Abstract
Background
Iatrogenic coronary artery injuries during percutaneous coronary interventions (PCI) often require emergent surgical management. Our study evaluated the early and long‐term outcomes in patients undergoing surgical treatment of iatrogenic PCI complications and identified the predictors of operative and long‐term mortality.
Methods and Results
Pre‐, intra‐ and post‐operative data and hospital outcomes of 168 consecutive patients undergoing cardiac surgical procedures for iatrogenic complications following PCI between December 1999 and July 2015, were prospectively collected in our computerized database. Logistic and Cox regression analyses were used to identify the independent predictors of operative and long‐term mortality. The mean age was 68.5±10.2 years and 35.7% were females. PCI complications included left anterior descending (38.7%), right coronary (29.2%), circumflex (13.1%), left main coronary artery injuries (19.0%), and acute myocardial infarction (66.7%), Type A aortic dissection (7.7%), cardiac tamponade (17.9%), and cardiogenic shock (CS) (46.4%). Operative mortality for corrective surgery was 20.8% and was independently predicted by critical preoperative state (odds ratio: 3.5; P=0.01). The 5‐ and 10‐year survival for all patients was 63.9±4.0% and 49.6±5.0%, which improved remarkably in hospital survivors (79.0±4.0% and 64.0±6.0%). Risk factors for long‐term mortality were critical preoperative state (hazard ratio: 3.5; P<0.0001) and coronary artery occlusion during PCI (hazard ratio: 2.6; P=0.002). The 5‐ and 10‐year freedom from major adverse cardiac and cerebrovascular events was 59.7±4.0% and 41.9±5.0%.
Conclusions
Iatrogenic injuries after PCI or coronary angiography requiring surgical correction are associated with a high operative and long‐term mortality. Patients developing acute coronary artery occlusion have a more guarded long‐term prognosis. Hospital survivors, however, have a superior long‐term survival.
Keywords: aortic surgery, complication, coronary artery bypass graft surgery, percutaneous coronary intervention, surgery
Subject Categories: Percutaneous Coronary Intervention, Cardiovascular Surgery, Revascularization, Complications, Mortality/Survival
Short abstract
See Editorial by Affronti and Ruel
Clinical Perspective
What Is New?
Percutaneous coronary interventions (PCI) are occasionally associated with complications that need surgical correction, the most reported one being coronary artery perforation.
Other complications such as Type A aortic dissection, coronary artery occlusion because of coronary artery dissection, thrombosis, stent dislocation, or an irretrievable guide wire have been seldom reported; our study is unique as it included all possible complications that could occur after PCI.
Additionally, it also assessed the long‐term outcomes of such patients, which have been unascertained to date.
What Are the Clinical Implications?
Iatrogenic dissection of coronary artery was the most common complication following PCI that needed urgent or emergent surgical intervention.
Patients who developed an acute coronary occlusion following 1 of the PCI‐related complications have the worst long‐term prognosis.
The preoperative clinical status of the patient, ie, the clinical condition after development of a PCI complication is the most crucial determinant of patient outcomes.
Introduction
Complications following percutaneous coronary intervention (PCI) are often successfully managed in the catheterization laboratory, but certain complications require emergent surgical intervention. One of the most dreadful, albeit rare, complications is coronary artery perforation, which occurs in the range from 0.1% to 3.0%.1, 2 It has been classified into 3 grades based on angiographic appearance: I, extraluminal crater without extravasation; II, epicardial fat or myocardial blushing; and III, extravasation through a perforation >1 mm diameter or cavity spilling. Although Grade III coronary perforations are the most catastrophic and dramatic complications of PCI and are associated with high mortality rates,1, 2, 3, 4 even low‐risk perforations can occasionally result in poor clinical outcomes.1 The need of surgical repair and/or revascularization has been reported to be in the range of 16% to 34% in such patients.3, 4 Other life threatening problems such as Type A aortic dissection following retrograde extension of a coronary artery dissection or occlusion of a coronary artery caused by stent dislocation or coronary artery dissection or thrombus formation around an irretrievable fragment of guide wire following rupture or a combination of these, adversely impacts in‐hospital outcomes.5 Such patients develop peri‐procedural myocardial infarction (MI) and consequently a poor clinical status that requires emergent surgery and high‐quality intensive care therapy (cardiopulmonary resuscitation [CPR], mechanical ventilation, inotropic and/or intra‐aortic balloon pump [IABP] and/or extracorporeal life support).5 Despite the exponential reduction in the incidence of emergent surgical interventions following coronary catheterization and PCI,6, 7 the increasing complexity of these procedures in the treatment of patients with severe multivessel and left main coronary artery disease (CAD)8 can potentially lead to complications that would require emergent surgery. Little information exists in literature regarding the outcomes, particularly the long‐term results, of surgical treatment of iatrogenic coronary artery injuries that occur during PCI.
Here we present our experience in the management of patients, who developed coronary artery or other iatrogenic injuries during coronary catheterization or PCI necessitating emergency surgery. We report the early and long‐term outcomes and the predictors of hospital and late mortality observed in these patients.
Methods
Data Source
A total of 161 200 coronary diagnostic and interventional procedures were performed in the catheterization laboratory in our institute between January 1999 and July 2015. Of these, 133 patients (0.08%) developed iatrogenic complications that necessitated emergent surgical rescue procedures. An additional 35 patients, transferred from other referral centers, also underwent emergent surgery for such complications, resulting in a total of 168 patients undergoing emergency corrective surgery in our institute for complications that occurred during diagnostic coronary angiography (CA) or PCI. The patients who developed such complications, but were managed successfully by bail‐out interventional procedures were not included in the present study. The demographic data, preoperative clinical parameters, operative and outcome data of all patients were prospectively collected in our computerized database. Additionally, individual patient charts were revisited to note the timing of the catheterization and surgical procedures. The study was approved by the university Ethics Committee. Individual patient informed consent was waived, because it was a retrospective study.
