Abstract
The aim of this study was to explore the neuroprotective effect and the underlying mechanism of erythropoietin (EPO) on the cortical neuronal cells insulted with oxygen and glucose deprivation (OGD). Different concentrations of EPO were used to determine the anti-apoptosis effect of EPO. In addition, PI3K inhibitor LY294002 and ERK1/2 inhibitor U0126 were added to explore the underlying mechanism of EPO. Cell apoptosis rate was measured by flow cytometry. The protein expression of Bax, Bcl-2, cleaved caspase-3, AKT, p-AKT, Erk1/2 and p-Erk1/2 wasmeasured by Western blot. Our results showed that EPO alleviates OGD-induced cell apoptosis in a dose-dependent manner; the neuroprotective effect of EPO was further confirmed by the fact that EPO treatment reversed the protein expression of cleaved caspase-3, as well as the Bcl-2/Bax ratio as compared with the OGD treatment. In the mechanism part, our results demonstrated that OGD and EPO nearly had no influence on the protein expression of AKT and Erk1/2 but altered the phosphorylation of them. Specifically, OGD decreased the expression of p-AKT and increased the expression of p-Erk1/2; while, EPO treatment reversed the expression of p-AKT and p-Erk1/2 as compared with OGD treatment. Interestingly, LY294002 decreased the expression of p-AKT and attenuated the neuroprotective effect of EPO; while, U0126 decreased the expression of p-Erk1/2 and enhanced the neuroprotective effect of EPO. Our study demonstrated that EPO protects neurons against apoptosis induced by OGD, which is closely related with activation of PI3K/AKT and inactivation of Erk1/2 signaling pathway.
Keywords: Erythropoietin, Neuroprotective agents, PI3K/AKT, Erk1/2
Introduction
Hypoxic-ischemic encephalopathy (HIE) is characterized with ischemic-induced brain damage and may cause neuropsychiatric symptoms (Grasso et al. 2015). In developing countries, HIE is one of the leading causes of neonatal mortality and may lead to neurodevelopmental disability in later life (Garg et al. 2017). Current therapies for HIE, including mild hypothermia, hyperbaric oxygen, positive pressure ventilation and blood purification, are all physical in nature; however, few drugs are available.
Erythropoietin (EPO) has been widely used for anemia treatment in clinical practice (Biggar and Kim 2017). Accumulating evidences revealed that, other than treatment for anemia, EPO also has a role in neuroprotection. In a randomized controlled study, EPO monotherapy significantly reduced the risk of death or disability in neonates with moderate or severe encephalopathy (Malla et al. 2017). A phase II clinical study suggested that a high dose of EPO administered with hypothermia may result in less brain injury in patients with HIE (Wu et al. 2016). These evidences suggested that EPO may be a promising agent for HIE treatment. In addition to clinical trials, lots of experimental studies also focused on the mechanism of EPO under condition of hypoxia–ischemia insult. For instance, Yan et al. reported that EPO improves hypoxic-ischemic encephalopathy in neonatal rats by enhancing angiogenesis (Yan et al. 2016). Souvenir et al. reported that EPO inhibits HIF-1α expression in an in vitro hypoxia–ischemia model (Souvenir et al. 2014). Previous evidences also demonstrated that many downstream signaling pathways of EPO receptor, including signal transducer and activator of transcription 5 (STAT-5), phosphatidylinositol 3 Kinase/protein kinase B (PI3K/Akt), extracellular signal-regulated kinase (Erk1/2) and protein kinase C (PKC) pathways were involved in the neuroprotection of EPO (Hernández et al. 2017; Ostrowski and Heinrich 2018; Suarez-Mendez et al. 2018). Among these pathways, PI3K/Akt and Erk1/2 pathways have attracted our most attention because a number of reports had contrarily described their role in neuroprotection. For example, Li et al. reported that irisin treatment protects against neuronal injury in middle cerebral artery occlusion (MCAO) and OGD models by activation of the Akt and ERK1/2 signaling pathways (Li et al. 2017), while, Yan et al. found that vanillin protects dopaminergic neurons against inflammation-mediated cell death via inhibiting Erk1/2, p38 and NF-κB signaling pathways (Yan et al. 2017).
