Abstract
When faced with an uncertain clinical pathosis in the oral cavity, identifying the color of the mucosal lesion helps to narrow down a differential diagnosis. Although less common than red and white lesions, yellow lesions encompass a small group of distinct mucosal pathologic entities. Adipose tissue, lymphoid tissue, and sebaceous glands are naturally occurring yellow constituents of the oral cavity and become apparent with associated developmental or neoplastic lesions. Reactive and inflammatory lesions can create a yellow hue due to purulence, necrosis, and calcification. Some systemic diseases are known to deposit yellow bi-products such as amyloid or bilirubin into the oral mucosa of an affected person, and while not always yellow, unusual entities like verruciform xanthoma and granular cell tumor fall under the umbrella of yellow lesions given their occasional propensity to demonstration the color. This chapter aims to explore the unique group that is yellow lesions presenting in the oral mucosa.
Keywords: Oral cavity, Yellow, Mucosal, Mouth, Oral manifestations, Systemic disease
Introduction
Within the oral mucosa, lesions that have a yellow hue comprise a small yet diverse group of pathologic entities with etiologies that include true neoplasms, developmental lesions, reactive/inflammatory conditions, calcified masses, and manifestations of systemic disease. From a physiologic standpoint, adipose tissue, lymphoid tissue, and sebaceous glands are the primary tissue types with a normal yellow hue. Naturally distributed throughout the oral cavity, adipose and lymphoid tissues become clinically evident as the result of cellular hyperplasia and often present as non-ulcerated, smooth surfaced, dome-shaped or bosselated masses that attenuate the overlying oral epithelium. Sebaceous glands are typically found in intimate association with hair follicles, but their aberrant presence in the oral cavity is frequently detected and considered a variation of normal. Yellow lesions in the oral cavity can also result from deposition of blood breakdown byproducts, inflammation, and necrosis. Within this subset of lesions, the mucosal surface may appear architecturally unaltered other than change in color, slightly raised with or without ulceration, or even fissured and harboring a nidus of foreign material. Finally, on rare occasion yellow lesions can indicate the presence of a systemic condition. This review will introduce and discuss the yellow appearing entities of the oral mucosa based on their generally accepted etiology (Table 1).
Table 1.
Yellow oral mucosal entities
| Developmental | Reactive inflammatory | Neoplastic | Oral manifestation of systemic disease |
|---|---|---|---|
| Fordyce granules | Verruciform xanthoma | Lipoma | Jaundice |
| Lymphoepithelial cyst | Abscess | Granular cell tumor | Pyostomatitis vegetans |
| Dermoid cyst | Sialolith and tonsillolith | Amyloid |
Developmental Yellow Lesions
Developmental lesions of the oral cavity include a group of conditions which are often evident in early childhood, remain clinically detectable over one’s lifetime, and at least in part, contain tissues native to the general anatomic site.
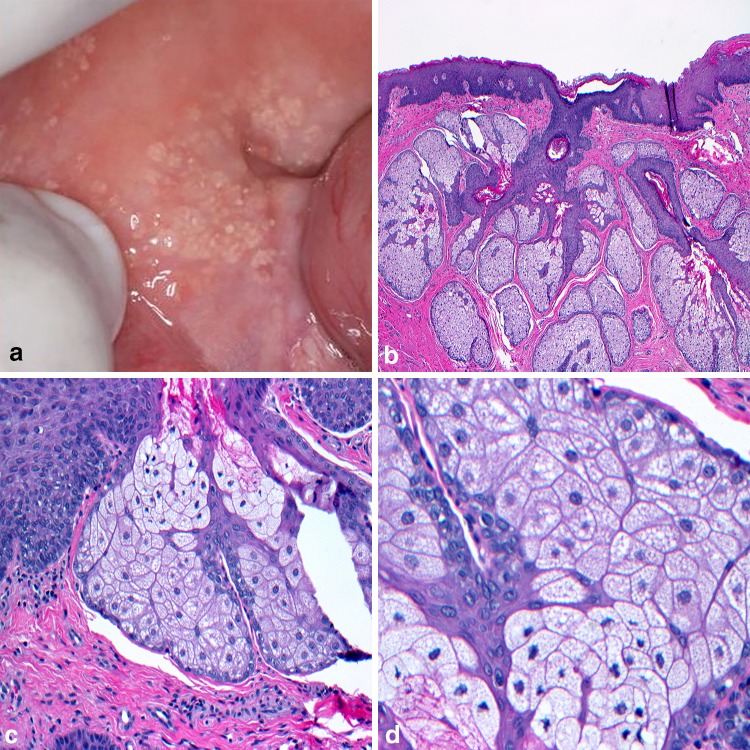
One yellow oral lesion that stretches the limitations of the above definition are Fordyce granules (FG). First described by John Addison Fordyce in 1896, these ectopic sebaceous glands can be found within the intraoral and labial mucosa, as well as on the genitalia [1]. The buccal mucosa and vermilion border of the upper lip are the most common head and neck sites [2] with isolated involvement of the anterior tonsillar pillars and retromolar pads also reported. Clinically described as yellow to cream-colored papules, FG appear as discrete clusters of granular-appearing cells that may impart a slight bumpy feel to the surface mucosa (Fig. 1a). With a prevalence approaching detection in 80% of the adult population, [3] FG are considered a variant of normal. Hyperlipidemia has been directly associated with individuals presenting with a higher density of FG and the increased risk factor for cardiovascular disease warrants examination [4]. Since FG is not a true pathologic entity [5] they are infrequently biopsied. If tissue is submitted, microscopic evaluation reveals a lobular aggregate of sebaceous acinar units immediately below the mucosal epithelium (Fig. 1b). Hair follicles are expectantly absent and ductal communication to the surface may be observed (Fig. 1c). The sebaceous lobules are comprised of large pale staining cells with a central nucleus and lipid-rich foamy cytoplasm (Fig. 1d). Azevedo and colleagues assessed Ki-67 expression and sebaceous lobule count in FG and established histologic distinction from sebaceous hyperplasia, and sebaceous adenoma based on a significant decrease in the numbers of lobules and a reduced Ki-67 proliferation rate in FG [6]. This distinction between sebaceous entities may be of importance for the early detection and initial diagnosis of Muir-Torre syndrome, a phenotypic subtype of Lynch syndrome [7]. Also known as hereditary nonpolyposis colorectal cancer syndrome, Lynch syndrome shows inherited defects in DNA mismatch repair (MMR) genes and association with sebaceous hyperplasia and sebaceous adenoma [8]. At least two studies have also suggested a clinical correlation between FG and the hereditary nonpolyposis colorectal cancer syndrome [9, 10] yet germline mutation of MMR genes has yet to be demonstrated in FG [8]. Finally, while treatment of FG is typically not indicated, the internet is replete with homoeopathic cures for those desiring removal for cosmetic concerns.
Fig. 1.
