Abstract
Background
High sodium intake elevates blood pressure and thereby raises cardiovascular diseases (CVDs). Sodium intake is high in northern China, including Shandong province where the SMASH (Shandong‐Ministry of Health Action on Sodium and Hypertension) is currently underway.
Methods and Results
Blood pressure values and sodium intake measurements using 24‐hour urinary excretion were collected from the 2011 SMASH baseline survey, which was conducted in 20 counties/districts using a multistage stratified cluster random sampling method. We derived cause‐specific mortality from the Shandong Death Registration System (SDRS) during the same year and used population‐attributable fraction to estimate annual CVDs deaths attributable to high sodium intake (mediated through elevated systolic blood pressure) and CVD deaths averted by achieving different sodium‐reduction targets. Overall, 16 100 (95% uncertainty intervals, 11 000–22 600) CVD deaths among adults aged 25 to 69 years, including 5600 (4000–6500) for ischemic heart disease and 9000 (6700–11 600) for stroke, were attributable to higher sodium intake (2000 mg/day or 5.0 g/day of salt as a reference) in Shandong in 2011, accounting for 19.9% (13.7–25.0%) of total CVD deaths. The benefit of CVD deaths from sodium reduction is considerable with 8800 (6400–13 600), 6700 (4900–11 600), and 8500 (6000–10 800) averted, respectively, if sodium intake was reduced from the 2011 baseline to 3500 mg/day, 4000 mg/day, or reduced by 30%.
Conclusions
Nearly 20% of CVD deaths among adults aged 25 to 69 years could be attributable to the systolic blood pressure–raising effect of high sodium intake in Shandong in 2011. Potential benefits from population reduction of sodium intake are considerable.
Keywords: blood pressure, cardiovascular disease prevention, population‐attributable fraction, salt intake, sodium
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors, Mortality/Survival
Clinical Perspective
What Is New?
We first assessed the numbers on life saved from sodium reduction using modeling approach with data from Shandong where the SMASH (Shandong‐Ministry of Health Action on Sodium and Hypertension) project is currently underway.
Nearly 20% of cardiovascular disease deaths among the adults aged 25 to 69 years were attributable to high sodium intake in Shandong in 2011.
What Are the Clinical Implications?
The potential benefits of reducing sodium intake are considerable.
The implementation of SMASH‐like interventions elsewhere in China, even other alike counties, is expected to be helpful in reduction of cardiovascular disease deaths.
High sodium intake is associated with elevated blood pressure (BP) and thereby raises the morbidity and mortality of cardiovascular and cerebrovascular diseases (CVDs).1, 2, 3 The 2010 GBD (Global Burden of Disease) study4 showed that from 1990 to 2010 attributed mortality to high BP increased from 7.29 to 9.40 million, including deaths attributed to high sodium intake, grew from 2.25 to 3.10 million globally. High BP was ranked first among 67 different risk factors as the leading contributor to the burden of disease in East Asia, as well as the world. When only deaths caused by CVDs are considered, high sodium intake is the fourth risk factor.4
According to national nutrition surveys conducted in China in 1982, 1992, 2002, and the national Chronic Disease Risk Factor Surveillance Survey (CDRFSS) in 2010, ≈80% of Chinese residents consumed more than 6 g/day of dietary salt per capita, with intake estimates of 9.1 g/day for urban and 11.5 g/day for rural residents in 2010.5 Unlike in Western countries, in China a larger dietary source of sodium derives from home cooking (76%). In addition, northern Chinese food sample has a 50% higher sodium content than southern Chinese food.6 As a northern Chinese province, Shandong residents experienced a higher dietary salt intake of 12.5 g/day in 2011 than the national average of 10.6 g/day and higher adult hypertension prevalence of 23.4% than the national average of 21.5%.5, 7, 8
A number of countries have successfully implemented cost‐effective sodium‐reduction measures, which have lowered relevant morbidity and mortality, CVD incidence, and medical costs dramatically,9, 10 such as Japan, the United Kingdom, United States, Finland, Canada, Netherlands, and Ireland.11 This is the first study that estimates deaths from CVDs attributable to high sodium intake in Shandong where the SMASH (Shandong‐Ministry of Health Action on Sodium and Hypertension) is currently underway7 and predicts the potential difference in mortality using different sodium reduction targets.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Following the updated Comparative Risk Assessment framework of the GBD,4, 12 we first measured the effects of high sodium intake on BP (ie, the BP values attributed to sodium intake above a reference level). Next, we quantified the attributed deaths from CVDs to elevated BP at present and with effects removed for high sodium intake in the first step. Finally, we calculated the number of CVDs deaths attributed to high sodium intake mediated through elevated BP. Uncertainty analysis was applied to address the potential random variations, system errors, and faults of input data from multiple sources. Following the same methods, we estimated CVDs deaths averted by achieving different sodium‐reduction targets.
Data Sources
Exposure data of sodium intake and BP
The 24‐hour urinary sodium excretion was used as the gold‐standard measure for sodium intake13, 14, 15 and systolic BP (SBP) was identified as the exposure proxy for BP4 consistent with the Comparative Risk Assessment framework of the GBD.
The 24‐hour urinary sodium data in 2011 for Shandong were obtained from the baseline survey of the SMASH study of 2061 participants aged 18 to 69 years who had been living locally for more than 6 months. Data were taken using the 4‐stage stratified cluster random sampling method from participants who were enrolled from 52 administrative villages/neighborhood committees, of 52 towns/subdistricts, of 20 counties/districts.7 Design weight sampling and population structure adjustments were involved to ensure the provincial representativeness of the study.