In the present study, we used oxygen and glucose deprivation (OGD) model to mimic the condition of HIE in vitro. Flow cytometry and Western blot were performed to confirm the neuroprotective effect and underlying mechanism of EPO on the cortical neuronal cells insulted with OGD.
Methods and materials
Isolation, culture and identification of cortical neuronal cells
The isolation, culture and identification of neuronal cells were performed according to the previous description (Guangyun Zhang et al. 2012). In brief, 7-day-old specific pathogen-free grade C57 mice were sacrificed; brains were collected and trypsinized; separated cells were cultured in neurobasal-A medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum and 1/100 penicillin–streptomycin at 37 °C in a humidified atmosphere. Four hours later, medium was replaced with serum-free neurobasal-A + B27 medium (Invitrogen, Carlsbad, CA, USA). Medium was replaced every day. Identification of isolated cells was performed by morphology observation and MAP2 staining.
Immunofluorescence staining
Neuronal cells, grown on glass coverslips, were fixed with 4% formaldehyde for 15 min and penetrated with 0.1% Triton X-100 for 15 min. Non-specific signal was blocked with 5% (w/v) normal goat serum for 1 h at room temperature. Cells were then incubated with primary antibody against MAP2 (1:100; Abcam, UK) overnight at 4 °C. Subsequently, cells were incubated with HRP-conjugated secondary antibody (1:100; Gene Tex, USA) and stained with DAPI. Coverslips were mounted and observed under a laser confocal scanning microscope (Olympus, Tokyo, Japan).
OGD model establishment and study design
For OGD model establishment, neuronal cells were incubated with glucose-deprived Earle’s Balanced Salt Solution (Sigma-Aldrich, St. Louis, MO, USA) and maintained for 1 h. Culture medium was then replaced with complete culture medium.
To confirm the neuroprotective effect of EPO, cells were divided into six groups: (1) control group; (2) OGD group; (3) 1.56 U/ml EPO group; (4) 3.1 U/ml EPO group; (5) 6.25 U/ml EPO group; and (6) 12.5 U/ml EPO group. For control group, neuronal cells were cultured with complete culture medium. For OGD group, neuronal cells were treated as the method of model establishment. For all EPO groups, OGD model was established for neuronal cells at first; different concentrations of EPO were then added. 24 h after treatment, cell apoptosis was determined using flow cytometry.
To determine the underlying mechanism of neuroprotective effect of EPO, cells were divided into five groups: (1) control group; (2) OGD group; (3) 12.5 U/ml EPO group; (4) LY294002 group and (6) U0126 group. For the former three groups, treatments were same as the above-mentioned methods. For LY294002 group, OGD model was established for neuronal cells at first; 12.5 U/ml EPO and 10 µM LY294002 (Calbiochem, San Diego, CA, USA) were then added. For U0126 group, the treatment was similar to the LY294002 group except that 50 µM U0126 (Calbiochem, San Diego, CA, USA), instead of LY294002, was added. 24 h after treatment, cell apoptosis was determined using flow cytometry. The protein expression of Bcl-2, Bax, cleaved caspase-3, AKT, p-AKT, Erk1/2 and p-Erk1/2 was determined using Western blot.
Flow cytometry
Cell apoptosis was measured by Annexin V/Propidium iodide (PI) staining assay using flow cytometry according to manufacturer’s instruction (BD Biosciences, CA, USA). Briefly, 24 h after treatment, approximately 2 × 105 cells were collected, rinsed twice with cold PBS and incubated with Annexin V for 30 min in dark at 4 °C. PI was then added and cells apoptosis was detected using a flow cytometer (BD Biosciences, CA, USA).
Western blot analysis
After treatment, cells were collected and lysed in RIPA lysis buffer (Beyotime, Shanghai, China). Protein concentration was measured by Bradford assay (Beyotime, Shanghai, China). Equal amount of proteins (30 µg/lane) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, 8–12%) and transferred to a PVDF membrane (Millipore, Bedford, MA, USA). Non-specific binding sites were blocked with 5% (w/v) non-fat milk at room temperature for 2 h. Primary antibodies against Bcl-2 (1:1000; Abcam, UK), Bax (1:1000; Abcam, UK), cleaved caspase-3 (1:1000; Gene Tex, USA), AKT (1:1000; Cell signaling Technology, Beverly, MA, USA), p-AKT (1:1000; Cell signaling Technology, Beverly, MA, USA), Erk1/2 (1:1000; Cell signaling Technology, Beverly, MA, USA) and p-Erk1/2 (1:1000; Cell signaling Technology, Beverly, MA, USA) were added and incubated with cells overnight at 4 °C. Horseradish peroxidase–conjugated goat anti-rabbit IgG (H + L) secondary antibody (1:2000; Cell signaling Technology, Beverly, MA, USA) was incubated at room temperature for 1 h. β-actin (1:1000; Sigma-Aldrich, St. Louis, USA) was used as the reference protein. The protein expression was detected by electrochemiluminescence assay (Bio-Red Laboratories, Cambridge, MA, USA).