Fordyce granules. a Ectopic sebaceous glands distributed along the buccal mucosa; b lobular aggregate of sebaceous acinar units immediately below the mucosal epithelium; c intimate association of lobules with ductal elements; d large ovoid sebaceous cells with lipid filled vacuoles and a centrally placed nucleus
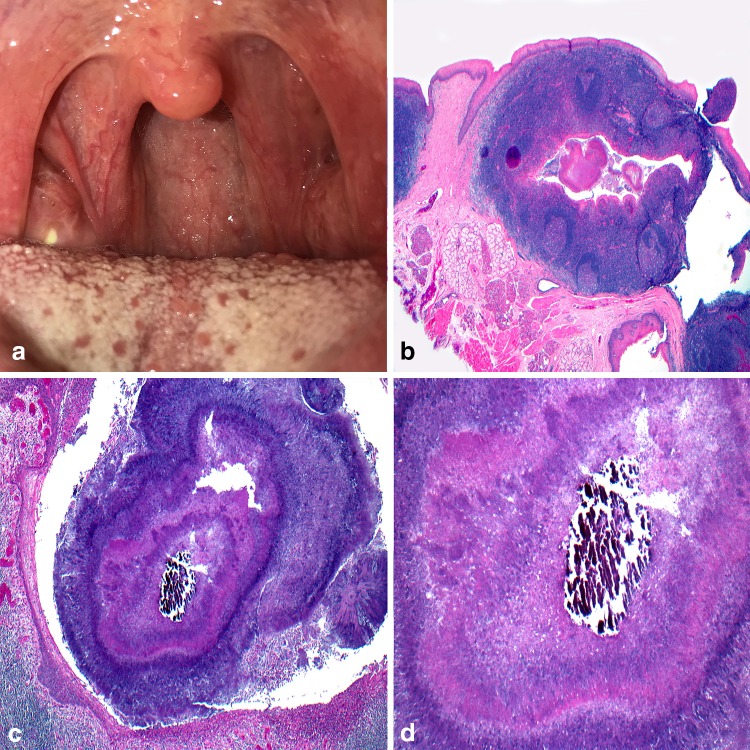
Originally referred to by Gold in 1962 as an intraoral branchial cyst, [11] the oral lymphoepithelial cyst (OLC) is a benign developmental lesion with a tendency for occurrence in the floor of the mouth and ventral-lateral tongue [12]. Within the oral cavity, aggregates of lymphoid tissue are naturally present in the area of Waldeyer’s ring, specifically the lingual, pharyngeal, and palatine tonsils. Accessory oral tonsillar tissue and lymphoid aggregates are occasionally detected in the floor of the mouth and ventral surface of the tongue [2]. While several theories of pathogenesis of OLC exist, squamous epithelium either invaginates or becomes implanted within the lymphoid tissue, undergoes cystic dilatation, and results in a clinically detectable yellow to yellow-white dome-shaped swelling (Fig. 2a). Rarely reaching beyond 1 cm in greatest dimension, OLC are frequently detected during the patient’s third and fourth decades. An increased predilection in females has also been reported [13, 14]. Histologic examination reveals a predominantly parakeratinized squamous epithelial cyst lining surrounded fully or in part by hyperplastic lymphoid tissue often in a follicular pattern containing germinal centers (Fig. 2b). Desquamated epithelium usually fills the cyst lumen (Fig. 2c) and the lymphoid tissue abuts the cystic lining (Fig. 2d). Rarely would follicular lymphoma become a diagnostic concern, but if entertained, the lymphoid germinal center of OLC would contain mitotic figures, tangible body macrophages, and stain strongly positive for CD20 yet demonstrate weak or negative staining with CD10 and consistently negative BCL-2 immunoreactivity [15–17]. This later result contrasts sharply with follicular lymphoma, especially those identified as Grade 1 and 2. Conservative excisional biopsy is considered adequate treatment.
Fig. 2.
Oral lymphoepithelial cyst. a Slightly raised, yellow nodule arising in the tonsillar pillars; b squamous epithelial cyst lining surrounded by hyperplastic lymphoid tissue containing follicular pattern containing germinal centers; c desquamated epithelium and cellular debris filling the lumen; d lymphoid tissue abuts the cystic lining.
Clinical photo courtesy of Dr. Kristin McNamara
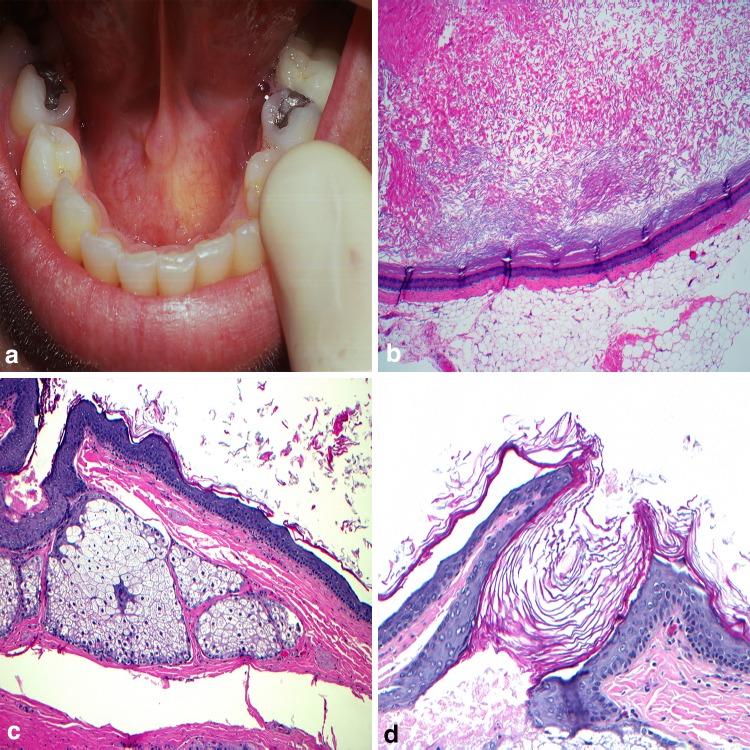
The term dysontogenetic, suggesting marked defective development, has been used to categorize a group of intraoral cysts that occur primarily along the midline of the floor of mouth or as an intralingual swelling [18]. Included in this group is the dermoid cyst (DC). Theories to the origin of the DC date back to the late nineteenth century [19] with acquired and congenital models formulated. Acquired lesions are purported to arise from traumatically implanted epithelium and are more likely to occur at the extremities [20]. Congenital cysts are thought to develop from entrapped ectodermal tissue along the body midline. This theory would appear to best explain intraoral DC with origins from aberrant epithelium during the fusion of the mandibular processes and/or branchial arches. While diagnosed over a broad age range exists, King and colleagues found the highest incidence of intraoral DC (27.6%) are reported in the initial five years of life [19]. Clinically the lesions will present as mucosal colored or have a yellow tinge. Typically asymptomatic at time of diagnosis, floor of mouth and intralingual cysts may impair mastication, speech, and cause difficulty breathing [21–23] (Fig. 3a). For appropriate diagnosis the histologic examination of DC must identify two germ cell layers; a typically uniformly thin keratinized squamous epithelial cyst lining (Fig. 3b) with sebaceous glands or hair follicles present within the connective tissues intimately associated with the epithelium [20, 21, 23, 24] (Fig. 3c, d). Surgical excision of DC is the most effective therapy, yet the exact approach is often site-specific [23]. In the cases along the midline of the neck, those presenting above the geniohyoid muscle may facilitate an intraoral approach while those below the muscle often necessitate extraoral removal [24].
Fig. 3.
Dermoid cyst. a Fluctuant swelling at midline of the floor of mouth; b orthokeratinized stratified squamous epithelial cyst lining with prominent keratohyalin granular cell layer; c sebaceous glands in the cyst wall; d dermal appendage elements intimately associated with the epithelium
Reactive and Inflammatory Yellow Lesions
Reactive and inflammatory responses are part of the body’s defense mechanism that are elicited to protect individuals from infection and injury. The response consists of an increase in permeability of the nearby blood vessels with the migration of fluid, proteins, phagocytic neutrophils, and macrophages to the site of tissue damage. The components of the response and repair process often present intraorally with a beige to yellow hue dependent in part on the type of substance encountered, the presence of purulence, or if a nidus of calcification has formed.