Definitions of Preoperative Explanatory Variables
Some specific core baseline preoperative explanatory variables collected in our database are defined below. Renal insufficiency was graded according to creatinine clearance (1=>90 mL/min per 1.73 m2, 2=60–89 mL/min per 1.73 m2, 3=30–59 mL/min per 1.73 m2, 4=15–29 mL/min per 1.73 m2, 5=<15 mL/min per 1.73 m2).9 Preoperative critical state was characterized by inotrope and/or IABP and/or ventilation requirement, the need for CPR, and presence of cardiogenic shock (CS) before surgery. Left ventricular ejection fraction was categorized as: >60%, ≤60%–≥30%, and <30%. CPR involved mechanical chest compressions with airway management and artificial mechanical ventilation with intravenous administration of inotropes through a central or peripheral venous catheter. Cardiac tamponade was defined as the presence of systolic blood pressure <90 mm Hg with echocardiographic evidence of pericardial effusion with features of tamponade such as significant dilatation of the inferior vena cava with loss of collapsibility on inspiration, or diastolic collapse of the right ventricular free wall.4 CS was defined as a systolic blood pressure <90 mm Hg for at least 30 minutes with a severe reduction in cardiac index (<1.8 L/min per m2 without support or <2.0–2.2 L/min per m2 with support) and elevated left or right ventricular filling pressures; the need for inotropes/IABP/extracorporeal membrane oxygenation (ECMO) to maintain a systolic blood pressure ≥90 mm Hg; or inadequate peripheral or end‐organ perfusion (pH <7.3, serum lactate >2 mmol/L, cool extremities, urine output <30 mL/h, altered mental status), acute pulmonary congestion, or edema; and a heart rate >90 bpm or the need for CPR.10 The criteria used for the diagnosis of an acute MI in Germany are based on the universal definition of MI.11
Management Strategy
Patients admitted to our hospital with mechanical complications that are produced iatrogenically during diagnostic CA and/or PCI are usually in a critical clinical status and require immediate corrective therapy. Such patients are commonly transferred to our department with high‐dose inotropic support and are often ventilated. In the worst case scenario, mechanical assist systems such as IABP or ECMO support are already introduced in the catheterization laboratory. The commonest complications encountered in such patients are coronary artery perforation, dissection or occlusion; Type A aortic dissection and cardiac tamponade due to excessive bleeding into the pericardium follwing coronary artery injury. Some patients may develop >1 complication as well. Therefore, our institution policy entails immediate surgery in the next available operating room. The type of surgery performed depends on the type and the extent of injury or complications, the severity of coronary artery disease and the hemodynamic status of the patient. Patients presenting with isolated coronary artery occlusion because of stent dislocation or dissection undergo emergent coronary artery bypass graft (CABG) and those with coronary artery perforation undergo emergent exploration, closure of the perforation and CABG. Such patients are treated with on‐ or off‐pump CABG depending on the patient's hemodynamic status and surgeon preference, although our departmental preference is off‐pump CABG in hemodynamic stable and beating‐heart on‐pump CABG in unstable patients.12 Patients developing a Type A aortic dissection undergo emergent replacement of the ascending aorta. The choice of partial or total arch replacement depends upon the distal extent of the dissection and the presence of a re‐entry tear in the aortic arch. Reconstruction or replacement of the aortic root is performed if the dissection extends proximally from the coronary ostium towards the aortic valve. Additionally, CABG is performed in the presence of coronary artery injury or if coronary artery lesions have not been treated with PCI.
Postoperative Outcomes
The primary outcome of this study was operative mortality which was defined as mortality occurring within the hospital stay of the patient or within 30 days following surgery. Long‐term survival and freedom from major adverse cardiac and cerebrovascular events, which included mortality, MI, stroke, and repeat revascularization, were the secondary outcomes. Follow‐up was obtained by personal or phone contact with patients and family members and mailed questionnaires, with supplemental information supplied by family physicians and referring cardiologists. The closing interval, that is, the time period during which all surviving patients included in the analysis were exclusively followed up for the present study, was from October 2017 through March 2018. The follow‐up was 90% complete with 17 patients lost to follow‐up. The follow‐up ranged from 1 day to 16.2 years with a mean follow‐up of 5.6±4.3 years.
Data Analysis
All statistical analyses were performed using SPSS 17.0 (Chicago, IL).Categorical variables are expressed as frequencies and percentages and continuous variables are expressed as mean±SD. All perioperative variables that had a univariate P<0.25 or those judged to be clinically important were submitted to a logistic regression model by stepwise selection. Multivariate logistic regression methods were used to determine the independent predictors of operative mortality and are expressed as odds ratios (OR) and 95% CI. Long‐term survival and freedom from major adverse cardiac and cerebrovascular events were calculated by Kaplan–Meier methods using 95% CI. Independent predictors of long‐term survival were determined with Cox proportional hazards analysis. P<0.05 were considered statistically significant.
Results
Demographic and Preoperative Data
The demographic data and preprocedural (before PCI) characteristics of all patients are depicted in Table 1.
Table 1.
Preoperative Data
| Preoperative Variables | All Patients, n=168 |
|---|---|
| Age, y (mean±SD) | 68.5±10.2 |
| Female sex, n (%) | 60 (35.7) |
| Cardiac risk factors, n (%) | |
| Diabetes mellitus | 54 (32.1) |
| Systemic hypertension | 158 (94.0) |
| Hyperlipidemia | 104 (61.9) |
| Smoker | 56 (33.3) |
| Comorbidities | |
| Chronic obstructive pulmonary disease, n (%) | 12 (7.0) |
| Stroke, n (%) | 15 (8.9) |
| Peripheral vascular disease, n (%) | 30 (17.9) |
| Glomerular filtration rate, mL/min per 1.73 m2 (mean±SD) | 71±37 |
| Cardiac parameters | |
| Previous myocardial infarction (>90 d), n (%) | 31 (18.5) |
| Left ventricular ejection fraction, % | 52±16 |
| Triple vessel disease, n (%) | 74 (44.0) |
| Left main disease, n (%) | 31 (18.4) |
| Primary indication for PCI or coronary angiography, n (%) | |
| Stable angina pectoris | 98 (58.3) |
| Acute coronary syndrome | 62 (36.9) |
| Asymptomatic at time of coronary angiography | 8 (4.8) |
| Site of intervention, n (%) | |
| In hospital | 133 (79.2) |
| Referral center | 35 (20.8) |
| Clinical and hemodynamic status following PCI complications, n (%) | |
| Myocardial infarction after PCI complication | 112 (66.7) |
| ST‐segment–elevation myocardial infarction | 87 (51.8) |
| Non–ST‐segment–elevation myocardial infarction | 25 (14.9) |
| Cardiac tamponade | 26 (15.5) |
| Inotropic support | 114 (67.8) |
| High dose of inotropic support | 65 (38.7) |
| Critical preoperative state | 96 (57.1) |
| Resuscitation | 34 (20.2) |
| Ventilation | 26 (15.6) |
| Cardiogenic shock | 78 (46.4) |
| Lactate level, mmols/L (mean±SD) | 3.3±4.1 |
| ECMO implantation | 6 (3.5) |
| IABP implantation | 19 (11.3) |
| Time interval from PCI to surgery, min (mean±SD) | 167±107 |
ECMO indicates extracorporeal oxygenation; IABP, intra‐aortic balloon pump implantation; PCI, percutaneous coronary implantation.
