Short abstract
Sickle cell disease (SCD) is an inherited disease caused by the production of abnormal hemoglobin (Hb) S, whose deoxygenation-induced polymerization results in red blood cell (RBC) sickling and numerous pathophysiological consequences. SCD affects approximately 300,000 newborns worldwide each year and is associated with acute and chronic complications, including frequent painful vaso-occlusive episodes that often require hospitalization. Chronic intravascular hemolysis in SCD significantly reduces vascular nitric oxide (NO) bioavailability, consequently decreasing intracellular signaling via cyclic guanosine monophosphate (cGMP), in turn diminishing vasodilation and contributing to the inflammatory mechanisms that trigger vaso-occlusive processes. Oxidative stress may further reduce NO bioavailability in SCD and can oxidize the intracellular enzyme target of NO, soluble guanylate cyclase (sGC), rendering it inactive. Increasing intracellular cGMP-dependent signaling constitutes an important pharmacological therapeutic approach for SCD with a view to augmenting vasodilation, and reducing inflammatory mechanisms, as well as for increasing the production of anti-polymerizing fetal Hb in erythroid cells. Pharmacological agents under pre-clinical and clinical investigation for SCD include NO-based therapeutics to augment NO bioavailability, as well as heme-dependent sGC stimulators and heme-independent sGC activators that directly stimulate native and oxidized sGC, respectively, therefore bypassing the need for vascular NO delivery. Additionally, the phosphodiesterases (PDEs) that degrade intracellular cyclic nucleotides with specific cellular distributions are attractive drug targets for SCD; PDE9 is highly expressed in hematopoietic cells, making the use of PDE9 inhibitors, originally developed for use in neurological diseases, a potential approach that could rapidly amplify intracellular cGMP concentrations in a relatively tissue-specific manner.
Impact statement
Sickle cell disease (SCD) is one of the most common inherited diseases and is associated with a reduced life expectancy and acute and chronic complications, including frequent painful vaso-occlusive episodes that often require hospitalization. At present, treatment of SCD is limited to hematopoietic stem cell transplant, transfusion, and limited options for pharmacotherapy, based principally on hydroxyurea therapy. This review highlights the importance of intracellular cGMP-dependent signaling pathways in SCD pathophysiology; modulation of these pathways with soluble guanylate cyclase (sGC) stimulators or phosphodiesterase (PDE) inhibitors could potentially provide vasorelaxation and anti-inflammatory effects, as well as elevate levels of anti-sickling fetal hemoglobin.
Keywords: cGMP, fetal hemoglobin, hemolysis, hydroxyurea, nitric oxide, phosphodiesterases, sickle cell disease, soluble guanylate cyclase, sGC stimulators
Introduction
Sickle cell disease (SCD) constitutes a group of genetic disorders, caused primarily by a mutation in the hemoglobin subunit beta gene (HBB; c.20A>T; glutamic acid-valine; rs334), producing altered sickle hemoglobin, HbS.1 Homozygosity for this mutation results in sickle cell anemia (SCA; HbSS), while compound heterozygosity for HbS in association with other hemoglobin variants or thalassemias results in SCD, where disease phenotype demonstrates similarities to that of SCA.1,2 It has been estimated that approximately 300,000 children are born with SCD in the world every year, of which the great majority are in Africa.3
The pathophysiology of SCD is caused by the polymerization of deoxygenated HbS, which can disrupt the flexibility and architecture of the red blood cell (RBC), causing it to become sickle shaped. The rate and extent of HbS polymerization depend on the HbS concentration in the erythrocytes, pH, temperature, and local oxygen tension.4,5 Alterations in the physical properties of the RBCs of SCD individuals can trigger the premature destruction of erythrocytes, leading to chronic hemolytic anemia, and induce a number of inflammatory pathways that culminate in the hemolytic and vaso-occlusive processes that characterize the pathophysiology of the disease.1,6 SCD displays a range of severity, but in general it is associated with high morbidity and a decreased life expectancy, with a wide range of acute and chronic complications, including acute painful vaso-occlusive episodes (VOEs) that often require hospitalization, stroke, acute chest syndrome (ACS), pulmonary hypertension, autosplenectomy, retinopathy, nephropathy, and leg ulcers.7–9
Present therapeutic options for SCD include cell-based therapies (hematopoietic stem cell transplantation and transfusion) and pharmacotherapy based largely on hydroxyurea therapy. At least 40 substances are currently in various stages of pre-clinical and clinical studies for the prevention or treatment of VOE in SCD, all of which have been developed based on the complex pathophysiology of the disease.6,10 We summarize herein evidence for dysregulation of nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling in SCD and describe cGMP-modulating pharmacotherapeutics under investigation with a view to use in SCD.