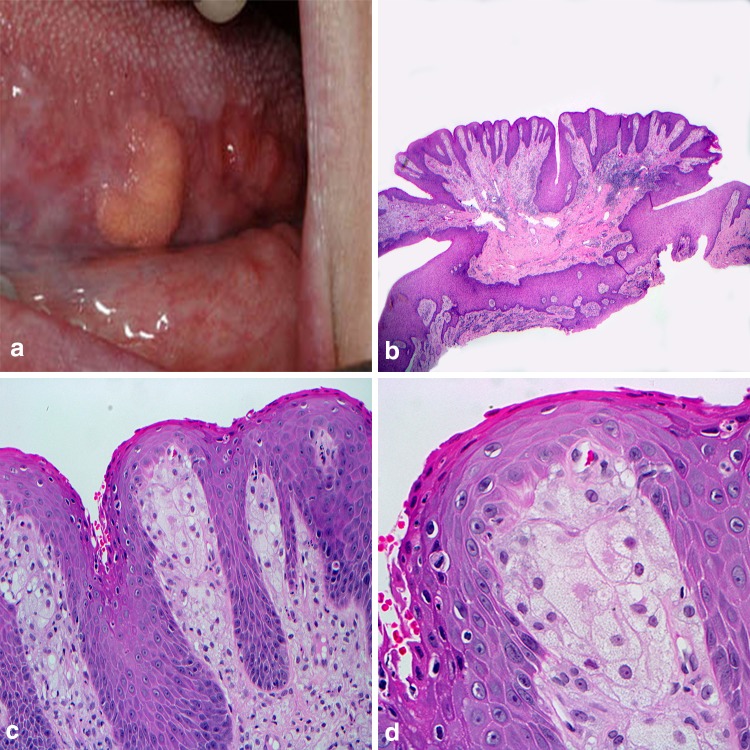
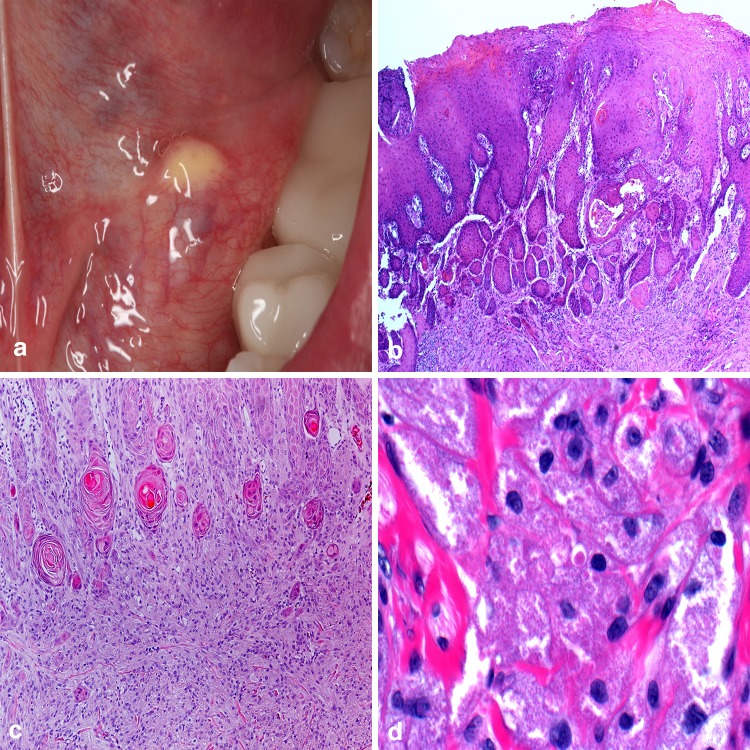
With mounting evidence for derivation as a localized reactive process, Shafer described the verruciform xanthoma (VX) as a benign oral lesion in 1971 [25]. Over the next decade, extraoral cases involving skin and the genitalia were added to the literature. Intraorally, VX appear to favor the masticatory mucosa with greater than 85% of cases reported occurring on the keratinized surfaces [26]. Typically presenting as a solitary lesion, VX is described as being slightly exophytic, having a pebbly or warty surface architecture, and can range from yellow to red-brown in color (Fig. 4a). The lesions are most common in the fifth and sixth decades of life and reported as asymptomatic at the time of discovery. Histologic examination reveals a parakeratinized hyperplastic epithelium displaying a verrucous-like appearance with uniformly elongated rete pegs (Fig. 4b). Large polygonal, foamy-appearing, “xanthoma” cells fill the connective tissue papillae and only rarely venture beyond the depth of the rete pegs [27] (Fig. 4c, d). The individual xanthoma cells exhibit strong positive staining with CD68, validating a macrophage lineage, and Periodic–Acid–Schiff stain shows reactivity in the granular contents of these foam cells [26]. Further evaluation with macrophage subpopulation antibody probes RM3/1, 25F9, and 27E10 helped demonstrate the majority of the xanthoma cells are of reparative and resident phenotype and help to characterize the VX as a chronic reactive process [28]. While considered exceedingly innocuous and warranting conservative surgical removal, the clinician should be aware that VX has been reported in association with numerous inflammatory conditions such as pemphigus vulgaris and lichen planus, in addition to premalignant and malignant neoplasms to include squamous cell carcinoma [29].
Fig. 4.
Verruciform xanthoma. a Yellow-orange mass along the ventral lateral border of the tongue; b pebbly or warty surface architecture arising from surface mucosa; c parakeratinized hyperplastic epithelium with uniformly elongated rete pegs; d large polygonal, foamy-appearing, “xanthoma” cells fill the connective tissue papillae
Abscess (AB) formation in the oral cavity can have a myriad of etiologies to include pulpal necrosis, bacterial or foreign material-driven gingival breakdown, or periodontal inflammation involving the tooth supporting bone. Though AB is not considered a rare finding, in a community screening of over 23,000 adult patients, it did not make the list of the most common oral lesions encountered [30]. In addition, a histologic review of 125 periapical lesions treated with periapical surgery by Schulz and his coauthors found only 5% of lesions diagnostic of AB [31]. Furthermore, Herrera and colleagues while reviewing the periodontics literature noted the low frequency of AB occurring in the periodontium while highlighting the importance of immediate recognition and management [32]. Clinically AB often presents with a cream-yellow colored central nidus of purulence surrounded by a reddish halo. This purulent inflammatory infiltrate is characterized by a collection of neutrophils, necrotic cells, and edematous fluid [33]. Treatment, which is frequently emergent, [34] is directed toward eliminating the cause of the infection with or without the prescribing of antibiotic medications [35].