The SBP data for Shandong were also extracted from the 2011 SMASH, which was conducted at 52 towns/subdistricts across 20 counties/districts of Shandong with provincial representativeness.7 Overall, 15 350 participants aged 18 to 69 years living locally more than 6 months were enrolled using the multistage stratified cluster random sampling method at each point. BP value was measured with a HEM‐7071 OMRON (OMRON Corporation, Kyoto, Japan) electronic BP meter 3 times, and the average of the last 2 was adopted in the final analysis. Sampling design weight and population structure adjustments were also considered to ensure provincial representativeness.16
In total, there were 1769 and 13 272 participants aged 25 to 69 years included in the final analysis for 24‐hour urinary sodium and BP, respectively.
Mortality data
Data of cause of death in 2011 were derived from the Shandong Death Registration System, which has covered the entire population across the whole province since 2010, and was adjusted with the annual population‐based under‐reporting investigation.17 Garbage codes of cause of death were redistributed according to the well‐established methods in the GBD study,18 and all the data mapped age‐, sex‐, and cause‐specific mortality.
Effects of high sodium intake on BP
Mozaffarian et al12 performed a new meta‐analysis of the dose‐response effects of sodium on BP based on 103 randomized controlled trials from 2 Cochrane studies1, 19 and obtained the linear model: y=3.735+0.105×(x1−50)+1.874×x2+2.489×x3, where y is the SBP increase (mm Hg) with each 100‐mmol/day increase of 24‐hour urinary sodium (or 2300 mg/day sodium) above the reference level; x1 is age or mid‐value of age group (years; mid‐value was used in this study); x2 is hypertensive status (normotensive [x2=0] or hypertensive [x2=1]); and x3 is race (nonblacks [x3=0] or blacks [x3=1], nonblacks used for Chinese; Table 1). A conservative level of 87.0 mmol/day for 24‐hour urinary sodium (or 2000 mg/day sodium) was defined as the reference in this model for the present study focused on CVDs,12 and also the reference level of 43.5 mmol/day (or 1000 mg/day) consistently with the GBD study,4 was utilized to replicate the estimates as a sensitivity analyses. The reference level connotes the minimum risk exposure to sodium intake on BP.
Table 1.
SBP Increase (mm Hg) With Each 100‐mmol/day Increase of 24 Hours Urinary Sodium
| Age (y) | HBP | SBP Increase | Age (y) | HBP | SBP Increase |
|---|---|---|---|---|---|
| 25 to 29 | No | 1.373 | 55 to 59 | No | 4.523 |
| 25 to 29 | Yes | 3.247 | 55 to 59 | Yes | 6.397 |
| 30 to 34 | No | 1.898 | 60 to 64 | No | 5.048 |
| 30 to 34 | Yes | 3.772 | 60 to 64 | Yes | 6.922 |
| 35 to 39 | No | 2.423 | 65 to 69 | No | 5.573 |
| 35 to 39 | Yes | 4.297 | 65 to 69 | Yes | 7.447 |
| 40 to 44 | No | 2.948 | 70 to 74 | No | 6.098 |
| 40 to 44 | Yes | 4.822 | 70 to 74 | Yes | 7.972 |
| 45 to 49 | No | 3.473 | 75 to 79 | No | 6.623 |
| 45 to 49 | Yes | 5.347 | 75 to 79 | Yes | 8.497 |
| 50 to 54 | No | 3.998 | 80+ | No | 7.148 |
| 50 to 54 | Yes | 5.872 | 80+ | Yes | 9.002 |
HBP indicates high blood pressure; SBP, systolic blood pressure.
Causal health outcomes of elevated BP and the relative risk
In accord with the Comparative Risk Assessment in the GBD study,4 ischemic heart disease, stroke, rheumatic heart disease, endocarditis, cardiomyopathy and myocarditis, aorta aneurysm, hypertensive heart disease, atrial fibrillation, peripheral vascular disease, and other circulatory diseases were identifed as the health outcomes of CVDs etiologically associated with elevated BP, and their relative risk (RR) per 10 mm Hg increase in SBP by age group (25 years and above) for each health outcome was used in our study (Table 2). Causal health outcomes of elevated BP and their RR were calculated by GBD study team using meta‐analysis based on randomized controlled trials, large cohort studies, and confounding factors were consistently adjusted. Regression dilution bias was also adjusted in the modeling of BP measures over time.4, 20
Table 2.