Pathophysiology of SCD
Alterations in RBC physiology, caused by HbS polymerization, result in a vicious circle of constant intravascular hemolytic and vaso-occlusive processes, in association with a chronic inflammatory state.6 Sickle RBCs are less deformable and, therefore, rupture more easily in the circulation; it has been estimated that up to 10% of the total RBC blood volume may be destroyed every day in an individual with SCD and that approximately 30% of this hemolysis may occur intravascularly.1,11 Hemolysis may have a huge impact on both NO biology and inflammatory processes in the vasculature. Upon intravascular RBC lysis, large amounts of cell-free hemoglobin (Hb) are released into the circulation.12 When not compartmentalized inside the RBC, Hb is extremely reactive and can rapidly release heme. Free heme has potent inflammatory effects, and is able to activate toll-like-receptor-(TLR) mediated cell signaling,13 induces macrophage inflammasome formation,14 activates complement, and stimulates neutrophil extracellular trap release, amongst other effects.15,16 In mice with SCD, heme infusion has been shown to induce TLR4-mediated endothelial activation and microvascular stasis, and trigger ACS.17 The proinflammatory effects of heme released during hemolysis, in in vivo situations, have though been questioned, since highly hydrophobic heme is rapidly bound to and neutralized by macromolecules, such as hemopexin or albumin, or lipids18; however, hemopexin levels are significantly depleted in SCD19,20 and it is possible that heme may modulate inflammation in more complex models of sequential priming and activation processes.18
Vaso-occlusive processes are a defining characteristic of SCD, where they occur principally in the microcirculation, reducing tissue oxygenation and inducing tissue damage. Inflammatory cell activation leads to the production and secretion of molecules such as cytokines and chemokines, growth factors, eicosanoids, and peptides that propagate the inflammatory state and further activate other cells in the vasculature. Numerous cytokines, produced from multiple cell types, are elevated in steady-state SCD, including the leukocyte-derived cytokine, tumor necrosis factor (TNF)-α, which may be generated as an early consequence of ischemia-reperfusion21 and has potent effects on both leukocytes and endothelial cells. Vaso-occlusion is a multicellular process that occurs as a consequence of these altered inflammatory and molecular pathways; in vitro studies and studies of the microvasculature of SCD mice models indicate that vaso-occlusion is initiated by the adhesion of activated leukocytes,22,23 RBCs,24,25 and platelets to the endothelium26 in a mechanism that is propagated by inflammatory processes. In turn, diminished blood flow may decrease local oxygenation and trigger RBC sickling, resulting in occlusion of the blood vessel. Decreased vascular NO bioavailability, as well as oxidative stress, endothelial dysfunction, and the expression of adhesive molecules on blood cells and on the endothelium1,6 all drive the vaso-occlusive process, and therefore represent potential therapeutic targets in SCD.