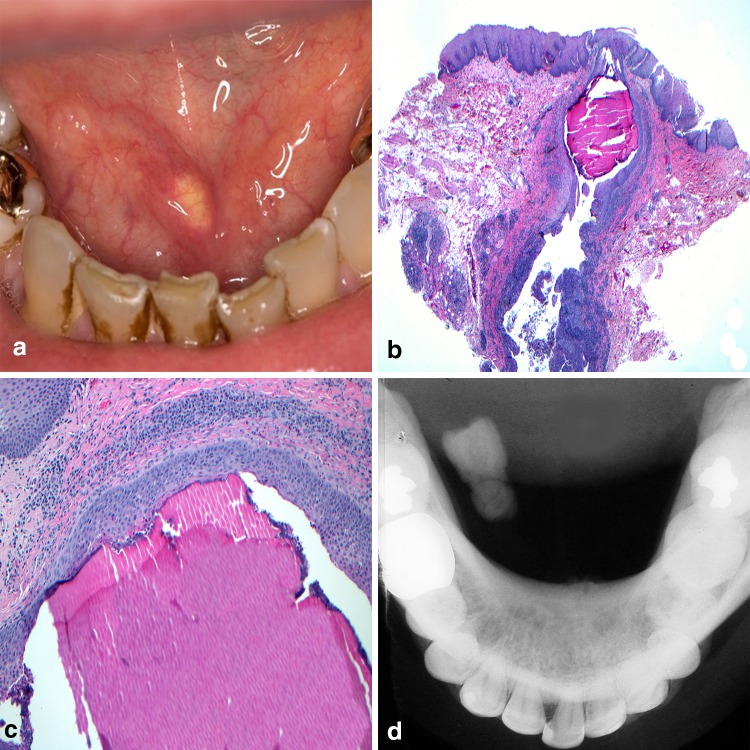
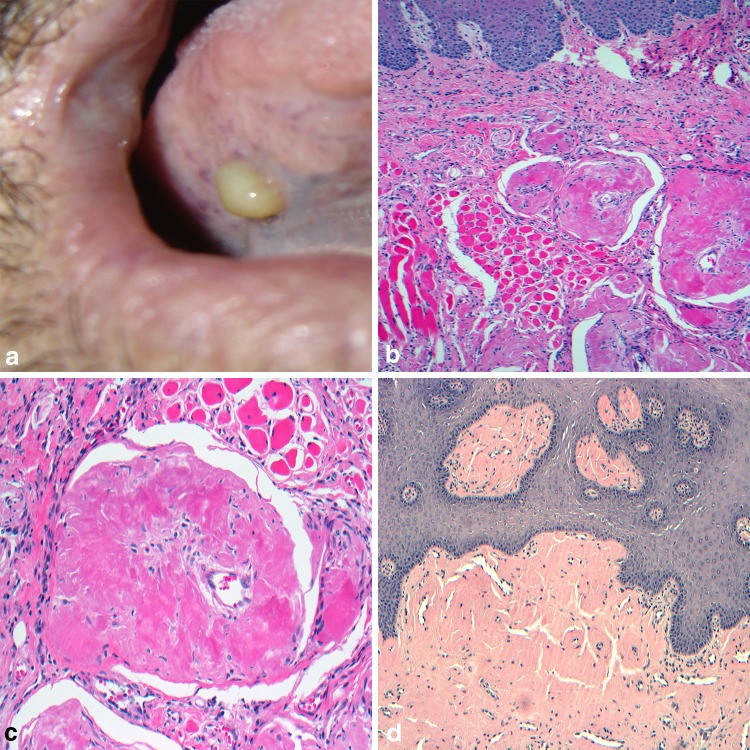
Bacterial inflammation and retained organic matter have been implicated as factors in the formation of “stone-like” deposits throughout the body [36] and the head and neck region is not exempt. Sialoliths (SL) and tonsilloliths (TL) are examples of stone formation, affecting the ducts of salivary glands and the tonsillar crypts respectively. In the case of SL, the most commonly affected location is the submandibular gland duct with is anti-gravitational uphill and tortuous course from gland parenchyma to the exit at Wharton’s duct. While the surrounding tissues can become red with inflammation, the SL and its associated bacterial debris, appear yellowish especially when present at or near the duct orifice (Fig. 5a). In addition to anatomic factors, high saliva calcium concentrations, and retrograde migration of bacteria and debris through the duct orifice have also been implicated in SL formation [37]. The SL itself is an aggregate of different calcium phosphates, mainly hydroxyapatite, along with inflammatory cells and organic material within the excretory duct lumen (Fig. 5b). The calcified mass may give the appearance of concentric deposition [36] (Fig. 5c). Patients will regularly report episodic swelling and pain often correlating with mealtimes and radiographs can be useful in confirming the presence of larger lesions (Fig. 5d). Foletti and colleagues [38] have proposed a decision-tree based approach to management of the calculi based upon the specific gland involved, portion of the excretory duct involved, and diameter of the SL in hopes of inviting more minimally invasive treatments. Findings by Lustmann, et al. suggest that patients with SL are more prone to develop nephrolithiasis [39]. Other reviews have failed to identify a correlative relationship between SL formation in patients additionally suffering from kidney stones or gallstones [40, 41]. A case controlled study recently found significantly more antibiotic use in patients with SL formation as compared to control populations, however no association with other systemic diseases, smoking, or alcohol consumption was discovered [42].
Fig. 5.
Sialolithiasis. a Calcified “stone” within the submandibular gland excretory duct; b sialolith and inflammatory debris within the excretory duct lumen; c calcified mass appearing as concentric deposition; d occlusal radiograph confirms the presence of calcified mass.
Clinical photo courtesy of Dr. Jerry Bouquot
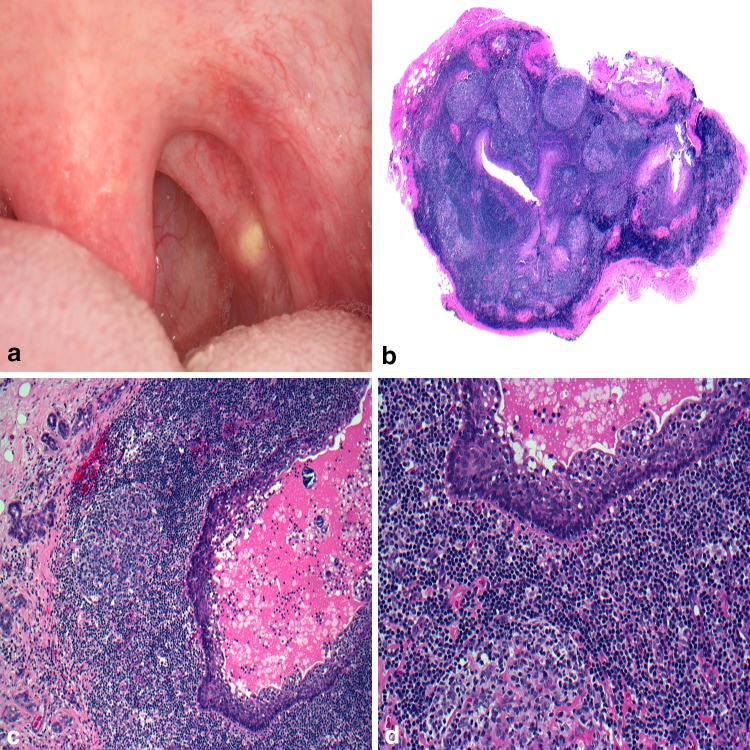
The pharyngeal tonsils with their irregularly branching crypts help introduce host immune cells to antigens from the oral cavity. Within these crypts keratinaceous debris, foreign material, and bacterial colonies accumulate and occasionally calcify to form TL [43]. Clinically TL are common, presenting as white or yellowish masses within the tonsillar folds (Fig. 6a). While TL are usually asymptomatic, occasional ailments include pain, dysphagia, sore throat, and halitosis. A review of plain film X-rays at the University of Iowa detected TL in over 8% of the patient population [44] while Takahashi and co-authors detected tonsillar calcification in 39.9% of patients using computed tomography [45] demonstrating in part, the increased sensitivity of 3D imaging. Treatments range from self-inflicted home remedies to curettage or laser therapy [46]. Tonsillectomy is reserved for symptomatic or recurrent cases. Examination of the submitted surgical tissue beyond gross inspection is dictated by local pathology laboratory policy [47, 48] and if performed would reveal a tonsillar crypt lined by stratified squamous epithelium and surrounded by reactive follicular hyperplasia (Fig. 6b). The crypt itself harbors the concentrically organized bacterial colonies with a central calcified nidus (Fig. 6c). Filamentous and coccus forms of bacterial are apparent (Fig. 6d).
Fig. 6.
Tonsillolith. a White-yellow mass noted protruding from a tonsillar crypt; b tonsillar crypt lined by stratified squamous epithelium and surrounded by reactive follicular hyperplasia; c crypt harboring concentrically organized bacterial colonies; d central calcified nidus surrounded by bacteria and neutrophils
Neoplastic Yellow Oral Mucosal Lesions
Detectable changes in oral mucosa morphology and color are often initial signs of neoplastic change, with color frequently employed to categorize the various pathologic entities for the purpose of generating a differential diagnosis. While neoplasia, benign or malignant, is not an uncommon phenomenon in the head and neck region, Medline® searches for yellow oral neoplasms/tumors typically yield but two entities; lipoma and granular cell tumor [49–51].
