Abstract
Background
This eHealth implementation study aimed to evaluate strategies to promote opportunistic atrial fibrillation (AF) screening using electronic screening prompts and improve treatment using electronic decision support (EDS) software.
Methods and Results
An electronic screening prompt appeared whenever an eligible patient's (aged ≥65 years, no AF diagnosis) medical record was opened in participating general practices. General practitioners and practice nurses offered screening using a smartphone ECG, with validated AF algorithm. Guideline‐based EDS was provided to assist treatment decisions. Deidentified data were collected from practices using a data extraction tool. General practices (n=8) across Sydney, Australia, screened for a median of 6 months. A total of 1805 of 11 476 (16%) eligible patients who attended were screened (44% men, mean age 75.7 years). Screening identified 19 (1.1%) new cases of AF (mean age, 79 years; mean CHA 2 DS 2‐VASc, 3.7; 53% men). General practitioners (n=30) performed 70% of all screenings (range 1–448 patients per general practitioner). The proportion of patients with AF who had CHA 2 DS 2‐VASc ≥2 for men or ≥3 for women prescribed oral anticoagulants was higher for those diagnosed during the study: 15 of 18 (83%) for screen‐detected and 39 of 46 (85%) for clinically detected, compared with 933 of 1306 (71%) patients diagnosed before the study (P<0.001). The EDS was accessed 111 times for patients with AF and for 4 of 19 screen‐detected patients.
Conclusions
The eHealth tools showed promise. Adherence to guideline‐based oral anticoagulant prescription was significantly higher in patients diagnosed during the study period, although the EDS was only used in a minority. While the proportion of eligible patients screened and EDS use was relatively low, further refinements may improve uptake in clinical practice.
Clinical Trial Registration
URL: www.anzctr.org.au. Unique identifier: ACTRN12616000850471.
Keywords: atrial fibrillation, eHealth, general practices, screening, stroke prevention
Subject Categories: Atrial Fibrillation, Secondary Prevention, Electrocardiology (ECG)
Clinical Perspective
What Is New?
This study demonstrates the feasibility and utility of a unique suite of automated eHealth tools to support atrial fibrillation screening in general practice.
These eHealth tools are integrated with practices’ electronic medical records and cover all stages of the screening process: identifying eligible patients, providing a screening prompt, recording screening results, providing decision support to clinicians, and including a data extract system for quality improvement and monitoring.
What Are the Clinical Implications?
The use of eHealth tools to support all stages of the screening process is a promising implementation strategy for atrial fibrillation screening in general practice.
Introduction
Atrial fibrillation (AF) is the most common heart arrhythmia worldwide.1 In Australia, the projected prevalence of AF by 2034 is 600 000.2 The risk of developing AF rapidly increases with age, exceeding 20% for those 80 years and older.3 Growing AF prevalence is a significant financial burden on the Australian healthcare system, contributing to over 60 000 hospitalizations each year4 with an annual cost of approximately AU$874 million.5
It has been estimated that between 1.4% and 1.6% of the population 65 years and older have undiagnosed AF,6 which can often be asymptomatic.7, 8 While AF can cause a 5‐fold increase in risk of stroke,9, 10 treatment with appropriate oral anticoagulant (OAC) therapy can reduce AF‐related stroke risk by 64%.11 Clinical guidelines include strong recommendations for OAC (including novel OAC) treatment for patients at high risk for stroke (as estimated by the CHA2DS2‐VASc12 or the similar “sexless” CHA2DS2‐VA score in the recently released Australian guidelines13). Large gaps between evidence and practice have been reported, with almost 40% of eligible patients not taking OACs.14
Opportunistic screening for AF in people 65 years and older by pulse palpation or ECG rhythm strip is now recommended by Australian and European guidelines and expert consensus.12, 13, 15 Importantly, these guidelines are referring to single time point opportunistic screening, rather than extended ECG monitoring, which will find more brief paroxysmal AF with a more benign prognosis. In terms of the risks of screen‐detected AF, it is thought that the prognosis of opportunistic single time point screening for AF is likely to be similar to incidentally detected AF in the absence of symptoms.15, 16 While screening is recommended in guidelines, it is rarely performed in practice. According to a recent survey of general practitioners (GPs) conducted by The Economist, Australian respondents had opportunistically screened only 11% of patients 65 years and older in the past fortnight.17
AF screening in general practices is well aligned with their role in providing ongoing chronic care for large numbers of older community‐dwelling patients. According to a recent report, over 35% of patient encounters in general practices were for chronic health issues and those 65 years and older (at greater risk of AF) accounted for over 30% of the total number of general practice encounters.18
A screening program ideally needs a system to accurately identify eligible patients and prompt staff to perform the screening. In the “opportunistic screening” arm of the SAFE (Screening for Atrial Fibrillation in the Elderly)6 cluster randomized trial, both paper and computer “flags” were used to encourage staff to take patients’ pulse rates during the consultation. Ideally, an automatic electronic flag or prompt could broaden uptake.