Statistical analysis
Data are presented as mean ± standard deviation (SD) and analyzed by one-way ANOVA assay using SPSS 19.0 software (SPSS, Chicago, IL, USA). Two-tailed p value less than 0.05 was considered statistically significant.
Results
Identification of neuronal cells
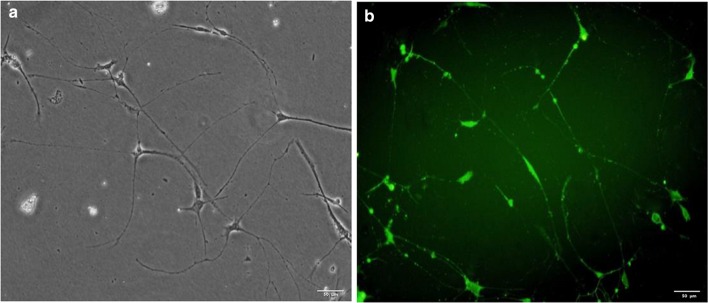
The morphology of isolated cells at the third passage was observed by microscope, which shows typical features of neuronal cells (Fig. 1a). Moreover, the cells were positive for MAP2 staining (Fig. 1b), which further confirmed that the cells were neuronal cells. Cells at the third to six passages were used for further experiments.
Fig. 1.
Identification of neuronal cells. a Representative picture of neuronal cells under microscope; b representative picture of MAP2 immunofluorescence staining for neuronal cells. Bar 50 µm; magnification × 200
EPO alleviated OGD-induced neuronal cell apoptosis in a dose-dependent manner
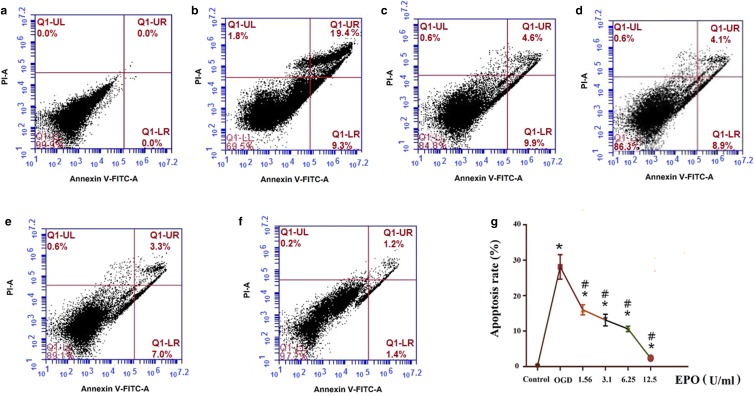
OGD significantly increased cell apoptosis rate (28.1 ± 3.44%) (Fig. 2b) when compared with normal cultured neuronal cells (0.30 ± 0.26%) (Fig. 2a) (p < 0.05). To confirm the neuroprotective effect of EPO, different concentrations of EPO were used to treat OGD cells. As expected, EPO significantly decreased OGD-induced cell apoptosis in a dose-dependent manner (Fig. 2c-g).
Fig. 2.
