71.2) | 99 (85.3) |
| Unknown | 39 (0.7) | 1 (0.9) |
| Radiotherapy field involving the heart | ||
| No chest radiotherapy | 4575 (78.3) | 78 (67.2) |
| Radiotherapy potentially involving the heart | 588 (10.1) | 9 (7.8) |
| Radiotherapy involving the heart <20 Gy | 275 (4.7) | 15 (12.9) |
| Radiotherapy involving the heart ≥20 Gy | 363 (6.2) | 14 (12.1) |
| Unknown | 44 (0.7) | 0 (0) |
| Recurrence | ||
| No | 4836 (82.7) | 87 (75.0) |
| Yes | 1009 (17.3) | 29 (25.0) |
| Follow‐up | ||
| Vital status | ||
| Alive | 5278 (90.3) | 87 (75.0) |
| Deceased | 567 (9.7) | 29 (25.0) |
| Attained age (y), median (min‐max) | 27.3 (5.1–65.2) | 23.8 (6.2–48.8) |
| ≤14 | 463 (7.9) | 15 (12.9) |
| 15 to 24 | 1949 (33.4) | 45 (38.8) |
| 25 to 34 | 2000 (34.2) | 38 (32.8) |
| 35 to 44 | 1129 (19.3) | 16 (13.8) |
| 45 to 54 | 267 (4.6) | 2 (1.7) |
| ≥55 | 37 (0.6) | 0 (0) |
| Follow‐up duration from primary cancer diagnosis (y), median (min‐max) | 19.9 (5.0–50.4) | 16.8 (5.0–36.8) |
| >5 to 9 | 480 (8.2) | 21 (18.1) |
| 19 to 10 | 2459 (42.1) | 48 (41.4) |
| 20 to 29 | 1791 (30.6) | 34 (29.3) |
| 30 to 39 | 965 (16.5) | 13 (11.2) |
| ≥40 | 150 (2.6) | 0 (0) |
| Source | ||
| LATER questionnaire | 3056 (52.3) | 58 (50.0) |
| General practitioner questionnaire | 773 (13.2) | 6 (5.2) |
| Medical chart | 2016 (34.5) | 52 (44.8) |
| Cardiac events | ||
| Type of validated symptomatic heart failure | ||
| Grade 3 | 61 (52.5) | 61 (52.5) |
| Grade 4 | 33 (28.5) | 33 (28.5) |
| Grade 5 | 22 (19.0) | 22 (19.0) |
| Other cardiac events | ||
| Cardiac ischemia | 21 (0.4) | 2 (1.7)§ |
| Pericarditis | 13 (0.2) | 1 (0.9)§ |
| Valvular disease | 22 (0.4) | 3 (2.6)§ |
| Arrhythmia | 41 (0.7) | 3 (2.6)§ |
CCS indicates childhood cancer survivor; CNS, central nervous system; CT, chemotherapy; DCOG‐LATER, Dutch Childhood Oncology Group Long‐term outcomes after cancer treatment; ICCC, International Classification of Childhood Cancer; IQR, interquartile range; RT, radiatiotherapy.
Percentage of the total DCOG‐LATER cohort.
n=94 anthracycline=yes, but dose missing.
n=7 mitoxantrone=yes, but dose missing.
Cardiac event before the onset of heart failure.
Among the 5845 eligible CCS with cardiac follow‐up, 116 CCS (2.0%) developed heart failure, 61 CCS were grade 3, 33 CCS grade 4, and 22 CCS grade 5. Among all cases, 35 (30.2%) had received both cardiotoxic chemotherapy and radiotherapy involving the heart, 75 (64.7%) had received cardiotoxic chemotherapy only, 2 cases (1.7%) had received radiotherapy involving the heart only, and the 4 remaining cases (3.4%) had received no known potential cardiotoxic treatment, or their treatment was unknown.
The 3 cases without known potential cardiotoxic treatment had conditions known to predispose to heart failure: Duchenne muscular dystrophy, noncompaction cardiomyopathy, and Tetralogy of Fallot. The details of the CCS with heart failure are presented in Table 1.
Cumulative Incidence by Treatment Groups
Table S1 presents the cumulative incidence of developing heart failure by time since childhood cancer diagnosis and for mutually exclusive treatment groups. The cumulative incidence of developing heart failure 40 years after childhood cancer diagnosis was 4.4% (95% CI 3.4% to 5.5%). The cumulative incidence of heart failure 40 years after diagnosis was much higher among CCS who received had 1 or more types of cardiotoxic treatment (10.6%, 95% CI 7.4% to 13.9%) than among CCS treated without cardiotoxic treatment (0.3%, 95% CI 0.0% to 0.7%) (Gray test of cardiotoxic treatment versus no cardiotoxic treatment, P<0.0001) (Table S1 and Figure 1). The cumulative incidence of heart failure 40 years after diagnosis was 27.8% (95% CI 5.1% to 50.6%) for CCS who had received both cardiotoxic chemotherapy and radiotherapy involving the heart, 10.5% (95% CI 6.4% to 14.4%) for CCS who had received only cardiotoxic chemotherapy, and 3.0% (95% CI 0.0% to 5.9%) for CCS treated with only radiotherapy involving the heart (Table S1, Figure S3).
