Abstract
Background
Although right ventricular (RV) volume was significantly decreased in symptomatic patients with repaired tetralogy of Fallot (rTOF) after pulmonary valve replacement (PVR), RV size was still enlarged along with RV dysfunction.
Methods and Results
A prospective case‐control study was conducted in a tertiary hospital; 81 asymptomatic repaired tetralogy of Fallot patients with moderate or severe pulmonary regurgitation were enrolled. The enrolled cohort was divided into 2 groups: PVR group (n=41) and medication group (n=40). Cardiac magnetic resonance, transthoracic echocardiography, and electrocardiography were scheduled after recruitment and 6 months after PVR or recruitment. Adverse events were recorded during follow‐up. Three deaths, 1 heart transplantation, 3 PVRs, and 2 symptomatic heart failures in medication group and 1 redo PVR in the PVR group were observed during follow‐up. Compared with the medication group, the PVR group had significantly lower adverse events rate (P=0.023; odds ratio, 0.086; 95% CI, 0.010–0.716), and RV function was significantly improved (P<0.05). Binary logistic regression analysis identified preoperative RV end‐systolic volume index (10‐mL/m2 increment, P=0.009; odds ratio, 0.64; 95% CI, 0.457–0.893) was an independent predictor of normalization of RV size after PVR. A preoperative RV end‐systolic volume index cut‐off value of 120 mL/m2 (area under curve, 0.819; sensitivity, 90.3%; specificity, 70%) was analyzed by receiver operating characteristic curves for normalized RV size after PVR.
Conclusions
PVR in asymptomatic repaired tetralogy of Fallot patients is appropriate and effective in reducing right ventricular size and preserving right ventricular function. The recommended criterion of RV end‐systolic volume index for PVR is 120 mL/m2.
Keywords: asymptomatic, magnetic resonance, pulmonary valve, tetralogy of Fallot
Subject Categories: Treatment, Cardiovascular Surgery, Mortality/Survival
Clinical Perspective
What Is New?
Pulmonary valve replacement in asymptomatic patients with moderate or great regurgitation after repaired tetralogy of Fallot repair is appropriate.
The recommended criterion of right ventricular end‐systolic volume index for pulmonary valve replacement in asymptomatic patients with moderate or great regurgitation after repaired tetralogy of Fallot repair is 120 mL/m2.
What Are the Clinical Implications?
Pulmonary valve replacement in asymptomatic patients with moderate or great regurgitation after repaired tetralogy of Fallot repair should be considered as a routine strategy following the recommended criteria.
Introduction
A favorable 30‐year survival rate of repaired tetralogy of Fallot (rTOF) is achieved following successful improvement in surgical techniques and perioperative care. Consequently, a growing population of adolescents and adult patients with rTOF is emerging.1 Classic surgical methods, especially transannular patches, may cause severe pulmonary regurgitation (PR) and significant right ventricular (RV) outflow tract (ROVT) hemodynamic dysfunction.2 Although PR is initially well tolerated in most patients, the long‐standing valve insufficiency may cause RV enlargement, tricuspid regurgitation, arrhythmia, RV failure, and eventually left ventricular (LV) failure. Approximately 40% of individuals with rTOF die of heart failure, and ≈10% suffer from sudden deaths, probably attributed to arrhythmias.3
Pulmonary valve replacement (PVR) is the gold‐standard treatment for PR. However, for patients with rTOF and PR, the optimal timing of PVR remains debatable. Currently, the recommended timing of PVR depends mainly on symptoms and specific biological/physiological variables.4, 5 Nevertheless, because of the discouraging outcomes in these patients, some surgeons advocated for early intervention for PR in asymptomatic patients. Therrien et al6, 7, 8 reported that RV enlargement and dysfunction might be prevented by early PVR. However, the optimal timing and indications of PVR for those asymptomatic rTOF patients are still sparking debate, which has catalyzed increasing research efforts over the past decade.6, 7, 8, 9
In this study, our hypothesis was that PVR in asymptomatic rTOF patients could decrease the adverse events rate, reduce the RV size to normal volume, and improve RV function. We aimed to evaluate the outcomes of PVR and attempted to detect a parameter that could be used to determine the best timing of PVR intervention.
Materials and Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
This study was a prospective case‐control study undertaken at the Fuwai Hospital. The Ethics Committee of the Fuwai Hospital approved the study protocol. All patients were provided written informed consent before enrollment.
Population
From June 2014 to April 2018, asymptomatic rTOF patients with moderate or severe PR examined by transthoracic echocardiography (TTE) during routine follow‐up at the Fuwai Hospital were recruited. Eighty‐one patients met the inclusion criteria and were enrolled in this study. Medical history and individual data were collected from medical records. Cardiac magnetic resonance (CMR), TTE, and ECG were examined at enrollment and reexamined 6‐months later after surgery (PVR group) or enrollment (medication group). Parameters were indexed to body surface area, if appropriate. Clinical status was obtained through outpatient follow‐up or phone conversation with patients or family members every 3 months after enrollment.