RR Values (95% CI) Per 10 mm Hg Increase in SBP on CVDs by Age Group
| Health Outcomes | ICD‐10 | Sex | Age (y) | RR (95% CI) |
|---|---|---|---|---|
| Ischemic heart disease | I20–I25 | Both | 25+ | 1.76 (1.64–1.89) |
| Both | 30+ | 1.70 (1.59–1.82) | ||
| Both | 35+ | 1.64 (1.55–1.75) | ||
| Both | 40+ | 1.59 (1.50–1.69) | ||
| Both | 45+ | 1.53 (1.46–1.62) | ||
| Both | 50+ | 1.48 (1.41–1.56) | ||
| Both | 55+ | 1.43 (1.37–1.50) | ||
| Both | 60+ | 1.38 (1.33–1.44) | ||
| Both | 65+ | 1.34 (1.29–1.39) | ||
| Both | 70+ | 1.29 (1.25–1.34) | ||
| Both | 75+ | 1.25 (1.22–1.29) | ||
| Both | 80+ | 1.19 (1.16–1.21) | ||
| Ischemic stroke | I63, I65–I67 (except I67.4), I69.3 | Both | 25+ | 2.17 (2.03–2.31) |
| Both | 30+ | 2.07 (1.95–2.20) | ||
| Both | 35+ | 1.97 (1.86–2.09) | ||
| Both | 40+ | 1.88 (1.79–1.99) | ||
| Both | 45+ | 1.80 (1.71–1.89) | ||
| Both | 50+ | 1.71 (1.64–1.79) | ||
| Both | 55+ | 1.64 (1.57–1.71) | ||
| Both | 60+ | 1.56 (1.50–1.62) | ||
| Both | 65+ | 1.49 (1.44–1.54) | ||
| Both | 70+ | 1.42 (1.38–1.46) | ||
| Both | 75+ | 1.36 (1.32–1.39) | ||
| Both | 80+ | 1.26 (1.24–1.29) | ||
| Hemorrhagic and other nonischemic stroke | I60–I62, I69.0–I69.2, I67.4 | Both | 25+ | 2.38 (2.06–2.72) |
| Both | 30+ | 2.26 (1.97–2.56) | ||
| Both | 35+ | 2.14 (1.89–2.41) | ||
| Both | 40+ | 2.03 (1.81–2.27) | ||
| Both | 45+ | 1.93 (1.73–2.14) | ||
| Both | 50+ | 1.83 (1.66–2.01) | ||
| Both | 55+ | 1.74 (1.58–1.89) | ||
| Both | 60+ | 1.65 (1.52–1.78) | ||
| Both | 65+ | 1.56 (1.45–1.68) | ||
| Both | 70+ | 1.48 (1.39–1.58) | ||
| Both | 75+ | 1.41 (1.33–1.48) | ||
| Both | 80+ | 1.30 (1.25–1.35) | ||
| Rheumatic heart disease | I01, I02.0, I05–I09 | Both | 25+ | 1.30 (1.20–1.41) |
| Both | 30+ | 1.28 (1.18–1.38) | ||
| Both | 35+ | 1.26 (1.17–1.36) | ||
| Both | 40+ | 1.24 (1.16–1.33) | ||
| Both | 45+ | 1.22 (1.14–1.30) | ||
| Both | 50+ | 1.20 (1.13–1.27) | ||
| Both | 55+ | 1.18 (1.12–1.25) | ||
| Both | 60+ | 1.16 (1.11–1.22) | ||
| Both | 65+ | 1.14 (1.10–1.20) | ||
| Both | 70+ | 1.13 (1.08–1.17) | ||
| Both | 75+ | 1.11 (1.07–1.15) | ||
| Both | 80+ | 1.08 (1.06–1.11) | ||
| Endocarditis, cardiomyopathy and myocarditis | I33, I40, I42 | Both | 25+ | 1.49 (1.39–1.61) |
| Both | 30+ | 1.45 (1.36–1.56) | ||
| Both | 35+ | 1.42 (1.33–1.52) | ||
| Both | 40+ | 1.39 (1.31–1.47) | ||
| Both | 45+ | 1.35 (1.28–1.43) | ||
| Both | 50+ | 1.32 (1.26–1.39) | ||
| Both | 55+ | 1.29 (1.23–1.35) | ||
| Both | 60+ | 1.26 (1.21–1.31) | ||
| Both | 65+ | 1.23 (1.18–1.28) | ||
| Both | 70+ | 1.20 (1.16–1.24) | ||
| Both | 75+ | 1.17 (1.14–1.20) | ||
| Both | 80+ | 1.13 (1.10–1.15) | ||
| Aortic aneurysm | I71 | Both | 25+ | 1.68 (1.58–1.77) |
| Both | 30+ | 1.62 (1.54–1.71) | ||
| Both | 35+ | 1.57 (1.49–1.65) | ||
| Both | 40+ | 1.53 (1.45–1.60) | ||
| Both | 45+ | 1.48 (1.41–1.54) | ||
| Both | 50+ | 1.43 (1.37–1.49) | ||
| Both | 55+ | 1.39 (1.34–1.44) | ||
| Both | 60+ | 1.35 (1.30–1.39) | ||
| Both | 65+ | 1.30 (1.27–1.34) | ||
| Both | 70+ | 1.26 (1.23–1.30) | ||
| Both | 75+ | 1.23 (1.20–1.25) | ||
| Both | 80+ | 1.17 (1.15–1.19) | ||
| Hypertensive heart disease | I11 | Both | 25+ | 3.67 (3.39–3.94) |
| Both | 30+ | 3.39 (3.15–3.63) | ||
| Both | 35+ | 3.13 (2.92–3.34) | ||
| Both | 40+ | 2.89 (2.71–3.07) | ||
| Both | 45+ | 2.68 (2.52–2.83) | ||
| Both | 50+ | 2.47 (2.34–2.60) | ||
| Both | 55+ | 2.29 (2.17–2.39) | ||
| Both | 60+ | 2.11 (2.02–2.20) | ||
| Both | 65+ | 1.95 (1.87–2.03) | ||
| Both | 70+ | 1.80 (1.74–1.86) | ||
| Both | 75+ | 1.67 (1.62–1.72) | ||
| Both | 80+ | 1.48 (1.45–1.52) | ||
| Atrial fibrillation, peripheral vascular disease, and other circulatory diseases | I48, I73, I70.2, I00, I02.9, I27–I28 (except I27.1), I30–I32 (except I31.2, I31.3), I34–I39, I47, I70.8, I72, I77–I80, I82–I84, I86–I98, G45 | Both | 25+ | 1.55 (1.49–1.62) |
| Both | 30+ | 1.51 (1.45–1.57) | ||
| Both | 35+ | 1.47 (1.42–1.53) | ||
| Both | 40+ | 1.43 (1.38–1.48) | ||
| Both | 45+ | 1.39 (1.35–1.44) | ||
| Both | 50+ | 1.36 (1.32–1.40) | ||
| Both | 55+ | 1.32 (1.29–1.36) | ||
| Both | 60+ | 1.29 (1.26–1.32) | ||
| Both | 65+ | 1.25 (1.23–1.28) | ||
| Both | 70+ | 1.22 (1.20–1.24) | ||
| Both | 75+ | 1.19 (1.17–1.21) | ||
| Both | 80+ | 1.14 (1.13–1.16) |
CVDs indicates cardiovascular diseases; ICD‐10, International Classification of Diseases Tenth Revision; RR, relative ration; SBP, systolic blood pressure.