Figure 1.
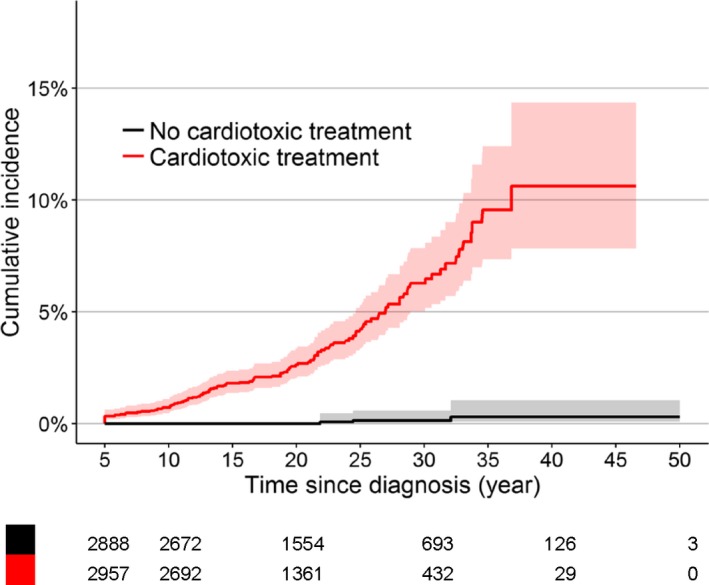
Cumulative incidence of heart failure for cardiotoxic treatment (anthracyclines, mitoxantrone, and radiotherapy involving the heart) with time since childhood cancer diagnosis as time scale. P‐value for Gray test is P<0.0001. Shaded areas indicate 95% CI.
The cumulative incidence of heart failure 20 years after diagnosis for CCS treated with mitoxantrone (±anthracyclines) was 11.4% (95% CI 3.6% to 19.1%). The cumulative incidence increased significantly in the CCS treated with higher anthracycline doses (Table S1 and Figure 2).
Figure 2.
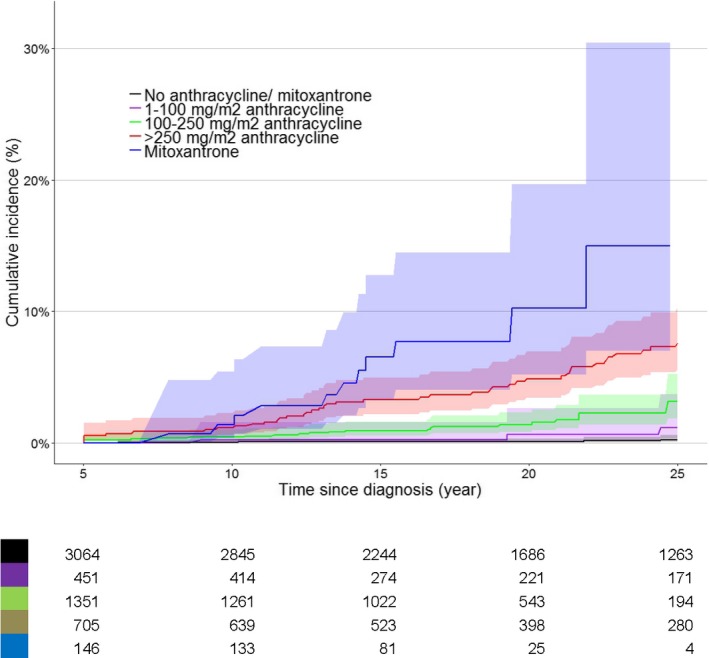
Cumulative incidence of heart failure (grades 3, 4, and 5) for 2 specific treatment groups: anthracyclines only (n=2598 cohort members, 96 cases) and mitoxantrone (with/without anthracyclines) (n=146 cohort members, 12 cases). All childhood cancer survivors who had radiotherapy involving the heart region were excluded from these analyses. Parwise comparisons found these degrees of significance: no anthracycline/mitoxantrone vs anthracycline 1 to 100 mg/m2, P=0.17; no anthracycline/mitoxantrone vs anthracycline 100 to 250 mg/m2, P<0.00001; no anthracycline/mitoxantrone vs anthracycline >250 mg/m2, P<0.00001; no anthracycline/mitoxantrone vs mitoxantrone, P<0.00001; anthracycline 1 to 100 mg/m2 vs anthracycline 100 to 250 mg/m2, P=0.007; anthracycline 1 to 100 mg/m2 vs anthracycline >250 mg/m2, P<0.00001; anthracycline 1 to 100 mg/m2 vs mitoxantrone, P<0.00001; anthracycline 100 to 250 mg/m2 vs anthracycline >250 mg/m2, P<0.00001; anthracycline 100 to 250 mg/m2 vs mitoxantrone, P<0.00001; anthracycline >250 mg/m2 vs mitoxantrone, P=0.02. Shaded areas indicate 95% CI.