EPO alleviates OGD-induced neuronal cell apoptosis in a dose-dependent manner. a Control group; b OGD group; c 1.56 U/ml EPO group; d 3.1 U/ml EPO group; e 6.25 U/ml EPO group; and f 12.5 U/ml EPO group; g Line graph of apoptosis results of all groups. Data are expressed as percent (%); all experiments were performed in triplicate. *p < 0.05, vs. the control group; #p < 0.05, vs. the OGD group
The neuroprotective effect of EPO was attenuated by LY294002 and enhanced by U0126
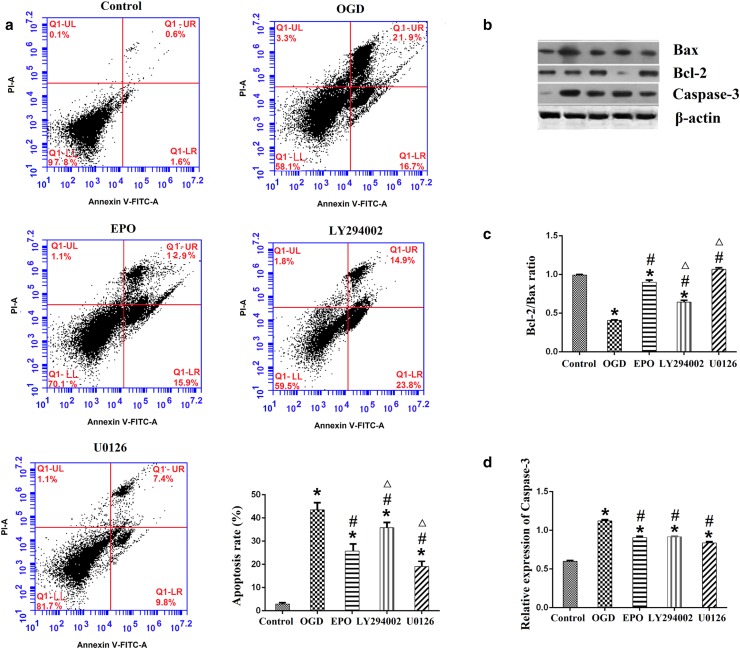
From Fig. 3a we can see that, OGD significantly increased the apoptosis rate of neuronal cells which was alleviated by EPO treatment; AKT inhibitor LY294002 significantly increased the apoptosis rate of neuronal cells; on the contrary, Erk1/2 inhibitor U0126 significantly decreased the apoptosis rate of neuronal cells as compared with EPO treatment.
Fig. 3.
LY294002 attenuated and U0126 enhanced the neuroprotective effect of EPO. a Representative flow cytometry pictures and bar graph of all groups; b representative pictures of Western blot for Bax, Bcl-2 and cleaved caspase-3; β-actin was used as a loading control; c bar graph of the Bcl-2/Bax ratio; d relative protein expression of the cleaved caspase-3. Data are expressed as mean ± SD; all experiments were performed in triplicate. *p < 0.05, vs. the control group; #p < 0.05, vs. the OGD group; ∆p < 0.05, vs. the EPO group
As we know, Bcl-2 is an anti-apoptotic protein and Bax is a pro-apoptotic protein, any factor that decreases the Bcl-2/Bax ratio may promote apoptosis (Xu et al. 2007). Our results showed that, OGD significantly decreased the Bcl-2/Bax ratio of neuronal cells which was reversed by EPO treatment. In addition, the Bcl-2/Bax ratio of neuronal cells was decreased by LY294002 treatment and increased by U0126 treatment as compared with EPO treatment alone (Fig. 3b, c).
Just like Bax, cleaved caspase-3 is another pro-apoptotic protein. Our results showed that, OGD significantly increased the protein expression of cleaved caspase-3 which was reversed by EPO treatment. The protein expression of cleaved caspase-3 was comparable in the EPO group, the LY294002 group and the U0126 group (Fig. 3b, d).
Taken together, our results confirmed that EPO has neuroprotective effect on cortical neuronal cells insulted with OGD, while LY294002 attenuated and U0126 enhanced this effect.