Statistical Analysis
Cardiovascular mortality attributable to elevated BP
PAF (population‐attributable fractions) was calculated to obtain the proportion of CVDs deaths attributable to elevated BP by comparing the observed distribution of SBP to a theoretical minimum or counterfactual distribution for each disease by sex and age group. The formula with BP treated as a continuous variable was:
where RR(x) is the relative risk at SBP level x; P(x) is the observed population distribution of SBP; P’(x) is the theoretical minimum SBP distribution (mean of 115 mm Hg with SD of 6 mm Hg) or other counterfactual distributions; and m is the maximum amount of SBP level.4 CVD mortality attributable to elevated BP for each disease was calculated by multiplying the PAF with the observed deaths of CVDs by sex and age group (≥25 years).
Cardiovascular mortality attributable to the SBP‐raising effect of high sodium intake
We assumed that PAF1 is the attributable fraction of CVDs deaths to elevated BP (SBP) at present or in an observed population. The formula was:
where RR(x) is the relative risk on etiologically associated diseases at SBP level x, which was derived from the GBD study based on meta‐analysis with pooled data worldwide involved; P1(x) is the observed or present population distribution of SBP by age group, which comes from Shandong province in 2011; P’(x) is the theoretical minimum SBP distribution (mean of 115 mm Hg with SD of 6 mm Hg); and m is the maximum amount of SBP level.
Then, we assumed PAF2 accounts for the attributable fraction of CVD deaths to elevated BP (SBP) after removing the effects of high sodium intake above the reference level in the same population. The formula was:
where RR(x) is the relative risk on etiologically associated diseases at SBP level x, which is same as that in the calculation of PAF1; P2(x) is the distribution of SBP by age group after removing the effects of high sodium intake above the reference level (2000 or 1000 mg/day) in the same population of Shandong in 2011; P’(x) is the theoretical minimum SBP distribution (mean of 115 mm Hg with SD of 6 mm Hg), which is same as that in the calculation of PAF1; and m is the maximum amount of SBP level.
Finally, the attributable CVDs deaths to the SBP‐raising effect of high sodium intake can be expressed as (PAF1−PAF2)×D, where PAF1−PAF2 represents the attributable fraction of CVDs deaths to the SBP‐raising effect of high sodium intake, and D is the total deaths of CVDs in this observed population of Shandong province in 2011.
Alternative exposure distribution setting of sodium intake
We applied 3 alternative sodium intake distributions to track the effects of sodium reduction on CVDs deaths by comparing with baseline distribution observed in 2011: (1) The 24‐hour urinary sodium mean for each sex and age group was decreased from the baseline level to 4000 mg/day (or ≈10 g/day of salt), which is the goal of SMASH,7 and the 24‐hour urinary sodium mean for each sex and age group was decreased from the baseline level to 3500 mg/day (or ≈9 g/day of salt), which is the goal of chronic disease control and prevention of China21; (2) the 24‐hour urinary sodium mean for each sex and age group was decreased by 30% compared with the baseline level, which is the global target for the prevention and control of noncommunicable diseases recommended by the World Health Organization.22
Uncertainty analysis
Monte Carlo simulation techniques were used to present uncertainty ranges around point estimates by assigning probability distributions to model variables with risk and generating random numbers based on those distributions.23 @RISK software (version 6.1 for Excel; Microsoft Corporation, Redmond, WA)24 was used to calculate uncertainty ranges. We defined risk‐factor exposure levels and RR value as the input variables (RR as a lognormal distribution and others as normal distributions). The attributable fractions, attributable CVDs deaths, were treated as the output variables. For each of the output variables, 95% uncertainty intervals were calculated using the 1000 iteration values generated between the 2.5th and 97.5th percentile.
Ethics Considerations
The baseline survey in SMASH survey received ethical approval from the ethics committee in Shandong. All enrolled participants gave written and informed consent for participation, and the records/information of participants was de‐identified before analysis.
Results
Urinary Sodium Excretion and SBP Level in Shandong
The means of 24‐hour urinary sodium by age among Shandong adults ranged from 225.48 to 264.13 mmol/day in men and 209.57 to 245.96 mmol/day in women. The mean level of 24‐hour urinary sodium was higher in men than women (Table 3).
Table 3.
Mean 24 Hours Urinary Sodium and SD With 95% CI (mmol/day) Among Shandong Adults by Age and Sex, 2011
| Age (y) | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | |||
| Lower | Upper | Lower | Upper | |||||
| 25 to 29 | 244.26 | 116.96 | 224.12 | 264.40 | 224.92 | 84.30 | 211.37 | 238.47 |
| 30 to 34 | 226.69 | 69.22 | 213.67 | 239.71 | 245.96 | 92.61 | 228.46 | 263.46 |
| 35 to 39 | 264.13 | 111.54 | 247.19 | 281.06 | 220.71 | 72.77 | 208.36 | 233.05 |
| 40 to 44 | 256.50 | 103.05 | 235.85 | 277.15 | 229.74 | 79.36 | 212.93 | 246.55 |
| 45 to 49 | 233.08 | 76.95 | 218.20 | 247.97 | 221.52 | 90.29 | 203.78 | 239.25 |
| 50 to 54 | 253.28 | 100.44 | 227.55 | 279.00 | 230.43 | 86.04 | 211.29 | 249.58 |
| 55 to 59 | 249.56 | 85.83 | 232.62 | 266.50 | 230.66 | 80.86 | 213.22 | 248.10 |
| 60 to 64 | 225.48 | 77.88 | 208.88 | 242.07 | 209.57 | 66.38 | 196.05 | 223.09 |
| 65 to 69 | 228.90 | 75.39 | 210.37 | 247.43 | 210.07 | 71.01 | 187.67 | 232.47 |
The mean SBP by age in Shandong ranged from 119.08 to 135.04 mm Hg among men and 107.15 to 137.91 mm Hg among women. The overall trend of the SBP means increased with age, and the mean SBP for males was generally higher than that for females in those aged <60 years, but lower among those aged >60 years (Table 4).