The cumulative incidence of developing heart failure by attained age is presented in Table S2 and Figure S4. At age 50 years, the cumulative incidence of developing heart failure in the whole cohort was 5.3% (95% CI 3.7% to 6.9%).
Cumulative Incidence by Treatment Era
Table 2 shows the cardiotoxic treatment survivors received for the different treatment periods (1960–1979, 1980–1989, and 1990–2001). We observed a statistically significant difference between survivors treated in the oldest treatment era (1960–1979) and in more recent treatment eras (1980–1989 and 1990–2001), especially for those survivors treated with anthracyclines (compared with those not treated with anthracyclines), and for those survivors treated with radiotherapy to the chest.
Table 2.
Description of Cardiotoxic Treatment for Different Cancer Treatment Periods
| Treatment Between 1960 and 1979 (n=990 CCS) | Treatment Between 1980 and 1989 (n=1853 CCS) | Treatment Between 1990 and 2001 (n=3002 CCS) | |
|---|---|---|---|
| Anthracycline | |||
| Median dose (IQR) | 180 (22.5–740) | 200 (18.0–1950) | 160 (6.89–668) |
| n (%) | n (%) | n (%) | |
| No anthracyclines | 745 (75.2) | 970 (52.3) | 1384 (46.1) |
| Anthracyclines any dose | 233 (23.6) | 867 (46.8) | 1607 (53.5) |
| Missing | 12 (1.2) | 16 (0.9) | 11 (0.4) |
| 1 to 100 mg/m2 | 75 (7.6) | 174 (9.4) | 212 (7.1) |
| 100 to 250 mg/m2 | 40 (4.0) | 324 (17.5) | 1059 (35.3) |
| >250 mg/m2 | 85 (8.6) | 342 (18.5) | 302 (10.1) |
| Missing | 33 (3.3) | 27 (1.5) | 34 (1.1) |
| Mitoxantrone | |||
| Median dose (IQR) | 50 (20–40) | 38 (22–46) | 39 (20–70) |
| n (%) | n (%) | n (%) | |
| No mitoxantrone | 976 (98.5) | 1822 (98.3) | 2863 (95.4) |
| Mitoxantrone any dose | 3 (0.3) | 15 (0.8) | 128 (4.3) |
| Missing | 12 (1.2) | 16 (0.9) | 11 (0.4) |
| 1 to 40 mg/m2 | 1 (33.4) | 9 (60.0) | 71 (55.5) |
| >40 mg/m2 | 2 (66.6) | 3 (20.0) | 53 (41.4) |
| Missing | 3 (20.0) | 4 (3.9) | |
| Radiotherapy | |||
| No chest radiotherapy | 650 (65.6) | 1447 (78.1) | 2478 (82.5) |
| Radiotherapy potentially involving the heart | 192 (19.4) | 190 (10.3) | 206 (6.9) |
| Radiotherapy involving the heart | 142 (14.3) | 204 (11.0) | 301 (10.0) |
| Missing | 7 (0.7) | 11 (0.6) | 17 (0.6) |
CCS indicates childhood cancer survivor; IQR, interquartile range.
The cumulative incidence of heart failure (grades 3, 4, and 5) increased for CCS treated in more recent years (Figure 3A). The cumulative risks at 20 years after diagnosis for CCS treated during 1970–1979, 1980–1989, and 1990–2001 were 0.5% (95% CI 0.01% to 0.9%), 1.6% (95% CI 1.0% to 2.2%), 1.5% (95% CI 0.9% to 2.0%), respectively. The risks of heart failure 20 years after diagnosis for CCS treated during 1990–2001 and between 1980 and 1989 were significantly higher than the risk of heart failure for CCS treated during 1970–1979 (Gray test 1970–1979 versus 1980–1989, P=0.01; 1970–1979 versus 1990–2001, P=0.03). Figure 3B displays the cumulative incidence per treatment period for fatal heart failure (grade 5). The risks of a fatal event 20 years after diagnosis for CCS treated during 1970–1979 and 1980–1989 were significantly higher than the risk of CCS treated during 1990–2001 (Gray test 1970–1979 versus 1990–2001, P=0.04; 1980–1989 versus 1990–2001, P=0.02). Because most childhood cancer treatment centers started in 1970 in the Netherlands, there is a possibility that children had been treated in adult cancer centers before that time; thus, we excluded children diagnosed with cancer before 1970 (n=90, 1.5%; n=2 children treated with anthracyclines).
Figure 3.