Importantly, a screening program should include a system to support evidence‐based treatment for patients with diagnosed AF.15 A range of interventions have been developed to increase effective prescribing of guideline‐recommended OACs in primary care settings, with mixed success. These include electronic decision support (EDS) tools,19 targeted GP‐education programs,20 consultant‐led primary care anticoagulation assessment clinics,21 and patient‐focused education interventions.22
Our pilot studies showed opportunistic AF screening in general practice to be feasible (including as an adjunct to influenza vaccination).23, 24 However, a number of barriers were identified, including the lack of remuneration, lack of time during flu vaccination, the need for a prompt to remind staff to screen by identifying eligible patients, and gaps in evidence‐based treatment for those identified with AF. This implementation study aimed to develop and test a suite of eHealth tools to overcome these barriers and to support opportunistic AF screening in general practice by nurses and GPs, including an automated electronic prompt and evidence‐based EDS for OAC prescription integrated with the 2 most commonly used electronic medical record (EMR) systems in Australian general practices.
Methods
This implementation study was conducted in a convenience sample of 8 general practices between November 2016 and July 2018 in Sydney, Australia. The study was approved by the University of Sydney's human research ethics committee (project number 2014/962) and registered with the Australia New Zealand Clinical Trial Registry (trial record ACTRN12616000850471). Practices gave written informed consent, and patients gave oral consent. The data, analytic methods, and study materials will not be made available to other researchers for the purpose of reproducing the results or replicating the procedure as data sharing is not permitted by our ethics approval committee. Researchers interested in the data, methods, or analysis can contact the corresponding author for more information.
eHealth Study Tools
Smartphone ECG
AliveCor KardiaMobile smartphone ECG (iECG) devices were used for screening. The iECG is a single‐lead ECG approved by the Australian Therapeutic Goods Administration as a medical device, class IIa. The device has a validated, automated algorithm for detecting AF, providing an immediate provisional diagnosis of either “normal,” “possible AF,” or “unclassified.”25
Screening prompt
An AF screening app and prompt was developed in collaboration with a third‐party software developer (PenCS). The AF app ran on the provider's point‐of‐care hosting platform, which extracts information from the general practice EMR and automates data population from the medical record, thus eliminating the need for manual data entry. The prompt was iteratively modified throughout the study based on user feedback. Specifically, the prominence of the prompt was improved, as initially it was not prominent, and occasionally was not visible on the point‐of‐care tool because of early space limitations.
In the final version, when an eligible patient's EMR is opened, a “pop‐up” prompt automatically appears for 5 seconds in the bottom right of the screen to recommend screening for AF. A small red “1” remains visible beside the app at the top of the screen (Figure 1). When screening is complete, staff are able to record the provisional iECG screening result within the AF app and click the embedded link to add any provisional diagnoses to the patient's medical record.
Figure 1.
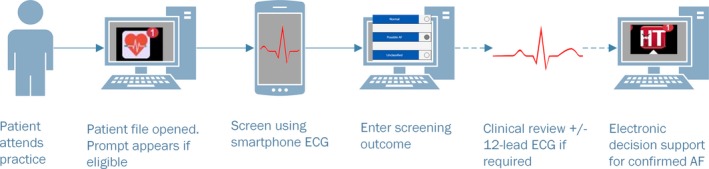
eHealth tools to assist atrial fibrillation (AF) screening.
AF EDS software
An AF treatment algorithm was designed in collaboration with the George Institute, Sydney, Australia and the University of Sydney, Australia, to bridge gaps between evidence and practice in AF risk management.26 The algorithm was built as an additional module of The George Institute's HealthTracker software, which provides information on overall cardiovascular risk and evidence‐based cardiovascular decision support.27 HealthTracker is also accessed through the third‐party point‐of‐care tool.
The AF EDS algorithm was developed based on the 2016 European Society of Cardiology AF guidelines,12 tested, and clinically validated. The new AF EDS software uses relevant data extracted from the patient's EMR to calculate individual stroke risk scores (CHA2DS2‐VASc). The EDS takes into consideration coexisting cardiac comorbidities and provides clinicians with evidence‐based recommendations regarding OAC and antiplatelet treatment, taking into consideration whether AF is present alone or together with vascular disease. The EDS also includes information on government pharmaceutical benefits scheme eligibility for medications, specific to each patient to support clinical decisions.