Evidence for the downregulation of NO-cGMP-dependent signaling in SCD pathophysiology
NO is a free radical signaling gas produced by the NO synthases (NOS) during the conversion of arginine to citrulline.27 Endothelial-derived NO, produced by endothelial NOS (eNOS), plays a pivotal role in vascular homeostasis,28 diffusing across the endothelial cell membrane into the adjacent smooth muscle and binding to the ferrous (Fe2+) heme of soluble guanylate cyclase (sGC). Activated sGC converts guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP), a second messenger that activates cGMP-dependent protein kinases (PKG) to promote a cascade of effects, resulting in calcium removal from smooth muscle cells, in turn relaxing blood vessels and increasing blood flow via vasodilation.29,30 In addition to promoting vasodilation, NO also maintains vascular homeostasis by inhibiting platelet activity, adhesion and aggregation31–34 and decreasing leukocyte adhesion and function34–37 in a cGMP-dependent fashion. NO also inhibits endothelial adhesion activation and adhesion molecule expression.38,39
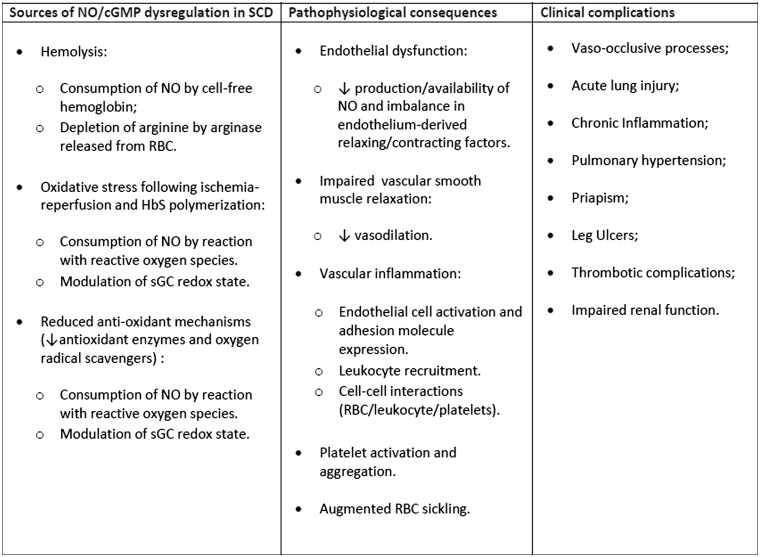
In SCD, and other hemolytic diseases, the bioavailability of NO is compromised (Figure 1), largely as a consequence of its reaction with cell-free Hb. Upon intravascular hemolysis, cell-free extracellular oxyHb reacts with NO, rapidly and irreversibly forming methemoglobin and nitrate.12,40 In addition, arginase, also released from the RBC during hemolysis, depletes arginine, reducing substrate availability for NO synthesis by eNOS.41 NO can also be consumed by reactions with reactive oxygen species (ROS), such as the superoxide radical, which are generated as a result of ischemia and reperfusion processes, and HbS autooxidation amongst other mechanisms.8,42,43 Furthermore, anti-oxidant defense mechanisms are impaired in SCD, due to reduced antioxidant enzyme and oxygen radical scavenger levels, also contributing to the consumption of NO by ROS.44
Figure 1.
Box figure of mechanisms leading to dysregulated NO-cGMP-dependent signaling and associated clinical complications in SCD. NO: nitric oxide: RBC; red blood cell: sGC; soluble guanylate cyclase.
Evidence for altered NO biology, and therefore altered cGMP-dependent signaling, in SCD pathophysiology has existed for some time (Figure 1); modulations in plasma NO metabolite levels were reported, in 1995, in SCD patients during acute VOEs,45 while inhibition of NO synthesis in rats infused with sickle RBC induced their adhesion to the cerebral microvasculature, resulting in vaso-occlusion.46 Lower levels of NO metabolites were later shown to correlate with higher pain scores in SCD patients hospitalized for acute VOEs.47 However, the primary role of hemolysis in reduced NO bioavailability in SCD and the potential consequences of this effect was first highlighted by Reiter et al. in 2002,12 who showed that NO-dependent increases in forearm blood flow were inhibited proportionately to cell-free Hb concentrations in SCD