Abstract
Background
Cardiac magnetic resonance imaging (CMR) provides useful information for characterizing cardiac masses, but there are limited data on whether CMR can accurately distinguish benign from malignant lesions. We aimed to describe the distribution and imaging characteristics of cardiac masses identified by CMR and to determine the diagnostic accuracy of CMR for distinguishing benign from malignant tumors.
Methods and Results
We examined consecutive patients referred for CMR between May 2008 and August 2013 to identify those with a cardiac mass. In patients for whom there was histological correlation, 2 investigators blinded to all data analyzed the CMR images to categorize the mass as benign or malignant. For benign masses, readers were also asked to specify the most likely diagnosis. Benign masses were defined as benign neoplastic or non‐neoplastic. Malignant masses were defined as primary cardiac or metastatic. Of 8069 patients (mean age: 58±16 years; 55% female) undergoing CMR, 145 (1.8%) had a cardiac mass. In most cases (142, 98%), there was a known cardiac mass before the CMR study. Among 145 patients with a cardiac mass, 93 (64%) had a known history of malignancy. Among 53 cases that had histological correlation, 25 (47%) were benign, 26 (49%) were metastatic, and 2 (4%) were malignant primary cardiac masses. Blinded readers correctly diagnosed 89% to 94% of the cases as benign versus malignant, with a 95% agreement rate (κ=0.83).
Conclusions
Although CMR can be highly effective in distinguishing benign from malignant lesions, pathology remains the gold standard in accurately determining the type of mass.
Keywords: benign, magnetic resonance imaging, malignant, outcome, tumor
Subject Categories: Magnetic Resonance Imaging (MRI), Diagnostic Testing
Clinical Perspective
What Is New?
This study represents the largest reported experience evaluating cardiac masses in adults using cardiac magnetic resonance imaging and is the largest to examine the utility of contemporary cardiac magnetic resonance imaging techniques to distinguish benign versus malignant lesions.
We found that cardiac magnetic resonance imaging has high accuracy for discriminating between benign and malignant lesions, with a high rate of interobserver agreement.
What Are the Clinical Implications?
Although a comprehensive cardiac magnetic resonance imaging protocol had high accuracy for identifying benign lesions, the ability to correctly diagnosis malignant cases in our study was more limited.
Pathology remains the gold standard in accurately determining the type of mass.
Introduction
Cardiac tumors are rare but can be associated with high morbidity and mortality. In a large series of 12 485 autopsies, the incidence of primary and secondary cardiac tumors was 0.056% and 1.23%, respectively.1 The goal of imaging cardiac tumors is to confirm the presence of the tumor and its location and to ascertain whether the tumor is benign or malignant. Among noninvasive imaging modalities, transthoracic echocardiography (TTE), transesophageal echocardiography, computed tomography, positron emission tomography, and cardiac magnetic resonance imaging (CMR) are complementary tools to guide the prognosis, management, and surveillance of cardiac tumors.2, 3, 4, 5
Although each imaging modality has inherent strengths and limitations for evaluating cardiac tumors, CMR is often used for its robust tissue characterization with high‐contrast resolution.6, 7, 8, 9 However, few studies have examined the ability of CMR to distinguish malignant versus benign masses with comparison to histology3, 7, 10, 11, 12 and with only limited outcome data.13, 14 Therefore, additional studies are needed to examine the accuracy of CMR to diagnose cardiac masses compared with histology and with association of these findings to patient outcomes.
The primary aim of this study was to describe the distribution and imaging characteristics of cardiac masses identified by CMR and, in cases with histological diagnoses, to determine the diagnostic accuracy of CMR for distinguishing between benign and malignant tumors.
Methods
Patient Population and Data Collection
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Requirement for informed consent was waived by the institutional review board for this retrospective study. We performed a retrospective study of patients found to have a cardiac mass on CMR between May 2008 and August 2013 at Brigham and Women's Hospital (Boston, MA). A search was carried out in our CMR database using the term mass and/or tumor to identify all patients with possible cardiac tumors.
The search identified 639 patients with the term mass or tumor in their CMR reports. The reports of all 639 patients were reviewed, and the following patients were then excluded: (1) patients with no cardiac tumor identified on CMR and (2) patients with a mediastinal mass but no evidence of pericardial or myocardial involvement. Ultimately, 145 patients with a cardiac mass were included in the final cohort (Figure 1).
Figure 1.
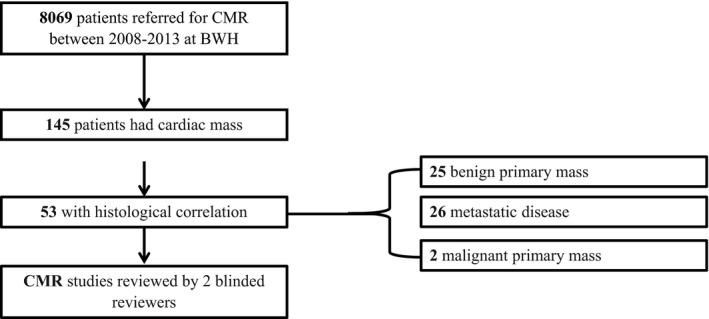
Study flowchart. BWH indicates Brigham and Women's Hospital; CMR, cardiac magnetic resonance imaging.
Each patient's electronic medical records were reviewed to ascertain demographic and clinical data, including the initial presenting signs and symptoms, mode of initial diagnosis, other imaging findings (ie, TTE, computed tomography, positron emission tomography, if available), source of tissue if a histological diagnosis was available, treatment, and clinical status at latest follow‐up.
Study data were collected and managed using REDCap (Research Electronic Data Capture), a secure web‐based application hosted at Brigham and Women's Hospital designed to support data capture for research studies.15 The study was approved by the Partners Healthcare institutional review board and was conducted in accordance with institutional guidelines.
CMR Image Acquisition
All patients were studied while in the supine position either in a 1.5‐T scanner (Signa CV/i; GE Healthcare) with an 8‐ or 12‐element cardiac phased‐array coil or in a 3.0‐T system (Magnetom TIM TRIO; Siemens) with a 16‐element phased‐array coil. Intravenous gadolinium chelate was administered in a dose appropriate for the patient's weight (0.1–0.2 mmol/kg). In all cases, a standard cardiac mass protocol was used for the assessment of cardiac masses, including morphological, functional, and tissue characterization (Appendix S1).
