Abstract
Black and brown pigmentation of the oral mucosa can occur due to a multitude of non-neoplastic causes. Endogenous or exogenous pigments may be responsible for oral discoloration which can range from innocuous to life-threatening in nature. Physiologic, reactive, and idiopathic melanin production seen in smoker’s melanosis, drug-related discolorations, melanotic macule, melanoacanthoma and systemic diseases are presented. Exogenous sources of pigmentation such as amalgam tattoo and black hairy tongue are also discussed. Determining the significance of mucosal pigmented lesions may represent a diagnostic challenge for clinicians. Biopsy is indicated whenever the source of pigmentation cannot be definitively identified based on the clinical presentation.
Keywords: Black, Brown, Pigmentation, Oral mucosa, Melanin, Melanotic, Biopsy
Introduction
Black and brown pigmented lesions of the oral cavity can occur from deposition of either exogenous materials or endogenous pigments. The etiology may be physiologic, reactive, neoplastic, idiopathic, or a sign of systemic disease. While the function of melanocytes in oral mucosa is uncertain, they are found within the basal layer and produce melanin which can be taken up by epithelial cells and/or spilled into the subjacent connective tissue [1]. Determining the significance of oral mucosal pigmentation requires clinical expertise and careful evaluation. This section focuses on non-neoplastic causes of brown and black lesions affecting the oral mucosa.
Physiologic Pigmentation
Physiologic pigmentation (racial pigmentation) presents clinically as macular pigmented areas of varying shapes and sizes in patients from many different ethnic backgrounds. It is seen more commonly in darker-skinned people and results from increased melanocytic activity rather than increased numbers of melanocytes [2]. No gender predilection has been reported [3, 4]. The pigmentation can be seen in any age person however prevalence increases with age [3, 5]. Increased intensity of pigment may occur when factors including smoking, hormonal changes and systemic medications are involved [5].
The color of pigmentation may vary from light tan to dark brown to almost black. Physiologic pigmentation may be seen in any oral location, but the attached gingiva is most common (Fig. 1a). Gingival pigmentation is highly variable in both pattern and color among people of different races. Additionally, variability within a single race is also noted. Other involved sites in decreasing order of frequency include the buccal mucosa, lips and palate [4, 5]. In nongingival locations, the pigmentation tends to be irregularly-shaped with less defined borders [6]. On the dorsal tongue, the pigmentation may involve just the tips of the fungiform papillae (Fig. 1b). When examined microscopically, physiologic pigmentation shows increased melanin within the basal epithelial layer and melanin incontinence within the superficial lamina propria [2]. Diagnosis of physiologic pigmentation is based on clinical examination and treatment is not necessary. Other entities that may exhibit similar features include smoker’s melanosis, medication-related pigmentation, Addison’s disease and possibly Peutz–Jeghers syndrome (PJS). Dark-skinned individuals are also prone to developing post-inflammatory pigmentation in the oral cavity, which can be seen with chronic trauma or inflammatory conditions [2, 5, 7, 8]. It is commonly seen within or adjacent to lesions of lichen planus (Fig. 1c). Taking a complete medical and social history as well as questioning the patient about the onset of pigmentation is of importance in determining the diagnosis.
Fig. 1.
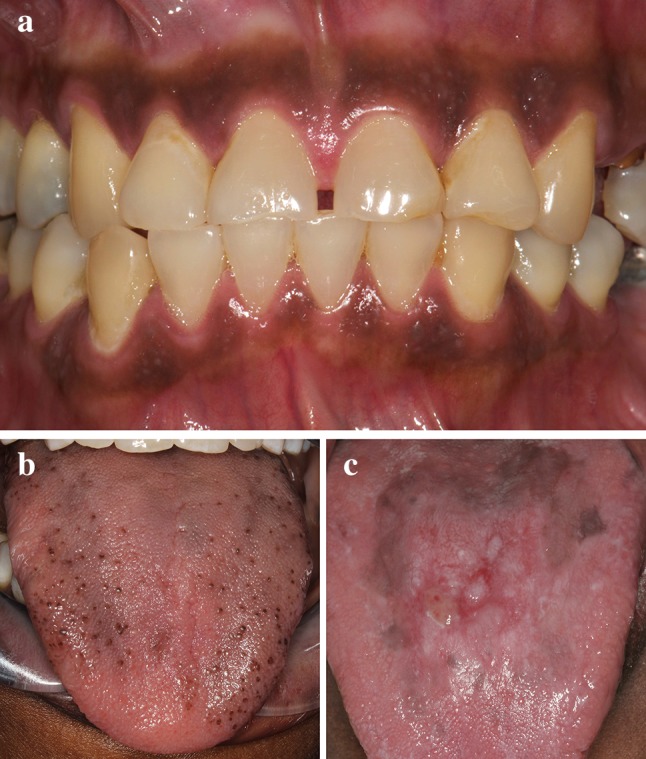
a Diffuse, homogenous brown pigmentation of the attached gingiva. b Physiologic pigmentation of the fungiform papillae. c Post-inflammatory pigmentation of the dorsal tongue in a patient with erosive lichen planus
Pigmentation Due to Systemic Disease
Oral and/or perioral pigmentation can be seen as a manifestation of certain systemic diseases. The pigmented lesions tend to be multifocal however the clinical presentation can vary from subtle to dramatic even within the same disease [5]. For each of the three conditions discussed hereafter, biopsy findings show the pigmentation is the result of increased melanin within the basal layer [5, 9–11].
Addison’s Disease (Hypoadrenocorticism)
Diffuse pigmentation of the oral cavity and skin is an early and highly specific manifestation of Addison’s disease, occurring in up to 92% of patients [8]. Addison’s disease is the result of inadequate production of cortisol and aldosterone due to destruction of the adrenal cortex [12]. A variety of mechanisms including infections (namely tuberculosis) and neoplasms can cause Addison’s disease however the most common cause is autoimmune adrenalitis [8, 12, 13]. In response to insufficient corticosteroid levels, the pituitary gland synthesizes and secretes increased amounts of adrenocorticotropic hormone (ACTH). As ACTH levels rise, there is a corresponding increase in α-melanocyte stimulating hormone; both of these promote melanogenesis and thus the characteristic mucocutaneous pigmentation [7, 12].