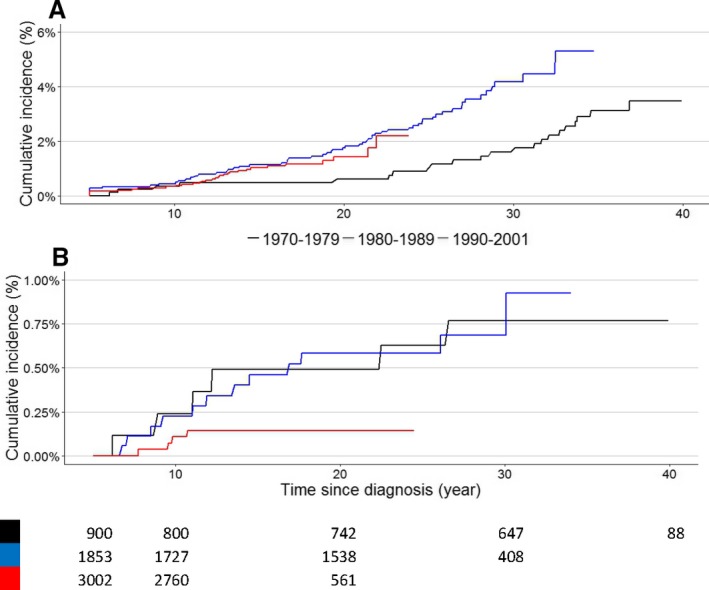
A, Cumulative incidence of heart failure (grades 3, 4, and 5) per treatment period, with time since childhood cancer diagnosis. P‐value for Gray test: 1970–1979 vs 1980–1989, P=0.011; 1970–1979 vs 1990–2001, P=0.03; 1980–1989 vs 1990–2001, P=0.81 B, Cumulative incidence of heart failure grade 5, fatal events, per treatment period with time since childhood cancer diagnosis. P‐value for Gray test: 1970–1979 vs 1980–1989, P=0.99; 1970–1979 vs 1990–2001, P=0.04; 1980–1989 vs 1990–2001, P=0.02. All childhood cancer survivors diagnosed between 1970 and 2001 were included in this figure.
Risk Factor Analyses
Table 3 presents the results of the multivariable model for the analysis of risk factors for heart failure (grade 3, 4, and 5). We found that younger age at childhood cancer diagnosis (per‐year hazard ratio [HR]=0.8, 95% CI 0.8–0.9), more recent year of childhood cancer diagnosis (HR=1.0, 95% CI 1.0–1.1), anthracyclines (per 1 mg/m2), mitoxantrone (per 1 mg/m²), cyclophosphamide (per 100 mg/m²) (the dose‐response curves and the actual HRs for anthracyclines, mitoxantrone, and cyclophosphamide are presented in Figure 4), and radiotherapy involving the heart (HR=2.0, 95% CI 1.1–3.6; HR=2.1, 95% CI 1.1–4.0) were significantly associated with heart failure risk. There was no influence of sex on the risk of developing heart failure. We did not find any statistically significant interaction between radiotherapy to the chest and cardiotoxic chemotherapy or among the different chemotherapy treatments.
Table 3.
Multivariable Cox Proportional Hazard Regression Model for the Analysis of Potential Determinants for Heart Failure (Grades 3, 4, 5): Age at Diagnosis, Sex, Period of Treatment, and Cancer Treatment
| Covariates* | REF (n)/Total (n) | Hazard Ratio, Median (IQR) | P Value | REF (n)/Events (n) |
|---|---|---|---|---|
| Age at primary childhood diagnosis (per y) | 0.8 (0.8–0.9) | <0.001 | ||
| Sex (REF=male) | 3257/5845 | 0.9 (0.6–1.3) | 0.64 | |
| Year of childhood cancer diagnosis (per y) | 1.0 (1.01–1.1) | 0.04 | ||
| Anthracycline (per 1 mg/m2, splines) | See Figure 4 | <0.001 | ||
| Mitoxantrone (per 1 mg/m2, splines) | See Figure 4 | <0.001 | ||
| Cyclophosphamide (per 100 mg/m2, splines) | See Figure 4 | 0.04 | ||
| Chest radiotherapy | ||||
| No chest radiotherapy | 4575/5845 | REF | 78/116 | |
| Radiotherapy potentially involving the heart | 588/5845 | 1.0 (0.4–2.0) | 0.96 | 9/116 |
| Radiotherapy involving the heart <20 Gy | 275/5845 | 2.0 (1.1–3.6) | 0.02 | 15/116 |
| Radiotherapy involving the heart ≥20 Gy | 363/5845 | 2.1 (1.1–4.0) | 0.02 | 14/116 |
| Cisplatin (per 1 mg/m2) | 1.0 (1.0–1.0) | 0.61 | ||
| Ifosfamide (per 1 mg/m2) | 1.0 (1.0–1.0) | 0.28 | ||
| Vincristine (per 1 mg/m2) | 1.0 (1.0–1.0) | 0.20 | ||
REF indicates reference category.