Majority of the patients had systemic hypertension and hyperlipidemia, whereas one third each was diabetics or smokers. Comorbidities were less prevalent. Almost two thirds of the patients had triple vessel or left main coronary artery disease and most patients had good left ventricular function. More than a third of patients underwent PCI or CA for acute coronary syndrome. The vast majority of patients referred to us following complications after CA or PCI were from our own department of cardiology. Two thirds of all these patients suffered an acute MI, most commonly ST‐segment–elevation MI (STEMI), following a complication, resulting in development of CS in almost half of them. More than a third of patients were on high‐dose inotropic support, with a few requiring IABP and/or ECMO as well. The overall acuity of patients was, therefore, high. The mean time‐interval between the beginning of the PCI procedure and the exact time of skin incision at the time of surgery for patients referred from other hospitals was 306±111 minutes and for those referred from within the hospital was 160±102 minutes (P<0.001). All but 1 patient had received ≥1 types of antiplatelet and anticoagulant agents (Table 2).
Table 2.
Anticoagulation Therapy Immediately Before Surgery
| Drugs, n (%) | All Patients, n=168 |
|---|---|
| Single drug | |
| Aspirin | 17 (10.1) |
| Heparin | 19 (11.3) |
| Newer oral anticoagulants | 5 (2.9) |
| Combination of drugs | |
| Aspirin+newer oral anticoagulants | 2 (1.2) |
| Aspirin+glycoprotein IIb/IIIa inhibitors | 3 (1.8) |
| Aspirin+P2Y12 inhibitors | 39 (23.2) |
| Aspirin+heparin | 39 (23.2) |
| Aspirin+glycoprotein IIb/IIIa inhibitors+P2Y12 inhibitors | 7 (4.2) |
| Aspirin+glycoprotein IIb/IIIa inhibitors+P2Y12 inhibitors+heparin | 7 (4.2) |
| Aspirin+P2Y12 inhibitors+heparin | 21 (12.5) |
| Aspirin+glycoprotein IIb/IIIa inhibitors+heparin | 4 (2.4) |
| Glycoprotein IIb/IIIa inhibitors+heparin | 4 (2.4) |
| No anticoagulation | 1 (0.6) |
The trends in incidence of complications over the time‐frame of the study are shown in Figure 1.
Figure 1.
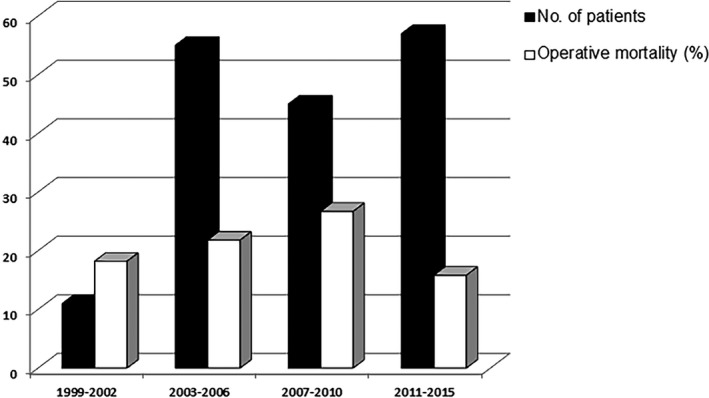
Trends in incidence of complications and operative mortality over the time‐frame of the study.
The details about the type of iatrogenic complication are mentioned in Table 3. Coronary artery dissection was the commonest complication, followed by occlusion and perforation. One third of patients had a combination of injuries. The details of native coronary lesions, the cause of complications and rescue procedures undertaken by the interventional cardiologist for managing complications following PCI/CA were reliably documented only in 133 patients, who underwent PCI/CA in our institute. The coronary artery lesions that were supposed to be treated by the intervention, but resulted in a complication were bifurcation stenoses in 14 patients and subtotal long lesions (Type B/C stenosis) or chronic total occlusions in 73 patients. The remaining 46 patients had a variety of lesions, which did not particularly pose an increased procedural risk. The coronary injury/perforation/occlusion was caused by the guide wire in 46 patients, stent implantation in 47 patients, intubation of a coronary vessel by an angiographic catheter in 7 patients and following balloon inflation before, during or after stent implantation in 26 patients. The catheterization laboratory reports of patients from referral centers lacked information and, therefore, are not mentioned here. The left anterior descending (LAD) artery was the most commonly affected vessel (n=65). More than 1 vessel was involved in a few patients. Less than one fifth of all patients developed a cardiac tamponade.
Table 3.
Indications for Emergency Surgery
| Indications, n (%) | All Patients, n=168 |
|---|---|
| Type A aortic dissection | 13 (7.7) |
| Hemopericardium causing cardiac tamponade | 30 (17.9) |
| Complications involving coronary arteries, n (%) | |
| Lesion location | |
| Left anterior descending artery territory, n (%) | 65 (38.7) |
| Right coronary artery territory, n (%) | 49 (29.2) |
| Left main stem territory, n (%) | 32 (19.0) |
| Circumflex artery territory, n (%) | 22 (13.1) |
| Multiple locations, n (%) | 21 (12.5) |
| Lesion type | |
| Dissection, n (%) | 126 (75.0) |
| Occlusion, n (%) | 32 (19.0) |
| Perforation, n (%) | 19 (11.3) |
| Stent dislocation, n (%) | 8 (4.8) |
| Irretrievable guide wire, n (%) | 3 (1.8) |
| Combination of lesions | 56 (33.3) |
Patients, who developed complications following PCI, were referred for surgical treatment only when the complications could either not be corrected or were found to be unsuitable for repair by interventional techniques. Failed recanalization of the native coronary vessel following dissection or flow limitation (TIMI [flow grades based on results of the Thrombolysis In Myocardial Infarction trial]=0–1) after PCI/CA was noted in 101 patients. It occurred because of inability to cross the lesions/injured segment with a guide wire or failure to establish a functional lumen. Failure to over‐stent a dissection or perforation to establish normal coronary blood flow or stop bleeding into the pericardial cavity, respectively, was observed in 34 patients. All patients with evidence of pericardial effusion (n=30) received a pericardial drain under echocardiographic guidance to prevent cardiac tamponade.