Table 4.
Mean SBP and SD (mm Hg) With 95% CI by Age and Sex Among Shandong Adults, 2011
| Age (y) | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | |||
| Lower | Upper | Lower | Upper | |||||
| 25 to 29 | 119.08 | 12.12 | 118.26 | 119.90 | 107.15 | 12.66 | 106.34 | 107.96 |
| 30 to 34 | 121.54 | 13.28 | 120.58 | 122.49 | 110.23 | 13.28 | 109.32 | 111.14 |
| 35 to 39 | 122.57 | 14.74 | 121.70 | 123.45 | 113.90 | 14.53 | 113.06 | 114.74 |
| 40 to 44 | 125.42 | 17.06 | 124.18 | 126.65 | 117.01 | 14.86 | 115.97 | 118.06 |
| 45 to 49 | 124.74 | 16.70 | 123.62 | 125.85 | 121.48 | 17.90 | 120.22 | 122.75 |
| 50 to 54 | 128.58 | 19.55 | 126.84 | 130.31 | 125.97 | 19.92 | 124.20 | 127.74 |
| 55 to 59 | 130.14 | 19.49 | 128.65 | 131.63 | 129.95 | 21.82 | 128.17 | 131.73 |
| 60 to 64 | 131.77 | 20.44 | 130.19 | 133.35 | 133.39 | 21.55 | 131.83 | 134.95 |
| 65 to 69 | 135.04 | 22.52 | 132.96 | 137.13 | 137.91 | 21.13 | 135.97 | 139.84 |
Cardiovascular Mortality Attributable to Elevated BP
Overall, there were 81 012 CVD deaths among those aged 25 to 69 years in Shandong in 2011, of which 41 600 (95%uncertainty interval, 27 700–55 800) could be attributed to elevated BP accounting for 51.3% of total CVD deaths (Table 5). The PAF is slightly higher in men than in women. The PAF for stroke is the highest (Figure).
Table 5.
CVD Deaths Attributable to Elevated BP by Sex in Shandong, 2011
| Health Outcomes | Males | Females | Both Sexes | |||
|---|---|---|---|---|---|---|
| Deaths (×1000) | 95% UI | Deaths (×1000) | 95% UI | Deaths (×1000) | 95% UI | |
| IHD | 8.8 | 5.7 to 12.3 | 4.5 | 3.2 to 6.1 | 13.3 | 8.6 to 18.2 |
| Stroke | 15.2 | 9.3 to 19.9 | 8.3 | 5.4 to 11.3 | 23.5 | 14.7 to 31.7 |
| Other CVD | 3 | 1.8 to 4.2 | 1.8 | 1.2 to 2.6 | 4.8 | 2.9 to 6.7 |
| All CVD | 26.9 | 17.7 to 36.3 | 14.7 | 8.7 to 20.4 | 41.6 | 27.7 to 55.8 |
| % total CVD deaths | 51.5 | 38.4 to 63.1 | 51.1 | 37.9 to 62.2 | 51.3 | 38.5 to 62.7 |
BP indicates blood pressure; CVD, cardiovascular diseases; IHD, ischemic heart disease; UI, uncertainty intervals.
Figure 1.
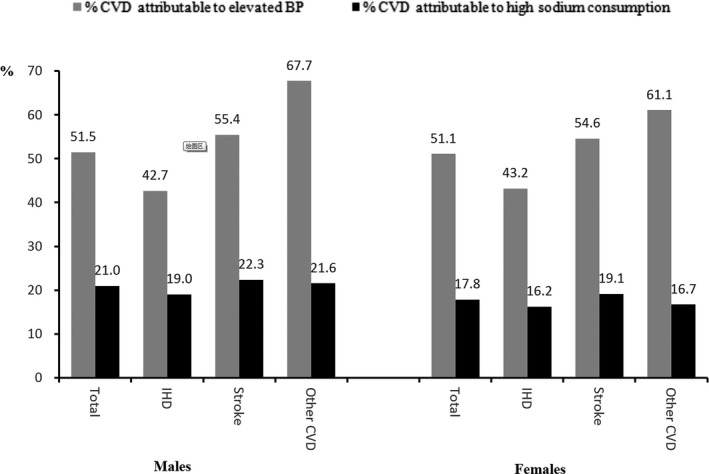
Population‐attributable fraction (%) of CVDs deaths to elevated BP and high sodium consumption (2000 mg/day as a reference) in 2011, Shandong. BP indicates blood pressure; CVDs, cardiovascular disease; IHD, ischemic heart disease.