Cine‐CMR was performed for anatomical and functional assessment using a steady‐state free‐precession sequence. The following sequences were also obtained: pre‐ and postcontrast T1‐weighted (T1W) double inversion recovery fast spin echo imaging with and without fat saturation through the mass, T2‐weighted (T2W) dual inversion recovery fast spin echo imaging with fat suppression, and first‐pass perfusion imaging (FPP) of the mass. Conventional left ventricular viability imaging using late gadolinium enhancement (LGE) was also performed. In addition, early and LGE imaging through the mass with long T1W imaging (600 ms) was obtained when a thrombus was suspected. Images were obtained in conventional and complementary cardiac planes or planes that best demonstrated each cardiac mass when necessary, and a physician experienced in cardiac imaging was present for the acquisition of all exams.
CMR Data Collection
The following magnetic resonance imaging tumor characteristics were recorded: (1) size, (2) location, (3) presence of pericardial effusion, (4) signal characteristics on pre‐ and postcontrast T1W and precontrast T2W images, (5) FPP imaging, and (6) delayed enhancement imaging (LGE). Signal intensity on T1W and T2W fast spin echo images were assessed as isointense/hypointense or hyperintense in reference to normal myocardium. The presence of fat was defined when there was increased signal on T1W images that was suppressed on fat‐suppression sequences. For each case, the most likely etiology of the mass was recorded based on the actual clinical interpretation, which was based on combining all CMR findings together with all available clinical information. Although no set of guidelines exists for definitive diagnosis of a malignant cardiac mass based on CMR, as previously published in the literature, the characteristics of a malignant cardiac mass used for differentiating a malignant from benign mass included history of extracardiac malignancy, infiltrative appearance, variable intensity on T1W and T2W imaging, and enhancement on LGE and perfusion imaging.
Blinded CMR Interpretation
To determine the accuracy of CMR in differentiating benign from malignant masses, we performed a dedicated blinded reanalysis of all patients who had a direct cardiac histological diagnosis. The CMR images of these 53 patients were reviewed by 2 experienced imagers with level 3 expertise who were blinded to all clinical history, prior test results, and the final histological diagnosis. Each reviewer was also blinded to the interpretation of the other reviewer. Each reviewer categorized his or her interpretation based on a predetermined case report form, which included the following items for assessment: (1) whether the reviewer thought the mass was benign or malignant; (2) if benign, the suspected diagnosis (eg, thrombus); and (3) the level of confidence for each diagnosis, rated using a 4‐point Likert scale (1=no confidence, 4=high confidence). The findings of both reviewers were then compared with the final histological diagnosis.
Histological Correlation
In 53 cases where there was a confirmed diagnosis from the cardiac mass sample, to validate the final diagnosis, a cardiac pathologist blinded to the prior pathology report reanalyzed all tissue samples to validate the final diagnosis.
Outcome Assessment
All‐cause death was ascertained through the Social Security Death Index and electronic medical records using the Partners Research Patient Data Registry. This centralized clinical data registry contains data from all institutions in the Partners HealthCare system.
Death analysis was stratified by (1) histology diagnosis in all patients with direct histology of the cardiac mass (n=53) and (2) a final clinical diagnosis of the mass type as benign or malignant, incorporating all available clinical data including a history of malignancy, extracardiac histology findings (available in 42 patients), CMR, and other image findings.
Statistical Analysis
Continuous data with normal distributions are presented as mean±SD and compared using the Student t test for 2 independent groups and 1‐way ANOVA for multiple groups. Continuous variables with non‐normal distributions are expressed as median with interquartile range [IQR; 25th to 75th percentile] and compared with the Wilcoxon rank sum test. Categorical variables are expressed as frequencies (percentages) and compared using the Pearson χ2 test or Fisher exact test for small group comparisons (observed frequency n<5 in any subgroup). Interobserver agreement between readers for distinguishing a benign from malignant tumor was analyzed using a linear‐weighted Cohen κ. To describe event‐free survival of malignant versus benign cardiac masses, we constructed Kaplan–Meier curves with survival comparison by log‐rank analysis. Cox proportional hazard ratios were estimated for all‐cause mortality. All analyses were performed using SAS (SAS Institute) and Stata (v13.1; StataCorp). A 2‐tailed P<0.05 was considered significant.
Results
Study Population and Clinical Presentation
Among 8069 patients referred for CMR during the study period, 145 (1.8%) met inclusion criteria and composed the study cohort. The baseline characteristics of the entire study population and the subset of patients with cardiac histology who had a benign (n=25) or malignant (n=28) cardiac mass are summarized in Table 1. The mean age of patients at the time of CMR was 58±16 years, and 55% were female. The majority of patients were asymptomatic (n=75, 52%) and had a known cardiac mass before CMR (n=142, 98%).
Table 1.
Baseline Characteristics Stratified by Histological Diagnosis
| All Patients (N=145) | Benign (n=25)a | Malignant (n=28)a | P Valuea | |
|---|---|---|---|---|
| Age at time of CMR, y, mean±SD | 58±16 | 56±19 | 54±15 | 0.75 |
| Male, n (%) | 65 (45) | 12 (48) | 17 (61) | 0.86 |
| Presenting symptoms, n (%)b | ||||
| Asymptomatic | 75 (52) | 10 (40) | 13 (46) | 0.64 |
| Chest pain | 26 (18) | 4 (16) | 8 (28) | 0.34 |
| Dyspnea/HF | 23 (16) | 5 (20) | 6 (21) | 0.90 |
| Palpitations/arrhythmia | 10 (7) | 2 (8) | 1 (4) | 0.60 |
| TIA/CVA | 7 (5) | 5 (20) | 1 (4) | 0.09 |
| Syncope/presyncope | 6 (4) | 1 (4) | 2 (7) | 0.99 |
| Unknown | 5 (4) | 0 (0) | 0 (0) | ··· |
| History of known malignancy before CMR, n (%) | 93 (64) | 11 (44) | 22 (79) | 0.01 |
CMR indicates cardiac magnetic resonance imaging; HF, heart failure; TIA/CVA, transient ischemic stroke/cerebrovascular accident.
Out of 53 patients with a known cardiac histological diagnosis.
Some patients had >1 symptom.