Data extraction tool and “clinical audit” data
Deidentified data from practices’ EMRs were collected using a third‐party data extraction tool, using a custom‐designed configuration for deidentified data collection. Data were available in CSV format, and provided a point‐in‐time snapshot of all adult “active” patients of the practice, ie, patients who had attended at least 3 times in the past 2 years and once in the past 6 months. Data included demographic and diagnostic information including AF and CHA₂DS₂‐VASc score, medications prescribed, iECG results as recorded by GPs/nurses (but not the actual iECG recordings), and number of times the EDS was accessed for each patient.
Screening Protocol
General practices were recruited and provided practice‐level written informed consent before participation. Practices were then set up with the electronic tools for the study. Licenses for the third‐party software were provided free to practices by government Primary Health Networks. Staff were trained in the use of the electronic study tools, and nurses were offered an evidence‐based AF training session, accredited for continuing professional development points.
Eligible patients were those attending the practice to see the practice nurse or GP and who were 65 years and older, with no recorded diagnosis of AF, and had not been screened with the iECG in the past 12 months. Patients unable to provide consent and those seen by GPs off site (home or residential aged care) were excluded from the study.
The nurse or GP explained the purpose and process of iECG screening and obtained oral consent from the patient. The nurse or GP then recorded the 30‐second iECG, visible in real‐time on the smartphone screen, and entered the automated interpretation into the AF app. For a “normal” result, no further action was required. For an “unclassified” result, follow‐up was at the GP's discretion. A 12‐lead ECG was often recommended, depending on the patient's history. For a “possible AF” result, further workup and management was also at the GP's discretion. A 12‐lead ECG was generally recommended to provide additional confirmation and to add extra leads. For patients with confirmed AF, GPs were encouraged to use the EDS system to assess stroke risk, CHA2DS2‐VASc score, and guideline‐recommended medication.
Reimbursement
Each practice was paid AU$1000 to cover IT setup costs plus AU$10 per patient for the first 500 patients screened. This amount was intended to reflect potential “real‐world” remuneration if screening were reimbursed by Medicare. Screening was free of charge for patients, although usual consultation fees applied.
Data Collection and Analysis
Deidentified clinical audit data extracts were taken at baseline, periodically throughout the study, and at completion. Following each extract, practices received feedback from researchers including a brief summary of screening numbers/results. The McNemar test was used to compare whether the OAC treatment rate was greater or lower for patients diagnosed before the study period than for patients diagnosed during the study period (either screen‐detected or otherwise‐detected). Two‐tailed P values of <0.05 were considered significant.
A sample size of 8 to 10 practices was chosen to provide a sufficient cross‐section of practices to test implementation of the eHealth tools.
Study Outcomes
Implementation success identified through process measures including fidelity to the protocol, and utilization of eHealth tools.
The proportion of eligible people attending the practice during the study period who were screened.
The proportion of people with new AF detected through screening.
Determination of guideline‐based OAC prescription rates for both patients with “screen‐detected” and “otherwise‐detected” AF during the study period, compared with the OAC prescription rates before study commencement.
Results
Nine general practices across Sydney, Australia, were recruited and 1 practice withdrew before commencing screening. The screening period was determined by each practice and ranged from 2 to 12 months (median 6 months). Across the 8 practices that completed the intervention, 11 476 eligible active patients 65 years and older attended during the study. A total of 1805 patients (16% of those attending; range 4–33% per practice) were screened (44% men; mean age, 75.7 years) (Figure 2). The automated iECG results, as entered by GPs/practice nurses into the AF app, were: 1563 normal (86.6%), 67 possible AF (3.7%), and 175 unclassified (9.7%).
Figure 2.