As already depicted in Table 1, patients who were in a critical preoperative state following PCI/CA complications because of development of hemodynamic deterioration with or without CS, and/or acute coronary syndrome, and/or cardiac tamponade or persistent bleeding into the pericardial cavity despite corrective interventional measures had a well‐defined indication for emergent surgery. However, hemodynamically stable patients with a significant blood flow limitation through a native coronary vessel (TIMI [flow grades based on results of the Thrombolysis In Myocardial Infarction trial] 0–1) attributable to an occlusion or dissection of the native vessel, or iatrogenic Type A aortic dissection or an irretrievable fractured guide wire that is trapped or jammed in the native coronary vessel were also considered surgical emergencies because of the concern of development of acute coronary syndrome, extension of the dissection or development of thrombus in the area of the trapped guide wire.
Intraoperative Data
The intraoperative details are shown in Table 4. The vast majority of patients underwent CABG. The technique used to perform CABG was almost equally divided between off‐pump, on‐pump beating heart, and on‐pump arrested heart CABG. Only 2 patients required conversion from off‐ to on‐pump CABG because of hemodynamic instability. The mean number of distal anastomoses in all patients who underwent CABG was 1.8±0.8. The LAD was grafted by a left internal mammary artery in 51 patients (78.5%). Surgical revascularization of all significant coronary stenoses was attempted, so that almost 90% of the patients underwent complete revascularization. Of the 18 patients who underwent incomplete revascularization, 13 had heavily calcified and/or occluded or extremely small vessels, which if not grafted, were not considered to have a negative impact on the outcomes, especially in acute situations when an expeditious operation was desired. The remaining 5 patients had a massive hematoma on the anterolateral wall following an injury, which made it impossible to find/identify the LAD. The diagonal branch was anastomosed in these patients.
Table 4.
Intraoperative Data
| Intraoperative Data | All Patients, n=168 |
|---|---|
| Length of surgery, min (mean±SD) | 173±98 |
| Cardiopulmonary bypass time, min (mean±SD) | 65±71 |
| Aortic cross‐clamp time, min (mean±SD) | 19±35 |
| Operative procedures, n (%) | |
| CABG surgery | 148 (88.1) |
| Off‐pump CABG | 51 (34.5) |
| On‐pump CABG, beating heart | 44 (29.7) |
| On‐pump CABG, arrested heart | 53 (35.8) |
| Conversion from off‐ to on‐pump CABG | 2 (1.4) |
| LIMA‐LAD bypass grafting | 95 (64.2) |
| Incomplete revascularization | 18 (12.2) |
| Aortic surgery | 13 (7.7) |
| Isolated ascending aorta replacement | 6 (3.6) |
| Partial/total aortic arch repair | 7 (4.2) |
| Reconstruction of the sinus Valsalva | 1 (0.6) |
| Valve surgery | 3 (1.8) |
| Aortic valve replacement | 2 (1.2) |
| Mitral valve replacement | 1 (0.6) |
| Exploratory sternotomy | 19 (11.4) |
| Suture repair of injured myocardium | 12 (7.2) |
CABG indicates coronary artery bypass graft; LAD, left anterior descending artery; LIMA, left internal mammary artery.
An isolated exploratory sternotomy and pericardiotomy to relieve cardiac tamponade and to find and address the site of injury was performed in 19 patients. Suture repair of the injured myocardium was performed in 12 patients. Additionally, 13 patients underwent replacement of the ascending aorta with/without partial or total arch repair for iatrogenic Type A aortic dissection. Of these, 2 (1.2%) patients underwent supracoronary ascending aorta replacement and 4 patients underwent aortic root replacement. Among patients undergoing arch surgery, 3 underwent partial‐ and 4 total‐arch replacement. Of the latter, 1 patient received a “conventional elephant trunk” procedure. Of the 2 patients undergoing aortic valve replacement, 1 underwent enlargement of the aortic annulus with patch reconstruction of the sinus of Valsalva for an iatrogenic aortic wall perforation. One patient underwent mitral valve replacement for severe mitral insufficiency.
Postoperative Outcomes
The postoperative outcomes are listed in Table 5. Overall 35 patients died in the early postoperative period. Of these, 29 were cardiac deaths, 1 patient died following multi‐organ system failure, 1 from sepsis, 2 because of neurological complications, and 1 from respiratory failure. The cause of death in 1 patient was unknown. The operative mortality was the lowest in the latest time cohort (Figure 1). One third of the patients developed low‐output syndrome, postoperatively. Most of them were managed with IABP and/or ECMO in addition to inotropic therapy.
Table 5.
Postoperative Outcomes
| Outcomes | All Patients, n=168 (%) |
|---|---|
| Operative mortality, n (%) | 35 (20.8) |
| Low‐output syndrome, n (%) | 54 (32.0) |
| Postoperative IABP implantation, n (%) | 36 (24.0) |
| Postoperative ECMO, n (%) | 20 (12.0) |
| Postoperative myocardial infarction, n (%) | 9 (54.0) |
| Stroke, n (%) | 16 (9.5) |
| Sepsis, n (%) | 4 (2.3) |
| New onset dialysis‐dependent renal failure | 31 (18.4) |
| Respiratory failure, n (%) | 43 (25.6) |
| Cardiopulmonary resuscitation, n (%) | 17 (10.1) |
| Gastrointestinal complications, n (%) | 15 (8.9) |
| Re‐exploration for bleeding, n (%) | 9 (5.4) |
| Platelet transfusion, mean±SD | 1.9±3.9 |
| Packed red blood cell transfusion, mean±SD | 8.6±13.7 |
| Fresh frozen plasma transfusion, mean±SD | 6.4±11.6 |
ECMO indicates extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump.
Predictors of hospital mortality
Univariate analysis revealed presence of a critical preoperative state (OR: 9.2; 95% CI: 1.3–62.7; P=0.02), CS (OR: 9.7; 95% CI: 1.2–81.2; P=0.04), pericardial effusion causing cardiac tamponade (OR: 6.1; 95% CI: 1.0–57.7; P=0.05), age at surgery (OR: 1.1; 95% CI: 1.0–1.3; P=0.02), and performance of PCI in our hospital (OR: 12.0; 95% CI: 1.9–70.0; P=0.01) to be the dependent factors of operative mortality. However, critical preoperative state (OR: 3.5; 95% CI: 1.3–9.2; P=0.01) was found to be the only independent predictor of operative mortality with good left ventricular ejection fraction being protective (OR: 0.9; 95% CI: 0.94–0.99; P=0.01).