Addison’s disease has no gender predilection and affects all age groups [13]. In the oral cavity, Addison’s disease generally manifests as patchy melanosis affecting multiple locations (Fig. 2). Intraoral pigmentation often precedes bronzing of the skin [13, 14]. Other clinical features include slowly progressive weakness, anorexia, mood disturbances, nausea, vomiting, diarrhea and weight loss [1, 5, 12, 13]. Hyperkalemia and hyponatremia resulting in a craving for salt are also observed [5, 7, 13]. Recognizing the early signs of Addison’s disease is critical because the condition can be fatal if not treated [12]. The diagnosis involves exogenous ACTH stimulation testing with subsequent measurement of serum cortisol and plasma ACTH levels [12, 14]. Treatment involves steroid replacement therapy which usually resolves the hyperpigmentation. The dosage may need to be temporarily increased prior to an extended medical or dental procedure due to the body’s increased need for corticosteroids during stressful occasions [14]. Patients who are managed properly can expect a normal life span [14].
Fig. 2.
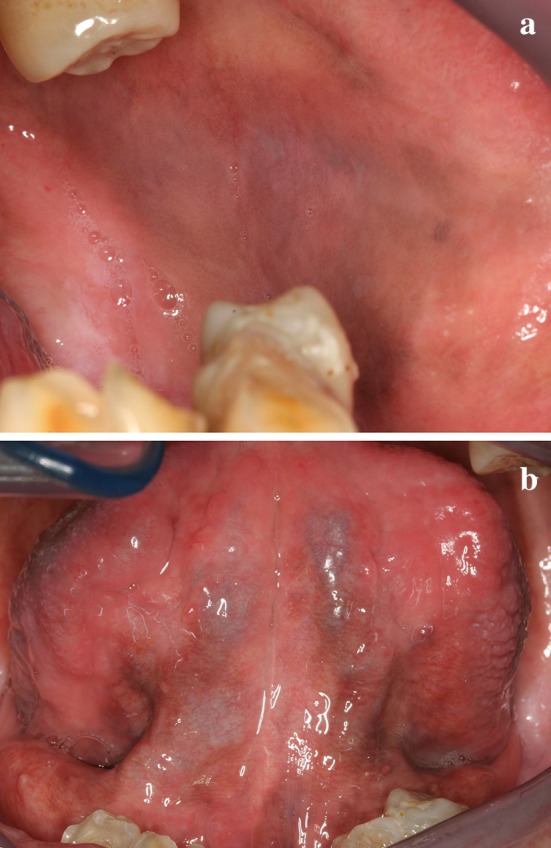
Diffuse light brown pigmentation affecting the buccal mucosa (a) and ventral tongue (b) in a patient with Addison’s disease
Peutz–Jeghers Syndrome
PJS is an autosomal dominant genetic condition due to mutation of the STK11 tumor suppressor gene [1, 7, 15]. Zhang et al. demonstrated a positive correlation between number of mutant STK11 copies and phenotypic severity of PJS [15]. Patients with the disease develop multiple hamartomatous intestinal polyps and have an increased likelihood of developing malignancies in multiple sites including the gastrointestinal tract, breast, thyroid, ovaries, testicles and pancreas [1, 5, 8, 15]. Intussusception and bowel obstruction also occur [11]. Males and females are equally affected by PJS [11].
Characteristic pigmentation of the lips and perioral skin resembling numerous dark freckles (Fig. 3) is one of the earliest manifestations of PJS, typically presenting during childhood or adolescence [5, 7, 15]. Pigmentation can also occur intraorally (particularly on the buccal mucosa) and on the skin of the extremities [7, 11, 14]. The differential diagnosis for this pattern of oral and perioral pigmentation includes Laugier–Hunziker syndrome (LHS), although the pigmentation in that condition does not occur until adulthood [7, 16]. Other clinical features of PJS include abdominal pain, anemia and gastrointestinal bleeding [15]. Treatment involves managing gastrointestinal symptoms and careful monitoring for the development of cancers [7, 14]. The oral and perioral pigmentation persists throughout life and does not require treatment however laser therapy can be performed if the patient elects cosmetic intervention [11].
Fig. 3.
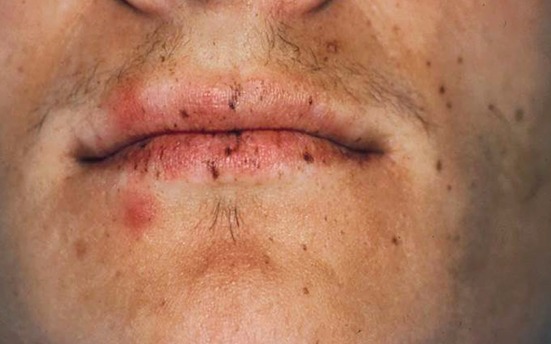
Multiple small, dark brown macules on the lip vermillion and perioral skin of a patient with Peutz–Jeghers syndrome (photo courtesy of Dr. Robert Brannon)
Laugier–Hunziker Syndrome (Idiopathic Lenticular Mucocutaneous Pigmentation)
LHS is characterized by multiple acquired, idiopathic pigmented macules on the oral mucosa and lips [10]. The condition is usually diagnosed in adulthood at an average age of 48 [16]. Females are affected twice as often as males [16]. While reported to be most common in Caucasians [5, 16], cases presenting in dark-skinned individuals may be misinterpreted as physiologic pigmentation. Lesions are most frequent on the buccal mucosa, lips, gingiva, palate and tongue (Fig. 4a) [5, 10, 16]. Other anatomic mucosae can also be affected [7, 16]. The fingernails and/or toenails exhibit longitudinal pigmented streaks (melanonychia) in 60–65% of patients (Fig. 4b) [10, 16]. Because LHS is a diagnosis of exclusion, patients with this clinical presentation should be referred to a gastroenterologist to rule out PJS [17]. The condition is gradually progressive but completely benign [16, 18]. Laser ablation can be effective in reducing the visibility of lesions in LHS [16, 19].
Fig. 4.