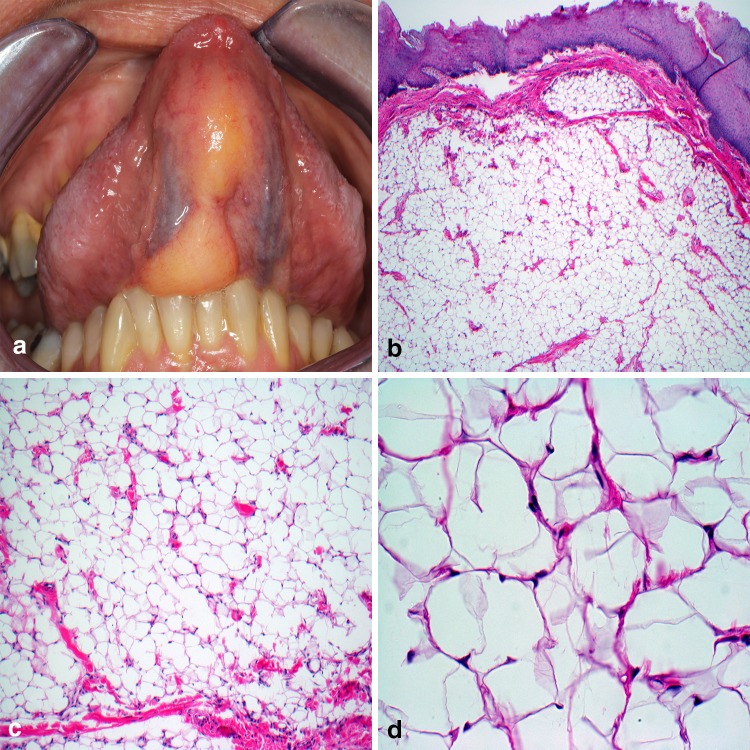
Considered the most frequently diagnosed soft tissue tumor in the body, roughly 20% of lipomas (LI) occur in the head and neck, though less than 4.4% constitute intraoral cases [50, 52]. Intraoral LI are typically located in the buccal mucosa, submandibular region, tongue, and floor of mouth [53] and when involving the superficial lamina propria LI impart an unmistakable yellow clinical appearance (Fig. 7a). Microscopic evaluation demonstrates a well-demarcated proliferation of mature adipocytes absent of cytologic atypia (Fig. 7b). Classic LI comprises the vast majority of histologic subtypes encountered with spindle cell, chondroid, and fibrolipoma variants also reported. A vague lobular appearance is produced by thin fibrous connective tissue septa containing small blood vessels (Fig. 7c). The individual lipocytes are large, varying little from normal fat, with a prominent lipid vacuole and peripherally placed nucleus (Fig. 7d). Sclerosis and presence of lipoblasts should alert concern for malignancy. Conservative surgical excision is considered curative.
Fig. 7.
Lipoma. a Soft, bosselated mass emanating from the ventral tongue; b well-circumscribed or encapsulated proliferation of mature adipose tissue; c thin fibrous strands containing inconspicuously scattered capillaries; d large vacuolated cells with peripherally displace nuclei.
Clinical photo courtesy of Dr. Sarah Aguirre
Although classified here as a soft tissue neoplasm, the granular cell tumor (GCT) has an unclear and debated histogenesis. Originally termed granular cell myoblastoma and Abrikossoff’s tumor, the plump granular cells of the GCT were at one point postulated to be of skeletal muscle origin. Current concepts support the tumor to be of neural origin, specifically Schwann cells, [54–57] though some still debate the classification of this lesion as a neoplasm arguing they are a product of metabolic stress or trauma [54]. GCTs can arise anywhere in the body but the majority occur in the head and neck region with an affinity for the dorsal tongue and a predilection for females [54–57]. Clinically, the lesions present as small firm yellow or pink nodules [57] (Fig. 8a). The overlying epithelium is typically nonulcerated and in approximately 50% of cases will, on histologic examination, demonstrate pseudoepitheliomatous hyperplasia which may be mistaken for squamous cell carcinoma [56] (Fig. 8b). The microscopic hallmark of this lesion is the presence of granular cells containing numerous autophagolysosomes imparting a course, gritty appearance to the cytoplasm [54] (Fig. 8d). These granular cells can appear syncytial or arranged in ribbons and sheets around fibrous connective tissue septa or muscle, often blending unperceptively along the epithelial interface [55] (Fig. 8c). While strong immunohistochemical expression of S-100 protein, CD57, CD68, and SOX10 is reported in the majority of the tumors, [58] a couple of authors have reported S-100 negative reactivity in GCT [59, 60]. Synchronous, metachronous, and malignant GCTs have been reported [57]. Surgical excision is the treatment of choice.
Fig. 8.
Granular cell tumor. a Firm, yellow dome-shaped mass in the floor of the mouth; b overlying epithelium is nonulcerated and demonstrates pseudoepitheliomatous hyperplasia; c unencapsulated tumor cells arranged in ribbons and sheets around fibrous connective tissue septa and muscle; d large polygonal cells containing numerous autophagolysosomes imparting a course, gritty appearance to the cytoplasm.
Clinical photo courtesy of Dr. Christine Harrington
Yellow Oral Manifestations of Systemic Diseases
Oral manifestations of systemic disease may present in a variety of manners to include bony and soft tissue growths, vesiculo-ulceration, deposition of cellular byproducts, and discrete or generalized areas of intra- and perioral discoloration. In many instances, these oral findings may precede the appearance of signs and symptoms at distant sites in the body.
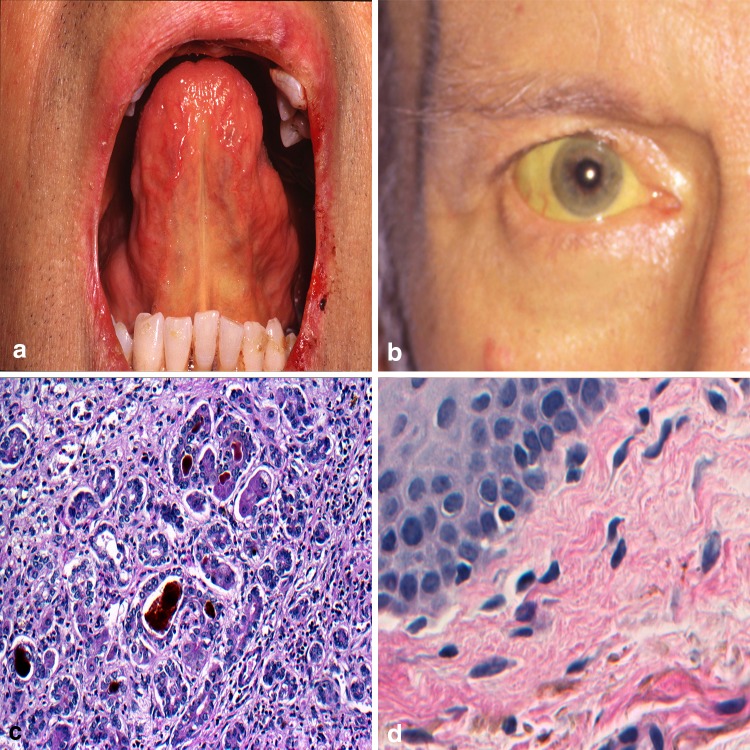
The etiology of altered mucosal coloration may be pathologic or physiologic and often the direct result of endogenous production or deposition of melanin, hemosiderin, or hemoglobin [61]. The term jaundice (JAU), refers to the yellow discoloration of the skin, sclera, or oral mucosa occurring as the result of excess bilirubin and its deposition in the dermis or submucosa [62]. With normal function the liver metabolizes and excretes bilirubin. In disease states such as cirrhosis, hepatitis, or pancreatic cancer, the liver fails to clear this waste material. Bilirubin shows an affinity for elastin fibers and concentrations of this yellowish-orange hemoglobin breakdown product tend to accumulate intraorally along the soft palate and floor of mouth in proximity to the lingual frenum [62, 63] (Fig. 9a). Extraoral head and neck sites include the sclera (Fig. 9b). The diagnosis workup of JAU usually includes a serum liver function panel and if formalin-fixed tissue from the liver is available, cholestasis is noted (Fig. 9c). Peripheral tissues can show deposits along elastin fibers (Fig. 9d). A Hall’s bilirubin stain will highlight deposits of bilirubin by converting the normally yellow-orange pigmented bile to olive or emerald green in color. As the seriousness of underlying causes of JAU vary significantly, the treatment and potential patient outcomes vacillate widely as well.