The initial imaging modality that identified the cardiac mass was chest computed tomography (n=60, 41%), TTE (n=60, 41%), whole‐body positron emission tomography (n=8, 6%), non‐CMR (n=5, 3%), abdominal computed tomography (n=2, 1%), transesophageal echocardiography (n=1, 0.7%), and chest roentgenogram (n=1, 0.7%). In 5 (3%) patients, the initial mode of diagnosis was unknown. For 3 (2%) patients, CMR was the initial mode of diagnosis for the cardiac mass, and in all 3 cases, the mass was benign.
The median time interval from the initial diagnosis of a cardiac mass to the CMR test was 774 days (IQR: 210–2252). Among 145 patients included in the study, 93 (64%) had a history of malignancy, with the most common malignancies being lymphoma (n=15, 10%), lung cancer (n=13, 9%), melanoma (n=10, 7%), and breast cancer (n=7, 5%). Pathological diagnosis was available for 95 (66%) patients, for whom 53 (58%) pathologies were taken directly from the cardiac mass. Of these 53 patients with direct pathological diagnosis, 28 had malignant and 25 had benign masses.
CMR Characteristics
Table 2 summarizes the CMR findings for all patients, stratified by benign versus malignant masses for cases with a histological diagnosis. Masses were identified in all cardiac chambers and the pericardium (Figure 2). The most common locations were intracavitary masses (n=56, 39%) with the right atrium being the most common chamber (n=31, 21%), followed by extrapericardial masses with cardiac extension (n=29, 20%). The median diameter of all masses was 3.1 cm (IQR: 1.9–5.2).
Table 2.
MRI Characteristics Stratified by Histological Diagnosis
| All Patients (N=145) | Benign (n=25)a | Malignant (n=28)a | P Valuea | |
|---|---|---|---|---|
| Mass diameter, cm, median (IQR) | 3.1 (1.9–5.2) | 3.2 (1.6–4.6) | 4.8 (3.3–6.5) | 0.01 |
| T1W characteristics, n (%) | ||||
| Hypointense | 30 (21) | 7 (28) | 2 (7) | 0.08 |
| Hyperintense | 22 (15) | 3 (12) | 1 (4) | |
| Isointense | 69 (48) | 13 (52) | 17 (61) | |
| Variable intensity | 15 (10) | 1 (4) | 6 (21) | |
| Indeterminate | 9 (6) | 1 (4) | 2 (7) | |
| T2W characteristics | ||||
| Hypointense | 25 (17) | 7 (28) | 3 (11) | 0.02 |
| Hyperintense | 77 (53) | 15 (60) | 10 (36) | |
| Isointense | 23 (16) | 2 (8) | 9 (32) | |
| Variable intensity | 12 (8) | 0 (0) | 4 (14) | |
| Indeterminate | 8 (6) | 1 (4) | 2 (7) | |
| LGE | ||||
| Yes | 82 (57) | 13 (54) | 22 (79) | 0.11 |
| No | 49 (34) | 8 (33) | 3 (11) | |
| Indeterminate | 13 (9) | 3 (13) | 3 (11) | |
| Not performed | 1 (1) | 1 (4) | 0 (0) | |
| Fat suppression | ||||
| Yes | 14 (10) | 2 (8) | 0 (0) | 0.41 |
| No | 115 (80) | 18 (72) | 23 (82) | |
| Indeterminate | 15 (10) | 5 (20) | 4 (14) | |
| Sequence not performed | 1 (1) | 0 (0) | 1 (4) | |
| FPP | ||||
| Yes | 69 (48) | 8 (32) | 20 (71) | 0.001 |
| No | 63 (43) | 15 (60) | 4 (14) | |
| Indeterminate | 13 (9) | 2 (8) | 4 (14) | |
| Associated pericardial effusion | ||||
| Yes | 36 (25) | 4 (16) | 10 (36) | 0.22 |
| Small (<1 cm) | 26 (72) | 2 (50) | 7 (70) | |
| Medium (1–2 cm) | 7 (19) | 0 (0) | 3 (30) | |
| Large (>2 cm) | 3 (8) | 2 (50) | 0 (0) | |
| No | 103 (71) | 20 (80) | 16 (57) | |
| Indeterminate | 6 (4) | 1 (4) | 2 (7) | |
Values are n (%) except as noted. P values reflect categorical comparisons for MRI characteristics with multiple subgroups. FPP indicates first‐pass perfusion; IQR, interquartile range; LGE, late gadolinium enhancement; MRI, magnetic resonance imaging; T1W, T1‐weighted; T2W, T2‐weighted.
Out of 53 patients with a known cardiac histological diagnosis.
Figure 2.
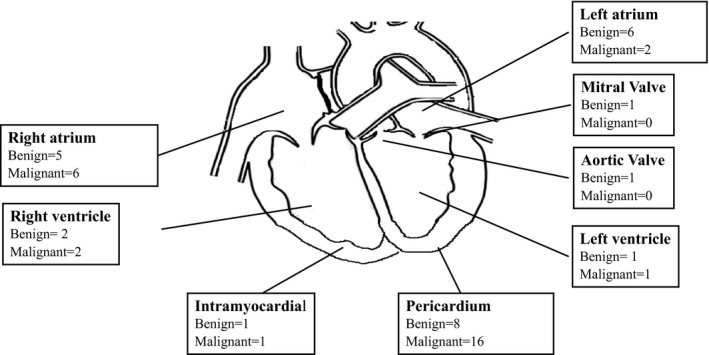
Location of cardiac masses in cases for which histological diagnosis available.
Benign Versus Malignant Mass
When examining the 53 patients with a histological diagnosis, benign masses were smaller than malignant masses (3.2 cm [IQR: 1.6–4.6] versus 4.8 cm [IQR: 3.3–6.5], P=0.01). Masses in the left heart were more commonly benign, whereas masses in the right heart and pericardium were more often malignant (Figure 2). Both benign and malignant masses were commonly isointense on T1W imaging, with no significant difference in T1W characteristics between groups (P=0.09). T2W characteristics demonstrated a significant difference between groups (P=0.02), as benign masses were more often hyperintense (n=15/25, 60%) and malignant masses demonstrated both hyperintense (10/28, 36%) or isointense (9/28, 32%) T2W signals. FPP was more frequent in malignant than benign tumors (71% versus 32%, P=0.002), whereas there was no difference between groups in fat signal (P=0.31).