We did not find a significant interaction term between anthracycline and radiotherapy involving the heart.
The bold values indicate the significant risk factors.
Figure 4.
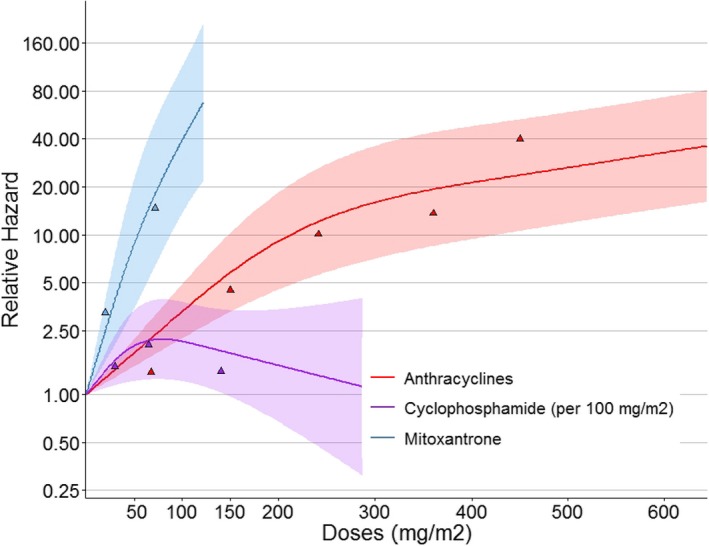
Dose‐response curves of the development of heart failure with anthracyclines, mitoxantrone, and cyclophosphamide. The colored triangles are the hazard ratios (HRs) from the model presented on a logarithmic scale to show the actual HRs. The model is also corrected for sex, age at diagnosis, year of childhood cancer diagnosis, and radiotherapy where the heart was in the field yes/no. Shaded areas indicate 95% CI.
Discussion
CCS are at a high risk of developing heart failure in young adulthood after cardiotoxic treatment. Important findings of this study are the increase in cumulative incidence of severe or life‐threatening heart failure in more recent treatment periods and the association of mitoxantrone and cyclophosphamide with symptomatic heart failure.
The current cohort study demonstrates that CCS treated with cardiotoxic treatment have a high risk of developing heart failure even at a relatively young age.
Previous research showed a reduction in (cardiac) mortality among CCS in recent periods of treatment, and the authors attributed this to a reduction in anthracycline dose in treatment regimens.8, 9 In line with this finding, our study identified a decreased risk of mortality due to heart failure in more recent treatment eras.
In our study we also identified an increased risk of heart failure with a broader definition (severe, life threatening, and death due to heart failure) and a decreased risk of heart failure (fatal) for CCS treated in more recent treatment years compared with survivors treated earlier. We identified this higher risk in the comparisons of the cumulative risk estimates as well as in the Cox proportional hazards model analysis. The cumulative incidence of heart failure remained low with anthracyclines at doses below 100 mg/m2, or at least below 250 mg/m2. However, most importantly, our results showed no safe dose for anthracyclines. This finding and the statistically significant association and dose‐response relationship of mitoxantrone with symptomatic heart failure underscore the need for primary prevention (such as avoiding cardiotoxic treatment), the use of lower doses of cardiotoxic treatments in children with cancer, and considering alternatives to mitoxantrone in new treatment protocols for children with cancer.
Several explanations for the increased risk of heart failure in more recent treatment periods can be considered. A first explanation could be the more frequent use of cardiotoxic treatment. The number of CCS treated with anthracyclines and mitoxantrone increased over the decades (Table 2).
Another possible explanation for the increased risk of heart failure among CCS treated more recently could be that CCS with heart failure in recent eras were diagnosed more precisely. Specialized outpatient late‐effects clinics were first established during the decade from 1990 to 2000, and among physicians, awareness of anthracycline‐induced heart failure increased. A third reason for the increased risk of heart failure over time could be a decrease in cardiac mortality. Individuals with cardiac dysfunction are expected to be referred more often to the cardiologist and are perhaps treated in an earlier phase. Similarly, treatment of heart failure has improved considerably over the past few decades.27 With the introduction of angiotensin‐converting enzyme inhibitors by the end of the 1980s28 into heart failure treatment and the addition of β‐blockers by the end of the 1990s,29 the mortality due to heart failure significantly decreased in the overall population of patients with heart failure.30
Despite the small number of CCS treated with mitoxantrone in our study, our results showed a statistically significant association of mitoxantrone with symptomatic heart failure in our cohort of CCS with a dose‐response relationship. We showed this both in the comparisons of the cumulative risk estimates—by time since treatment—as well as in the Cox proportional hazards model analysis (with attained age on the time scale and adjusted for follow‐up time). Our findings are in line with previous studies that have described a mitoxantrone association with cardiac dysfunction and symptomatic heart failure in CCS.19, 31 It has been suggested that mitoxantrone has different cardiotoxic mechanisms from the anthracyclines.32, 33 In the current study we showed that timing of presentation for mitoxantrone‐associated heart failure seems different from that of anthracycline‐associated heart failure, and that there are differences with respect to dose‐response relationship. CCS treated with mitoxantrone have a high risk for heart failure, and targeted follow‐up is needed.34 Further data on (childhood cancer) patients treated with mitoxantrone need to be replicated in studies with larger study populations. We found a significant association between cyclophosphamide and heart failure. Acute cardiac damage from cyclophosphamide has been suggested by other studies,35, 36 but, to our knowledge, late cardiac damage has not been previously reported. Further and more extensive research into the role of cyclophosphamide in the development of heart failure is needed.