Data Definitions
According to Khaled Alfakih's study,10 regardless of sex, normal RV size and RV function were defined as RV end‐diastolic volume index (EDVI) ≤114 mL/m2 and RV EDVI ≤114 mL/m2 plus RV ejection fraction (EF) ≥48%, respectively, by steady‐state free precession imaging sequences. Asymptomatic patients were defined as those who maintained a functional status in the New York Heart Association class I or II, without occurrence of syncope caused by arrhythmia or symptoms/signs of heart failure. Adverse events included death, cardiac transplantation, syncope caused by arrhythmias, PVR, or symptomatic heart failure during follow‐up. Echocardiographic criterion for the assessment of tricuspid and pulmonary regurgitation severity was graded as follows: Grade 0 (absent), Grade 1 (trivial+), Grade 2 (mild+), Grade 3 (moderate+), and Grade 4 (severe+).
Inclusion and Exclusion Criteria
According to the indications for PVR in asymptomatic patients with moderate or severe PR after TOF repair recommended by Boston Children's Hospital in 2013,11 our center established the following criteria.
Inclusion Criteria
Asymptomatic patients with moderate or severe pulmonary valve regurgitation after TOF repair with ≥2 of the following: (1) RV EDVI >150 mL/m2; (2) RV end‐systolic volume index (ESVI) >80 mL/m2; (3) RV EF <47%; (4) LV EF <55%; (5) large RVOT aneurysm; (6) QRS duration >160 ms; (7) Sustained tachyarrhythmia related to right‐sided heart volume load; and (8) other hemodynamically significant abnormalities.
Exclusion Criteria
Exclusion criteria included the following: (1) those with obvious symptoms such as right heart insufficiency, syncope caused by arrhythmia; (2) severe right ventricle outflow tract obstruction (gradient ≥60 mm Hg); (3) RV pressure equal to or exceeding LV pressure; (4) severe arrhythmia or implantable cardioverter‐defibrillator inserted; (5) history of pulmonary valve replacement; (6) contraindications for magnetic resonance imaging; and (7) surgery contraindications.
Treatment
PVR group
PVRs were performed on a cardiopulmonary bypass with mild hypothermia or on a beating heart (in the absence of right‐to‐left shunt). A longitudinal incision was performed in the pulmonary trunk or a previously placed patch. Either bioprosthetic valve (bovine pericardial; Edwards Lifesciences, Irvine, CA) or homograft valve was inserted in an orthotropic position. The concomitant procedures are summarized in Table 1.
Table 1.
Demographic Data and Operative Characteristics
| Variable | PVR Group (n=41) | Medication Group (N=40) | P Value |
|---|---|---|---|
| Age at enrollment, y | 21.00 (17.00) | 17.00 (11) | 0.023 |
| Sex (male) | 18 | 21 | 0.383 |
| Age at primary repair, y | 2.67 (4.00) | 2.00 (3.38) | 0.510 |
| Time interval between TOF repair and enrollment, y | 17 (6.30) | 13.83 (9.34) | 0.112 |
| Time interval between CMR, mo | 17.24±10.22 | 28.36±10.09 | 0.000 |
| Mean follow‐up time, mo | 33.76±10.49 | 32.08±9.65 | 0.455 |
| Transannular patch | 33 | 32 | 0.207 |
| Cardiopulmonary bypass time, min | 175.27±74.95 | ··· | ··· |
| Cross‐clamp time, min | 96.00±37.67 | ··· | ··· |
| Mean pulmonary valve diameter, mm | 26.61±3.42 | ··· | ··· |
| Concomitant procedures | |||
| RVOT aneurysm resection | 11 | ··· | ··· |
| Tricuspid ring annuloplasty | 9 | ··· | ··· |
| RV outflow tract muscle resection | 4 | ··· | ··· |
| Tricuspid valve surgery | 2 | ··· | ··· |
| Pulmonary artery patch | 3 | ··· | ··· |
| Residual ventricular septal defect closure | 2 | ··· | ··· |
| ASD or patent foramen ovale closure | 2 | ··· | ··· |
| Mitral valve surgery | 1 | ··· | ··· |
| Ligation of patent ductus arteriosus | 1 | ··· | ··· |
| Major aortopulmonary collateral arteries occlusion | 1 | ··· | ··· |
| Valve types | |||
| Bioprosthesis | 30 | ··· | ··· |
| Homograft | 11 | ··· | ··· |
| Heart incision types at TOF repair | |||
| RA+RV+PA | 35 | 31 | |
| RA+RV | 6 | 9 | 0.362 |
ASD indicates atrial septal defect; CMR, cardiac magnetic resonance; PA, pulmonary artery; PVR, pulmonary valve replacement; RA, right atrial; RV, right ventricular; RVOT, right ventricular outflow tract; TOF, tetralogy of Fallot.