The most likely diagnoses for all cardiac masses are listed in Table 3, incorporating clinical history, results of prior testing and CMR findings, and histology in the subset of patients with direct biopsies. Among all patients, the most common cause of cardiac mass was metastatic (n=62, 44%), followed by benign primary cardiac masses (n=54, 37%). Histological diagnosis based on cardiac tissue was available for 53 (37%) patients. The most likely diagnosis according to pathology was metastatic disease from a primary noncardiac malignancy (n=26, 49%), followed by a benign primary cardiac mass (n=17, 32%; Table 3).
Table 3.
Final Clinical Diagnosis Following CMR and Correlation With Histological Diagnosis
| Based on Clinical Diagnosis | Based on Pathology | ||
|---|---|---|---|
| All Patients (N=145) | Benign (n=25)a | Malignant (n=28)a | |
| Benign | |||
| Benign primary cardiac | 54 (37) | 17 (68) | |
| Thrombus | 15 (28) | 2 (8) | |
| Lipomatous hypertrophy of IAS | 12 (22) | 0 (0) | |
| Myxoma | 11 (20) | 11 (44) | |
| Papillary fibroelastoma | 4 (7) | 2 (8) | |
| Lipoma | 2 (4) | 0 (0) | |
| Vegetation | 2 (4) | 1 (4) | |
| Intracardiac cyst | 1 (2) | 0 (0) | |
| Infection/abscess | 1 (2) | 0 (0) | |
| Severe mitral annular calcification | 1 (2) | 0 (0) | |
| Intramural hematoma | 1 (2) | 0 (0) | |
| Fibroma | 0 (0) | 1 (4) | |
| Pericardial | 12 (8) | 8 (32) | |
| Pericardial cyst | 9 (75) | 2 (8) | |
| Pericardial thrombus | 2 (17) | 3 (12) | |
| Inflammatory pseudotumor | 1 (8) | 1 (4) | |
| Pericardium with reactive mesothelium | 0 (0) | 2 (8) | |
| Malignant | |||
| Malignant primary cardiac | 3 (2) | 2 (7) | |
| Angiosarcoma | 1 (33) | 2 (7) | |
| Undifferentiated Sarcoma | 1 (33) | 0 (0) | |
| Unknown | 1 (33) | 0 (0) | |
| Metastatic | 62 (44) | 26 (93) | |
| Not specified | 5 (3) | ||
| Not specified | 9 (6) | ||
Data are shown as n (%). CMR indicates cardiac magnetic resonance imaging; IAS, interatrial septum.
Out of 53 patients with a known cardiac histological diagnosis.
Echocardiographic Characteristics
Among 145 patients in the study cohort, 124 (86%) had dedicated TTE. Of these, a cardiac mass was identified in 103 (83%) patients. When considering the type of cardiac mass based on TTE, the most common diagnosis based on TTE interpretation was “unknown if benign or malignant” (n=50, 49%), “benign mass” (n=25, 24%), “metastatic mass” (n=18, 17%), thrombus (n=6, 6%) and “malignant mass type unknown” (n=3, 3%). The cardiac mass was not reported in the remaining 21 (17%) patients. A review of the cases that were not visualized on TTE identified that such cases were commonly extracavitary masses involving the pericardium.
Diagnostic Accuracy
Two reviewers blinded to histological diagnosis correctly diagnosed 89% (47/53) and 94% (50/53) of the cases as benign versus malignant, respectively. The readers achieved 95% and 100% agreement rates for benign and malignant masses, respectively (weighted κ=0.83). In addition, the reviewers correctly classified all 25 (100%) benign cases. Of the 28 malignant cases, reader 1 correctly diagnosed 22 cases (79%) as malignant, and reader 2 correctly diagnosed 25 cases (89%) as malignant (Table 4).
Table 4.
Reader Agreement Where Histological Diagnosis Available
| Reader 1 | Reader 2 | Agreement | |
|---|---|---|---|
| Benign cardiac mass, n=25 (47%) | 100 | ||
| Correct diagnosis | 25 (100) | 25 (100) | |
| Incorrect diagnosis | 0 (0) | 0 (0) | |
| Malignant mass, n=28 (53%) | 95 | ||
| Correct diagnosis | 22 (79) | 25 (89) | |
| Incorrect diagnosis | 6 (21) | 3 (11) |
Collectively, there were 6 cases of an incorrect diagnosis by reader 1 and 3 cases by reader 2 (Table S1). When reviewing these studies after unblinding the results, these cases all had atypical appearance or signal characteristics or an atypical location of the mass on CMR.
There were 3 cases for which both readers misdiagnosed a malignant mass as benign: Case 1 was a metastatic lymphoma in the posterior right atrium near the junction of the superior vena cava, incorrectly diagnosed as myxoma by reader 1 and thrombus by reader 2 (Figure S1). Case 2 was a spindle cell sarcoma in the left atrium adherent to the anterolateral aspect of the mitral annulus that was thought to be a myxoma by both readers (Figure S2). Case 3 had pathology demonstrating both thrombus and metastatic small cell carcinoma in the right atrium, and both readers categorized the mass as thrombus only (Figure S3). This right atrial mass was moving across the tricuspid valve into the right ventricular cavity during ventricular diastole and extended into the superior vena cava. In 2 of these 3 cases, both LGE and perfusion imaging were negative.
There were 3 additional cases of a misdiagnosis by reader 1 only. In all 3 cases, the CMR signal characteristics or mass location were atypical: Case 1 was a metastatic renal cell cancer to the myocardium with pericardial extension, misdiagnosed as hemangioma (Figure S4). Case 2 was a mediastinal spindle cell sarcoma with extension to the left atrium through the pulmonary veins incorrectly diagnosed as myxoma (Figure S5). Case 3 was a metastatic paraganglioma to the atrioventricular groove, misdiagnosed as an infectious process (Figure 3, top section).
Figure 3.