Follow‐Up
An additional 33 patients died during follow‐up. The overall survival at 1, 5, 10, and 12 years was 71.8±4.0%, 63.9±4.0%, 49.6±5.0%, and 44.6±6.0%, respectively (Figure 2).
Figure 2.
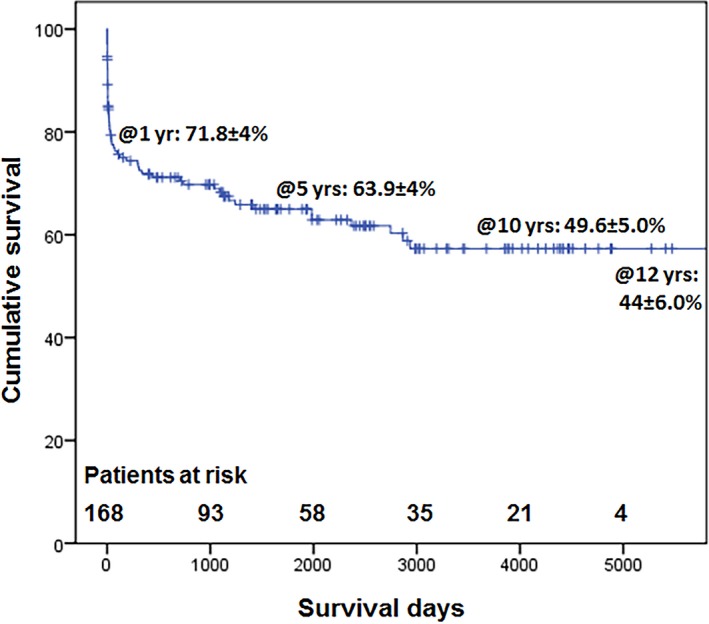
Cumulative survival for all patients.
On exclusion of operative mortality from the analysis, the corresponding survival was 89.8±3.0%, 79.0±4.0%, 64.0±6.0%, and 60.0±6.0% (Figure 3).
Figure 3.
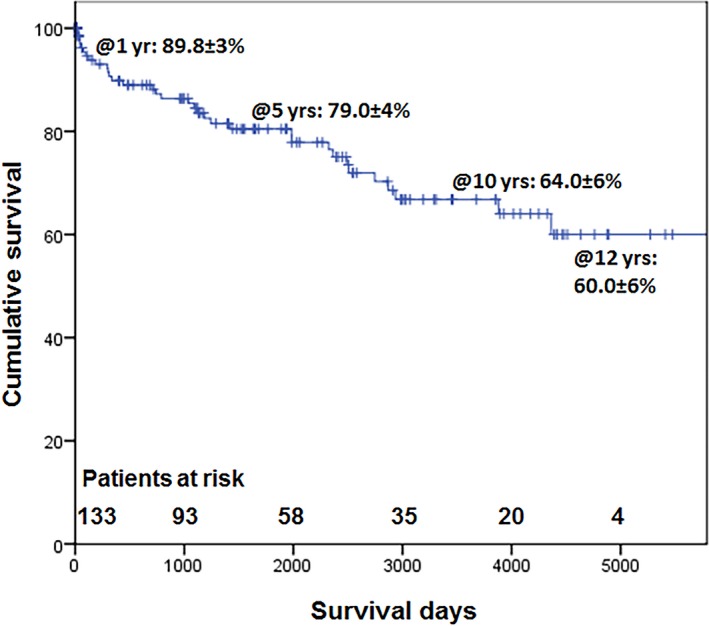
Cumulative survival for survivors (after exclusion of operative mortality).
Freedom from major adverse cardiac and cerebrovascular events for all patients was 71.3±4.0%, 59.7±4.0%, 41.9±5.0% and 35.8±5.0%, respectively (Figure 4).
Figure 4.
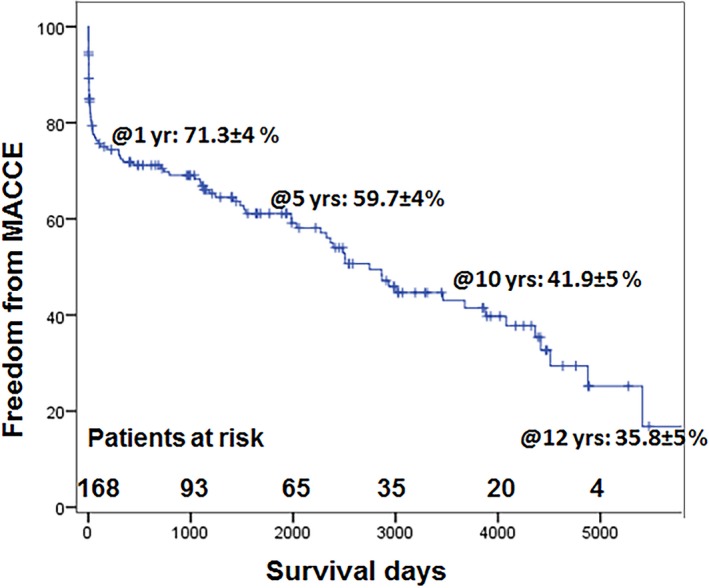
Freedom from major cardiac and cerebrovascular events for all patients. MACCE indicates major adverse cardiac and cerebrovascular events.
Cox regression analysis, with all patients included, identified critical preoperative state (hazard ratio [HR]: 3.5; 95% CI: 1.9–6.2; P<0.0001) and coronary artery occlusion during PCI (HR: 2.6; 95% CI: 1.4–4.7; P=0.002) as independent predictors of long‐term mortality, whereas that performed only for survivors revealed preoperative stroke (HR: 4.5; 95% CI: 0.98–20.5; P=0.05) and occlusion of the coronary artery during PCI (HR: 3.4; 95% CI: 1.5–7.7; P=0.004) as independent predictors. Good left ventricular ejection fraction (HR: 0.9; 95% CI: 0.95–0.98; P<0.0001) was a predictor for longer survival. More than half of survivors have reported good exercise tolerance (NYHA: New York Heart Association Class I=55.6%, NYHA [New York Heart Association] Class II=31.6%), and freedom from angina pectoris ( CCS: Canadian Cardiovascular Society I=62.0%, CCS: Canadian Cardiovascular Society II=31.6%) at the time of follow‐up.