The phosphorylation of AKT and Erk1/2 was altered after EPO treatment
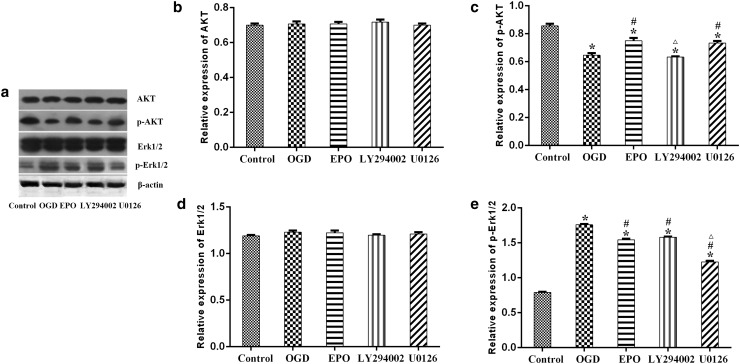
Our results showed that, the protein expression of AKT and Erk1/2 was almost unchanged in all groups (Fig. 4a, b, d). OGD treatment significantly decreased the expression of p-AKT which was reversed by EPO treatment; LY294002 treatment significantly decreased the expression of p-AKT as compared with EPO treatment alone (Fig. 4a, c). As for p-Erk1/2, OGD treatment induced a distinct increase of p-Erk1/2 which was reversed by EPO treatment; U0126 treatment further decreased the expression of p-Erk1/2 as compared with EPO treatment alone (Fig. 4a, e).
Fig. 4.
The phosphorylation of AKT and Erk1/2 was altered after EPO treatment. a Representative pictures of Western blot for AKT, p-AKT, Erk1/2, p-Erk1/2; b relative protein expression of AKT; c relative protein expression of p-AKT; d relative protein expression of Erk1/2; e relative protein expression of p-Erk1/2. Data are expressed as mean ± SD; all experiments were performed in triplicate. *p < 0.05, vs. the control group; #p < 0.05, vs. the OGD group; ∆p < 0.05, vs. the EPO group
Discussion
In the present study, we demonstrated that EPO protects cortical neurons from apoptosis induced by OGD insult. The protective effect of EPO was dependent on the activation of the PI3K/Akt signal pathway and inactivation of the Erk1/2 signal pathway. We also demonstrated that the PI3K inhibitor LY294002 attenuated the protective effect of EPO; on the contrary, the Erk1/2 inhibitor U0126 enhanced this effect.
Oxygen–glucose deprivation mimics the condition of HIE in vitro (Mikrogeorgiou et al. 2017), making it a suitable cell model for studying HIE. Our results showed that cell apoptosis rate was increased after OGD, which was consistent with previous study (Chen et al. 2017). EPO is a glycoprotein cytokine secreted by the kidney in response to cellular hypoxia. Increasing evidences have indicated that EPO could protect neuronal cells from apoptosis induced by OGD (Castillo et al. 2017; Garg et al. 2017; Jeong et al. 2017), which are in line with our results. Apoptosis involves a series of gene activation, expression and regulation. Among them, Bcl-2 family proteins, including pro-apoptotic members (such as Bax) and anti-apoptotic members (such as Bcl-2) play a pivotal role in the regulation of apoptosis. Cleaved caspase-3, known to act downstream of Bax/Bcl-2, also plays a key role in the execution of apoptosis (Salakou et al. 2007). Bax, Bcl-2 and caspase-3, as well as the Bcl-2/ Bax ratio, are often evaluated in apoptosis studies. Our study demonstrated that, OGD treatment increased the protein expression of Bax and cleaved caspase-3; on the contrary, the protein expression of Bcl-2, as well as the Bcl-2/Bax ratio was decreased after OGD. EPO treatment reversed the protein expression of Bcl-2, Bax and cleaved caspase 3, as well as the Bcl-2/Bax ratio as compared with the OGD treatment. Taken together, our results confirmed the neuroprotective effect of EPO in the OGD model.
The PI3K/AKT pathway is a key signaling pathway that participates in various cellular processes such as cell proliferation, survival, differentiation and apoptosis. Previous evidences suggested that PI3K/AKT activation is involved in EPO neuroprotection. For instance, Jia et al. reported that EPO-dependent activation of PI3K/Akt/FoxO3a signaling mediates neuroprotection in both in vitro and in vivo models of Parkinson’s disease (Jia et al. 2014). In a study performed by Ding et al., the authors found that both EPO and CEPO (carbamylated erythropoietin, an EPO derivative) exhibit neuroprotective effects in OGD model and hypoxic brain by triggering the CD131-GDNF-AKT pathway. Blockage of AKT pathway with LY294002 abolished the neuroprotective effects of EPO and CEPO (Ding et al. 2017). Similar observations were found by Yoo et al. who reported that both EPO and MK-X (an EPO-derived peptide) had neuroprotective effects in MCAO mouse model and glutamate-induced neurons model. The neuroprotective effects of MK-X and EPO were mediated by activation of JAK2 and its downstream PI3K/AKT and ERK1/2 signaling pathways (Yoo et al. 2017). The present study revealed that EPO promoted the phosphorylation of AKT and this activation was abrogated by PI3K inhibitor LY294002, which was in agreement with the results obtained by Ding et al. (Ding et al. 2017) and Yoo et al. (Yoo et al. 2017). These results suggested that the protective effect of EPO under OGD situation was dependent on the activation of PI3K/AKT pathway.