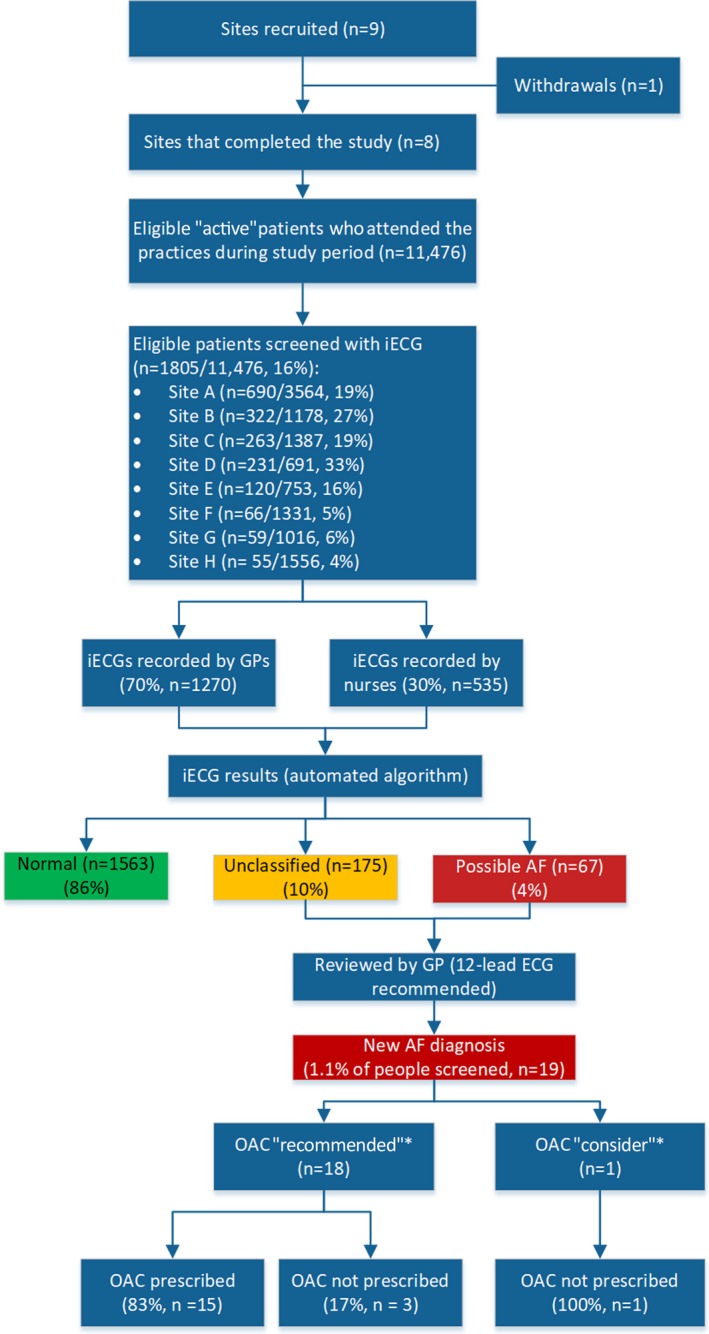
Study flowchart. AF indicates atrial fibrillation; GP, general practitioner; iECG, smartphone lead I ECG. *Oral anticoagulant (OAC) recommendations as per European Society of Cardiology 2016 guidelines.12
GPs (n=30) performed 70% of all screenings (range, 1–448 screenings per GP; median, 7.5 screenings per GP), with the remaining 30% performed by nursing staff (n=16; range, 1–82 screenings per nurse [median, 30.5 screenings per nurse]). Eight GPs accounted for 62% of all screenings, of whom 3 of 8 were from the same practice.
New AF
Of screened patients, 19 (1.1%) new cases of AF were confirmed (patient mean age, 78.6±7.8 years; mean CHA2DS2‐VASc, 3.7±0.9 [53% men]). Of these patients, 18 of 19 (95%) had a class 1 recommendation for OAC according to European Society of Cardiology guidelines12 (ie, CHA₂DS₂‐VASc score men ≥2 and women ≥3), and 15 of 18 (83%) were prescribed OAC therapy.
Guideline‐Based Treatment of AF Cases
The proportion of patients treated according to European Society of Cardiology guidelines significantly improved during the study period (Table). For patients with AF 65 years and older with a class 1 OAC recommendation, OAC prescription increased from 71% (933/1306) for AF diagnosed before the study to 84% (54/64) for patients diagnosed during the study (screen‐detected and clinically detected) (P<0.001). The EDS was accessed 111 times for patients with AF, and for only 4 of 19 screen‐detected patients.
Table 1.