individuals, indicating that the decompartmentalization of Hb into plasma diverts NO from homeostatic vascular function in these patients. The induction of acute intravascular hemolysis in an animal model was later shown to produce dose-dependent systemic vasoconstriction and impair renal function, secondary to the stoichiometric oxidation of endogenous NO by cell-free Hb.48 The vasoconstriction caused by this acute hemolytic process could be attenuated by the inhalation of NO gas in this experimental model.48
Consumption of NO by cell-free Hb and, therefore, dysregulation of cGMP-dependent signaling could have further vascular consequences. There is evidence to suggest that NO can inhibit HbS polymer formation in RBC by abolishing the excess positive charge of HbS and increasing its oxygen affinity; therefore, reduced NO could augment RBC sickling.49 Exposure of mice with SCD to hypoxia reduces cGMP in the lungs of animals, in association with elevated xanthine oxidase (an enzyme that generates ROS), impaired eNOS function, and acute lung injury.50 Interestingly, RBCs possess NOS and inhibition of this NOS activity by oxidative stress may diminish RBC deformability.51
There is also substantial evidence that reduced NO bioavailability may augment cell–cell interactions and vascular inflammatory processes in SCD. NO inhibits sickle RBC adhesion to the endothelium,52 and hypoxia and low NO bioavailability may synergistically augment sickle RBC adhesion to endothelium via upregulation of endothelial P-selectin.53 Leukocytes present augmented adhesive properties in SCD and both pharmacological NO donation and sGC stimulation are reported to inhibit the adhesion of SCD neutrophils to ligands found on the vascular wall.54 Furthermore, the induction of acute hemolytic processes in mice, to yield similar levels of plasma cell-free Hb/heme to those seen in mice with SCD, results in substantial systemic and vascular inflammation, leading to cellular recruitment mechanisms resembling those observed in the SCD mouse microcirculation.55 This inflammation was found to be associated with the modulation of plasma NO metabolites and could be inhibited by the co-administration of NO donor agents in an apparently sGC-dependent manner.55
Thus, reduced NO in SCD can culminate in an imbalance between vasodilation and vasoconstriction, leading to endothelial dysfunction, which in the case of SCD may contribute to vaso-occlusive processes and ultimately acute and chronic complications, such as pulmonary hypertension.9 Furthermore, in addition to facilitating vasodilation, NO-cGMP signaling pathways are important for preventing leukocyte recruitment and the adhesive interactions of both platelets and endothelial cells in blood vessels (Figure 1);40 therefore, it would seem reasonable to conclude that modulation of levels of NO and/or its intracellular second messenger, cGMP, could represent an effective approach to reducing vaso-occlusive processes in SCD (Figure 2).
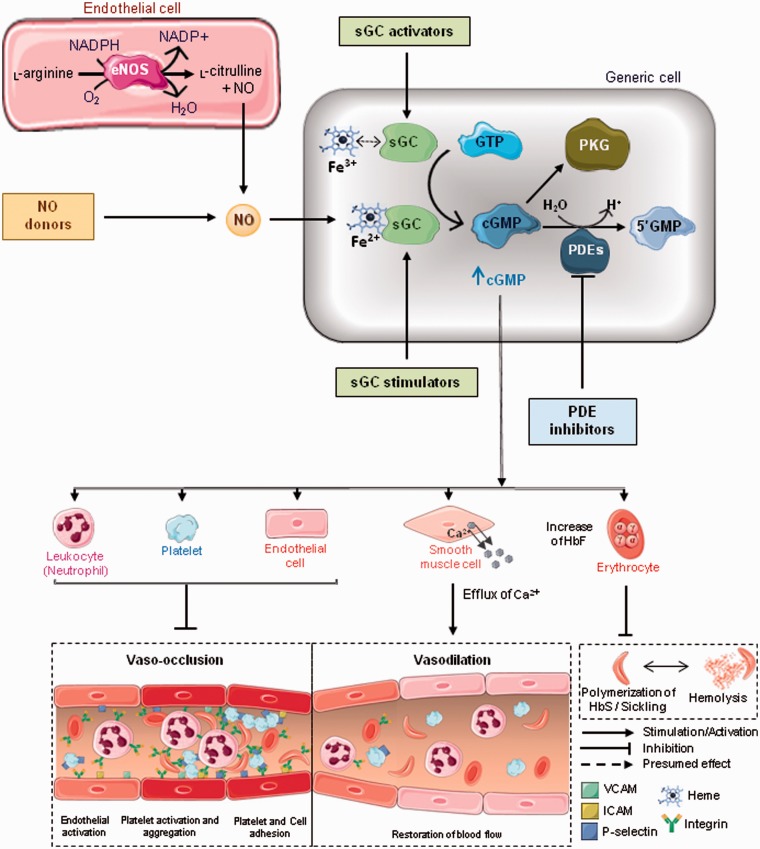
Figure 2.