Discussion
Few publications exist in literature that report the outcomes of surgical management of iatrogenic complications produced during CA or PCI.1, 2, 3, 4 According to published literature, emergency surgery is rarely required in patients following PCI complications.1, 2, 3, 4, 5, 6, 13 The results of a French multicenter registry revealed that the observed need for emergency bypass surgery performed within 24 hours of percutaneous transluminal angioplasty for years 1995 and 1996 was 0.38% and 0.32%, respectively,7 of which 101 (0.18%) procedures were for PCI complications. More contemporary reports by Al Lamee and coworkers and Shimony et al revealed that only 9 of 24 465 (0.04%) and 4 of 9568 (0.04%) patients, respectively, undergoing coronary interventions required emergency surgery for complications.4, 14 Similarly, in 1 of the most recent series by Parsh and colleagues, only 22 of the total of 181 590 (0.01%) patients undergoing coronary interventions needed CABG for coronary perforations.15 Our study, that spans almost 2 decades, also demonstrated a low rate of emergent surgical intervention (0.08%) for treating iatrogenic complications following diagnostic CA or PCI. The complication rates necessitating surgery ranged from a minimum of 1 to a maximum of 18 in 1 calendar year. The lower rate of rescue surgery in patients with complications following coronary interventions is probably multifactorial. Availability of more sophisticated hardware such as guide wires and stents in more recent times as compared with those used in the 1990s, the increasing success in the use of bail‐out stent procedures in the event of complications, and the large experience of interventional cardiologists in high‐volume centers are most likely responsible for the lower rate of complications during coronary interventions and the successful management of such complications if they do occur.5, 6, 7 Therefore, surgical treatment is needed only in cases of catastrophic unpredictable events that cannot be adequately managed by interventional methods, such as over‐stenting for localized coronary artery dissection, coronary artery balloon occlusion in cases of Ellis Class I or II coronary perforations, or pericardiocentesis for a hemopericardium.1, 2, 3 Grade III coronary perforations, usually caused by a rupture of the coronary artery, necessitate emergent corrective surgery in 20% to 40% of patients,3 because such patients commonly develop sudden hemodynamic collapse following cardiac tamponade because of rapid extravasation of blood into the pericardial cavity.1 Nonetheless, Al‐Lamee and coworkers reported that only 2 out of 56 patients (3.7%) with grade III perforations required CABG with repair of the perforation.4 They attributed their findings to the introduction of newer techniques such as covered stent implantation and experienced operators. In the present series, emergency surgical procedures were required in only 0.08% of the total of 161 200 patients, who underwent coronary interventions in our institution. We cannot calculate the complication rate for the entire cohort of patients analyzed in the current report, because we neither have the total number of patients undergoing coronary procedures at all our referral centers nor do we know whether all patients who developed complications were exclusively referred to our hospital. The variation observed in the necessity of surgery for managing complications during coronary procedures is also partly attributable to the small size of studies published, so that a small change in absolute numbers precipitates large differences in proportions.
The patients included in our study were heterogeneous with regard to the cardiac structure that was injured, which resulted in a wide variability in the type of the iatrogenic injuries observed. However, we included only those patients who underwent surgery for complications occurring during coronary interventions. Patients undergoing CABG for a failed PCI without any injury to the coronary tree or aorta were not included. The commonest indication for emergent surgery was coronary artery dissection, followed by occlusion and perforation (Table 3). In contrast, an old report from the French multicenter registry found stent complications such as thrombosis or lost stent to be the most prevalent indications for emergency CABG, when patients with percutaneous transluminal angioplasty failure as an indication were excluded.7 Few patients had coronary dissections, which according to the authors, was probably because of downsizing of guiding catheters that reduce the risk of coronary damage. The incidence of coronary perforation was, however, similar.
The most remarkable aspect of our study was the inclusion of all possible complications after PCI, ranging from coronary artery perforations to aortic dissections. The preoperative clinical status of our patients was, therefore, often critical, which is evident from the fact that almost half of them were in CS with >50% of patients suffering an acute STEMI because of the complication, one fifth and one sixth required CPR and mechanical ventilation, respectively, and mean lactate levels were 3.3±4.1 mmol/L before corrective surgery. The acuity of our patients was, therefore, much higher than that reported in other studies.5, 6, 7, 13, 16 The incidence of patients with CS and left ventricular failure in the French multicenter registry was only 11% and 15%, respectively.7 Additionally, our patients were older and had a higher incidence of multivessel and left main coronary artery disease as compared with other published series.5, 6, 7, 13, 16 The high incidence of acute MI could obviously be because of the sudden closure of coronary arteries following coronary artery dissection or occlusion, which may jeopardize a large area of myocardium depending upon the vessel involved and location of injury. The left main and the LAD coronary arteries, being responsible for blood supply to the majority of the myocardial territory in most patients, were the most frequently injured vessels (47.7%) in our series. Furthermore, if the injured coronary artery does not have a severe stenosis before the PCI complication, the myocardial damage may even be greater because of the lack of the protective effect of collateral circulation that usually develops in areas supplied by highly stenosed or occluded vessels. A more recent study by Slottosch et al, which described their experience of 52 patients with coronary artery injuries, also reported that the LAD and left main coronary arteries were involved in 50% of their patient cohort and the incidence of CS was 42.3%.17 The majority of complications were either dissections or occlusions of coronary vessels, which was similar to our observations (Table 3).