OAC Treatment of AF Detected Before the Study Versus During the Study
| Patients With AF (Aged ≥65 y) | Class 1 OAC Recommendation Group | CHA2DS2‐VASc, Mean | Age, Mean, y | Male Sex, % | ||||
|---|---|---|---|---|---|---|---|---|
| Total, No. | Class 1 OAC Recommendation, No. (%)* | Prescribed OAC, No. (%) | Prescribed Antiplatelet Only, No. (%) | No Therapy, No. (%) | ||||
| AF diagnosed before study | 1346 | 1306 (97) | 933 (71)† | 213 (16) | 160 (12) | 3.9 | 80.0 | 53 |
| AF diagnosed during study | 66 | 64 (97) | 54 (85)† | 3 (5) | 7 (11) | 3.8 | ||
| Screen‐detected AF | 19 | 18 (95) | 15 (83) | 1 (6) | 2 (11) | 3.8 | 78.6 | 53 |
| Otherwise‐detected AF | 47 | 46 (98) | 39 (85) | 2 (4) | 5 (11) | 3.8 | 79.6 | 45 |
AF indicates atrial fibrillation; CHA2DS2‐VASc: C, congestive heart failure/left ventricular dysfunction; H, high blood pressure; A2, age older than 75 years; D, diabetes mellitus; S2, stroke/transient ischemic attack/thromboembolism; V, vascular disease (coronary artery disease, myocardial infarction, peripheral artery disease, aortic plaque); A, age 65 to 74 years; Sc, sex category female.
Oral anticoagulant (OAC) recommendations as per European Society of Cardiology 2016 guidelines.12
Between‐group difference (P<0.001).
Discussion
This study demonstrates the feasibility and utility of a unique suite of automated eHealth tools to support AF screening in general practice. We are not aware of any other study with integrated eHealth tools covering all stages of the screening process—from identifying eligible patients, providing a prompt for screening, recording screening results data, providing decision support to clinicians, and including a custom‐designed data extract system for quality improvement and tracking of the screening program. Importantly, these tools are integrated with practices’ EMRs, allowing for real‐time analysis of the patient's medical data. The screening prompt was iteratively and gradually modified during the study based on user feedback and thus improved in reliability and visibility. This approach to improve the prompt is similar to that taken in a Canadian study evaluating an EMR toolkit for AF management, which took a “human factors” approach to iteratively refine a user‐centered quality improvement toolkit.28
Overall, the final version of the prompt showed promise. The real‐time ECG trace and immediate results of the iECG device made screening quick and efficient. The screening program identified 1.1% of patients with new AF. Importantly, guideline‐based OAC prescription increased during the study, from 71% (prestudy) to 83% (screen‐detected and otherwise‐detected).
The OAC treatment rate of 83% during our study is substantially higher than the previously reported rates in Australia of ≈60%.29, 30 This may be reflective of recent trends where the treatment gap appears to be closing, with some European studies reporting higher rates of OAC treatment, likely the result of the introduction of novel OACs.31, 32, 33 Despite this, a proportion of people are still inappropriately treated, with many still prescribed antiplatelets, and the EDS can assist with further improving guideline‐based treatment aimed at preventing strokes.34
Engagement in the screening process (and, in particular, the percentage of eligible patients screened) varied between the practices, despite all practices using the same eHealth tools. A small number of GPs and practice nurses were highly engaged, and many of these came from a single practice. This practice, along with 2 or 3 others in the study, had 1 or more “champion” (a GP and/or practice manager) driving the program. The importance of having both a “project champion” and an “organizational change champion” (who may be the same person) to implement and, importantly, maintain quality improvement programs in general practice has been previously described.35 It was our experience that practice champions, together with a team approach that gathers enthusiasm, was critical to success at the individual practice level.
Use of the customized clinical audit data, together with an audit and feedback system appeared to increase motivation and engagement. We believe this could be further improved through a more automated system for practices to receive frequent feedback during the study as required. Audit and feedback systems have been shown to improve clinical performance by a Cochrane review,36 and are currently under trial as part of a “toolkit” to improve OAC treatment for AF in primary care in Canada.37
Limitations and Further Development of eHealth Tools
The main limitation of the study was the reliability of the third‐party platform at practices, meaning the screening prompt and EDS apps could not be accessed for periods during the study, and GPs/nurses were not able to screen patients. The individual and complex nature of the computer setup at each practice meant that problems were sometimes difficult to resolve quickly, and thus screening may have become less habitual, perhaps persisting after the problem was resolved. Resolving reliability of the third‐party platform, better seamless integration with the practice medical software, and more frequent audit feedback to practices may resolve these issues and improve uptake.
The EDS was not accessed for all patients with AF, possibly as it was in a separate app and platform to the EMR, and some GPs used alternative sources/websites for calculating risk scores. Nevertheless, OAC prescription was higher compared with patients who received a diagnosis previously in the practice.
The practice data that were collected, while detailed, were limited to “active” patients only, as a result of the design of the data collection tool. This may have excluded patients in better health, eg, who did not have chronic conditions necessitating more frequent GP consultations.