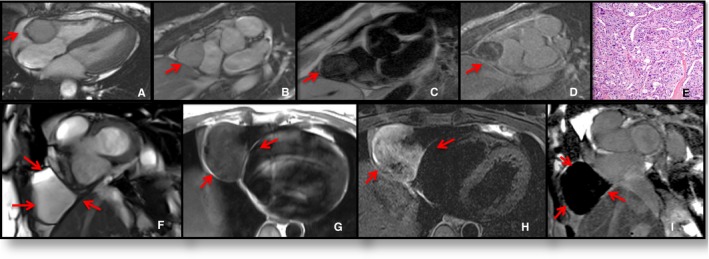
Examples of pericardial masses. Top, Atrioventricular groove paraganglioma. Four‐chamber (A) and short‐axis (B) steady‐state free precession imaging locates the mass in the atrioventricular groove. The mass had variable signal intensity at T1‐weighted (T1W) imaging (C) and heterogeneous contrast agent uptake at late gadolinium enhancement imaging (D). Additional images (not shown) demonstrated the mass to be isointense on T2‐weighted (T2W) sequence. Pathology (E) showed cellular nests (zellballen) and trabeculae of neuroendocrine cells within a prominent vascular network without necrosis or mitotic figures, diagnostic of a paraganglioma. Hematoxylin and eosin–stained section, ×200 original magnification. Bottom, Pericardial cyst. Short‐axis cine (F) demonstrates the mass in the right pericardiophrenic angle. The mass had isointense signal intensity at T1W imaging (G) and very high signal intensity at T2W imaging (H). There was no contrast agent uptake at phase‐sensitive inversion recovery sequence (I).
Both readers correctly diagnosed all 25 benign masses as benign (100%). However, when asked to define the type of benign mass, neither reader could identify the correct diagnosis in all cases. Reader 1 was able to correctly diagnose the type of the benign mass in 19 cases (19/25, 76%), whereas reader 2 was able to reach a correct identification in 20 cases (20/25, 80%; Table S2). As an example, Figure 4 (bottom section) depicts a right ventricular fibroelastoma identified as myxoma by both readers. Figure 5 shows 2 examples of metastatic cardiac masses.
Figure 4.
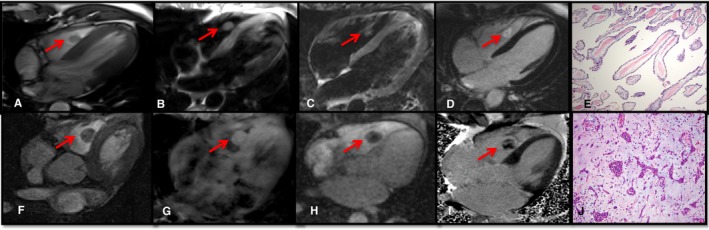
Examples of benign neoplastic cardiac masses. Top, Right ventricular (RV) fibroelastoma. Four‐chamber cine (A) locates the mass in the fight ventricle. The mass had isointense signal intensity at T1‐weighted (T1W) and T2‐weighted imaging (B and C) and heterogeneous contrast agent uptake at late gadolinium enhancement (LGE) imaging (D). Pathology (E) showed a sea anemone–like gross appearance, with microscopic features of fibroelastotic papillary cores lined by hyperplastic endothelial cells diagnostic of a papillary fibroelastoma. Hematoxylin and eosin–stained section, ×100 original magnification. Bottom, RV myxoma. Axial cine (F) demonstrates the mass in the right ventricle. The mass had isointense signal intensity at T1W imaging (G) and heterogeneous contrast agent uptake at perfusion (H) and LGE imaging (I). Pathology (J) showed syncytial‐like bland‐appearing myxoma cells rimming around vessels, in clusters and singly within a myxoid matrix, diagnostic of a cardiac myxoma. Hematoxylin and eosin–stained section, ×200 original magnification.
Figure 5.
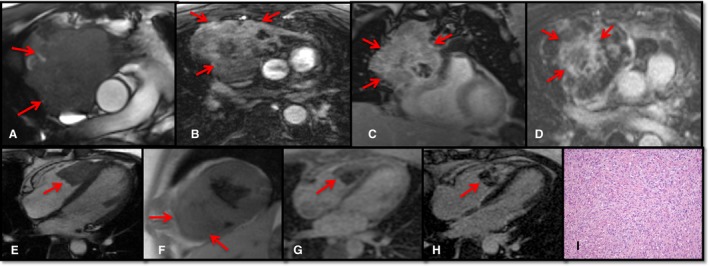
Examples of metastatic masses. Top, Metastatic thymoma. Axial cine (A) demonstrates a large mediastinal mass invading the right atrium. The mass had variable signal intensity at postcontrast T1‐weighted (T1W) imaging (B) and heterogeneous contrast agent uptake at perfusion (C) and late gadolinium enhancement (LGE) imaging (D). Bottom, Metastatic sarcoma. Four‐chamber cine (E) illustrates the mass in the right ventricle. The mass had isointense signal intensity at T1W imaging (F) and heterogeneous contrast agent uptake at perfusion (G) and LGE imaging (H). Pathology (I) showed a high‐grade spindle cell neoplasm with abundant mitotic figures and focal necrosis, consistent with spread from the patient's known sarcoma. Hematoxylin and eosin–stained section, ×200 original magnification.
Clinical Course and Patient Outcomes
Among the entire cohort, 50 (34%) patients had no interventions for the cardiac mass, 47 (32%) were treated with radiation and/or chemotherapy, and 43 (30%) underwent mass resection. Over a median follow‐up of 723 days (IQR: 191–3204), among patients with cardiac pathology and available follow‐up (N=53), 18 (72%) patients with a benign mass were alive and 7 (28%) died, whereas 5 (18%) patients with a malignant mass were alive and 23 (82%) died. As shown in Figure 6, patients with malignant masses had decreased long‐term survival compared with patients with benign masses (P<0.001 between groups). Figure 7 demonstrates similar event‐free survival among all patients undergoing CMR with available follow‐up, stratified by clinical diagnosis. The reduced long‐term survival in patients with malignant masses was also seen when adjusted for age and sex (Table 5).
Figure 6.
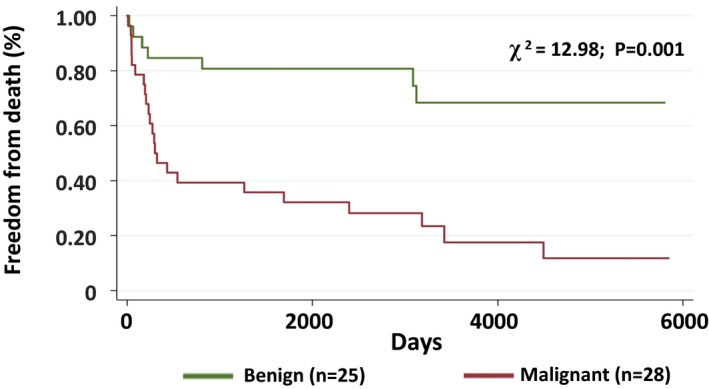
Freedom from death for benign vs malignant cardiac masses (patients with biopsy). Kaplan–Meier analysis demonstrating freedom from death among patients with a benign (n=25) vs malignant (n=28) cardiac mass confirmed by direct histology.