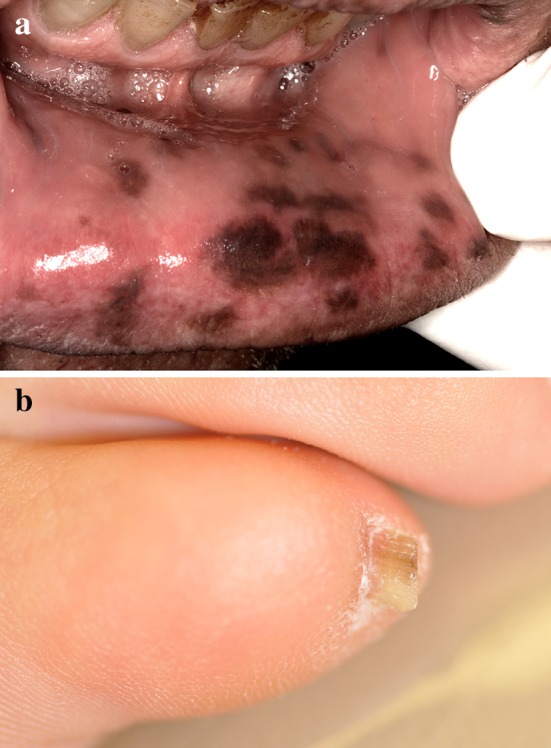
a Multiple brown macules on the labial mucosa of a patient with Laugier–Hunziker syndrome (photo courtesy of Dr. Carl Allen) and b toenail exhibiting melanonychia
Drug-Related Discolorations
Numerous systemic medications have been implicated in causing oral mucosal pigmentation by either inducing melanin synthesis or by deposition of the drug or its metabolites. Medications which have been most frequently implicated include antimalarials, hormones, oral contraceptives, phenothiazines, chemotherapeutics, amiodarone, minocycline, zidovudine, clofazimine and ketoconazole [2, 5, 7, 8, 20, 21]. Any patient taking these medications long term should be monitored for the development of oral pigmentation [22]. Hard palate, gingiva and buccal mucosa are the most common locations affected [8, 9]. Clinically, the discoloration is flat and can be focal, multifocal or diffuse. The color may be black, gray, blue or brown. Pigmentation restricted to the hard palate is classically seen with antimalarials, which are commonly prescribed in the treatment of rheumatoid arthritis and systemic lupus erythematosus [23]. A somewhat symmetric area of involvement centered on the midline may be seen (Fig. 5). Minocycline induces blue–black discoloration of gnathic bones in 20% or more of individuals who have taken the medication more than 4 years [22]. While this can make the overlying tissue appear bluish clinically, it does not represent true mucosal pigmentation [7]. However, pigmentation of oral mucosa resulting from increased melanin production has been reported with minocycline [20, 22, 24].
Fig. 5.
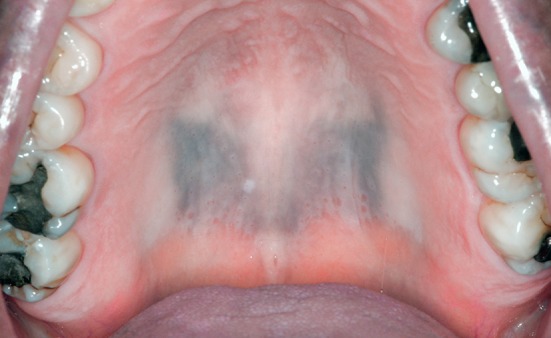
Drug-related discoloration: symmetric gray/black pigmentation of the posterior hard palate in a patient taking hydroxychloroquine for the treatment of systemic lupus erythematosus
Diagnosis is based on temporally correlating the pigmentation with initiation of a drug known to cause this adverse effect. Significant fading of the lesions can occur after discontinuation of the medication [5, 20]. Biopsy may be necessary in some instances to confirm the diagnosis given that these discolorations tend to occur in the same locations where oral melanoma most often develops. Depending on the medication, the histopathologic features either mimic those of melanotic macule or present as fine brown–yellow granules in the lamina propria interpreted as deposition of drug or drug products [20].
While uncommon, oral mucosal pigmentation can be a manifestation of metallic poisoning [20]. Systemic exposure to heavy metals can occur due to accidental ingestion, occupational exposure, or usage as a medical treatment. Lead poisoning (plumbism) results in formation of a blue discoloration of the marginal gingiva (Burton line) due to the action of oral bacteria producing lead sulfide precipitate [1, 5, 8, 14]. Similar gingival discoloration can be seen with mercury, silver and bismuth intoxication [14].
Melanotic Macule
Melanotic macule is one of the most common pigmented lesions involving the oral cavity. It is a benign lesion resulting from increased melanin production with occasional increase in melanocyte number [2]. Lesions have been reported in patients age 1–98 years, with a mean of approximately 42 years [25–28]. Lip lesions tend to be diagnosed at a younger age than intraoral lesions, possibly resulting from ease of patient visualization of lip vermillion versus intraoral sites [27]. The majority of melanotic macules are found in female patients. Most investigators report an average 2:1 female to male ratio, however Gupta et al. reported a 5:1 female to male ratio in a series of 79 cases [25–27, 29, 30]. Melanotic macules may be seen in patients of any race. Kaugers et al. reported that white patients were more likely to have lower lip lesions, while black patients more often had buccal mucosa lesions [25].
Clinically, melanotic macule presents as a solitary, well-defined, evenly-pigmented, flat lesion varying from tan to dark brown in color (Fig. 6a). Multiple lesions have been reported [5, 29, 31]. Lesions vary in size from 0.1 to 2.0 cm with the majority being less than 1 cm [2, 26]. The lip vermillion is the most common location in all series reviewed, with the lower lip being most common [25–28, 30]. Unlike the ephelis, melanotic macules do not darken with sun exposure [31, 32]. The most common intraroral locations include gingiva, palate and buccal mucosa (Fig. 6b). Both labial and intraoral lesions are typically asymptomatic. Some patients are unaware of the presence of the pigmented macules while others report noting their appearance for weeks to years [26, 29, 32].
Fig. 6.