The proportion of iECGs with an “unclassified” interpretation by the device was almost 10%. This is to be expected given that the automated algorithm was developed for use by individual patients, and it cannot report as “normal” conditions such as sinus bradycardia or sinus tachycardia, which are the usual reasons for the unclassified diagnosis. However, the number of unclassified results did require extra time for practice nurses/GPs to follow up. The research team was not able to see the iECGs, and therefore could not report the sensitivity and specificity of the device's automated algorithm. However, the sensitivity and specificity in this study may be less than the original validation figures, as this was a “real‐world” study, for which we have previously shown reduced specificity with sensitivity retained.30
The incidence of new AF identified during the study may be an underestimate, as some AF diagnoses may not have been recorded as a condition in the patient's EMR (eg, if only written in free text notes) and therefore would not appear in our clinical audit data. Only “active patients” were included in our clinical audit data, and some patients with paroxysmal AF may not have been identified by a single time point assessment.
Conclusions
Overall, the use of eHeatlh tools to support all stages of the screening process seem to be a promising implementation strategy for AF screening in general practice.
The proportion of patients with new AF identified during the screening period (by screening or otherwise) with CHA₂DS₂‐VASc ≥2 men or ≥3 women who were prescribed OAC therapy as recommended by European Society of Cardiology/EDS guidelines was significantly higher than for those who received a diagnosis before the study, although the EDS was only used in a minority. While the proportion of eligible patients screened was relatively low, further refinements to the screening process and electronic tools may improve uptake. Methods to increase engagement and increase adoption of screening and use of the tools should be further investigated. To be effective, eHealth tools need to influence behavior change, and future studies are required to provide further insight on key requirements to effect change.
Author Contributions
Freedman, Neubeck, Zwar, Ferguson, Lowres, Webster, and Gallagher contributed to the conception or design of the work. Orchard, Webster, and Li contributed to the acquisition of the data. Freedman, Neubeck, Lowres, Orchard, and Li contributed to the data analysis and interpretation of the data for the work. Orchard drafted the article. Freedman, Neubeck, Gallagher, Webster, Zwar, Ferguson, and Lowres critically revised the article. All authors gave final approval and agree to be accountable for all aspects of work, ensuring integrity and accuracy.
Sources of Funding
This work is supported by a Heart Foundation/New South Wales Health Cardiovascular Research Network Research Development Project Grant (101133). AliveCor has provided free smartphone ECG covers for study purposes. Orchard is supported by an Australian Government Research Training Program Scholarship. Lowres is funded by a New South Wales Health Early Career Fellowship (H16/52168). Webster is funded by a National Health and Medical Research Council Early Career Fellowship (APP1125044).
Disclosures
Orchard and Lowres report an investigator‐initiated grant from Pfizer. Neubeck reports grants from Pfizer/Bristol‐Myers Squibb and Bayer and honoraria from Pfizer/Bristol‐Myers Squibb, Bayer, and Boehringer Ingelheim. Freedman reports prior fees and advisory board honoraria from Bayer Pharma AG, Boehringer Ingelheim, Daiichi‐Sankyo, and Pfizer/Bristol‐Myers Squibb but for the past year has removed himself from pharmaceutical advisory boards and receives speaker fees only for accredited educational meetings. Ferguson has received an honorarium from Pfizer for teaching. Webster reports that George Health Enterprises, the social enterprise arm of The George Institute is actively involved in commercialization activities for their decision support products. The remaining authors have no disclosures to report.
Acknowledgments
The authors gratefully acknowledge Anushka Patel and Elizabeth Denney‐Wilson, and all of the practices that participated in the study.
(J Am Heart Assoc. 2019;8:e010959 DOI: 10.1161/JAHA.118.010959.)
This work was presented on the Digital Health Stage and as a poster at the European Society of Cardiology Congress, August 25–29, 2018, in Munich, Germany, and at the Cardiac Society of Australia and New Zealand Scientific Meeting, August 2–5, 2018, in Brisbane, Australia.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ball J, Thompson DR, Ski CF, Carrington MJ, Gerber T, Stewart S. Estimating the current and future prevalence of atrial fibrillation in the Australian adult population. Med J Aust. 2015;202:32–35. [DOI] [PubMed] [Google Scholar]
- 3. Magnani JW, Wang N, Benjamin EJ, Garcia ME, Bauer DC, Butler J, Ellinor PT, Kritchevsky S, Marcus GM, Newman A, Phillips CL, Sasai H, Satterfield S, Sullivan LM, Harris TB. Atrial fibrillation and declining physical performance in older adults: the Health, Aging, and Body Composition Study. Circ Arrhythm Electrophysiol. 2016;9:e003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gallagher C, Hendriks J, Giles L, Elliott A, Middeldorp M, Mahajan R, Lau D, Sanders P, Wong C. Twenty‐year national trends in hospitalisations due to atrial fibrillation in Australia: a relentless rise. Heart Lung Circ. 2017;26:S175. [Google Scholar]
- 5. PricewaterhouseCoopers . Commissioned by the National Stroke Foundation, The Economic Costs of Atrial Fibrillation in Australia. 2010.