The findings of this study need to be considered subject to the limitation of not having information on the absorbed radiation dose to the heart. However, based on the current results and those reported previously,11 the association between radiotherapy involving the heart and heart failure is less strong than the association between chemotherapy and heart failure.
The strengths of the current study include the near complete follow‐up (94.8%, 84.7% until 2013) of our entire nationwide cohort of CCS, the nearly complete collection of treatment data, and the validation of all cases of heart failure by extracting information from the medical charts or from the treating physicians using an extraction‐flowchart method.24 These strengths will increase the validity of the study.
Our study findings can inform new treatment protocols for children with cancer. In addition, other treatment options—if available—should be considered in current childhood cancer treatment protocols, and cardiotoxic doses should be limited because heart failure also develops after low doses of anthracyclines and/or mitoxantrone.
It is also important to realize that the risk of heart failure is high even at a young attained age, and therefore, CCS at risk of heart failure might benefit from early intervention. Previous literature suggests that early treatment can lead to better survival in comparable study populations.37 Thus, our results also warrant the need for appropriate cardiac surveillance of CCS and can therefore inform the current recommendations34 for cardiomyopathy surveillance by suggesting the need to provide separate recommendations for survivors treated with mitoxantrone and anthracyclines.
In addition, future studies are needed to evaluate risk factors models for heart failure that include variables that change value over time, such as smoking history, current BMI, and presence of other heart diseases.
In conclusion, CCS are at high risk of developing severe life‐threatening or fatal heart failure even 40 years after their diagnosis at a relatively young age, and CCS treated with anthracyclines and mitoxantrone are most at risk. Although mortality due to heart failure decreases in more recent treatment periods, the incidence of severe or life‐threatening heart failure increases. Primary prevention to diminish the risk of heart failure for CCS is needed.38
Sources of Funding
This work was supported by the European Union's Seventh Framework Programme for research, technological development, and demonstration (Grant Agreement No. 257505; PanCareSurFup). Cecile Ronckers is supported by grant funding from the Dutch Cancer Society.
Disclosures
None.
Supporting information
Data S1. Supplemental Methods
Data S2. Supplemental Results
Table S1. Cumulative Incidence of Heart Failure (≥Grade 3) Over Time Since Diagnosis (Follow‐Up) in Childhood Cancer Survivors According to the Nonparametric Estimator of Cause‐Specific Cumulative Incidence, With Death From Any Cause as Competing Risk
Table S2. Cumulative Incidence of Heart Failure (≥Grade 3) at Attained Age in Childhood Cancer Survivors According to the Nonparametric Estimator of the Cause‐Specific Cumulative Incidence, With Death From Any Other Cause as Competing Risk
Figure S1. Flowchart of the cardiac data collection.
Figure S2. Flowchart heart failure.1
Figure S3. Cumulative incidence of heart failure at time since childhood cancer diagnosis for 4 mutually exclusive treatment groups.
Figure S4. Cumulative incidence of heart failure at attained age for cardiotoxic treatment and no cardiotoxic treatment.
Acknowledgments
We thank Lideke van der Steeg, Andrica de Vries, Gea Huizinga, Margreet Veening, Marloes Louwerens, and Lilian Batenburg for their contributions to this study. We are also thankful to all the data managers in the 7 participating centers, especially Ingeborg Lange and Aslihan Mantici for obtaining the data for this study.
The DCOG‐LATER Study Group also includes the following collaborators:
W. Dolsma, University of Groningen/University Medical Center Groningen, The Netherlands; M.A. Grootenhuis. Princess Maxima Center for Pediatric Oncology, Utrecht, The Netherlands; J.G. den Hartogh, Dutch Childhood Cancer Parent Organization (VOKK), Nieuwegein, The Netherlands; M.W.M. Jaspers, Academic Medical Center, Amsterdam, The Netherlands; A. Postma, Dutch Childhood Oncology Group, The Hague, The Netherlands; N. Hollema, Dutch Childhood Oncology Group, The Hague, The Netherlands; J.L. Kok, Emma Children's Hospital/Academic Medical Center, Amsterdam, The Netherlands; J.C. Teepen, Emma Children's Hospital/Academic Medical Center, Amsterdam, The Netherlands; J.G. de Ridder, Dutch Childhood Oncology Group, The Hague, The Netherlands; H.N. Caron, Emma Children's Hospital/Academic Medical Center, Amsterdam, The Netherlands; P. van der Meer, University of Groningen/University Medical Center Groningen, The Netherlands.