Figure 7.
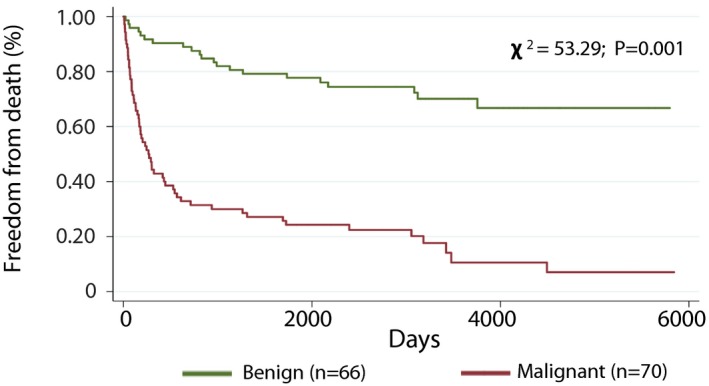
Freedom from death for benign vs malignant cardiac masses (all patients). Kaplan–Meier analysis demonstrating freedom from death among patients with a benign (n=66) vs malignant (n=70) cardiac mass incorporating all available clinical data including a history of malignancy, extracardiac histology findings, cardiac magnetic resonance imaging, and other image findings.
Table 5.
HR for Primary Outcome (All‐Cause Mortality)
| Variable | Unadjusted HR (95% CI) | P Value | Adjusted HR (95% CI)* | P Value |
|---|---|---|---|---|
| Age (per decile) | 1.1 (1.0–1.2) | 0.012 | ··· | ··· |
| Male (n=65) | 1.0 (0.6–1.5) | 0.90 | 0.9 (0.6–1.4)† | 0.71 |
| History of known malignancy (n=93) | 3.0 (1.7–5.2) | <0.001 | 2.9 (1.7–5.1)‡ | <0.001 |
| All patients (n=136)§ | ||||
| Benign, clinical diagnosis (n=66) | Reference | |||
| Malignant, clinical diagnosis (n=70) | 5.6 (3.4–9.3) | <0.001 | 4.9 (2.8–8.4) | <0.001 |
| Patients with direct histology (n=53) | ||||
| Benign, direct pathology (n=25) | Reference | |||
| Malignant, direct pathology (n=28) | 4.2 (1.8–9.8) | 0.001 | 3.9 (1.6–9.6) | 0.003 |
| CMR features (all patients) | ||||
| Mass diameter, per cm | 1.1 (1.0–1.2) | 0.001 | 1.1 (1.1–1.2) | <0.001 |
| First pass perfusion (+) | 3.3 (2.0–5.4) | <0.001 | 2.6 (1.5–4.3) | <0.001 |
| Extrapericardium with invasion (+) | 2.9 (1.8–4.7) | <0.001 | 2.6 (1.6–4.2) | <0.001 |
| LGE (+) | 2.6 (1.5–4.5) | <0.001 | 2.0 (1.2–3.5) | 0.01 |
| Nonmobile | 2.1 (1.2–3.6) | 0.01 | 1.9 (1.1–3.3) | 0.03 |
| Pericardial effusion (+) | 1.6 (1.0–2.7) | 0.04 | 1.5 (0.9–2.5) | 0.10 |
| Fat suppression (+) | 0.7 (0.3–1.5) | 0.35 | 0.8 (0.4–1.7) | 0.54 |
| T2W, hyperintense (+) | 0.9 (0.6–1.4) | 0.70 | 0.9 (0.5–1.4) | 0.56 |
| T1W, hyperintense (+) | 0.8 (0.4–1.5) | 0.42 | 0.9 (0.5–1.7) | 0.68 |
| Management | ||||
| Complete resection (all patients, n=43) | 0.7 (0.4–1.1) | 0.11 | 0.7 (0.4–1.1) | 0.11 |
| Complete resection, malignant (n=19) | 0.5 (0.3–0.9) | 0.02 | 0.5 (0.3–1.1) | 0.07 |
| Complete resection, benign (n=24) | 0.8 (0.3–2.1) | 0.64 | 0.6 (0.2–1.6) | 0.30 |
| Chemotherapy (n=28/70, malignant) | 0.8 (0.5–1.4) | 0.52 | 1.0 (0.6–1.7) | 0.89 |
| Radiotherapy (n=18/70, malignant) | 1.3 (0.7–2.4) | 0.99 | 1.3 (0.7–2.3) | 0.41 |
CI indicates confidence interval CMR, cardiac magnetic resonance imaging; HR, hazard ratio; LGE, late gadolinium enhancement; T1W, T1‐weighted; T2W, T2‐weighted.
Adjusted for age (at time of CMR), sex, and prior malignancy unless noted.
Adjusted for age only.
Adjusted for age and sex only.
Among 145 patients, 9 were excluded because the final clinical diagnosis was unspecified or uncertain.
Discussion
This study represents the largest reported experience evaluating cardiac masses in adults using CMR and is the largest to examine the utility of contemporary CMR techniques to distinguish benign from malignant lesions. We found that CMR has high accuracy for discriminating between benign and malignant lesions, with a high rate of interobserver agreement. As expected, patients with malignant tumors had decreased long‐term survival compared with patients with benign masses.
Although echocardiography is often the initial imaging modality used for evaluating most cardiac masses, it is common for imagers and clinicians to face uncertainty regarding whether a cardiac mass is benign or malignant. In such cases, our findings support the use of CMR to better characterize tissue and to distinguish between benign and malignant masses. Nevertheless, although certain CMR characteristics such as FPP are more commonly seen in malignant masses, no single feature or combination of findings can be used to determine that a mass is benign. Indeed, among biopsy‐confirmed malignant masses in our study, 3 (6%) cases were misclassified as benign by reader 1 and 6 (11%) cases were misclassified by reader 2. Consequently, when there is uncertainty regarding the diagnosis, a pathological assessment of cardiac masses should be considered if feasible.