Medication group
Digoxin and diuretics were taken in the medication group. Determination of dose was based on body weight of children, whereas digoxin 0.25 mg Qd, furosemide 20 mg Qd, and spironolactone 25 mg Qd were given at the conventional dose in adults. Electrolytes and serum digoxin levels were measured routinely.
Cardiac magnetic resonance
CMR was performed on a 1.5 Tesla magnetic resonance scanner (Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany). All imaging acquisitions were captured under breath control with the steady‐stage precession sequence technique. Late gadolinium enhancement images were acquired 15 minutes after intravenous administration of 0.2 mmol/kg of gadolinium‐DTPA (Magnevist; Schering, Berlin, Germany) using a phase‐sensitive inversion recovery/spoiled gradient echo sequence. Late gadolinium enhancement was performed only when patients were enrolled. RVOT aneurysm was defined as outward movement during systole of part of the ventricular wall or its reconstructed outflow tract. Images were reviewed and analyzed by 2 CMR experts. RV and LV volumes were measured. LV EF, RV‐corrected EF, and pulmonary regurgitation fraction were calculated. PRF=pulmonary regurgitant flow/pulmonary forward flow×100, and corrected EF=(pulmonary forward flow−regurgitant flow)/RV end‐diastolic volume.
Statistical Analysis
Categorical data are described as numbers with frequency and compared with the chi‐square test or the Fisher's exact test. Continuous variables are presented as the mean±SD or the median (interquartile range), and comparisons between groups were performed using the Student t test. The Mann–Whitney U test was used for non‐normally distributed variables. Freedom from adverse events between groups was analyzed using the Kaplan–Meier method followed by the log‐rank test. Binary logistic regression model was used to identify independent predictors of normalization of RV size in PVR group. Only variables statistically significant (P<0.05) in univariate analysis were included in the multivariate analysis (forward: likelihood ratio). The proportional hazard assumption of the Cox model was tested using the time‐dependent covariate test, and no clear violation was detected (P>0.05); when variables were statistically significant (P<0.05) in univariate Cox regression analysis, multivariate Cox regression analysis (forward: likelihood ratio) was conducted to identify the independent risk factors of adverse events in the medication group. Identification of a preoperative threshold in RV EDVI, RV end‐systolic volume index (ESVI), and QRS duration for normalization of RV EDVI was analyzed with the use of receiver operating characteristic (ROC) curves. Statistical analysis was performed using SPSS software (IBM SPSS Statistics for Macintosh, version 23.0; IBM Corp., Armonk, NY). A 2‐sided P<0.05 was considered to indicate statistical significance.
Results
Demographic data and operative characteristics are summarized in Table 1, and the aforementioned examination variables are summarized in Tables 2 and 3. Baseline characteristics were well balanced between groups. After a mean 33.1±10.0 months’ follow‐up, 3 deaths, 1 heart transplantation, 3 PVRs, and 2 symptomatic heart failures were recorded in the medication group during follow‐up, whereas only 1 redo PVR was recorded attributed to deterioration of the pulmonary valve in the PVR group. Tricuspid regurgitation improvement in the PVR group was significantly better than that in the medication group after treatment (1.08±0.89 to 2.74±1.87; P=0.000) at latest TTE follow‐up. Freedom from adverse events between the groups analyzed by the Kaplan–Meier method was significantly different between groups (P=0.006; Figure 1).
Table 2.
Enrollment Examination Between the Groups
| Parameters | PVR Group | Medication Group | P Value |
|---|---|---|---|
| CMR | |||
| PRF, % | 37.73±8.22 | 39.02±7.91 | 0.367 |
| Corrected RV EF, % | 20.71±5.43 | 22.97±5.63 | 0.070 |
| LV EF, % | 47.88±7.93 | 46.88±10.26 | 0.869 |
| RV EDVI, mL/m2 | 157.87±40.39 | 155.70 (72.10) | 0.702 |
| RV ESVI, mL/m2 | 104.43±34.00 | 90.65 (65.60) | 0.824 |
| RV EDD, mm | 43.38±8.33 | 44.74±8.56 | 0.721 |
| TTE | |||
| LV EF, % | 60.0 (10.9) | 60.26±9.29 | 0.650 |
| RV EDD, mm | 41.82±6.64 | 40.26±8.31 | 0.417 |
| TR | 2.15±1.27 | 2.47±1.62 | 0.354 |
| ECG | |||
| QRS duration, ms | 139.24±30.41 | 147.03±30.04 | 0.260 |
| Chest x‐ray | |||
| Cardiothoracic ratio | 0.57±0.07 | 0.57±0.08 | 0.572 |
CMR indicates cardiac magnetic resonance; EDD, end‐diastolic diameter; EDVI, end‐diastolic volume index; EF, ejection fraction; ESVI, end‐systolic volume index; LV, left ventricular; PRF, pulmonary regurgitation fraction; RV, right ventricular; TR, tricuspid regurgitation; TTE, transthoracic echocardiography.