Fig. 9.
Jaundice. a Diffuse yellow discoloration to the mucosa along the lingual frenum; b involvement of the sclera a common finding; c intraluminal plugging of ducts with bile with spillage into surrounding tissue; d peripheral tissues showing deposits along elastin fibers.
Clinical photos courtesy Dr. Christopher Fielding
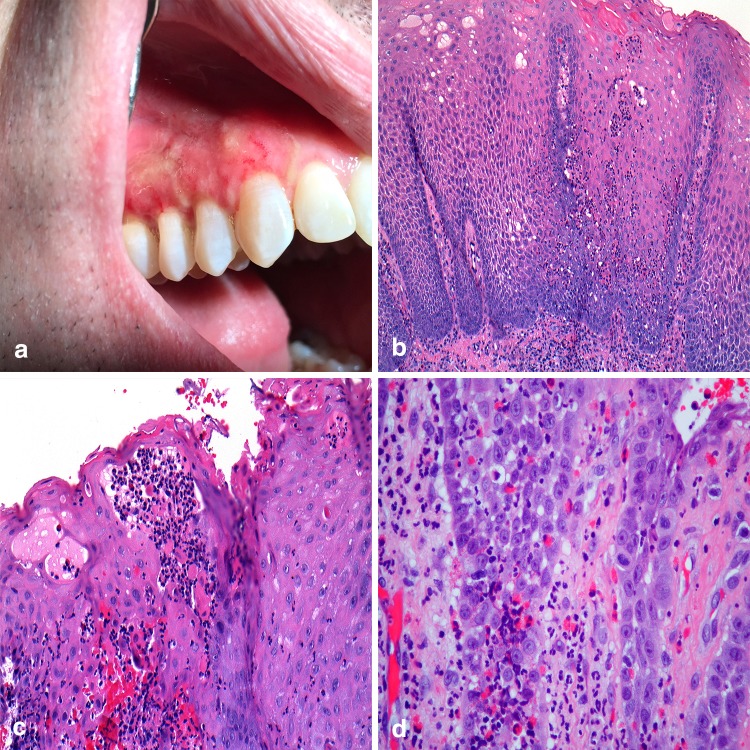
The term inflammatory bowel disease (IBD) encompasses as group of digestive disorders that may produce concomitant oral lesions including aphthous ulcers, pyostomatitis vegetans (PV), and drug induced lichenoid mucositis [64]. Recognized as the oral counterpart of pyoderma gangrenosum, PV has been shown to demonstrate a strong association with IBD, most commonly ulcerative colitis [65]. Clinically, small yellow-white pustules on erythematous mucosa that coalesce and break to form a “snail track” pattern characterize the lesions [64–66] (Fig. 10a). The labial gingiva, soft and hard palate, buccal mucosa, and labial mucosa are the most commonly reported sites of occurrence with tongue and floor of mouth involvement considered rare events [64–66]. Histopathologically, the affected epithelium shows acanthosis (Fig. 10b) with intraepithelial and subepithelial microabscesses comprised of neutrophils and eosinophils (Fig. 10c, d). The presence of PV should alert a clinician to refer the patient for evaluation by a gastroenterologist, as the lesions might be an initial sign of undiagnosed IBD. In patients previously diagnosed with IBD, the oral lesions may be an indicator of relapse [65].
Fig. 10.
Pyostomatitis vegetans. a Irregular linear “snail” tracts along the maxillary alveolar mucosa; b papillary, acanthotic epithelium with inflammation; c intraepithelial microabscesses; d pronounced eosinophilia in lamina propria.
Clinical photo courtesy of Dr. Paul Freedman
Within the oral cavity, the discovery of extracellular amyloid (AA) deposition can represent a localized occurrence or be a component of a widespread systemic phenomenon with significant associated morbidity and mortality. In addition to the variability in presentation, the pathogenesis of the amyloid fibrils also can differ. Immunoglobulin light chain-related proteins and inflammatory driven serum amyloid A derived proteins are considered the major contributors to primary and secondary amyloidosis, respectively [67]. While the most common head and neck site of involvement is the laryngeal and subglottic region, intraoral lesions most often occur in the tongue [68]. Presenting as localized nodules or plaque, or diffusely with resultant macroglossia, the AA can impart a yellow, orange, or even red tone to the overlying mucosa (Fig. 11a). Microscopically one expects to find nodular aggregates of an amorphous eosinophilic material displacing the lamina propria and submucosa with an overlying intact surface epithelium (Fig. 11b). The amyloid often closely approximates with muscle, native blood vessels, and occasionally surrounds adjacent minor salivary gland [69] (Fig. 11c). Staining with Congo red highlights the AA as pale red under direct light microscopy (Fig. 11d) and will impart an apple green birefringence of the deposits under polarized light. Most intraoral cases represent a localized process, requiring surgical intervention for diagnosis, and improved functional considerations. However, an appropriate workup to rule out systemic factors is warranted.
Fig. 11.
Amyloidosis. a Firm nodule on right ventral lateral tongue; b extracellular amorphous deposits of eosinophilic material in connective tissues; c affinity for muscle and small vessels; d highlighted by Congo red stain
Conclusion
While admittedly, few lesions appear yellow in the oral cavity, those that do have quite diverse and unique etiologies. The derivation of the yellow color in these lesions may be the result of the proliferation of normal occurring tissues like adipose or sebaceous elements, a bi-product of inflammation and calcified debris, or rare tissue deposits associated with systemic conditions. Despite this variation, substantiate conditions do exist that warrant early detection, and due to the limited number of yellow lesions in existence and the distinctiveness of their characteristics, identifying an unknown oral entity as yellow enhances the precision of a healthcare provider’s differential diagnosis.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
As no human participants were involved in a study, informed consent was not required.