Overall operative mortality in the present study was 20.8%, which was higher than that reported in literature for patients undergoing CABG for PCI complications.7, 17 One of the factors responsible for this difference could be the inclusion of complications other than coronary injuries in our study. Of 13 patients with iatrogenic Type A aortic dissection undergoing aortic repair, 5 (38.5%) patients died. Our group has already shown that the in‐hospital mortality for iatrogenic type A aortic dissection developing during cardiac surgery is 42%.18 Secondly, among the patients with coronary injuries, one fifth (n=32) had total occlusion of the coronary artery. Such patients also had a high‐operative mortality (25%). Additionally, one third of all patients had a combination of various coronary injuries, which could have contributed to the higher mortality. Furthermore, the LAD was involved in almost 40% of the injuries. A third key factor was the higher prevalence of critical clinical status (57%) of patients after the occurrence of PCI complications but before surgery in our series (Table 1). It was the only independent predictor of operative mortality. Pericardial effusions causing cardiac tamponade and eventually CS, both of which partly define critical preoperative state and were associated with significantly higher operative mortality on univariate analysis, could explain the above finding. Our group has already demonstrated that emergency CABG in patients with acute MI complicated by CS is associated with high in‐hospital mortality (33%).19 Patients with high‐dose inotropic support, which was observed in almost 40% of our patients, are also assumed to be in a critical preoperative state. Lazar et al revealed the presence of inotropic support as an independent predictor for in‐hospital mortality in a study involving 117 patients with complications during intervention.16 Some patients with critical preoperative state are wheeled into the operating room in extremely low cardiac output or under CPR. Such patients face a prohibitively high mortality of up to 32% and 47%, respectively, as shown by Carey et al.6
An important factor to consider is the time delay between the occurrence of PCI complication and surgery. The mean time interval between PCI and the start of surgery in our study was 167±107 minutes. It was significantly longer for patients referred to us from other hospitals (306±111 minutes) as compared with those referred from within the hospital (160±102 minutes) (P<0.001). One would expect that the latter group of patients would have better outcomes because of earlier implementation of corrective measures. However, univariate analysis showed that in‐hospital patients had a 12 times increased risk of mortality; this could be because of the referral of relatively stable patients with lower risk profile from external centers. Patients with severe hemodynamic instability refractory to resuscitation or stabilization measures would have either been referred to other nearby hospitals with cardiac surgical facilities or would have died before transfer. Slottosch et al attributed the lack of difference in in‐hospital cardiac mortality between patients undergoing PCI externally (11.4%) and on‐site (17.6%) (P=0.7) to the exclusion of patients with CS or those requiring ongoing resuscitation, because of the inability to transfer such patients.17 Establishment of ECMO‐based transfer of patients in refractory CS could be able to salvage many of these patients.20
Once the patients were transferred to our division, they were operated upon in the next available operating room to reduce the duration of myocardial ischemia and reduced perfusion to other end‐organs. Our hospital policy regarding the operative technique of CABG in patients with acute MI and CS have been described in detail in previous publications.12, 19, 21 The use of left internal mammary artery to the LAD was used in only 64.5% of patients. This could either be because of the fact that the LAD was not diseased or involved in the complication or in some cases the surgeons wanted to avoid additional ischemic time involved in the harvesting of left internal mammary artery.6, 13 Furthermore, in a salvage situation under high dose inotropes, a vein graft can provide more reliable immediate flow than arterial grafts. A major apprehension in performing such corrective surgeries is the extremely high probability of postoperative bleeding attributable to multiple anticoagulant and antiplatelet medications administered in these patients during PCI or at the time of rescue procedures after the complication has occurred. In the present series, the majority of patients received 2 and some even 3 anticoagulant and antiplatelet medications (Table 2). This resulted in a higher incidence of postoperative re‐exploration for bleeding, which was twice that observed in our patients undergoing CABG within 24 hours of non‐STEMI.19 The consumption of red blood cells, platelets and fresh frozen plasmas was also 2 times higher.
To the best of our knowledge, our study is probably the only one that reports the long‐term outcomes in patients undergoing emergency surgery for complications following PCI. The 1‐, 5‐, and 10‐year survival for all patients was 71.8±4.0%, 63.9±4.0%, and 49.6±5.0%, respectively. However, when operative mortality was excluded the corresponding survival rates were 89.8±3.0%, 79.0±4.0%, and 64.0±6.0%, which demonstrates that the maximum number of deaths occurred in the perioperative period. Lemmert et al depicted a similar trend in their study involving isolated coronary perforations.22 Their 30‐day and 1‐year mortality were 10.7% and 7.1%, respectively. The relatively lower mortality in their series was because the study involved only isolated coronary perforations, the majority of which were treated interventionally. Thus, the complexity of the injury and the consequences thereof were relatively benign compared with those in our series. Similarly, the overall 1‐, and 5‐year survival in this series was inferior to that observed in our study involving patients with non‐STEMI undergoing CABG (86.2±1% and 73.1±2%, respectively),19 but much better than that noted in our patients with acute MI complicated by CS (5‐year survival: 42.6±2%).21 However, after exclusion of operative mortality from the analysis, it was comparable if not better than that seen in non‐STEMI patients following CABG. This demonstrates that the most crucial period that determines the survival in patients undergoing corrective surgery is the perioperative period, particularly the time‐interval between the occurrence of a complication after PCI and surgery. This is further confirmed by the emergence of critical preoperative state and occlusion of a coronary vessel as significant independent predictors of late mortality. The latter usually results in a STEMI, the size and severity of which depend on the vessel and location of occlusion. Considering that 40.6% of discharged patients with coronary occlusion died at follow‐up, one can assume that it must have produced large‐sized infarcts, which are always associated with a higher incidence of CS perioperatively and poor survival at follow‐up. When Cox regression analysis was repeated only for survivors, preoperative stroke and coronary artery occlusion predicted late mortality. Preoperative stroke rate in this series could be considered as a marker of poor hemodynamic status, because it could have occurred during CPR, which was not uncommon (20.2%) in these patients. Contrary to survival, freedom from major adverse cardiac and cerebrovascular events appears to drop at a steady rate throughout follow‐up (Figure 3). Thus, these patients are highly susceptible to adverse events emphasizing the negative long‐term impact of iatrogenic complications occurring during PCI.
Study Limitations
The present study is retrospective in nature and, therefore, is susceptible to all the inherent weaknesses thereof. Selection bias is difficult to eliminate in such retrospective studies. This was probably truer for patients referred to us from external centers. However, our institute policy is to treat all patients with iatrogenic lesions irrespective of age and comorbidities, thereby reducing selection bias to a great extent. Although the sample size is relatively small, it is probably the largest study involving patient undergoing corrective cardiac surgery for complications occurring during PCI. It is a descriptive study with no control group. Because of the nature of the problem at hand, performing a randomized trial in such clinical scenarios as described in our study is near impossible. Therefore, retrospective studies like ours offer the best possible evidence.