Role of cGMP-dependent signaling and potential for cGMP-modulation therapies in SCD. Nitric oxide (NO) is generated from the conversion of L-arginine to L-citrulline by nitric oxide synthases (NOS), particularly endothelial NOS (eNOS). Once diffused across the endothelium, NO binds to the heme moiety of soluble guanylate cyclase (sGC) in smooth muscle cells and other cell types, promoting the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), which in turn activates cGMP-dependent protein kinases (PKG). The cascade of reactions triggered by this pathway plays a pivotal role in vascular homeostasis. Calcium removal from smooth muscle cells promotes relaxation of blood vessels and increases the blood flow. Besides promoting vasodilation, the cGMP-signaling pathway inhibits platelet activation and aggregation, endothelial activation, and adhesion molecule expression, decreasing adhesive interactions between platelets, erythrocytes, leukocytes and the endothelium. Additionally, sGC activation upregulates the expression of the gene encoding γ-globin (HBG) in erythroid cells, increasing fetal hemoglobin (HbF) levels in the erythrocytes and preventing HbS polymerization and hemolysis in sickle cell diseases (SCD). Since NO bioavailability is decreased in SCD due to hemolysis, NO inhalation, nitrite administration, or hydroxyurea are therapies that directly restore intravascular NO levels in varying efficacies, while supplementation with L-arginine provides the substrate for NO synthesis. sGC stimulators (heme-dependent) and activators (heme-independent) are a class of drugs that modulate sGC activity independently of NO. sGC activators increase the activity of sGC only in its oxidized Fe3+heme or heme-free (apo) state and are particularly efficient for activating sGC under oxidative stress conditions. Additionally, the inhibition of phosphodiesterases (PDEs), a group of enzymes that regulate cGMP by catalyzing its hydrolysis and degradation, has been investigated as therapy for SCD. Sildenafil is a selective inhibitor of PDE5, expressed in smooth muscle cells, platelets and corpus cavernosum, whereas IMR-687, BAY 73–6691 and PF04447943 inhibit PDE9, an isoform highly expressed in hematopoietic cells. HbS: hemoglobin S; VCAM: vascular cell adhesion molecule-1; ICAM: intercellular adhesion molecule-1. (A color version of this figure is available in the online journal.)
Role of cGMP-dependent signaling in fetal hemoglobin modulation
High levels of fetal hemoglobin (HbF) in the RBC are known to ameliorate the pathophysiology of SCD. HbF, formed by the combination of two α-globin and two γ-globin proteins, is normally synthesized during fetal life, where switching to the production of adult Hb, HbA (α2β2), or HbS (α2βS2) in the case of SCD individuals, occurs during the first year of life.56,57 The levels of HbF are modulated genetically in SCD and those individuals with high levels of HbF generally display milder disease57 as HbF cannot form part of the HbS polymer and therefore reduces Hb polymerization and red cell sickling. HbF switching to adult Hb is regulated by a complex mechanism of gene regulation, in which the zinc-finger transcriptional factor, BCL11A plays an important role in the repression of fetal Hb production.58
However, despite the complex transcriptional regulation of γ-globin gene expression, evidence exists that cGMP-dependent signaling in erythroid cells can modulate this gene’s expression.59,60 sGC activators or cGMP analogs were found to increase the expression of the γ-globin gene (HBG) in both erythroleukemic cells and primary erythroblasts from healthy subjects and patients with beta-thalassemia.59 Moreover, HbF induction by hemin and butyrate could be abolished by inhibiting sGC or PKG, suggesting that the sGC-PKG pathway can regulate HBG expression.59 sGC activity can be identified in RBCs and the enzyme apparently remains responsive to NO and sGC stimulation even in patients with endothelial dysfunction.61,62 cGMP levels have been reported as significantly higher in RBC of patients with SCA than healthy individuals, and RBC cGMP levels correlated with fetal Hb (HbF) levels in SCA, but not with reticulocyte count, indicating that augmentation of cGMP levels by NO in erythroid cells may constitute a mechanism for induction of HbF.61 Further reports indicate that cross talk between cGMP and cyclic adenosine monophosphate (cAMP)-dependent pathways may also contribute to HbF regulation, where these signaling molecules may share a common induction pathway, with evidence that cAMP dependent signaling is associated with downregulation of BCL11A expression, indicating a mechanism by which these signaling pathways may repress β-globin gene expression.63,64