References
- 1.Fordyce JA. A peculiar affection of the mucous membranes of the lips and the oral cavity. J Cutan Genito-Urin Dis. 1896;14:413–419. [Google Scholar]
- 2.Neville BW, Damm DD, Allen CM, Chi AC. Developmental defects of the oral and maxillofacial region. Oral and maxillofacial pathology. 4. St. Louis: Elsevier; 2016. pp. 1–48. [Google Scholar]
- 3.Batsakis JG, el-Naggar AK. Sebaceous lesion of salivary glands and oral cavity. Ann Otol Rhinol Laryngol. 1990;99:414–418. doi: 10.1177/000348949009900517. [DOI] [PubMed] [Google Scholar]
- 4.Gaballah KY, Rahimi I, et al. Can presence of oral Fordyce’s granules serve as a marker for hyperlipidemia. Dent Res J. 2014;11:553–558. [PMC free article] [PubMed] [Google Scholar]
- 5.Antonio N. Oral mucosa. Ten Cate’s oral histology. 9. St. Louis: Elsevier; 2017. pp. 260–288. [Google Scholar]
- 6.Azevedo RS, Almeida OP, Netto JNS, Miranda AM, et al. Comparative clinicopathological study of intraoral sebaceous hyperplasia and sebaceous adenoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:100–104. doi: 10.1016/j.tripleo.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Bhaijee F, Brown AS. Muir-Torre syndrome. Arch Pathol Lab Med. 2014;138:1685–1689. doi: 10.5858/arpa.2013-0301-RS. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Flores A, Peralto JLR. Mismatch repair protein expression in Fordyce granules. Appl Immunohistochem Mol Morphol. 2017;25:209–212. doi: 10.1097/PAI.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 9.De Felice C, Parrini S, Chitano G, Gentile M, et al. Fordyce granules and hereditary non-polyposis colorectal cancer syndrome. Gut. 2005;54:1279–1282. doi: 10.1136/gut.2005.064881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponti G, Meschieri A, Pollio A, Ruini C, et al. Fordyce granules and hyperplastic mucosal sebaceous glands as distinctive stigmata in Muir-Torre syndrome patients: characterization with reflectance confocal microscopy. J Oral Pathol Med. 2015;44:552–557. doi: 10.1111/jop.12256. [DOI] [PubMed] [Google Scholar]
- 11.Gold C. Branchial cleft cyst located in the floor of the mouth. Report of a case. Oral Surg Oral Med Oral Pathol. 1962;15:1118–1120. doi: 10.1016/0030-4220(62)90311-0. [DOI] [PubMed] [Google Scholar]
- 12.Leite RB, Severo MLB, Oliveira PT, Medeiros AMC, et al. Lymphoepithelial cyst on the tongue: case report at unusual location. J Bras Patol Med Lab. 2017;53:273–275. doi: 10.5935/1676-2444.20170043. [DOI] [Google Scholar]
- 13.Sykara M, Ntovas P, Kalogirou EM, Tosios KI, et al. Oral lymphoepithelial cyst: a clinicopathological study of 26 cases and review of the literature. J Clin Exp Dent. 2017;9:e1035–e1043. doi: 10.4317/jced.54072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Ow A, Zhang CP, Wang L, et al. Clinical analysis of 120 cases of intraoral lymphoepithelial cyst. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:448–452. doi: 10.1016/j.tripleo.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 15.Hanemann JAC, de Carli ML, Dendena ER, Filho CE, et al. Rare case report of an aggressive follicular lymphoid hyperplasia in maxilla. Oral Maxillofac Surg. 2017;21:475–481. doi: 10.1007/s10006-017-0661-y. [DOI] [PubMed] [Google Scholar]
- 16.Stacy RC, Jakobiec FA, Schoenfield L, Singh AD. Unifocal and multifocal reactive lymphoid hyperplasia vs follicular lymphoma of the ocular adnexa. Am J Ophthalmol. 2010;150:412–426. doi: 10.1016/j.ajo.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Jham BC, Binmadi NO, Scheper MA, Zhao XF, et al. Follicular lymphoid hyperplasia in palate: case report and literature review. J Cranio-Maxillofac Surg. 2009;37:79–82. doi: 10.1016/j.jcms.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Meyer I. Dermoid cysts (dermoids) of the floor of the mouth. Oral Surg Oral Med Oral Pathol. 1955;8:1149–1164. doi: 10.1016/0030-4220(55)90380-7. [DOI] [PubMed] [Google Scholar]
- 19.King RC, Smith BR, Burk JL. Dermoid cyst in the floor of the mouth: review of the literature and case reports. Oral Surg Oral Med Oral Pathol. 1994;78:567–576. doi: 10.1016/0030-4220(94)90166-X. [DOI] [PubMed] [Google Scholar]
- 20.Erich JB. Sebaceous, mucous, dermoid, and epidermoid cysts. Am J Surg. 1940;50:672–677. doi: 10.1016/S0002-9610(40)90463-9. [DOI] [Google Scholar]
- 21.Dimtsas S, Theologie-Lygidakis N, Iatrou I. Intralingual dermoid cyst in an infant presenting swallowing and sleeping difficulties. J Clin Pediatr Dent. 2010;34:335–338. doi: 10.17796/jcpd.34.4.jhxtw61076u8150t. [DOI] [PubMed] [Google Scholar]
- 22.MacNeil SD, Moxham JP. Review of floor of mouth dysontogenic cysts. Ann Otol Rhinol Laryngol. 2010;119:165–173. doi: 10.1177/000348941011900304. [DOI] [PubMed] [Google Scholar]
- 23.Pryor SG, Lewis JE, Weaver AL, Orvida LJ. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938–942. doi: 10.1016/j.otohns.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Dillon JR, Avillo AJ, Nelson BL. Dermoid cyst of the floor of the mouth. Head Neck Pathol. 2015;9:376–378. doi: 10.1007/s12105-014-0576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shafer WG. Verruciform xanthoma. Oral Surg Oral Med Oral Pathol. 1971;31:784–789. doi: 10.1016/0030-4220(71)90134-4. [DOI] [PubMed] [Google Scholar]
- 26.Marques YM, de Andrade CR, Machado de Sousa SC, Navarro CM. Oral verruciform xanthoma: a case report and literature review. Case Rep Pathol. 2014;2014:641015. doi: 10.1155/2014/641015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philipsen HP, Reichart PA, Takata T, Ogawa I. Verruciform xanthoma—biological profile of 282 oral lesions based on a literature survey with nine new cases from Japan. Oral Oncol. 2003;39:325–336. doi: 10.1016/S1368-8375(02)00088-X. [DOI] [PubMed] [Google Scholar]
- 28.Rawal SY, Kalmar JR, Tatakis DN. Verruciform xanthoma: immunohistochemical characterization of xanthoma cell phenotypes. J Periodontol. 2007;78:504–509. doi: 10.1902/jop.2007.060196. [DOI] [PubMed] [Google Scholar]
- 29.Mannes KD, Dekle CL, Requena L, Sangueza OP. Verruciform xanthoma associated with squamous cell carcinoma. Am J Dermatopathol. 1999;21:66–69. doi: 10.1097/00000372-199902000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Bouquot JE. Common oral lesions found during a mass screening examination. J Am Dental Assoc. 1986;112:50–57. doi: 10.14219/jada.archive.1986.0007. [DOI] [PubMed] [Google Scholar]
- 31.Schulz M, von Arx T, Altermatt HJ, Bosshardt D. Histology of periapical lesions obtained during apical surgery. J Endod. 2009;35:634–642. doi: 10.1016/j.joen.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Herrara D, Retamal-Valdes B, Alonso B, Feres M. Acute periodontal lesions (periodontal abscess and necrotizing periodontal diseases) and endo-periodontal lesions. J Periodontol. 2018;89:s85–s102. doi: 10.1002/JPER.16-0642. [DOI] [PubMed] [Google Scholar]
- 33.Kumar V, Abbas AK, Aster JC. Acute inflammation. Robbins and cotran pathologic basis of disease. 9. Philadelphia: Elsevier Saunders; 2015. p. 91. [Google Scholar]
- 34.Herrera D, Roldán S, Sanz M. The periodontal abscess: a review. J Clin Periodontol. 2000;27:377–386. doi: 10.1034/j.1600-051x.2000.027006377.x. [DOI] [PubMed] [Google Scholar]
- 35.Cope A, Francis N, Wood F, Mann MK, Chestnutt IG. Systemic antibiotics for symptomatic apical periodontitis and acute apical abscess in adults. Cochrane Database Syst Rev. 2014;6:CD010136. doi: 10.1002/14651858.CD010136.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Grases F, Santiago C, Simonet BM, Costa-Bauza A. Sialolithiasis: mechanism of calculi formation and etiologic factors. Clin Chim Acta. 2003;334:131–136. doi: 10.1016/S0009-8981(03)00227-4. [DOI] [PubMed] [Google Scholar]