Table 3.
Reexamination Variables Between the Groups
| Parameters | PVR Group | Medication Group | P Value |
|---|---|---|---|
| CMR | |||
| PRF, % | 2.10 (6.43) | 43.32±8.15 | 0.000 |
| Corrected RV EF, % | 37.94±9.49 | 22.87±9.21 | 0.000 |
| LV EF, % | 54.22±6.75 | 45.67±11.43 | 0.017 |
| RV EDVI, mL/m2 | 104.05±29.04 | 142.50 (69.3) | 0.000 |
| RV ESVI, mL/m2 | 57.82 (34.92) | 94.40 (73.97) | 0.000 |
| RV EDD, mm | 34.00 (9.00) | 48.00 (12.75) | 0.000 |
| TTE | |||
| LV EF, % | 64.49±7.40 | 62.50 (15.95) | 0.384 |
| RV EDD, mm | 27.00 (6.50) | 30.00 (8.75) | 0.031 |
| TR | 1.08±0.89 | 2.74±1.87 | 0.000 |
| ECG | |||
| QRS duration, ms | 138.37±31.76 | 150.67±32.08 | 0.144 |
| Chest x‐ray | |||
| Cardiothoracic ratio | 0.53±0.07 | 0.58±0.06 | 0.008 |
CMR indicates cardiac magnetic resonance; EDD, end‐diastolic diameter; EDVI, end‐diastolic volume index; EF, ejection fraction; ESVI, end‐systolic volume index; LV, left ventricular; PRF, pulmonary regurgitation fraction; RV, right ventricular; TR, tricuspid regurgitation; TTE, Transthoracic echocardiography.
Figure 1.
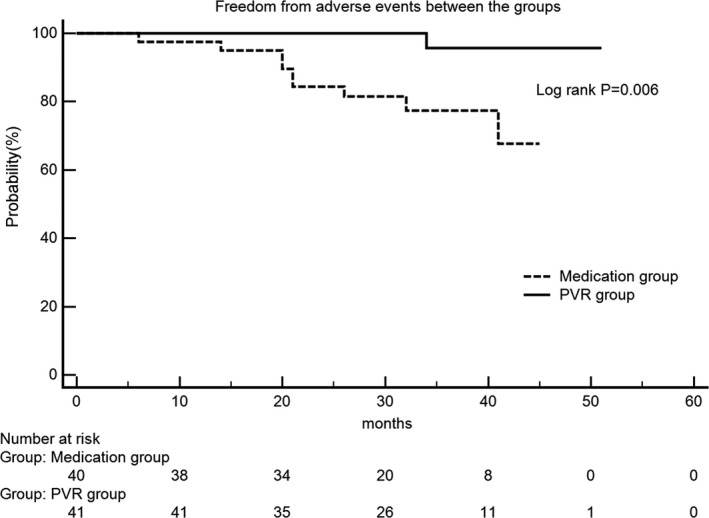
Freedom from adverse events between groups. Patients in the PVR group had a lower adverse events rate (log rank test, P=0.006). PVR indicates pulmonary valve replacement.
Medication Group
Examinations were performed when participants were enrolled and reperformed after a mean duration of 28.36±10.09 months. Thirty‐two of 40 patients had severe PR, and the remaining 8 patients had moderate PR, preoperatively. Myocardial fibrosis, severe or absent left pulmonary artery stenosis, and RVOT aneurysm were found in 5, 5, and 6 patients by CMR, respectively. Two patients with normal RV size were observed; 1 returned to normal with pre–RV EDVI 126 mL/m2, whereas the other developed a larger RV size within its normal range. Causes of death in 3 cases included 2 sudden deaths and 1 heart failure. Heart transplantation was performed in another hospital 18 months after enrollment. According to the criteria of PVR for symptomatic rTOF patients in our institution (moderate or severe pulmonary regurgitation with ≥1 of the following: [1] New York Heart Association class III/IV, [2] sustained tachyarrhythmia related to right‐sided heart volume load, [3] syncope caused by arrhythmias, and [4] significant hemodynamic abnormalities, such as severe aortic valve regurgitation, severe tricuspid valve regurgitation, large RVOT aneurysm, etc), 3 patients developed into symptomatic and underwent PVR 20, 26, and 40 months after enrollment, separately. Two patients developed symptomatic heart failure 14 and 21 months after enrollment without PVR, separately. The analysis revealed that there was no significant difference in examination results between enrollment and follow‐up period. Cox regression analysis identified that arrhythmia and preoperative RV ESVI were the independent risk factors of adverse events (Table 4).
Table 4.