Regarding the incidence and characteristics of the tumors, our findings are similar to previous studies,16, 17 as cardiac masses were most commonly metastatic and primary masses were most often benign. Although certain characteristics on CMR such as vascularity and hyperenhancement are often predictive of a pathologic diagnosis, these characteristics do not always accurately distinguish between different types of highly vascular benign masses (eg, hemangiomas or vascular malformations) and malignant masses (eg, malignant angiosarcoma or various malignant neuroendocrine tumors). In one case, an atrioventricular groove malignant paraganglioma was misdiagnosed as a benign tumor by one reader who was blinded to the patient's history of a pheochromocytoma. This was categorized as a benign tumor, as the mass had smooth borders, was not highly vascular, and had minimal contrast enhancement (Figure 3). However, paragangliomas are considered to be malignant only when metastasis is demonstrated.18
Several factors may influence the ability of CMR to distinguish between benign and malignant lesions, including image quality, reader experience, and use of a systematic approach to image acquisition and interpretation that integrates all available clinical history and prior testing. Of these, a consistent approach to the CMR examination of cardiac masses is essential. From a technical perspective, possible magnetic resonance pitfalls are more common when assessing small mobile masses, such as those attached to valves.
Although this study did not evaluate blinded‐image analysis in comparison to clinical history–guided image analysis, in other cases a clinical history of malignancy may aid in the diagnosis of a mass as benign or malignant.19 In our study, one case of a metastatic tumor was surrounded by thrombus and read by both readers as thrombus only when blinded to the patient's history of malignancy. Among 6 patients with malignant masses misdiagnosed by reader 1 as benign, a prior history of malignancy was present in 4. Even in cases where a history of malignancy is distant, prior radiotherapy or chemotherapy could have increased the risk of a secondary malignancy with cardiac metastasis. For instance, survivors of childhood Hodgkin lymphoma with prior chest radiotherapy are at a higher risk of lung or breast cancer, both of which in turn have the potential for cardiac extension.19, 20, 21, 22
Multimodality imaging provides complementary information in the initial evaluation and surveillance of patients with cardiac masses. However, each imaging modality has inherent limitations, and in some cases direct biopsy may be warranted to reach a correct diagnosis. Although TTE is the most frequently used initial test to evaluate cardiac masses and contrast echocardiography offers improving characterization of tumors, CMR is more useful in patients with suboptimal TTE image quality.23 Furthermore, CMR can provide better characterization of the lesion, presence of tumor invasion, and tissue signal. Accordingly, 105 (73%) patients in our study had TTE before CMR. In our cohort, the tumor was not reported in 22 (17%) patients who underwent an initial TTE. Of those cases where the tumor was seen, the tumor type was classified as unknown in approximately a third of cases (n=51, 50%). In a subgroup analysis, a blinded expert echocardiographer (M.K.C.) re‐read these 22 studies, in which the cardiac mass initially had not been reported. Interestingly, picture quality on the Likert scale was not significantly different between the group for which the mass was visualized and the group for which the mass was initially “unreported.” The majority of these unreported cases on echocardiography appeared to involve masses with predominant pericardial involvement. These data emphasize the limitations of echocardiography in identifying and characterizing cardiac tumors, even with good‐quality images.
Prior studies in adult patients have evaluated characteristics of cardiac masses by CMR with histopathology correlation. Hoffman et al7 evaluated 55 cases with pathology correlation between 1988 and 2001 utilizing a 0.5‐T magnet with less frequent use of gadolinium and no perfusion sequences. In this study, 2 blinded readers were able to correctly diagnose malignancy with an area under the receiver operating characteristic curve between 0.88 and 0.92.
Conversely, in a study of 116 patients by Pazos‐López et al, the ability of CMR to distinguish a benign from malignant tumor was only moderate.12 In this study, the highest accuracy of CMR features to distinguish a malignant versus benign tumor was 79%. These features included mass diameter ≥4.2 cm (73% accuracy), mass area ≥13.4 cm2 (69%), FPP (73%), and LGE (79%).
In our study, although 2 independent blinded observers achieved a high agreement rate of 95%, no single CMR feature was sufficient to correctly distinguish between benign and malignant tumors in all cases. We found that although CMR provides important clinical information on cardiac masses, malignancy cannot always be definitively excluded, requiring a tissue diagnosis in certain cases.
Study Limitations
Given the retrospective nature of the study, the imaging protocol was not uniform in all patients, as expected in clinical practice. T1 and T2 mapping sequences were not available for the majority of cases included in our study, but further research is needed regarding whether these techniques may enhance the ability of CMR to distinguish between benign and malignant masses.
Moreover, the timing of the CMR with respect to diagnosis and treatment may have influenced our findings. For instance, undergoing chemotherapy and/or radiotherapy before CMR could have affected the size of a tumor, its vascularity, or the presence of an accompanying pericardial effusion. Given the inherent study design required to define the accuracy of CMR against a gold standard, we examined only cases with tissue‐proven histopathology. Consequently, cases managed by observation alone were not included and may have influenced the frequency of benign versus malignant masses as well as underestimated the accuracy of CMR for distinguishing between benign and malignant pathology. In addition, because all readers were blinded to clinical history, the true accuracy of magnetic resonance imaging for distinguishing between benign and malignant masses could change accordingly, as knowledge regarding prior metastatic disease and correlation with alternative imaging would significantly affect the pretest probability of having a malignant mass.
Conclusion
In the diagnostic workup of an unknown cardiac mass, it is often challenging to distinguish benign from malignant lesions on the basis of noninvasive imaging alone. Although a comprehensive CMR protocol had a high accuracy for identifying benign lesions, the ability to correctly diagnosis malignant cases in our study was more limited. Although CMR can be highly effective in distinguishing benign from malignant lesions, pathology is often required for determining the type of mass.
Disclosures
None.
Supporting information
Table S1. Details of 6 Cases Misdiagnosed as Benign by Readers
Table S2. Details of Cases Correctly Identified as Benign But Histological Diagnosis Incorrectly Specified by Readers
Figure S1. Metastatic lymphoma.
Figure S2. Spindle cell sarcoma.
Figure S3. Metastatic squamous cell carcinoma.
Figure S4. Metastatic renal cell carcinoma.
Figure S5. Metastatic mediastinal spindle cell sarcoma.