- 37.Marchal F, Kurt AM, Dulguerov P, Lehmann W. Retrograde theory in sialolithiasis formation. Arch Otolaryngol Head Neck Surg. 2001;127:66–68. doi: 10.1001/archotol.127.1.66. [DOI] [PubMed] [Google Scholar]
- 38.Foletti JM, Graillon N, Avignon S, Guyot L, et al. Salivary calculi removal by minimally invasive techniques: a decision tree based on the diameter of the calculi and their position in the excretory duct. J Oral Maxillofac Surg. 2018;76:112–118. doi: 10.1016/j.joms.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Lustmann J, Regev E, Melamed Y. Sialolithiasis—survey on 245 patients and a review of the literature. Int J Oral Maxillofac Surg. 1990;19:135–138. doi: 10.1016/S0901-5027(05)80127-4. [DOI] [PubMed] [Google Scholar]
- 40.Zenk J, Constantinidis J, Kydles S, Hornung J, Iro H. Clinical and diagnostic findings of sialolithiasis. HNO. 1999;47:963–969. doi: 10.1007/s001060050476. [DOI] [PubMed] [Google Scholar]
- 41.Huoh KC, Eisele DW. Etiologic factors in sialolithiasis. Otolaryngol Head Neck Surg. 2011;145:935–939. doi: 10.1177/0194599811415489. [DOI] [PubMed] [Google Scholar]
- 42.Kraaij S, Karagozoglu KH, Kenter YAG, Pijpe J, et al. Systemic diseases and the risk of developing salivary stones: a case control study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:539–543. doi: 10.1016/j.oooo.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Neville BW, Damm DD, Allen CM, Chi AC. Bacterial infections. Oral and maxillofacial pathology. 4. St. Louis: Elsevier; 2016. pp. 164–190. [Google Scholar]
- 44.Bamgbose BO, Ruprecht A, Hellsein J, Timmons, et al. The prevalence of tonsilloliths and other soft tissue calcification in patients attending oral and maxillofacial radiology clinic of the University of Iowa. ISRN Dent. 2014 doi: 10.1155/2014/839635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi A, Sugawara C, Kudoh T, Uchida D, et al. Prevalence and imaging characteristics of palatine tonsilloliths detected by CT in 2,873 consecutive patients. Sci World J. 2014 doi: 10.1155/2014/940960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krespi YP, Kizhner V. Laser tonsil cryptolysis: in-office 500 cases review. Am J Otolaryngol Head Neck Med Surg. 2013 doi: 10.1016/j.amjoto.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Alvi A, Vartanian AJ. Microscopic examination of routine tonsillectomy specimens: is it necessary. Otolaryngol Head Neck Surg. 1998;119:361–363. doi: 10.1016/S0194-5998(98)70079-8. [DOI] [PubMed] [Google Scholar]
- 48.Williams MD, Brown HM. The adequacy of gross pathological examination of routine tonsils and adenoids in patients 21 years old and younger. Hum Pathol. 2003;34:1053–1057. doi: 10.1053/S0046-8177(03)00408-8. [DOI] [PubMed] [Google Scholar]
- 49.Gomez I, Varela P, Romero A, Garcia MJ, et al. Yellowish lesions of the oral cavity. Suggestion for a classification. Med Oral Patol Oral Cir Bucal. 2007;12:e272–e276. [PubMed] [Google Scholar]
- 50.Mohammed F, Thapasum A, Mohamed S, Shamaz H, et al. Yellow lesions of the oral cavity: diagnostic appraisal and management strategies. Brunei Int Med J. 2013;9:290–301. [Google Scholar]
- 51.Tosios K, Rallis G, Villianatou D, Vlachodimitropoulos D. Yellow-white tumor of the floor of the mouth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:701–704. doi: 10.1016/j.tripleo.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 52.Naruse T, Yanamoto S, Yamada S, Rokutanda S, et al. Lipomas of the oral cavity: clinicopathological and immunohistochemical study of 24 cases and review of the literature. Ind J Otolaryngol Head Neck Surg. 2015;67:67–73. doi: 10.1007/s12070-014-0765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Furlong MA, Fanburg-Smith JC, Childers EL. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:441–450. doi: 10.1016/j.tripleo.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 54.Rejas RA, Campos MS, Cortes AR, Pinto DD, et al. The neural histogenetic origin of the oral granular cell tumor: an immunohistochemical evidence. Med Oral Patol Oral Circ Bucal. 2011;16:e6–e10. doi: 10.4317/medoral.16.e6. [DOI] [PubMed] [Google Scholar]
- 55.Vered M, Carpenter WM, Buchner A. Granular cell tumor of the oral cavity: updated immunohistochemical profile. J Oral Pathol Med. 2009;38:150–159. doi: 10.1111/j.1600-0714.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 56.Ferreira JCB, Oton-Leite AF, Guidi R, Mendonca EF. Granular cell tumor mimicking a squamous cell carcinoma of the tongue: a case report. BMC Res Notes. 2017 doi: 10.1186/s13104-016-2325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van de Loo S, Thunnissen E, Postmus P, Van der Waal I. Granular cell tumor of the oral cavity; a case series including a case of metachronous occurrence in the tongue and the lung. Med Oral Patol Oral Cir Bucal. 2015;20:e30–e33. doi: 10.4317/medoral.19867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.An S, Jang J, Kim MS, Park H, et al. Granular cell tumor of the gastrointestinal tract: histologic and immunohistochemical analysis of 98 cases. Hum Pathol. 2015;46:813–819. doi: 10.1016/j.humpath.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Solomon LW, Velez I. S-100 negative granular cell tumor of the oral cavity. Head Neck Pathol. 2016;10(3):367–373. doi: 10.1007/s12105-015-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rawal YB, Dodson TB. S-100 negative granular cell tumor (so-called primitive polypoid non-neural granular cell tumor) of the oral cavity. Head Neck Pathol. 2017;11:404–412. doi: 10.1007/s12105-016-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sreeja C, Ramakrishnan K, Vijayalakshmi D, Devi M, et al. Oral pigmentation: a review. J Pharm Bioallied Sci. 2015;7:s403–s408. doi: 10.4103/0975-7406.163471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daley TD, Armstrong JE. Oral manifestations of gastrointestinal diseases. Can J Gastroenterol. 2007;21:241–244. doi: 10.1155/2007/952673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neville BW, Damm DD, Allen CM, Chi AC. Oral manifestations of systemic diseases. Oral and maxillofacial pathology. 4. St. Louis: Elsevier; 2016. pp. 761–800. [Google Scholar]
- 64.Kumar KPM, Nachiammai N, Madhushankari GS. Association of oral manifestations in ulcerative colitis: a pilot study. J Oral Maxillofac Pathol. 2018;22:199–203. doi: 10.4103/jomfp.JOMFP_223_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jajam M, Bozzolo P, Niklander S. Oral manifestations of gastrointestinal disorders. J Clin Exp Dent. 2017;9:e1242–e1248. doi: 10.4317/jced.54008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atarbashi-Moghadam S, Lotfi A, Atarbashi-Moghadam F. Pyostomatitis vegetans: a clue for diagnosis of silent Crohn’s disease. J Clin Diag Res. 2016;10:zd12–zd13. doi: 10.7860/JCDR/2016/22573.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoopler ET, Sollecito TP, Chen SY. Amyloid deposition in the oral cavity: a retrospective study and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:674–680. doi: 10.1067/moe.2003.136. [DOI] [PubMed] [Google Scholar]
- 68.Silva WPP, Wastner BF, Bohn JC, Jung JE, et al. Unusual presentation of oral amyloidosis. Contemp Clin Dent. 2015;6:s282–s284. doi: 10.4103/0976-237X.166814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angiero F, Seramondi R, Magistro S, Crippa R, et al. Amyloid deposition in the tongue: clinical and histopathological profile. Anticancer Res. 2010;30:3009–3014. [PubMed] [Google Scholar]