Cox Regression Analysis for Adverse Events in the Medication Group
| Predictors | P Value of PH Assumption | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Exp (B) (95% CI) | P Value | Exp (B) (95% CI) | P Value | ||
| Arrhythmia | 0.419 | 8.497 (1.895, 38.100) | 0.005 | 20.372 (2.232, 185.924) | 0.008 |
| Corrected RV EF, % | 0.301 | 0.839 (0.745, 0.946) | 0.004 | ··· | ··· |
| RV ESVI (10‐mL/m2 increment) | 0.636 | 1.190 (1.086, 1.304) | 0.000 | 1.151 (1.029, 1.287) | 0.014 |
| LV EF, % | 0.689 | 0.880 (0.815, 0.951) | 0.001 | ··· | ··· |
| LV ESVI (10‐mL/m2 increment) | 0.879 | 1.264 (1.103, 1.448) | 0.001 | ··· | ··· |
| TR degree | 0.424 | 3.797 (1.644, 8.770) | 0.002 | ··· | ··· |
| QRS duration (10‐ms increment) | 0.488 | 1.122 (1.044, 1.205) | 0.002 | ··· | ··· |
| Cardiothoracic ratio, % | 0.445 | 1.498 (1.115, 2.013) | 0.007 | ··· | ··· |
EF indicates ejection fraction; ESVI, end‐systolic volume index; LV, left ventricular; PH, proportional hazard; PRF, pulmonary regurgitation fraction; RV, right ventricular; TR, tricuspid regurgitation.
PVR Group
Examinations were performed with a mean duration of 3.68±4.50 months preoperatively and repeated at a mean duration of 17.24±10.22 months postoperatively. Thirty‐nine of 41 patients had severe PR, and the remaining 2 patients had moderate PR, preoperatively. Myocardial fibrosis, absent or severe left pulmonary artery stenosis, and RVOT aneurysm were found in 1, 4, and 11 patients by CMR, respectively. After PVR, there were no in‐hospital and late deaths, whereas 1 redo PVR was performed because of valve failure during follow‐up. At latest TTE follow‐up, 2 trivial, 4 mild, and 2 moderate pulmonary regurgitation were found in the PVR group. Tricuspid regurgitation significantly decreased from 2.15±1.27 to 1.08±0.89 (P=0.000). The mean residual gradient of RVOT after PVR was 16.33±9.02 mm Hg, and no residual obstruction or RVOT aneurysm was found after PVR. Functional status of all patients was New York Heart Association class I. Thirty‐one patients achieved normal RV EDVI, and 7 achieved normal RV EF.
In CMR, LV EF and corrected RV EF were significantly increased after PVR (P<0.05). Pulmonary regurgitation fraction, RV EDVI, RV ESVI, and RV EDD were significantly decreased after PVR (P<0.05), whereas LV EDVI and LV ESVI did not change significantly after operation. LV EF, RV EDD, and tricuspid regurgitation detected by TTE improved significantly after PVR (P<0.05). Postoperative cardiothoracic ratio was significantly decreased. QRS duration was not significantly changed postoperatively.
Predictors of Normalization of RV Size and Cut‐Off Value Predicting Normalization of RV Size
Results of the binary logistics regression analysis for predictors of normalization of RV size are shown in Table 5. Preoperative RV ESVI (10‐mL/m2 increment, P=0.009; odds ratio, 0.64; 95% CI, 0.457–0.893) was the independent predictor of normalization of RV size after PVR in multivariate analysis.
Table 5.
Predictors of Normalization of RV Size
| Predictors | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Exp (B) (95% CI) | P Value | Exp (B) (95% CI) | P Value | |
| Preoperative RV EDVI (10‐mL/m2 increment) | 0.732 (0.561, 0.937) | 0.014 | ··· | ··· |
| Preoperative RV ESVI (10‐mL/m2 increment) | 0.639 (0.457, 0.893) | 0.009 | 0.639 (0.457, 0.893) | 0.009 |
| RVOT aneurysm | 0.142 (0.031, 0.653) | 0.012 | ··· | ··· |
| QRS duration (10‐ms increment) | 0.663 (0.457, 0.933) | 0.019 | ··· | ··· |
EDVI indicates end‐diastolic volume index; ESVI, end‐systolic volume index; RV, right ventricular; RVOT, right ventricular outflow tract.
Thirty‐one (31 of 41; 75.6%) patients returned to normal RV size, and 7 (7 of 41; 17%) returned to normal RV function. Patients with normalized RV size after surgery had a lower preoperative RV EDVI (144.91±31.29 versus 179.29±30.64 mL/m2; P=0.004), a lower preoperative RV ESVI (92.63±25.99 versus 123.25±25.46 mL/m2; P=0.002), and a shorter QRS duration (133.10±28.99 versus 158.30±27.986 ms; P=0.021) than that of patients without a normalized RV size in PVR group. The preoperative RV EDVI cut‐off value of 155 mL/m2 (71% sensitivity, 80% specificity), preoperative RV ESVI cut‐off value of 120 mL/m2 (90.3% sensitivity, 70% specificity), and preoperative QRS duration cut‐off value of 121 ms (35.5% sensitivity, 100% specificity) were calculated by ROC curves for normalization of RV size, and the areas under the curve was 0.795, 0.819, and 0.695, respectively (Figure 2).