(J Am Heart Assoc. 2019;8:e009122 DOI: 10.1161/JAHA.118.009122)
Contributor Information
E. A. M. (Lieke) Feijen, Email: e.a.feijen@amc.uva.nl.
the DCOG‐LATER Study Group:
W. Dolsma, M.A. Grootenhuis, J.G. den Hartogh, M.W.M. Jaspers, A. Postma, N. Hollema, J.L. Kok, J.C. Teepen, J.G. de Ridder, H.N. Caron, and P. van der Meer
References
- 1. Curry HL, Parkes SE, Powell JE, Mann JR. Caring for survivors of childhood cancers: the size of the problem. Eur J Cancer. 2006;42:501–508. [DOI] [PubMed] [Google Scholar]
- 2. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life‐long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina NM, Hobbie W, Kadan‐Lottick NS, Schwartz C, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. [DOI] [PubMed] [Google Scholar]
- 4. Geenen MM, Cardous‐Ubbink MC, Kremer LC, van der Bos C, van der Pal HJ, Heinen RC, Jaspers MWM, Koning CC, Oldenburger F, Langeveld NE, Hart AAM, Bakker PJ, Caron HN, van Leeuwen FE. Medical assessment of adverse health outcomes in long‐term survivors of childhood cancer. JAMA. 2007;297:2705–2715. [DOI] [PubMed] [Google Scholar]
- 5. Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, Sklar CA, Srinivasan SR, Robison LL. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garwicz S, Anderson H, Olsen JH, Winther JF, Sankila R, Langmark F, Tryggvadottir L, Moller TR; Association of the Nordic Cancer Registries; Nordic Society for Pediatric Hematology Oncology . Late and very late mortality in 5‐year survivors of childhood cancer: changing pattern over four decades experience from the Nordic countries. Int J Cancer. 2012;131:1659–1666. [DOI] [PubMed] [Google Scholar]
- 7. Gudmundsdottir T, J FW, de Fine Licht S, T GB, P HA, Tryggvadottir L, Anderson H, Wesenberg F, Malila N, Hasle H; Olsen JH; ALiCCS Study Group . Cardiovascular disease in adult life after childhood cancer in Scandinavia: a population‐based cohort study of 32,308 one‐year survivors. Int J Cancer. 2015;137:1176–1186. [DOI] [PubMed] [Google Scholar]
- 8. Fidler MM, Reulen RC, Henson K, Kelly J, Cutter DJ, Levitt GA, Frobisher C, Winter DL, Hawkins MM. Population‐based long‐term cardiac‐specific mortality among 34,489 five‐year survivors of childhood cancer in Great Britain. Circulation. 2017;135:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, Stovall M, Oeffinger KC, Bhatia S, Krull KR, Nathan PC, Neglia JP, Green DM, Hudson MM, Robison LL. Reduction in late mortality among 5‐year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, Leisenring WM. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Pal HJ, van Dalen EC, van Delden E, van Dijk IW, Kok WE, Geskus RB, Sieswerda E, Oldenburger F, Koning CC, van Leeuwen FE, Caron HN, Kremer LC. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. [DOI] [PubMed] [Google Scholar]
- 12. van der Pal HJH, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van der Bos C, Oldenburger F, Koning CC, van Leeuwen FE, Kremer LC. Cardiac function in 5‐year survivors of childhood cancer. Arch Intern Med. 2010;170:1247–1255. [DOI] [PubMed] [Google Scholar]
- 13. Adams M, Lipshultz S, Schwartz C, Fajardo L, Coen V, Constine L. Radiation‐associated cardiovascular disease: manifestations and management. Semin Radiat Oncol. 2003;13:346–356. [DOI] [PubMed] [Google Scholar]
- 14. Green DM, Grigoriev YA, Takashima JR, Norkool PA, D'Angio GJ, Breslow NE. Congestive heart failure after treatment for Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol. 2001;19:1926–1934. [DOI] [PubMed] [Google Scholar]
- 15. Haddy N, Diallo S, El‐Fayech C, Schwartz B, Pein F, Hawkins M, Veres C, Oberlin O, Guibout C, Pacquement H, Munzer M, N'Guyen TD, Bondiau PY, Berchery D, Laprie A, Scarabin PY, Jouven X, Bridier A, Koscielny S, Deutsch E, Diallo I, de Vathaire F. Cardiac diseases following childhood cancer treatment: cohort study. Circulation. 2016;133:31–38. [DOI] [PubMed] [Google Scholar]
- 16. Hudson MM, Rai SN, Nunez C, Merchant TE, Marina NM, Zalamea N, Cox C, Phipps S, Pompeu R, Rosenthal D. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25:3635–3643. [DOI] [PubMed] [Google Scholar]
- 17. Lipshultz S, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, Orav EJ, Gelber RD, Colan SD. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. [DOI] [PubMed] [Google Scholar]
- 18. Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. [DOI] [PubMed] [Google Scholar]