(J Am Heart Assoc. 2019;8:e007829 DOI: 10.1161/JAHA.117.007829)
References
- 1. Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20‐year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–1031. [PubMed] [Google Scholar]
- 2. Nensa F, Tezgah E, Poeppel TD, Jensen CJ, Schelhorn J, Kohler J, Heusch P, Bruder O, Schlosser T, Nassenstein K. Integrated 18F‐FDG PET/MR imaging in the assessment of cardiac masses: a pilot study. J Nucl Med. 2015;56:255–260. [DOI] [PubMed] [Google Scholar]
- 3. Patel R, Lim RP, Saric M, Nayar A, Babb J, Ettel M, Axel L, Srichai MB. Diagnostic performance of cardiac magnetic resonance imaging and echocardiography in evaluation of cardiac and paracardiac masses. Am J Cardiol. 2016;117:135–140. [DOI] [PubMed] [Google Scholar]
- 4. Scheffel H, Baumueller S, Stolzmann P, Leschka S, Plass A, Alkadhi H, Schertler T. Atrial myxomas and thrombi: comparison of imaging features on CT. AJR Am J Roentgenol. 2009;192:639–645. [DOI] [PubMed] [Google Scholar]
- 5. Rinuncini M, Zuin M, Scaranello F, Fejzo M, Rampin L, Rubello D, Faggian G, Roncon L. Differentiation of cardiac thrombus from cardiac tumor combining cardiac MRI and 18F‐FDG‐PET/CT imaging. Int J Cardiol. 2016;212:94–96. [DOI] [PubMed] [Google Scholar]
- 6. Pun SC, Plodkowski A, Matasar MJ, Lakhman Y, Halpenny DF, Gupta D, Moskowitz C, Kim J, Steingart R, Weinsaft JW. Pattern and prognostic implications of cardiac metastases among patients with advanced systemic cancer assessed with cardiac magnetic resonance imaging. J Am Heart Assoc. 2016;5:e003368 DOI: 10.1161/JAHA.116.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffmann U, Globits S, Schima W, Loewe C, Puig S, Oberhuber G, Frank H. Usefulness of magnetic resonance imaging of cardiac and paracardiac masses. Am J Cardiol. 2003;92:890–895. [DOI] [PubMed] [Google Scholar]
- 8. Sakaguchi M, Minato N, Katayama Y, Nakashima A. Cardiac angiosarcoma with right atrial perforation and cardiac tamponade. Ann Thorac Cardiovasc Surg. 2006;12:145. [PubMed] [Google Scholar]
- 9. Nascimento AF, Winters GL, Pinkus GS. Primary cardiac lymphoma: clinical, histologic, immunophenotypic, and genotypic features of 5 cases of a rare disorder. Am J Surg Pathol. 2007;31:1344–1350. [DOI] [PubMed] [Google Scholar]
- 10. Beroukhim RS, Prakash A, Buechel ERV, Cava JR, Dorfman AL, Festa P, Hlavacek AM, Johnson TR, Keller MS, Krishnamurthy R. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol. 2011;58:1044–1054. [DOI] [PubMed] [Google Scholar]
- 11. Fussen S, De Boeck BW, Zellweger MJ, Bremerich J, Goetschalckx K, Zuber M, Buser PT. Cardiovascular magnetic resonance imaging for diagnosis and clinical management of suspected cardiac masses and tumours. Eur Heart J. 2011;32:1551–1560. [DOI] [PubMed] [Google Scholar]
- 12. Pazos‐López P, Pozo E, Siqueira ME, García‐Lunar I, Cham M, Jacobi A, Macaluso F, Fuster V, Narula J, Sanz J. Value of CMR for the differential diagnosis of cardiac masses. JACC Cardiovasc Imaging. 2014;7:896–905. [DOI] [PubMed] [Google Scholar]
- 13. Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, Schaff HV. Survival after resection of primary cardiac tumors: a 48‐year experience. Circulation. 2008;118(14 suppl):S7–S15. [DOI] [PubMed] [Google Scholar]
- 14. Chan AT, Plodkowski AJ, Pun SC, Lakhman Y, Halpenny DF, Kim J, Goldburg SR, Matasar MJ, Moskowitz CS, Gupta D. Prognostic utility of differential tissue characterization of cardiac neoplasm and thrombus via late gadolinium enhancement cardiovascular magnetic resonance among patients with advanced systemic cancer. J Cardiovasc Magn Reson. 2017;19:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burke A, Jeudy J, Virmani R. Cardiac tumours: an update. Heart. 2008;94:117–123. [DOI] [PubMed] [Google Scholar]
- 17. McAllister HA, Hall RJ, Cooley DA. Tumors of the heart and pericardium. Curr Probl Cardiol. 1999;24:59–116. [PubMed] [Google Scholar]
- 18. DeLellis R, Lloyd RV, Heitz P, Eng C. Pathology and Genetics of Tumours of Endocrine Organs. World Health Organization: IARC; 2004. [Google Scholar]
- 19. Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, Robison LL, Yasui Y. Cause‐specific late mortality among 5‐year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aleman BM, van den Belt‐Dusebout AW, Klokman WJ, Van't Veer MB, Bartelink H, van Leeuwen FE. Long‐term cause‐specific mortality of patients treated for Hodgkin's disease. J Clin Oncol. 2003;21:3431–3439. [DOI] [PubMed] [Google Scholar]
- 21. Hoppe RT. Hodgkin's disease: complications of therapy and excess mortality. Ann Oncol. 1997;8(suppl 1):115–118. [PubMed] [Google Scholar]
- 22. Ng AK, Bernardo MP, Weller E, Backstrand KH, Silver B, Marcus KC, Tarbell NJ, Friedberg J, Canellos GP, Mauch PM. Long‐term survival and competing causes of death in patients with early‐stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol. 2002;20:2101–2108. [DOI] [PubMed] [Google Scholar]
- 23. Araoz PA, Mulvagh SL, Tazelaar HD, Julsrud PR, Breen JF. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics. 2000;20:1303–1319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Details of 6 Cases Misdiagnosed as Benign by Readers
Table S2. Details of Cases Correctly Identified as Benign But Histological Diagnosis Incorrectly Specified by Readers
Figure S1. Metastatic lymphoma.
Figure S2. Spindle cell sarcoma.
Figure S3. Metastatic squamous cell carcinoma.
Figure S4. Metastatic renal cell carcinoma.
Figure S5. Metastatic mediastinal spindle cell sarcoma.