Figure 2.
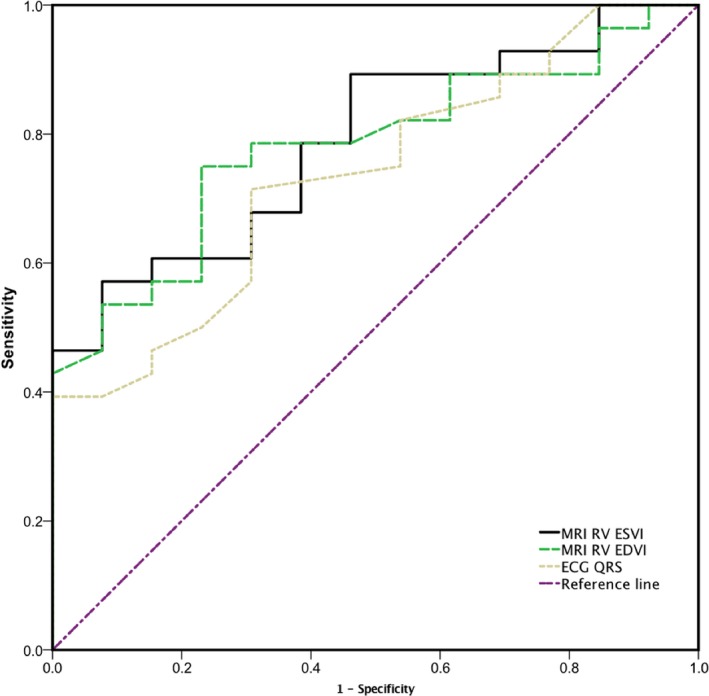
Preoperative RV EDVI, RV ESVI, and QRS duration were analyzed by receiver operating characteristic curves for normalized RV size after pulmonary valve replacement. EDVI indicates end‐diastolic volume index; ESVI, end‐systolic volume index; MRI, magnetic resonance imaging; RV, right ventricular.
Discussion
Our study demonstrated a beneficial effect of PVR in asymptomatic rTOF patients with moderate or great pulmonary regurgitation on RV size and function, and normalization of RV size after PVR had a correlation with preoperative RV ESVI.
Optimal timing of PVR has sparked debate, which has catalyzed increasing research efforts over the past decade. Several studies6, 12, 13 revealed that minimal benefit was achieved in some of the sickest patients after PVR, indicating that it was too late to reverse RV size and function. Burchill et al14 pointed out that PVR should be performed in patients who were aged <18 years according to improvement of exercise capacity. In contrast, some studies15, 16 demonstrated that young age at PVR was associated with an increased risk of prosthetic valve failure and dysfunction. Chen et al suggested the recommended age for PVR was ≥15 years. In our study, patients who underwent PVR maintained heart function in New York Heart Association class I, and no valve failure and dysfunction were found at the latest follow‐up. Previous studies demonstrated that mortality rate nearly tripled during the third decade after TOF repair,17 which implies that PVR should be performed within 20 years after TOF repair to prevent occurrence of adverse outcomes. In the current study, PVR was underwent after a duration of 17 (6.30) years after TOF repair, which indicates that the timing for PVR was acceptable. Compared with the PVR group, incidence of adverse events was higher in the medication group after a duration of 13.83 (9.34) years after TOF repair, although in the face of younger enrollment age. Furthermore, compared with symptomatic rTOF patients, RV function in asymptomatic rTOF patients was maintained in the compensatory stage, indicating an increased likelihood of restoration of RV function after PVR.
Although RV size decreases after PVR, RV normalization does not occur very often.4 Several studies8, 18, 19 found that RV size does not return to normal after PVR unless preoperative RV EDVI was <160 to 170 mL/m2 or RV ESVI was <85 to 90 mL/m2. However, normalization of RV size did occur in 3 patients with the preoperative RV EDVI >200 mL/m2, suggesting that normalization was possible even in patients with a massive RV volume. According to our results, it appears that there is no determined upper threshold for normalization, which agrees with previous studies.20 However, it should be pointed out that these 3 patients in our study did not present arrhythmia, RVOT aneurysm, or myocardial fibrosis, which have been confirmed as risk factors of RV dysfunction by previous studies.21, 22 In this study, logistics regression analysis found that preoperative RV ESVI was the independent predictor of normalization of RV size. ROC curve analysis calculated preoperative RV EDVI and RV ESVI cut‐off values of 155 and 120 mL/m2 for normalization of RV size, respectively. It is worthwhile to emphasize that the cut‐off value was calculated by an ROC curve with a maximum value of the Youden index, and sensitivity and specificity levels should be carefully selected during clinical decision making.