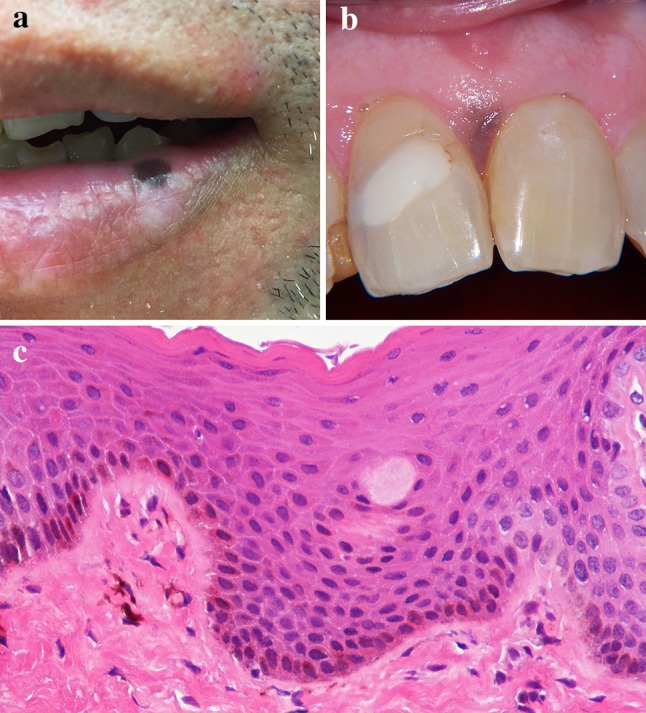
a Melanotic macule at the wet–dry line of the lower lip. b Melanotic macule of the interdental papilla between #8 and #9. c Histopathologic features of melanotic macule demonstrating increased melanin within the basal layer and incontinent melanin within the lamina propria
Microscopically, melanotic macules exhibit increased melanin pigmentation within melanocytes and keratinocytes located at the basal layer of otherwise normal stratified squamous epithelium (Fig. 6c) [2, 31–33]. Melanin pigmentation has been reported to be most intense at the tips of the rete ridges [31, 34]. Mild hyperkeratosis and/or mild acanthosis of the spinous layer may be observed however elongation of the rete ridges is absent [1, 5, 26, 31, 33]. Typically, no increase in numbers of melanocytes is reported [7, 27, 35] although Page et al. reported melanocytic hyperplasia in 24% of cases in the first publication regarding the oral melanotic macule [33]. Additionally, Sexton and Maize found a statistically significant increase in the number of melanocytes present within the basilar epithelial layers of labial melanotic macules when compared to control tissue of clinically normal adult lip [34]. The superficial lamina propria may exhibit incontinent melanin (Fig. 6c), melanophages, and varying amounts of chronic inflammation [25, 26, 31–33]. Lesions should not exhibit atypia or nesting of melanocytes. In the series by Ho et al. of 36 labial melanotic macules, all were negative with immunohistochemical marker HMB-45 [31].
A biopsy is usually required for a definitive diagnosis of melanotic macule. If presenting with classic features on the lower lip, a clinical diagnosis may be established with recommendation of periodic monitoring by a dental or medical provider. Lambertini et al. suggested sequential photography for melanotic macules < 0.5 cm and biopsy of lesions > 0.5 cm [36]. Uribe et al. reported in vivo reflectance confocal microscopy to be useful in differentiating between labial melanotic macules and melanoma however, biopsy was still necessary for definitive diagnosis [37]. If labial lesions have border irregularity, uneven pigment, color change, ulceration or other suspicious features, regardless of size, biopsy is recommended to rule out melanoma. As there are no clinical criteria to distinguish intraoral melanotic macules from melanoma, it is recommended that all oral cavity lesions be biopsied, particularly those of the palate as this is the most common site of intraoral melanoma [2, 33]. No further treatment is necessary once a biopsy proven diagnosis of melanotic macule has been rendered.
Melanoacanthoma
Melanoacanthoma is an uncommon, acquired, benign, rapidly evolving pigmented lesion of oral mucosa [7, 27, 38]. The cause of this lesion is unknown, however, a traumatic or reactive etiology has been suggested [1, 5, 27]. It typically occurs in black females in the third to fourth decades of life, but may be observed in other populations [2, 5, 39, 40]. Clinically, melanoacanthoma presents as a rapidly enlarging dark brown to black pigmentation of the oral mucosa that can mimic melanoma (Fig. 7a) [7, 27, 38]. Melanoacanthoma is usually unilateral but can be bilateral. It may be flat or slightly elevated and is usually well demarcated from the surrounding tissue [5, 39, 40]. The most commonly involved site is the buccal mucosa; however, this condition has also been reported on the lip, palate, and gingiva [5, 7, 40–42].
Fig. 7.
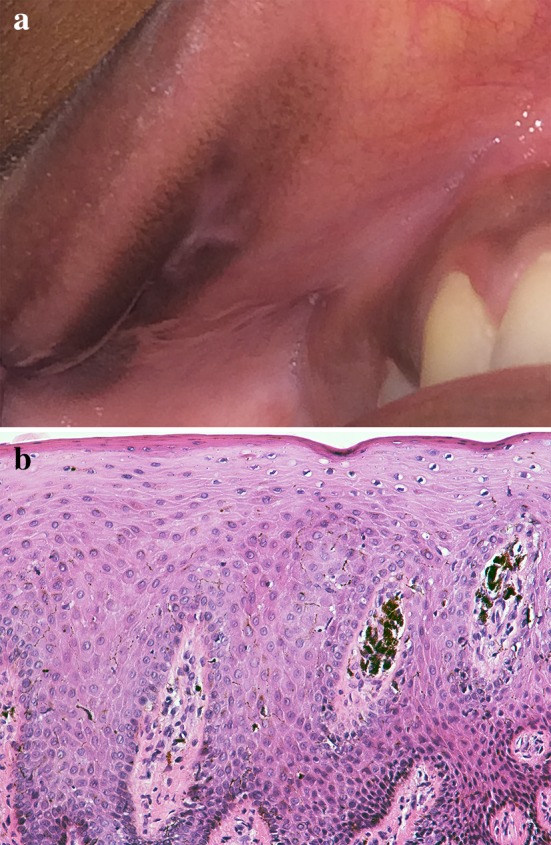
a Melanoacanthoma of the upper labial mucosa. b Histopathologic features of melanoacanthoma showing dendritic melanocytes dispersed throughout acanthotic stratified squamous epithelium and incontinent melanin within the lamina propria
Microscopically, melanoacanthoma exhibits hyperkeratotic, acanthotic, spongiotic epithelium with dendritic melanocytes dispersed throughout the layers of epithelial cells (Fig. 7b) [2, 27, 35, 39]. The presence of an inflammatory cell infiltrate may be observed in the superficial connective tissue and supports a reactive etiology [2, 43]. Due to the rapidly progressing nature of this condition, incisional biopsy is necessary to rule out oral melanoma [27, 36, 38]. Melanoacanthomas typically involute following incisional biopsy [7, 27]. If resolution does not occur, topical steroids may be beneficial [36].