Conclusions
Iatrogenic coronary artery injuries after PCI or coronary angiography requiring surgical correction are associated with a high operative and long‐term mortality despite immediate surgical treatment. Patients developing an acute coronary occlusion during PCI have a more guarded long‐term prognosis. Development of a critical clinical status before surgery is strongly associated with higher odds of early and late postoperative death, thereby showing that the perioperative period is the most crucial determinant of patient outcomes, which is further confirmed by superior long‐term survival in hospital survivors.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e010940 DOI: 10.1161/JAHA.118.010940)
References
- 1. Ellis SG, Ajluni S, Arnold AZ, Popma JJ, Bittl JA, Eigler NL, Cowley MJ, Raymond RE, Safian RD, Whitlow PL. Increased coronary perforation in the new device era: incidence, classification, management, and outcome. Circulation. 1994;90:2725–2730. [DOI] [PubMed] [Google Scholar]
- 2. Ajluni SC, Glazier S, Blankenship L, O'Neill WW, Safian RD. Perforations after percutaneous coronary interventions: clinical, angiographic, and therapeutic observations. Cathet Cardiovasc Diagn. 1994;32:206–212. [DOI] [PubMed] [Google Scholar]
- 3. Javaid A, Buch AN, Satler LF, Kent KM, Suddath WO, Lindsay J Jr, Pichard AD, Waksman R. Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2006;98:911–914. [DOI] [PubMed] [Google Scholar]
- 4. Al‐Lamee R, Ielasi A, Latib A, Godino C, Ferraro M, Mussardo M, Arioli F, Carlino M, Montorfano M, Chieffo A, Colombo A. Incidence, predictors, management, immediate and long‐term outcomes following grade III coronary perforation. JACC Cardiovasc Interv. 2011;4:87–95. [DOI] [PubMed] [Google Scholar]
- 5. Reinecke H, Fetsch T, Roeder N, Schmid C, Winter A, Ribbing M, Berendes E, Block M, Schield HH, Breithardt G, Kerber S. Emergency coronary bypass grafting after failed coronary angioplasty: what has changed in a decade? Ann Thorac Surg. 2000;70:1997–2003. [DOI] [PubMed] [Google Scholar]
- 6. Carey JA, Davies S, Balcon R, Layton C, Magee P, Rothman MT, Timmis AD, Wright JE, Walesby RK. Emergency surgical revascularization for coronary angioplasty complications. Br Heart J. 1994;72:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loubeyre C, Morice M‐C, Berzin B, Virot P, Commeau P, Drobinski G, Ethevenot G, Moquet B, Marco J, Labrunie P, Cattan S, Coste P, Aubry P, Ferrier A. Emergency coronary artery bypass surgery following coronary angioplasty and stenting. Results of a French multicenter registry. Catheter Cardiovasc Interv. 1999;48:441–448. [DOI] [PubMed] [Google Scholar]
- 8. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 9. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association for Clinical Chemistry . 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. [DOI] [PubMed] [Google Scholar]
- 10. Reynolds HR, Hochman JS. Cardiogenic shock: current concepts and improving outcomes. Circulation. 2008;117:686–697. [DOI] [PubMed] [Google Scholar]
- 11. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035.22923432 [Google Scholar]
- 12. Rastan AJ, Eckenstein JI, Hentschel B, Funkat AK, Gummert JF, Doll N, Walther T, Falk V, Mohr FW. Emergency coronary artery bypass surgery for acute coronary syndrome: beating heart versus conventional cardioplegic cardiac arrest strategies. Circulation. 2006;114:I477–I485. [DOI] [PubMed] [Google Scholar]
- 13. Barakate MS, Bannon PG, Hughes CF, Horton MD, Callaway A, Hurst T. Emergency surgery after unsuccessful coronary angioplasty: a review of 15 years’ experience. Ann Thorac Surg. 2003;75:1400–1405. [DOI] [PubMed] [Google Scholar]
- 14. Shimony A, Zahger D, Van Straten M, Shalev A, Gilutz H, Ilia R, Cafri C. Incidence, risk factors, management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2009;104:1674–1677. [DOI] [PubMed] [Google Scholar]
- 15. Parsh J, Seth M, Green J, Sutton NR, Chetcuti S, Dixon S, Grossman PM, Khandelwal A, Dupree JM, Gurm HS. Coronary artery perforations after contemporary percutaneous coronary interventions: evaluation of incidence, risk factors, outcomes, and predictors of mortality. Catheter Cardiovasc Interv. 2017;89:966–973. [DOI] [PubMed] [Google Scholar]
- 16. Lazar HL, Jacobs AK, Aldea GS, Shapira OM, Lancaster D, Shemin RJ. Factors influencing mortality after emergency coronary artery bypass grafting for failed percutaneous transluminal coronary angioplasty. Ann Thorac Surg. 1997;64:1747–1752. [DOI] [PubMed] [Google Scholar]
- 17. Slottosch I, Liakopoulos O, Kuhn E, Deppe AC, Scherner M, Mader N, Choi YH, Wahlers T. Outcome after coronary bypass grafting for coronary complications following coronary angiography. J Surg Res. 2017;210:69–77. [DOI] [PubMed] [Google Scholar]
- 18. Leontyev S, Borger M, Legare J‐F, Merk D, Hahn J, Seeburger J, Lehmann S, Mohr FW. Iatrogenic type A aortic dissection during cardiac procedures: early and late outcome in 48 patients. Eur J Cardiothorac Surg. 2012;41:641–646. [DOI] [PubMed] [Google Scholar]
- 19. Davierwala PM, Leontyev S, Verevkin A, Rastan AJ, Mohr M, Bakhtiary F, Misfeld M, Mohr FW. Temporal trends in predictors of early and late mortality after emergency coronary artery bypass grafting for cardiogenic shock complicating acute myocardial infarction. Circulation. 2016;134:1224–1237. [DOI] [PubMed] [Google Scholar]
- 20. Dini CS, Lazzeri C, Chiostri M, Gensini GF, Valente S. A local network for extracorporeal membrane oxygenation in refractory cardiogenic shock. Acute Card Care. 2015;17:49–54. [DOI] [PubMed] [Google Scholar]
- 21. Davierwala PM, Verevkin A, Leontyev S, Misfeld M, Borger MA, Mohr FW. Does timing of coronary artery bypass surgery affect early and long‐term outcomes in patients with non‐ST‐segment‐elevation myocardial infarction? Circulation. 2015;132:731–740. [DOI] [PubMed] [Google Scholar]
- 22. Lemmert ME, van Bommel RJ, Diletti R, Wilschut JM, de Jaegere PP, Zijlstra F, Daemen J, Van Mieghem NM. Clinical characteristics and management of coronary artery perforations: a single‐center 11‐year experience and practical overview. J Am Heart Assoc. 2017;6:e007049 DOI: 10.1161/JAHA.117.007049. [DOI] [PMC free article] [PubMed] [Google Scholar]