Cardiovascular Mortality Attributable to the SBP‐Raising Effect of High Sodium Intake
An estimated 16 100 (95% uncertainty interval, 11 000–22 600) CVD deaths among those aged 25 to 69 years (11 000 men and 5100 women) were attributable to the SBP‐raising effect of high sodium intake (2000 mg/day as a reference) in Shandong, accounting for 19.9% (95% uncertainty interval, 13.7–25.0%; 21.0% for males, 17.8% for females) of total CVD deaths. CVD deaths attributable to the SBP‐raising effect of high sodium intake mainly resulted from stroke, followed by ischemic heart disease, in both men and women (Table 6). The PAF is slightly higher in men than that in women. The PAF for stroke is the highest (Figure).
Table 6.
CVDs Deaths Attributable to High Sodium Consumption >2000 mg/day by Sex in Shandong, 2011
| Health Outcomes | Males | Females | Both Sexes | |||
|---|---|---|---|---|---|---|
| Deaths (×1000) | 95% UI | Deaths (×1000) | 95% UI | Deaths (×1000) | 95% UI | |
| IHD | 3.9 | 2.5 to 4.4 | 1.7 | 1.2 to 2.2 | 5.6 | 4.0 to 6.5 |
| Stroke | 6.1 | 4.0 to 8.4 | 2.9 | 2.0 to 4.4 | 9.0 | 6.7 to 11.6 |
| Other CVDs | 0.9 | 0.7 to 1.6 | 0.5 | 0.3 to 0.9 | 1.5 | 1.1 to 2.1 |
| All CVDs | 11.0 | 7.4 to 13.5 | 5.1 | 3.3 to 7.3 | 16.1 | 11.0 to 22.6 |
CVDs indicates cardiovascular diseases; IHD, ischemic heart disease; UI, uncertainty intervals.
Lowering the reference level from 2000 to 1000 mg/day of sodium increased CVD deaths among residents aged 25 to 69 years attributed to the SBP‐raising effect of high sodium intake by ≈37.9% to 22 200 (13 400–31 200) in Shandong (Table 7).
Table 7.
CVD Deaths Attributable to High Sodium Consumption >1000 mg/day by Sex in Shandong, 2011
| Health Outcomes | Males | Females | Both Sexes | |||
|---|---|---|---|---|---|---|
| Deaths (×1000) | 95% UI | Deaths (×1000) | 95% UI | Deaths (×1000) | 95% UI | |
| IHD | 5.2 | 3.1 to 6.9 | 2.4 | 1.4 to 3.1 | 7.7 | 5.1 to 10.4 |
| Stroke | 8.4 | 4.7 to 12.1 | 4.1 | 2.8 to 6 | 12.5 | 8.1 to 18.1 |
| Other CVD | 1.2 | 0.9 to 1.8 | 0.7 | 0.4 to 1 | 2 | 1.2 to 3 |
| All CVD | 14.9 | 9.4 to 20.6 | 7.2 | 4.4 to 9.7 | 22.2 | 13.4 to 31.2 |
| % total CVD deaths | 28.5 | 20.6 to 40.1 | 25.3 | 17.2 to 35.4 | 27.6 | 20 to 38.7 |
CVD indicates cardiovascular diseases; IHD, ischemic heart disease; UI, uncertainty intervals.
If sodium intake for each sex and age group was reduced from the baseline level in 2011 to 3500 mg/day, 4000 mg/day, or reduced by 30%, respectively, in Shandong, the potential avoidable CVD deaths attributable to the SBP‐raising effect of high sodium intake were 8800 (6400–13 600), 6700 (4900–11 600), and 8500 (6000–10 800; Table 8).
Table 8.
Shift of CVDs Deaths Attributable to High Sodium Consumption >2000 mg/day With Alternative Exposure Scenarios (24 Hours Urinary Sodium) by Sex, Shandong
| Exposure Scenarios | Males | Females | Both Sexes | |||
|---|---|---|---|---|---|---|
| Deaths (×1000) | 95% UI | Deaths (×1000) | 95% UI | Deaths (×1000) | 95% UI | |
| Baseline (B) | 11.0 | 7.4 to 13.5 | 5.1 | 3.3 to 7.3 | 16.1 | 11 to 22.6 |
| Decreased to 3.5 g (i)a | 4.7 | 3.2 to 6.2 | 2.6 | 1.7 to 3.8 | 7.3 | 4.6 to 9 |
| Decreased to 4.0 g (ii)b | 5.9 | 4 to 7.3 | 3.3 | 2.1 to 4.3 | 9.4 | 6.1 to 11 |
| Decreased by 30% (iii)c | 5.3 | 3.5 to 7.9 | 2.3 | 1.6 to 3.9 | 7.6 | 5.0 to 11.8 |
| Deaths shifts | ||||||
| (B)‐(i) | 6.3 | 4.2 to 7.3 | 2.6 | 1.6 to 3.5 | 8.8 | 6.4 to 13.6 |
| (B)‐(ii) | 5.1 | 3.4 to 6.2 | 1.8 | 1.2 to 3.0 | 6.7 | 4.9 to 11.6 |
| (B)‐(iii) | 5.7 | 3.9 to 5.6 | 2.8 | 1.7 to 3.4 | 8.5 | 6.0 to 10.8 |
UI indicates uncertainty intervals.
Decreased to 3.5 g is the goal of chronic disease control and prevention of China on sodium intake.
Decreased to 4.0 g is the goal of Shandong‐Ministry of Health Action on Sodium and Hypertension (SMASH) on sodium intake.
Decreased by 30% is the global target for the prevention and control of noncommunicable diseases recommended by World Health Organization (WHO).
Discussion
Consistently with the GBD Comparative Risk Assessment framework, we collected local data and estimated ≈19.9% of total CVD deaths among adults aged 25 to 69 years (16 100) in 2011 was attributed to the SBP‐raising effect of high sodium intake >2000 mg/day in Shandong. The estimate for Shandong in our study is higher than the result in China from Mozaffarian et al (15.3%), also at the reference level of 1000 mg/day.12 Our results are lower than the estimates from the GBD 2010,25 but higher than that from Liu et al,26 probably because we used local data available in modeling. Nevertheless, the percentages of total CVD deaths attributed to the SBP‐raising effect of high sodium intake in Shandong is much higher than the average level worldwide (9.5%).12 For the first time, we estimated the burden of disease attributable to high sodium intake at a provincial level.