Smoker’s Melanosis
Smoker’s melanosis is a common, benign, reactive condition resulting in increased pigmentation of the oral mucosa from cigarette or pipe smoking. This process is thought to be a result of either the noxious chemicals in cigarette smoke or heat stimulating melanocytes to protectively produce melanin [2, 5, 7, 44]. This condition typically occurs in adults and has been reported in 21.5–30% of smokers [1, 39, 40]. Women are more commonly affected, which has led some authors to suggest a possible hormonal link [2, 39]. The most common location for smoker’s melanosis is the anterior labial mandibular gingiva, however, buccal mucosa, lip, hard palate, and tongue can also be affected [1, 2, 36, 45]. Typically, multiple brown macules are present and may appear light brown to brown–black, depending on duration and amount of tobacco smoking (Fig. 8) [40].
Fig. 8.
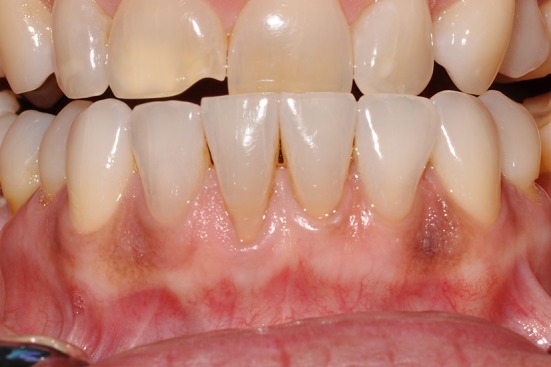
Light brown pigmentation of the mandibular anterior gingiva in a Caucasian female who reported smoking one pack of cigarettes per day for 40 years
Microscopically, melanin production can be observed in the basal layer of the epithelium. Incontinent melanin and melanophages may be noted in the superficial connective tissue [1, 40, 45]. The histopathology of smoker’s melanosis is microscopically identical to physiologic pigmentation or melanotic macule, thus clinical correlation is necessary to definitely diagnose this condition [40]. The combination of clinical presentation and smoking history are usually sufficient for diagnosis, however, if a lesion is elevated or occurs in an unusual site, a biopsy to rule out melanoma is necessary [35, 36, 45]. While this condition requires no treatment, discussion with the patient regarding health risks associated with smoking is suggested. Smoking cessation may result in gradual disappearance of the pigmented areas [1, 38]. For patients with aesthetic concerns, surgical excision or laser ablation may result in resolution of the pigmented areas; however, the pigmentation may recur if the habit persists [46].
Amalgam Tattoo/Foreign Body Tattoo
Mucosal pigmentation can occur due to deposition of exogenous foreign materials such as dental amalgam, tattoo pigment, or graphite. By far the most common of these is unintended implantation of amalgam, known as amalgam tattoo, which affects 3.3% of the US adult population [47]. This can occur during placement or removal of a restoration where fragments can enter through an abrasion, extraction site or the gingival sulcus. Amalgam tattoo can occur in anyone with a history of amalgam restorations. It presents as a flat discoloration which can be gray, blue, or black (Fig. 9a). The borders may be well-defined, irregular or diffuse. The majority of amalgam tattoos are 6 mm or less [48]. Enlargement can occur, presumably due to macrophage migration, resorption of underlying bone, or continued corrosion [49]. The most common location is gingiva or alveolar mucosa however other sites including buccal mucosa and floor of mouth are often affected [48]. Multiple lesions can be seen.
Fig. 9.
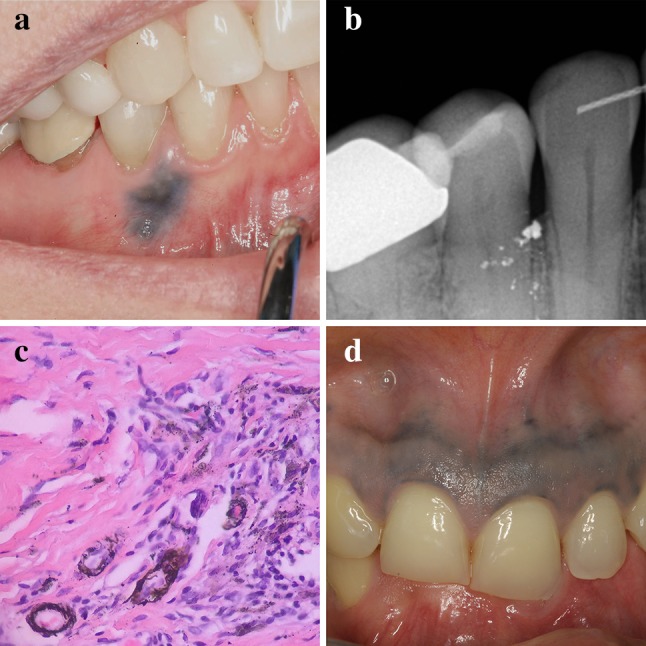
a Clinical appearance of an amalgam tattoo. b Radiographic findings in same patient from a. c Histopathology of amalgam tattoo showing staining of reticulin fibers around small blood vessels. d Intentional gingival tattooing in an African female
Evidence of radiopaque metallic fragments can help diagnose an amalgam tattoo (Fig. 9b) however many cases have no radiographic findings [14]. Microscopic examination of amalgam tattoo shows numerous fine black granules within the connective tissue exhibiting affinity for reticulin fibers (Fig. 9c). Larger aggregates of amalgam may elicit a chronic inflammatory response including lymphocytes, macrophages and multinucleated giant cells. Extensive grayish-black pigmentation of the anterior maxillary gingiva is seen with intentional tattooing, which is most common in certain African cultures (Fig. 9d) [14]. No treatment is necessary for amalgam or foreign body tattoo. Biopsy is indicated if a lesion cannot be diagnosed either clinically or radiographically. Extensive lesions in the cosmetic zone may be successfully treated with surgical excision and gingival grafting [50].
Black Hairy Tongue
Black hairy tongue is a benign, acquired condition affecting the dorsal tongue characterized by darkly discolored, elongated filiform papillae. No large population studies exist on black hairy tongue specifically however data regarding hairy tongue has been published. In a Turkish study, 581/5150 patients (11.3%) were diagnosed with hairy tongue; frequency increased with male gender, advancing age, smoking, drinking large amounts of hot black tea and poor oral hygiene [51]. In contrast, Shulman et al. reported only 51 cases of hairy tongue in 17,235 patients (0.0029%) age 17 and older [47]. Other studies from the US, Finland and Iran report prevalences varying from 0.06 to 8.4% of study patients exhibiting hairy tongue, with the majority stating males were more commonly affected [52–54].