- 19. van Dalen EC, van der Pal HJ, Bakker PJ, Caron HN, Kremer LC. Cumulative incidence and risk factors of mitoxantrone‐induced cardiotoxicity in children: a systematic review. Eur J Cancer. 2004;40:643–652. [DOI] [PubMed] [Google Scholar]
- 20. Steliarova‐Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103:1457–1467. [DOI] [PubMed] [Google Scholar]
- 21. Feijen EA, Leisenring WM, Stratton KL, Ness KK, van der Pal HJ, Caron HN, Armstrong GT, Green DM, Hudson MM, Oeffinger KC, Robison LL, Stovall M, Kremer LC, Chow EJ. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol. 2015;33:3774–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Children's Oncology Group . Long term follow‐up guidelines for survivors of childhood, adolescent and young adult cancers. Version 4.0. Monrovia, CA: Children's Oncology Group; 2013. [Google Scholar]
- 23. NCI . Common Terminology Criteria for Adverse Events (CTCAE). Washington, DC: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 24. Feijen EAM, van der Pal HJ, van Dalen EC, Mulder RL, Bardi E, Kuehni C, Tissing WJ, Kremer LC. A new method to facilitate valid and consistent grading cardiac events in childhood cancer survivors using medical records. PLoS One. 2014;9:e100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geskus RB. Data Analysis with Competing Risks and Intermediate States. Amsterdam: CRC Press, Taylor & Francis Group; 2016. [Google Scholar]
- 26. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 27. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Group Cts . Effects of enelapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316:1429–1435. [DOI] [PubMed] [Google Scholar]
- 29. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of cardvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 30. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KKL, Murabito JM, Vasan RS. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 31. Behar C, Such S, Benoit Y, Robert A, Vilmer E, Boutard P, Bertrand P, Lutz P, Ferster A, Tokaji E, Manel A, Solbu G, Otten J. Mitoxantrone‐containing regimen for treatment of childhood acute leukemia (AML) and analysis of prognostic factors: results of the EORTC Children Leukemia Cooperative Study 58872. Med Pediatr Oncol. 1996;26:173–179. [DOI] [PubMed] [Google Scholar]
- 32. Novak RF, Kharasch ED. Mitoxantrone: propensity for free radical formation and lipid peroxidation–implications for cardiotoxicity. Investig New Drugs. 1985;3:95–99. [DOI] [PubMed] [Google Scholar]
- 33. Rossato LG, Costa VM, Dallegrave E, Arbo M, Silva R, Ferreira R, Amado F, Dinis‐Oliveira RJ, Duarte JA, de Lourdes Bastos M, Palmeira C, Remiao F. Mitochondrial cumulative damage induced by mitoxantrone: late onset cardiac energetic impairment. Cardiovasc Toxicol. 2014;14:30–40. [DOI] [PubMed] [Google Scholar]
- 34. Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, Nathan PC, Tissing WJE, Shankar S, Sieswerda E, Skinner R, Steinberger J, van Dalen EC, van der Pal H, Wallace WH, Levitt G, Kremer LCM. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goldberg MA, Antin JH, Guinan EC, Rappeport JM. Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood. 1986;68:1114–1118. [PubMed] [Google Scholar]
- 36. Gottdiener JS, Appelbaum FR, Ferrans VJ, Deisseroth A, Ziegler J. Cardiotoxicity associated with high dose cyclophosphamide therapy. Arch Intern Med. 1981;141:758–763. [PubMed] [Google Scholar]
- 37. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 38. van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011;6:CD003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods
Data S2. Supplemental Results
Table S1. Cumulative Incidence of Heart Failure (≥Grade 3) Over Time Since Diagnosis (Follow‐Up) in Childhood Cancer Survivors According to the Nonparametric Estimator of Cause‐Specific Cumulative Incidence, With Death From Any Cause as Competing Risk
Table S2. Cumulative Incidence of Heart Failure (≥Grade 3) at Attained Age in Childhood Cancer Survivors According to the Nonparametric Estimator of the Cause‐Specific Cumulative Incidence, With Death From Any Other Cause as Competing Risk
Figure S1. Flowchart of the cardiac data collection.
Figure S2. Flowchart heart failure.1
Figure S3. Cumulative incidence of heart failure at time since childhood cancer diagnosis for 4 mutually exclusive treatment groups.
Figure S4. Cumulative incidence of heart failure at attained age for cardiotoxic treatment and no cardiotoxic treatment.