- 6. Fitzmaurice DA, Hobbs FD, Jowett S, Mant J, Murray ET, Holder R, Raftery JP, Bryan S, Davies M, Lip GY, Allan TF. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J, Reyes BA, McManus DD, Maitas O, Chon KH. Atrial fibrillation detection using an iPhone 4S. IEEE Trans Biomed Eng. 2013;60:203–206. [DOI] [PubMed] [Google Scholar]
- 8. Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol. 2000;4:369–382. [DOI] [PubMed] [Google Scholar]
- 9. Hughes M, Lip GY; Guideline Development Group—NICE . Stroke and thromboembolism in atrial fibrillation: a systematic review of stroke risk factors, risk stratification schema and cost effectiveness data. Thromb Haemost. 2008;99:295–304. [DOI] [PubMed] [Google Scholar]
- 10. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 11. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 12. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; Group ESCSD . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 13. Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, Connell C, Freedman B, Ferguson C, Hall T, Haqqani H, Hendriks J, Hespe C, Hung J, Kalman JM, Sanders P, Worthington J, Yan TD, Zwar N. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ. 2018;27:1209–1266. [DOI] [PubMed] [Google Scholar]
- 14. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638–645.e4. [DOI] [PubMed] [Google Scholar]
- 15. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, Albert CM, Anderson CS, Antoniou S, Benjamin EJ, Boriani G, Brachmann J, Brandes A, Chao TF, Conen D, Engdahl J, Fauchier L, Fitzmaurice DA, Friberg L, Gersh BJ, Gladstone DJ, Glotzer TV, Gwynne K, Hankey GJ, Harbison J, Hillis GS, Hills MT, Kamel H, Kirchhof P, Kowey PR, Krieger D, Lee VW, Levin LA, Lip GY, Lobban T, Lowres N, Mairesse GH, Martinez C, Neubeck L, Orchard J, Piccini JP, Poppe K, Potpara TS, Puererfellner H, Rienstra M, Sandhu RK, Schnabel RB, Siu CW, Steinhubl S, Svendsen JH, Svennberg E, Themistoclakis S, Tieleman RG, Turakhia MP, Tveit A, Uittenbogaart SB, Van Gelder IC, Verma A, Wachter R, Yan BP; AF‐Screen Collaborators . Screening for atrial fibrillation: a report of the AF‐SCREEN International Collaboration. Circulation. 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 16. Orchard J, Lowres N, Neubeck L, Freedman B. Atrial fibrillation: is there enough evidence to recommend opportunistic or systematic screening? Int J Epidemiol. 2018;47:1372–1378. [DOI] [PubMed] [Google Scholar]
- 17. The Economist Intelligence Unit . Preventing stroke: uneven progress. A global policy research programme. Economist. 2017:1–28. [Google Scholar]
- 18. Britt H, Miller GC, Henderson J, Bayram C, Harrison C, Valenti L, Pan Y, Charles J, Pollack AJ, Wong C, Gordon J. General Practice Activity in Australia 2015–16: BEACH: Bettering the Evaluation and Care of Health. Sydney: Sydney University Press; 2016. [Google Scholar]
- 19. Eckman MH, Lip GY, Wise RE, Speer B, Sullivan M, Walker N, Kissela B, Flaherty ML, Kleindorfer D, Baker P, Ireton R, Hoskins D, Harnett BM, Aguilar C, Leonard AC, Arduser L, Steen D, Costea A, Kues J. Impact of an atrial fibrillation decision support tool on thromboprophylaxis for atrial fibrillation. Am Heart J. 2016;176:17–27. [DOI] [PubMed] [Google Scholar]
- 20. Jackson SL, Peterson GM, Vial JH. A community‐based educational intervention to improve antithrombotic drug use in atrial fibrillation. Ann Pharmacother. 2004;38:1794–1799. [DOI] [PubMed] [Google Scholar]
- 21. Das M, Panter L, Wynn GJ, Taylor RM, Connor N, Mills JD, Kirchhof P, Gupta D. Primary Care Atrial Fibrillation Service: outcomes from consultant‐led anticoagulation assessment clinics in the primary care setting in the UK. BMJ Open. 2015;5:e009267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McAlister FA, Man‐Son‐Hing M, Straus SE, Ghali WA, Anderson D, Majumdar SR, Gibson P, Cox JL, Fradette M; Decision Aid in Atrial Fibrillation (DAAFI) Investigators . Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: a cluster randomized trial. CMAJ. 2005;173:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orchard J, Freedman SB, Lowres N, Peiris D, Neubeck L. iPhone ECG screening by practice nurses and receptionists for atrial fibrillation in general practice: the GP‐SEARCH qualitative pilot study. Aust Fam Physician. 2014;43:315–319. [PubMed] [Google Scholar]