Although the name implies the color change is black, variations of yellow, tan, brown and even green are observed. The filiform papillae are elongated and overlap one another, forming an almost mat-like appearance on the dorsal tongue (Fig. 10) [14, 54]. These hair-like projections can range from 3 to 20 mm in length [55–57]. Patients do not report pain with the condition; some may be unaware of its presence while others seek care due to the unusual appearance. The papillary elongation can be due to decreased desquamation and/or increased production of keratin. Manabe et al. reported these elongated spires of keratin were produced by epithelial cells expressing hair-type keratins, located specifically at the tips of the filiform papillae [58]. Dietary intake can impact the thickness of keratin on the dorsal tongue, with coarse/crunchy foods aiding in desquamation. A diet composed of primarily soft foods, lack of proper oral hygiene and general debilitation can lead to increased keratin accumulation [14]. Excess production of keratin can be triggered by irritants including smoking, drinking excessive hot liquids and use of oxidizing mouthwashes [55]. Discoloration of the keratin has been reported in conjunction with smoking, electronic cigarette usage, colonization with chromogenic bacteria or yeast, use of oxidizing mouthwashes, radiation therapy, chemotherapy and a broad spectrum of medications including but not limited to: bismuth, chlorpromazine, linezolid, olanzapine, penicillin, ranitidine and tetracycline [57, 59–62]. Many of the factors listed above can also lead to xerostomia which is implicated in causation of hairy tongue. In some cases, the discoloration occurs on the tips of otherwise normal (not elongated) filiform papillae; this change has been referred to as pseudo-black hairy tongue [57]. This most commonly occurs when bismuth sulfide accumulates on the tongue, resulting from use of bismuth containing medications reacting with trace sulfur present in saliva [14].
Fig. 10.
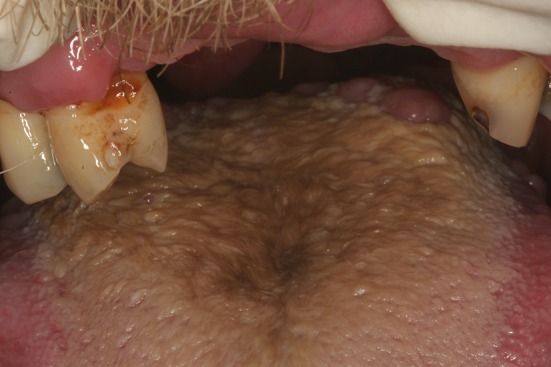
Hairy tongue with brown pigmentation
Diagnosis of hairy tongue, black or otherwise, is based on clinical findings. Biopsy is not necessary, however if performed the histopathologic features include markedly hyperparakeratotic, hyperplastic stratified squamous epithelium demonstrating a papillary architecture with accumulation of elongated, hair-like spires of keratin [14, 63]. The keratin is frequently colonized by bacterial organisms. No treatment is necessary for black hairy tongue, although when patients seek care it is typically for cosmetic reasons or halitosis. The management of the condition begins with determination of potential causative factors [57]. Cessation of habits, improving oral hygiene, increasing dietary roughage and elimination of implicated medications, if possible, are recommended. Ultimately, gentle tongue scraping or brushing with a toothbrush may be all that is required to eliminate the condition.
Summary
Black and brown mucosal pigmentation can result from a variety of sources ranging from harmless conditions to life-threatening diseases. When clinicians identify these lesions in their patients, a thorough clinical history should be obtained including onset, duration, and presence of local or systemic symptoms. Although some lesions can be diagnosed clinically or by laboratory testing, a biopsy is necessary whenever the cause of the pigmentation cannot be definitively determined.
Acknowledgments
Funding
None.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Meleti M, Vescovi P, Mooi WJ, van der Waal I. Pigmented lesions of the oral mucosa and perioral tissues: a flow-chart for the diagnosis and some recommendations for the management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(5):606–616. doi: 10.1016/j.tripleo.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 2.Muller S. Melanin-associated pigmented lesions of the oral mucosa: presentation, differential diagnosis, and treatment. Dermatol Ther. 2010;23(3):220–229. doi: 10.1111/j.1529-8019.2010.01319.x. [DOI] [PubMed] [Google Scholar]
- 3.Amir E, Gorsky M, Buchner A, Sarnat H, Gat H. Physiologic pigmentation of the oral mucosa in Israeli children. Oral Surg Oral Med Oral Pathol. 1991;71(3):396–398. doi: 10.1016/0030-4220(91)90325-7. [DOI] [PubMed] [Google Scholar]
- 4.Gorsky M, Buchner A, Moskona D, Aviv I. Physiologic pigmentation of the oral mucosa in Israeli Jews of different ethnic origin. Community Dent Oral Epidemiol. 1984;12(3):188–190. doi: 10.1111/j.1600-0528.1984.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 5.Eisen D. Disorders of pigmentation in the oral cavity. Clin Dermatol. 2000;18(5):579–587. doi: 10.1016/s0738-081x(00)00148-6. [DOI] [PubMed] [Google Scholar]
- 6.Mallikarjuna K, Gupta S, Shukla S, Chaurasia S. Unusual extensive physiologic melanin pigmentation of the oral cavity: a clinical presentation. J Indian Soc Pedod Prev Dent. 2013;31(2):121–125. doi: 10.4103/0970-4388.115718. [DOI] [PubMed] [Google Scholar]
- 7.Alawi F. Pigmented lesions of the oral cavity: an update. Dent Clin N Am. 2013;57(4):699–710. doi: 10.1016/j.cden.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sreeja C, Ramakrishnan K, Vijayalakshmi D, Devi M, Aesha I, Vijayabanu B. Oral pigmentation: a review. J Pharm Bioallied Sci. 2015;7(Suppl 2):403–408. doi: 10.4103/0975-7406.163471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaeta GM, Satriano RA, Baroni A. Oral pigmented lesions. Clin Dermatol. 2002;20(3):286–288. doi: 10.1016/s0738-081x(02)00225-0. [DOI] [PubMed] [Google Scholar]