The Erk1/2 signaling pathway has a close relationship with neuronal cells differentiation and proliferation; however, previous reports exhibited controversial observations about the role of Erk1/2 in the process of neuroprotection. Studies from Gong et al (2014) and Tian et al. (2018) showed that activation of Erk1/2 after OGD or OGD/PR was observed characterized by increased expression of p-ERK1/2. In contrast, some studies demonstrated that Erk1/2 activation contributed to the protective effects of some neuroprotectants. For example, Savoia et al. demonstrated that the neuroprotective effect of rosuvastatin in cortical neurons exposed to OGD/reoxygenation was partially due to Erk1/2 pathway activation (Savoia et al. 2011). In the present study, we demonstrated that Erk1/2 was activated after OGD and attenuated by EPO treatment. Moreover, Erk1/2 inhibitor U0126 enhanced the efficacy of EPO. Our results suggested that Erk1/2 pathway inactivation promoted the neuroprotection function of EPO under OGD situation.
Conclusions
Our study demonstrated that EPO protects neurons against apoptosis induced by OGD, which is closely related with activation of PI3K/AKT and inactivation of Erk1/2 signaling pathway. However, further studies are warranted to confirm this speculation in animal models.
Acknowledgements
This study was sponsored by the Guizhou Science and Technology Cooperation Plan (Qian Ke He LH Zi [2014]7022).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Biggar P, Kim GH. Treatment of renal anemia: erythropoiesis stimulating agents and beyond. Kidney Res Clin Pract. 2017;36:209–223. doi: 10.23876/j.krcp.2017.36.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo C, et al. Neuroprotective effect of a new variant of Epo nonhematopoietic against oxidative stress. Redox Biol. 2017;14:285–294. doi: 10.1016/j.redox.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, et al. Sirt1Sirt3 axis regulates human bloodbrain barrier permeability in response to ischemia. Redox Biol. 2017;14:229–236. doi: 10.1016/j.redox.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, et al. Neuroprotection and CD131/GDNF/AKT pathway of carbamylated erythropoietin in hypoxic neurons. Mol Neurobiol. 2017;54:5051–5060. doi: 10.1007/s12035-016-0022-0. [DOI] [PubMed] [Google Scholar]
- Garg BD, Sharma D, Bansal A. Systematic review seeking erythropoietin role for neuroprotection in neonates with hypoxic ischemic encephalopathy: presently where do we stand. J Matern Fetal Neonatal Med. 2017 doi: 10.1080/14767058.2017.1366982. [DOI] [PubMed] [Google Scholar]
- Gong G, et al. Tetramethylpyrazine suppresses transient oxygen-glucose deprivation-induced connexin32 expression and cell apoptosis via the ERK1/2 and p38 MAPK pathway in cultured hippocampal neurons. PLoS One. 2014;9:e105944. doi: 10.1371/journal.pone.0105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G, Alafaci C, Buemi M. Erythropoietin in traumatic brain injury: an answer will come soon. World Neurosurg. 2015;84:1491–1492. doi: 10.1016/j.wneu.2015.05.056. [DOI] [PubMed] [Google Scholar]
- Guangyun Zhang XH, Ping Y, Wang J. Establishment of in vitro model of neonatal mouse cortical neuronal injury induced by glutamate. J Neurol Anat. 2012;4:363–367. [Google Scholar]
- Hernández CC, Burgos CF, Gajardo AH, Silva-Grecchi T, Gavilan J, Toledo JR, Fuentealba J. Neuroprotective effects of erythropoietin on neurodegenerative and ischemic brain diseases: the role of erythropoietin receptor. Neural Regen Res. 2017;12:1381. doi: 10.4103/1673-5374.215240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JE, Park JH, Kim CS, Lee SL, Chung HL, Kim WT, Lee EJ. Neuroprotective effects of erythropoietin against hypoxic injury via modulation of the mitogen-activated protein kinase pathway and apoptosis. Korean J Pediatr. 2017;60:181–188. doi: 10.3345/kjp.2017.60.6.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, et al. EPO-dependent activation of PI3K/Akt/FoxO3a signalling mediates neuroprotection in in vitro and in vivo models of Parkinson’s disease. J Mol Neurosci. 2014;53:117–124. doi: 10.1007/s12031-013-0208-0. [DOI] [PubMed] [Google Scholar]