- 24. Orchard J, Lowres N, Freedman SB, Ladak L, Lee W, Zwar N, Peiris D, Kamaladasa Y, Li J, Neubeck L. Screening for atrial fibrillation during influenza vaccinations by primary care nurses using a smartphone electrocardiograph (iECG): a feasibility study. Eur J Prev Cardiol. 2016;23:13–20. [DOI] [PubMed] [Google Scholar]
- 25. Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, Albert DE, Freedman SB. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165:193–194. [DOI] [PubMed] [Google Scholar]
- 26. Peiris D, Usherwood T, Panaretto K, Harris M, Hunt J, Patel B, Zwar N, Redfern J, Macmahon S, Colagiuri S, Hayman N, Patel A. The Treatment of cardiovascular Risk in Primary care using Electronic Decision supOrt (TORPEDO) study‐intervention development and protocol for a cluster randomised, controlled trial of an electronic decision support and quality improvement intervention in Australian primary healthcare. BMJ Open. 2012;2:e002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peiris DP, Joshi R, Webster RJ, Groenestein P, Usherwood TP, Heeley E, Turnbull FM, Lipman A, Patel AA. An electronic clinical decision support tool to assist primary care providers in cardiovascular disease risk management: development and mixed methods evaluation. J Med Internet Res. 2009;11:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tran K, Leblanc K, Valentinis A, Kavanagh D, Zahr N, Ivers NM. Evaluating the usability and perceived impact of an electronic medical record toolkit for atrial fibrillation management in primary care: a mixed‐methods study incorporating human factors design. JMIR Hum Factors. 2016;3:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alamneh EA, Chalmers L, Bereznicki LR. The Tasmanian atrial fibrillation study: transition to direct oral anticoagulants 2011–2015. Cardiovasc Ther. 2017;35:e12254. [DOI] [PubMed] [Google Scholar]
- 30. Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallenhorst C, Lau JK, Brieger DB, Sy RW, Freedman SB. Feasibility and cost‐effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH‐AF study. Thromb Haemost. 2014;111:1167–1176. [DOI] [PubMed] [Google Scholar]
- 31. Gadsbøll K, Staerk L, Fosbøl EL, Sindet‐Pedersen C, Gundlund A, Lip GYH, Gislason GH, Olesen JB. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J. 2017;38:899–906. [DOI] [PubMed] [Google Scholar]
- 32. Rodríguez‐Bernal CL, Hurtado I, García‐Sempere A, Peiró S, Sanfélix‐Gimeno G. Oral anticoagulants initiation in patients with atrial fibrillation: real‐world data from a population‐based cohort. Front Pharmacol. 2017;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cowan JC, Wu J, Hall M, Orlowski A, West RM, Gale CP. A 10 year study of hospitalized atrial fibrillation‐related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J. 2018;39:2975–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Freedman B. Major progress in anticoagulant uptake for atrial fibrillation at last: does it translate into stroke prevention? Eur Heart J. 2018;39:2984–2986. [DOI] [PubMed] [Google Scholar]
- 35. Shaw EK, Howard J, West DR, Crabtree BF, Nease DE, Tutt B, Nutting PA. The role of the champion in primary care change efforts. J Am Board Fam Med. 2012;25:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jamtvedt G, Young JM, Kristoffersen DT, Thomson O'Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2003:CD000259. [DOI] [PubMed] [Google Scholar]
- 37. Lee TM, Ivers NM, Bhatia S, Butt DA, Dorian P, Jaakkimainen L, Leblanc K, Legge D, Morra D, Valentinis A, Wing L, Young J, Tu K. Improving stroke prevention therapy for patients with atrial fibrillation in primary care: protocol for a pragmatic, cluster‐randomized trial. Implement Sci. 2016;11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]