- 10.Mignogna MD, Lo Muzio L, Ruoppo E, Errico M, Amato M, Satriano RA. Oral manifestations of idiopathic lenticular mucocutaneous pigmentation (Laugier–Hunziker syndrome): a clinical, histopathological and ultrastructural review of 12 cases. Oral Dis. 1999;5(1):80–86. doi: 10.1111/j.1601-0825.1999.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffari HR, Rezaei F, Sharifi R, Mirbahari SG. Seven-year follow-up of Peutz–Jeghers syndrome. Case Rep Dent. 2016;2016:6052181. doi: 10.1155/2016/6052181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieman LK, Chanco Turner ML. Addison’s disease. Clin Dermatol. 2006;24(4):276–280. doi: 10.1016/j.clindermatol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar SB, Sarkar S, Ghosh S, Bandyopadhyay S. Addison’s disease. Contemp Clin Dent. 2012;3(4):484–486. doi: 10.4103/0976-237X.107450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neville B, Damm D, Allen C, Chi A. Oral and maxillofacial pathology. 4. St. Louis: Elsevier; 2016. [Google Scholar]
- 15.Zhang Y, Ke Y, Zheng X, Liu Q, Duan X. Correlation between genotype and phenotype in three families with Peutz–Jeghers syndrome. Exp Ther Med. 2017;13(2):507–514. doi: 10.3892/etm.2016.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikitakis NG, Koumaki D. Laugier–Hunziker syndrome: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):e52–e58. doi: 10.1016/j.oooo.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Rangwala S, Doherty CB, Katta R. Laugier–Hunziker syndrome: a case report and review of the literature. Dermatol Online J. 2010;16(12):9. [PubMed]
- 18.Wang WM, Wang X, Duan N, Jiang HL, Huang XF. Laugier–Hunziker syndrome: a report of three cases and literature review. Int J Oral Sci. 2012;4(4):226–230. doi: 10.1038/ijos.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abduljabbar T, Vohra F, Akram Z, Ghani SMA, Al-Hamoudi N, Javed F. Efficacy of surgical laser therapy in the management of oral pigmented lesions: a systematic review. J Photochem Photobiol B. 2017;173:353–359. doi: 10.1016/j.jphotobiol.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Tosios KI, Kalogirou EM, Sklavounou A. Drug-associated hyperpigmentation of the oral mucosa: report of four cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(3):e54–e66. doi: 10.1016/j.oooo.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Yuan A, Woo SB. Adverse drug events in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(1):35–47. doi: 10.1016/j.oooo.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Eisen D, Hakim MD. Minocycline-induced pigmentation. Incidence, prevention and management. Drug Saf. 1998;18(6):431–440. doi: 10.2165/00002018-199818060-00004. [DOI] [PubMed] [Google Scholar]
- 23.Lerman MA, Karimbux N, Guze KA, Woo SB. Pigmentation of the hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(1):8–12. doi: 10.1016/j.tripleo.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Treister NS, Magalnick D, Woo SB. Oral mucosal pigmentation secondary to minocycline therapy: report of two cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(6):718–725. doi: 10.1016/j.tripleo.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Kaugars GE, Heise AP, Riley WT, Abbey LM, Svirsky JA. Oral melanotic macules. A review of 353 cases. Oral Surg Oral Med Oral Pathol. 1993;76(1):59–61. doi: 10.1016/0030-4220(93)90295-f. [DOI] [PubMed] [Google Scholar]
- 26.Buchner A, Hansen LS. Melanotic macule of the oral mucosa. A clinicopathologic study of 105 cases. Oral Surg Oral Med Oral Pathol. 1979;48(3):244–249. doi: 10.1016/0030-4220(79)90011-2. [DOI] [PubMed] [Google Scholar]
- 27.Buchner A, Merrell PW, Carpenter WM. Relative frequency of solitary melanocytic lesions of the oral mucosa. J Oral Pathol Med. 2004;33(9):550–557. doi: 10.1111/j.1600-0714.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 28.Shen ZY, Liu W, Bao ZX, Zhou ZT, Wang LZ. Oral melanotic macule and primary oral malignant melanoma: epidemiology, location involved, and clinical implications. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(1):e21–e25. doi: 10.1016/j.tripleo.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 29.Gupta G, Williams RE, Mackie RM. The labial melanotic macule: a review of 79 cases. Br J Dermatol. 1997;136(5):772–775. [PubMed] [Google Scholar]
- 30.Tavares TS, Meirelles DP, de Aguiar MCF, Caldeira PC. Pigmented lesions of the oral mucosa: a cross-sectional study of 458 histopathological specimens. Oral Dis. 2018 doi: 10.1111/odi.12924. [DOI] [PubMed] [Google Scholar]
- 31.Ho KK, Dervan P, O’Loughlin S, Powell FC. Labial melanotic macule: a clinical, histopathologic, and ultrastructural study. J Am Acad Dermatol. 1993;28(1):33–39. doi: 10.1016/0190-9622(93)70005-e. [DOI] [PubMed] [Google Scholar]
- 32.Weathers DR, Corio RL, Crawford BE, Giansanti JS, Page LR. The labial melanotic macule. Oral Surg Oral Med Oral Pathol. 1976;42(2):196–205. doi: 10.1016/0030-4220(76)90124-9. [DOI] [PubMed] [Google Scholar]
- 33.Page LR, Corio RL, Crawford BE, Giansanti JS, Weathers DR. The oral melanotic macule. Oral Surg Oral Med Oral Pathol. 1977;44(2):219–226. doi: 10.1016/0030-4220(77)90272-9. [DOI] [PubMed] [Google Scholar]
- 34.Sexton FM, Maize JC. Melanotic macules and melanoacanthomas of the lip. A comparative study with census of the basal melanocyte population. Am J Dermatopathol. 1987;9(5):438–444. doi: 10.1097/00000372-198710000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Kauzman A, Pavone M, Blanas N, Bradley G. Pigmented lesions of the oral cavity: review, differential diagnosis, and case presentations. J Can Dent Assoc. 2004;70(10):682–683. [PubMed] [Google Scholar]
- 36.Lambertini M, Patrizi A, Ravaioli GM, Dika E. Oral pigmentation in physiologic conditions, post inflammatory affections and systemic diseases. G Ital Dermatol Venereol. 2017 doi: 10.23736/S0392-0488.17.05619-X. [DOI] [PubMed] [Google Scholar]