Strokes account for the largest number of deaths from CVDs attributed to the SBP‐raising effect of high sodium intake in Shandong, followed by ischemic heart disease. This also reflects the overall patterns of vital statistics given that stroke is the leading cause of death.18
Besides high sodium intake, the other potential risk factors of elevating BP involve obesity, physical inactivity, insufficient intake of fruit and vegetables, and excessive drinking. However, high sodium intake remains a major risk factor.4, 25 Because of the substantial burden of disease attributable to high sodium intake, many countries have taken measures to reduce sodium intake to bring down BP. For instance, the United Kingdom has obtained successful results by setting up the action group “Consensus Action on Salt and Health,” recommending ≤6 g/day (or 2400 mg/day of sodium) of salt intake throughout the country and by urging supermarkets and food companies to reduce additive amounts of sodium by 10% to 15%.11, 13 Likewise, Finland has achieved favorable results by initiating a systematic approach, including mass media campaigns, cooperation with food companies, and legislation of salt labeling to reduce salt intake among its people.13 Other countries, including Japan, Australia, Ireland, Canada, Netherlands, the United States, etc, also have achieved good effectiveness. However, most countries, especially developing countries, have not carried out effective sodium reduction projects.13 Responding to the World Health Organization's campaign on chronic disease prevention and control, the Chinese government initiated “China Healthy Lifestyles for All” in 2007,27 advocating a low‐salt diet and setting 9 g/day of dietary salt intake as the goal of chronic disease control and prevention in China. In addition, in 2011, the Government of Shandong Province and the National Health and Family Planning Commission (formerly Ministry of Health) collaboratively launched the SMASH project, introducing interventions to reduce sodium intake and prevent and control hypertension across the province.7 It is expected that our study may provide a basis for evaluating the effectiveness of the salt reduction strategies implemented in Shandong province, or SMASH‐like interventions that may be introduced elsewhere in China or even across the whole country.
Research has demonstrated that salt reduction projects implemented among populations are cost‐effective.28, 29 Our study found that 8800, 6700, and 8500 CVDs deaths would be saved among adults aged 25 to 69 years in Shandong if the salt reduction targets of 9 g/day in 2015 for chronic disease control and prevention planning of China,21 10 g/day in 2015 for SMASH,7 and 30% for the World Health Organization recommendation22 could be achieved, respectively. Calculation of the number of deaths avoided or lives saved under different targets using the same methods, along with data on intervention costs, can be used in cost‐effectiveness analysis and cost‐benefits analysis, which would provide a basis for policy priority development.
There are limitations in our study. First, the quantitative effects of high sodium intake on BP and RR values for SBP on CVD were all directly obtained from Cochrane studies and the GBD study (mainly meta‐analysis), respectively. The effect estimates we applied here may not able to represent the true effect in Shandong or the Chinese population, given that they were calculated based on the pooled global data mainly with white people involved. However, it will not affect the results of longitudinal comparison within the same region as well as the horizontal comparison among a population with similar characteristics. Second, the 24‐hour urinary sodium, SBP values, and mortality data used in this study were derived from survey or surveillance, and data quality would be inevitably impacted by the field work, although quality‐control measures and analytical adjustments implemented.
In conclusion, the number of CVD deaths attributable to the SBP‐raising effect of high sodium intake in Shandong is high. The potential benefits of reducing sodium intake are considerable. The implementation of SMASH‐like interventions elsewhere in China, even other alike counties, is expected to be helpful in reduction of CVD deaths.
Author Contributions
Liu, Xu, and Zhang conceptualized the manuscript, conducted the statistical analysis, and drafted the manuscript. Guo, Lu, Tang, and Li contributed to interpretation of the results and critical review and revision of the manuscript.
Sources of Funding
This research was supported by the National Key R&D Program of China (2017YFC1310902) from the Ministry of Science and Technology of the People's Republic of China, and a provincial technical development plan grant (2012GSF11828) from the People's Government of Shandong Province, Jinan, China.
Disclosures
None.
Acknowledgments
The authors thank staff at the 20 County/District Centers for Disease Control and Prevention (CDC) of the SMASH project in Shandong province.
(J Am Heart Assoc. 2019;8:e010737 DOI: 10.1161/JAHA.118.010737)
Contributor Information
Aiqiang Xu, Email: aqxuepi@163.com.
Shiwei Liu, Email: shiwei_liu@aliyun.com.