Some studies23, 24 found that RV EF did not improve significantly after PVR. We assumed that the reason for this phenomenon was that the noncorrected preoperative RV EF was over‐rated. In our study, the corrected RV EF, which only calculated the net pulmonary flow, was used to evaluate RV function instead of the conventional RV EF. A dramatic increase from 20.71% to 37.94% in the corrected RV EF was found, suggesting that the surgery was beneficial for recovery of RV function.
Several studies25, 26, 27, 28 identified QRS duration as an important predictor of RV remodeling and noted that QRS duration shortens after an operation. However, QRS duration did not change significantly in the current study, and the reason might be that all these patients were preoperatively asymptomatic. The preoperative QRS duration cut‐off value calculated by the ROC curve is hardly of practical significance because of the low sensitivity.
The study has a number of important limitations; no rigid protocol or guideline for PVR in these asymptomatic rTOF patients was followed. Inclusion and exclusion criteria were derived from the Consensus, which was mainly based on former retrospective studies. The small study population was another important limitation. Cardiac function in our study lacked of objective evaluation. Hence, the cardiopulmonary exercise testing or 6‐minute walk test was arranged for the subsequent patients. An extended follow‐up time span should be adopted.
Conclusion
In the current study, we confirmed a beneficial effect of PVR in asymptomatic rTOF patients with moderate or great pulmonary regurgitation on RV size and function. Our findings indicated that the criterion for ESVI should be updated because the RV ESVI >120 mL/m2 is related to less RV normalization.
Sources of Funding
This study was supported by the National Key R&D Program of China (2017YFC1308100).
Disclosures
None.
Acknowledgments
We would like to acknowledge Dr Wei Li for the assistance with the statistical analysis.
(J Am Heart Assoc. 2019;8:e010689 DOI: 10.1161/JAHA.118.010689.)
References
- 1. Webb G, Mulder BJ, Aboulhosn J, Daniels CJ, Elizari MA, Hong G, Horlick E, Landzberg MJ, Marelli AJ, O'Donnell CP, Oechslin EN, Pearson DD, Pieper EP, Saxena A, Schwerzmann M, Stout KK, Warnes CA, Khairy P. The care of adults with congenital heart disease across the globe: current assessment and future perspective: a position statement from the International Society for Adult Congenital Heart Disease (ISACHD). Int J Cardiol. 2015;195:326–333. [DOI] [PubMed] [Google Scholar]
- 2. Frigiola A, Hughes M, Turner M, Taylor A, Marek J, Giardini A, Hsia TY, Bull K. Physiological and phenotypic characteristics of late survivors of tetralogy of Fallot repair who are free from pulmonary valve replacement. Circulation. 2013;128:1861–1868. [DOI] [PubMed] [Google Scholar]
- 3. Avila P, Chaix MA, Mondesert B, Khairy P. Sudden cardiac death in adult congenital heart disease. Card Electrophysiol Clin. 2017;9:225–234. [DOI] [PubMed] [Google Scholar]
- 4. Ferraz Cavalcanti PE, Sá MP, Santos CA, Esmeraldo IM, de Escobar RR, de Menezes AM, de Azevedo OM Jr, de Vasconcelos Silva FP, Lins RF, Lima Rde C. Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta‐analysis and meta‐regression of 3,118 patients from 48 studies. J Am Coll Cardiol. 2013;62:2227–2243. [DOI] [PubMed] [Google Scholar]
- 5. van der Wall EE, Mulder BJ. Pulmonary valve replacement in patients with tetralogy of Fallot and pulmonary regurgitation: early surgery similar to optimal timing of surgery? Eur Heart J. 2005;26:2614–2615. [DOI] [PubMed] [Google Scholar]
- 6. Therrien J, Siu SC, McLaughlin PR, Liu PP, Williams WG, Webb GD. Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J Am Coll Cardiol. 2000;36:1670–1675. [DOI] [PubMed] [Google Scholar]
- 7. Therrien J, Siu SC, Harris L, Dore A, Niwa K, Janousek J, Williams WG, Webb G, Gatzoulis MA. Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation. 2001;103:2489–2494. [DOI] [PubMed] [Google Scholar]
- 8. Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–782. [DOI] [PubMed] [Google Scholar]
- 9. Tweddell JS, Simpson P, Li SH, Dunham‐Ingle J, Bartz PJ, Earing MG, Pelech AN. Timing and technique of pulmonary valve replacement in the patient with tetralogy of Fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2012;15:27–33. [DOI] [PubMed] [Google Scholar]
- 10. Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady‐state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. [DOI] [PubMed] [Google Scholar]