- 37.Uribe P, Collgros H, Scolyer RA, Menzies SW, Guitera P. In vivo reflectance confocal microscopy for the diagnosis of melanoma and melanotic macules of the lip. JAMA Dermatol. 2017;153(9):882–891. doi: 10.1001/jamadermatol.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatch CL. Pigmented lesions of the oral cavity. Dent Clin N Am. 2005;49(1):185–201, ix–x. [DOI] [PubMed]
- 39.Tarakji B, Umair A, Prasad D, Alsakran Altamimi M. Diagnosis of oral pigmentations and malignant transformations. Singap Dent J. 2014;35C:39–46. doi: 10.1016/j.sdj.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Gondak RO, da Silva-Jorge R, Jorge J, Lopes MA, Vargas PA. Oral pigmented lesions: clinicopathologic features and review of the literature. Med Oral Patol Oral Cir Bucal. 2012;17(6):e919–e924. doi: 10.4317/medoral.17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohilla K, Ramesh V, Balamurali P, Singh N. Oral melanoacanthoma of a rare intraoral site: case report and review of literature. Int J Clin Pediatr Dent. 2013;6(1):40–43. doi: 10.5005/jp-journals-10005-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters SM, Mandel L, Perrino MA. Oral melanoacanthoma of the palate: an unusual presentation of an uncommon entity. JAAD Case Rep. 2018;4(2):138–9. [DOI] [PMC free article] [PubMed]
- 43.Cantudo-Sanagustin E, Gutierrez-Corrales A, Vigo-Martinez M, Serrera-Figallo MA, Torres-Lagares D, Gutierrez-Perez JL. Pathogenesis and clinicohistopathological characteristics of melanoacanthoma: a systematic review. J Clin Exp Dent. 2016;8(3):e327–e336. doi: 10.4317/jced.52860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassona Y, Sawair F, Al-Karadsheh O, Scully C. Prevalence and clinical features of pigmented oral lesions. Int J Dermatol. 2016;55(9):1005–1013. doi: 10.1111/ijd.13133. [DOI] [PubMed] [Google Scholar]
- 45.Mirbod SM, Ahing SI. Tobacco-associated lesions of the oral cavity: Part I. Nonmalignant lesions. J Can Dent Assoc. 2000;66(5):252–256. [PubMed] [Google Scholar]
- 46.Monteiro LS, Costa JA, da Camara MI, Albuquerque R, Martins M, Pacheco JJ, Salazar F, Figueira F. Aesthetic depigmentation of gingival smoker’s melanosis using carbon dioxide lasers. Case Rep Dent. 2015;2015:510589. doi: 10.1155/2015/510589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shulman JD, Beach MM, Rivera-Hidalgo F. The prevalence of oral mucosal lesions in U.S. adults: data from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Dent Assoc. 2004;135(9):1279–1286. doi: 10.14219/jada.archive.2004.0403. [DOI] [PubMed] [Google Scholar]
- 48.Buchner A, Hansen LS. Amalgam pigmentation (amalgam tattoo) of the oral mucosa. A clinicopathologic study of 268 cases. Oral Surg Oral Med Oral Pathol. 1980;49(2):139–147. doi: 10.1016/0030-4220(80)90306-0. [DOI] [PubMed] [Google Scholar]
- 49.Owens BM, Schuman NJ, Johnson WW. Oral amalgam tattoos: a diagnostic study. Compendium 1993;14(2):210, 212, 214 passim. [PubMed]
- 50.Thumbigere-Math V, Johnson DK. Treatment of amalgam tattoo with a subepithelial connective tissue graft and acellular dermal matrix. J Int Acad Periodontol. 2014;16(2):50–54. [PubMed] [Google Scholar]
- 51.Avcu N, Kanli A. The prevalence of tongue lesions in 5150 Turkish dental outpatients. Oral Dis. 2003;9(4):188–195. doi: 10.1034/j.1601-0825.2003.02933.x. [DOI] [PubMed] [Google Scholar]
- 52.Kullaa-Mikkonen A, Mikkonen M, Kotilainen R. Prevalence of different morphologic forms of the human tongue in young Finns. Oral Surg Oral Med Oral Pathol. 1982;53(2):152–156. doi: 10.1016/0030-4220(82)90281-x. [DOI] [PubMed] [Google Scholar]
- 53.Motallebnejad M, Babaee N, Sakhdari S, Tavasoli M. An epidemiologic study of tongue lesions in 1901 Iranian dental outpatients. J Contemp Dent Pract. 2008;9(7):73–80. [PubMed] [Google Scholar]
- 54.Redman RS. Prevalence of geographic tongue, fissured tongue, median rhomboid glossitis, and hairy tongue among 3,611 Minnesota schoolchildren. Oral Surg Oral Med Oral Pathol. 1970;30(3):390–395. doi: 10.1016/0030-4220(70)90320-8. [DOI] [PubMed] [Google Scholar]
- 55.Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20(31):10845–10850. doi: 10.3748/wjg.v20.i31.10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mangold AR, Torgerson RR, Rogers RS., III Diseases of the tongue. Clin Dermatol. 2016;34(4):458–469. doi: 10.1016/j.clindermatol.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 57.Schlager E, St Claire C, Ashack K, Khachemoune A. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18(4):563–569. doi: 10.1007/s40257-017-0268-y. [DOI] [PubMed] [Google Scholar]
- 58.Manabe M, Lim HW, Winzer M, Loomis CA. Architectural organization of filiform papillae in normal and black hairy tongue epithelium: dissection of differentiation pathways in a complex human epithelium according to their patterns of keratin expression. Arch Dermatol. 1999;135(2):177–181. doi: 10.1001/archderm.135.2.177. [DOI] [PubMed] [Google Scholar]
- 59.Akcaboy M, Sahin S, Zorlu P, Senel S. Ranitidine-induced black tongue: a case report. Pediatr Dermatol. 2017;34(6):e334–e336. doi: 10.1111/pde.13309. [DOI] [PubMed] [Google Scholar]
- 60.Farinha H, Martins V. Lingua villosa nigra associated with the use of electronic cigarette. Acta Med Port. 2015;28(3):393. doi: 10.20344/amp.5528. [DOI] [PubMed] [Google Scholar]
- 61.Lawoyin D, Brown RS. Drug-induced black hairy tongue: diagnosis and management challenges. Dent Today 2008;27(1):60, 62, 3; quiz 93, 58. [PubMed]
- 62.Nisa L, Giger R. Black hairy tongue. Am J Med. 2011;124(9):816–817. doi: 10.1016/j.amjmed.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 63.Winzer M, Gilliar U, Ackerman AB. Hairy lesions of the oral cavity. Clinical and histopathologic differentiation of hairy leukoplakia from hairy tongue. Am J Dermatopathol. 1988;10(2):155–159. doi: 10.1097/00000372-198804000-00010. [DOI] [PubMed] [Google Scholar]