- Li D-J, Li Y-H, Yuan H-B, Qu L-F, Wang P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism. 2017;68:31–42. doi: 10.1016/j.metabol.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Malla RR, Asimi R, Teli MA, Shaheen F, Bhat MA. Erythropoietin monotherapy in perinatal asphyxia with moderate to severe encephalopathy: a randomized placebo-controlled trial. J Perinatol. 2017;37:596–601. doi: 10.1038/jp.2017.17. [DOI] [PubMed] [Google Scholar]
- Mikrogeorgiou A, et al. Dedifferentiated fat cells as a novel source for cell therapy to target neonatal hypoxic-ischemic encephalopathy. Dev Neurosci. 2017;39:273–286. doi: 10.1159/000455836. [DOI] [PubMed] [Google Scholar]
- Ostrowski D, Heinrich R. Alternative erythropoietin receptors in the nervous system. J Clin Med. 2018;7:24. doi: 10.3390/jcm7020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salakou S, et al. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo. 2007;21:123–132. [PubMed] [Google Scholar]
- Savoia C, Sisalli MJ, Di Renzo G, Annunziato L, Scorziello A. Rosuvastatin-induced neuroprotection in cortical neurons exposed to OGD/reoxygenation is due to nitric oxide inhibition and ERK1/2 pathway activation. Int J Physiol Pathophysiol Pharmacol. 2011;3:57–64. [PMC free article] [PubMed] [Google Scholar]
- Souvenir R, Flores JJ, Ostrowski RP, Manaenko A, Duris K, Tang J. Erythropoietin inhibits HIF-1alpha expression via upregulation of PHD-2 transcription and translation in an in vitro model of hypoxia-ischemia. Transl Stroke Res. 2014;5:118–127. doi: 10.1007/s12975-013-0312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Mendez S, Tovilla-Zárate CA, Juárez-Rojop IE, Bermúdez-Ocaña DY. Erythropoietin: a potential drug in the management of diabetic neuropathy. Biomed Pharmacother. 2018;105:956–961. doi: 10.1016/j.biopha.2018.06.068. [DOI] [PubMed] [Google Scholar]
- Tian T, Zeng J, Zhao G, Zhao W, Gao S, Liu L. Neuroprotective effects of orientin on oxygen-glucose deprivation/reperfusion-induced cell injury in primary culture of rat cortical neurons. Exp Biol Med (Maywood NJ) 2018;243:78–86. doi: 10.1177/1535370217737983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics. 2016 doi: 10.1542/peds.2016-0191. [DOI] [PubMed] [Google Scholar]
- Xu G, Gong Z, Yu W, Gao L, He S, Qian Z. Increased expression ratio of Bcl-2/Bax is associated with crocin-mediated apoptosis in bovine aortic endothelial cells. Basic Clin Pharmacol Toxicol. 2007;100:31–35. doi: 10.1111/j.1742-7843.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- Yan F, et al. Erythropoietin improves hypoxic-ischemic encephalopathy in neonatal rats after short-term anoxia by enhancing angiogenesis. Brain Res. 2016;1651:104–113. doi: 10.1016/j.brainres.2016.09.024. [DOI] [PubMed] [Google Scholar]
- Yan X, et al. Vanillin protects dopaminergic neurons against inflammation-mediated cell death by inhibiting ERK1/2, p38 and the NF-κB signaling pathway. Int J Mol Sci. 2017;18:389. doi: 10.3390/ijms18020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-J, et al. The erythropoietin-derived peptide MK-X and erythropoietin have neuroprotective effects against ischemic brain damage. Cell Death Dis. 2017;8:e3003. doi: 10.1038/cddis.2017.381. [DOI] [PMC free article] [PubMed] [Google Scholar]