- 11. Geva T. Indications for pulmonary valve replacement in repaired tetralogy of Fallot: the quest continues. Circulation. 2013;128:1855–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sabate Rotes A, Eidem BW, Connolly HM, Bonnichsen CR, Rosedahl JK, Schaff HV, Dearani JA, Burkhart HM. Long‐term follow‐up after pulmonary valve replacement in repaired tetralogy of Fallot. Am J Cardiol. 2014;114:901–908. [DOI] [PubMed] [Google Scholar]
- 13. Westhoff‐Bleck M, Girke S, Breymann T, Lotz J, Pertschy S, Tutarel O, Roentgen P, Bertram H, Wessel A, Schieffer B, Meyer GP. Pulmonary valve replacement in chronic pulmonary regurgitation in adults with congenital heart disease: impact of preoperative QRS‐duration and NT‐proBNP levels on postoperative right ventricular function. Int J Cardiol. 2011;151:303–306. [DOI] [PubMed] [Google Scholar]
- 14. Burchill LJ, Wald RM, Harris L, Colman JM, Silversides CK. Pulmonary valve replacement in adults with repaired tetralogy of Fallot. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2011;14:92–97. [DOI] [PubMed] [Google Scholar]
- 15. Lindsey CW, Parks WJ, Kogon BE, Sallee D III, Mahle WT. Pulmonary valve replacement after tetralogy of Fallot repair in preadolescent patients. Ann Thorac Surg. 2010;89:147–151. [DOI] [PubMed] [Google Scholar]
- 16. Chen XJ, Smith PB, Jaggers J, Lodge AJ. Bioprosthetic pulmonary valve replacement: contemporary analysis of a large, single‐center series of 170 cases. J Thorac Cardiovasc Surg. 2013;146:1461–1466. [DOI] [PubMed] [Google Scholar]
- 17. Geva T. Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006;9:11–22. [DOI] [PubMed] [Google Scholar]
- 18. Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, Mulder BJ. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551. [DOI] [PubMed] [Google Scholar]
- 19. Geva T, Gauvreau K, Powell AJ, Cecchin F, Rhodes J, Geva J, del Nido P. Randomized trial of pulmonary valve replacement with and without right ventricular remodeling surgery. Circulation. 2010;122(11 Suppl):S201–S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quail MA, Frigiola A, Giardini A, Muthurangu V, Hughes M, Lurz P, Khambadkone S, Deanfield JE, Tsang V, Taylor AM. Impact of pulmonary valve replacement in tetralogy of Fallot with pulmonary regurgitation: a comparison of intervention and nonintervention. Ann Thorac Surg. 2012;94:1619–1626. [DOI] [PubMed] [Google Scholar]
- 21. Khairy P, Aboulhosn J, Gurvitz MZ, Opotowsky AR, Mongeon FP, Kay J, Valente AM, Earing MG, Lui G, Gersony DR, Cook S, Ting JG, Nickolaus MJ, Webb G, Landzberg MJ, Broberg CS; Alliance for Adult Research in Congenital Cardiology (AARCC) . Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi‐institutional study. Circulation. 2010;122:868–875. [DOI] [PubMed] [Google Scholar]
- 22. Wald RM, Haber I, Wald R, Valente AM, Powell AJ, Geva T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation. 2009;119:1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vliegen HW, van Straten A, de Roos A, Roest AA, Schoof PH, Zwinderman AH, Ottenkamp J, van der Wall EE, Hazekamp MG. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of Fallot. Circulation. 2002;106:1703–1707. [DOI] [PubMed] [Google Scholar]
- 24. Bigdelian H, Mardani D, Sedighi M. The effect of pulmonary valve replacement (PVR) surgery on hemodynamics of patients who underwent repair of tetralogy of Fallot (TOF). J Cardiovasc Thorac Res. 2015;7:122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paech C, Dähnert I, Riede FT, Wagner R, Kister T, Nieschke K, Wagner F, Gebauer RA. QRS width as a predictor of right ventricular remodeling after percutaneous pulmonary valve implantation. Pediatr Cardiol. 2017;38:1277–1281. [DOI] [PubMed] [Google Scholar]
- 26. Shanmugam N, Yap J, Tan RS, Le TT, Gao F, Chan JX, Chong D, Ho KL, Tan BY, Ching CK, Teo WS, Tan JL, Liew R. Fragmented QRS complexes predict right ventricular dysfunction and outflow tract aneurysms in patients with repaired tetralogy of Fallot. Int J Cardiol. 2013;167:1366–1372. [DOI] [PubMed] [Google Scholar]
- 27. Scherptong RW, Hazekamp MG, Mulder BJ, Wijers O, Swenne CA, van der Wall EE, Schalij MJ, Vliegen HW. Follow‐up after pulmonary valve replacement in adults with tetralogy of Fallot: association between QRS duration and outcome. J Am Coll Cardiol. 2010;56:1486–1492. [DOI] [PubMed] [Google Scholar]
- 28. McRae ME, Coleman B, Atz TW, Kelechi TJ. Patient outcomes after transcatheter and surgical pulmonary valve replacement for pulmonary regurgitation in patients with repaired tetralogy of Fallot: a quasi‐meta‐analysis. Eur J Cardiovasc Nurs. 2017;16:539–553. [DOI] [PubMed] [Google Scholar]