References
- 1. He FJ, Li J, Macgregor GA. Effect of longer‐term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013;(4):CD004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 2009;54:482–488. [DOI] [PubMed] [Google Scholar]
- 3. He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta‐analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16:761–770. [DOI] [PubMed] [Google Scholar]
- 4. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker‐Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan‐Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez‐Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez‐Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. China CDC . Report on Chronic Disease Risk Factor Surveillance in China 2010. Beijing, China: Military Medical Science Press; 2012. [Google Scholar]
- 6. Anderson CA, Appel LJ, Okuda N, Brown IJ, Chan Q, Zhao L, Ueshima H, Kesteloot H, Miura K, Curb JD, Yoshita K, Elliott P, Yamamoto ME, Stamler J. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J Am Diet Assoc. 2010;110:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bi Z, Liang X, Xu A, Wang L, Shi X, Zhao W, Ma J, Guo X, Zhang X, Zhang J, Ren J, Yan L, Lu Z, Wang H, Tang J, Cai X, Dong J, Zhang J, Chu J, Engelgau M, Yang Q, Hong Y, Wang Y. Hypertension prevalence, awareness, treatment, and control and sodium intake in Shandong Province, China: baseline results from Shandong‐Ministry of Health Action on Salt Reduction and Hypertension (SMASH), 2011. Prev Chronic Dis. 2014;11:E88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma YQ, Mei WH, Yin P, Yang XH, Rastegar SK, Yan JD. Prevalence of hypertension in Chinese cities: a meta‐analysis of published studies. PLoS One. 2013;8:e58302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bibbins‐Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He FJ, MacGregor GA. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis. 2010;52:363–382. [DOI] [PubMed] [Google Scholar]
- 11. Trieu K, Neal B, Hawkes C, Dunford E, Campbell N, Rodriguez‐Fernandez R, Legetic B, McLaren L, Barberio A, Webster J. Salt reduction initiatives around the world—a systematic review of progress towards the global target. PLoS One. 2015;10:e0130247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group . Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. [DOI] [PubMed] [Google Scholar]
- 13. Clark AJ, Mossholder S. Sodium and potassium intake measurements: dietary methodology problems. Am J Clin Nutr. 1986;43:470–476. [DOI] [PubMed] [Google Scholar]
- 14. Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, Mertz W, Smith JC Jr. Sodium and potassium intake and balance in adults consuming self‐selected diets. Am J Clin Nutr. 1984;40:786–793. [DOI] [PubMed] [Google Scholar]
- 15. Mickelsen O, Makdani D, Gill JL, Frank RL. Sodium and potassium intakes and excretions of normal men consuming sodium chloride or a 1:1 mixture of sodium and potassium chlorides. Am J Clin Nutr. 1977;30:2033–2040. [DOI] [PubMed] [Google Scholar]
- 16. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G; 2010 China Noncommunicable Disease Surveillance Group . Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. [DOI] [PubMed] [Google Scholar]
- 17. Chu J, Zhou C, Guo X, Sun J, Xue F, Zhang J, Lu Z, Fu Z, Xu A. Female breast cancer mortality clusters in Shandong Province, China: a spatial analysis. Sci Rep. 2017;7:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, Li Y, Wang L, Liu Y, Yin P, Liu J, Yu S, Tan F, Barber RM, Coates MM, Dicker D, Fraser M, González‐Medina D, Hamavid H, Hao Y, Hu G, Jiang G, Kan H, Lopez AD, Phillips MR, She J, Vos T, Wan X, Xu G, Yan LL, Yu C, Zhao Y, Zheng Y, Zou X, Naghavi M, Wang Y, Murray CJ, Yang G, Liang X. Cause‐specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:251–272. [DOI] [PubMed] [Google Scholar]
- 19. Graudal NA, Hubeck‐Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2011;(11):CD004022. [DOI] [PubMed] [Google Scholar]
- 20. Ezzati M, Lopez AD, Rodgers A, Murray CJ. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Vol. 1. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 21. Chronic Disease Control and Prevention Planning of China (2012–2015). Ministry of Health, National Development and Reform Commission, Ministry of Education, Ministry of Science and Technology, Ministry of Industry and Information Technology, Ministry of Civil Affairs, Ministry of Finance, Ministry of Human Resources and Social Security, Ministry of Environmental Protection, Ministry of Agriculture, Ministry of Commerce, State Administration of Radio, Film and Television, State Food and Drug Administration, General Administration of Sports, General Administration of Press and Publication; 2012. Available at: http://www.nhfpc.gov.cn/jkj/s5878/201205/167d45ff9ec7492bb9a4e2a5d283e72c.shtml. Accessed September 26, 2014.
- 22. World Health Organization (WHO) . Draft Comprehensive Global Monitoring Framework and Targets for the Prevention and Control of Noncommunicable Diseases. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 23. Xu G, Sui X, Liu S, Liu J, Liu J, Li Y, Huang S, Wang Z, Blair SN. Effects of insufficient physical activity on mortality and life expectancy in Jiangxi province of China, 2007–2010. PLoS One. 2014;9:e109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palisade Corporation . @RISK software version 6.1 for Excel. Available at: http://www.palisade.com/risk/. Accessed September 10, 2013.
- 25. Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, Vos T, Wang H, Lopez AD, Murray CJ. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu S, Cai X, Hong Y, Yang Q, Engelgau M, Ge Z, Cai Y, Li Y, Wang L, Ma J, Zhou M. Cardiovascular disease deaths and high sodium consumption in Shandong province and China: a modelling analysis. Lancet. 2015;386:S80. [Google Scholar]
- 27. Zhang J, Astell‐Burt T, Seo DC, Feng X, Kong L, Zhao W, Li N, Li Y, Yu S, Feng G, Ren D, Lv Y, Wang J, Shi X, Liang X, Chen C. Multilevel evaluation of ‘China Healthy Lifestyles for All’, a nationwide initiative to promote lower intakes of salt and edible oil. Prev Med. 2014;67:210–215. [DOI] [PubMed] [Google Scholar]
- 28. Murray CJ, Lauer JA, Hutubessy RC, Niessen L, Tomijima N, Rodgers A, Lawes CM, Evans DB. Effectiveness and costs of interventions to lower systolic blood pressure and cholesterol: a global and regional analysis on reduction of cardiovascular‐disease risk. Lancet. 2003;361:717–725. [DOI] [PubMed] [Google Scholar]
- 29. Frieden TR, Briss PA. We can reduce dietary sodium, save money, and save lives. Ann Intern Med. 2010;152:526–527, W182. [DOI] [PubMed] [Google Scholar]