NO donor and sGC activation properties of hydroxyurea
Hydroxyurea, also known as hydroxycarbamide, is the only drug approved by both the FDA and EMA for the prevention of recurrent painful vaso-occlusive crises in pediatric and adult patients suffering from symptomatic SCD. Pharmaceutical grade L-glutamine, an amino acid with antioxidant properties, was recently approved by the FDA for use in SCD, but hydroxyurea remains the mainstay for SCD pharmacotherapy, at this time. Hydroxyurea therapy significantly elevates HbF and reduces the incidence of acute VOEs, hospital admissions, ACS, and the need for blood transfusions in patients with SCD.65–69 Hydroxyurea is generally well tolerated by patients, however, there are some concerns regarding the effects of the drug on spermatogenesis70 and a recent Cochrane Review concluded that evidence of the long-term benefits of hydroxyurea for the prevention of the chronic complications of the disease is insufficient at present.71
Hydroxyurea is a cytostatic agent that is also found endogenously at varying concentrations in the plasma and tissues of humans and animals, possibly acting as a natural defense agent against infections.72–74 This molecule has NO donor and sGC activating properties and can be metabolized and oxidized by free Hb and heme, resulting in NO production from the –NHOH portion of hydroxyurea (Figure 2).75–78 Hydroxyurea also induces NO production by enhancing eNOS phosphorylation and activity in endothelial cells79 and may react with peroxidases to rapidly form NO in the presence of hydrogen peroxide (H2O2).80,81 Additionally, evidence suggests that hydroxyurea can interact directly with the Fe2+-heme of sGC, promoting the iron nitrosylation of sGC, and consequently activating cGMP production.82
Hydroxyurea induces cGMP elevation and gamma-globin gene expression in K562 erythroleukemic cells and human erythroid progenitor cells in a mechanism that can be abolished by the inhibition of sGC.60 It has also been suggested that these cGMP-dependent molecular mechanisms may also be involved in the cytostatic effects of hydroxyurea in erythroid progenitor cells.82 Furthermore, hydroxyurea administration increases plasma NO metabolites and cGMP levels in SCA patients (in steady state and experiencing VOE), in a temporal manner.77,83 Elevated RBC cGMP in hydroxyurea-treated SCA patients has been shown to correlate with individual HbF levels,61 although cAMP production is also reportedly required for full induction of HbF by hydroxyurea in human CD34(+) erythroid cell cultures (Table 1).63
Table 1.
cGMP modulating drugs and drug candidates for SCD therapy.
| Classification of agents | Mechanism of action | Agents | Pre-clinical evidence in SCD | Clinical evidence in SCD | Limitations |
|---|---|---|---|---|---|
|
Nitric oxide donors |
Improvement in NO bioavailability. |
NO inhalation |
Improvement in survival and lung injury following exposure to hypoxia and hypoxia-reperfusion in SCD animal model84,85 |
Improvement in pain scores during VOC86,87 |
No effects on Hb oxygen affinity; failure to improve outcomes of VOC compared with placebo; no efficacy for the treatment of ACS84,85,86 |
|
L-arginine supplementation |
Increase of NO metabolite levels; reduction of lipid peroxidation; increase of antioxidant levels in SCD animal model88 |
Benefits on pulmonary hypertension in SCD; amplification of NO response when co-administered with HU; reduction in pain scores and opioid use in children experiencing VOC89–91 |
Failure to demonstrate long-term clinical efficacy; alterations in redox potential of RBCs; no effects on hospital length of stay during VOC91,92 |
||
|
Hydroxyurea |
Evidence for cGMP-dependent effects:Anti-inflammatory effects via an NO-sGC dependent mechanism in SCD animal model.55 Induction of γ-globin expression in human erythroid progenitor cells via a sGC-dependent pathway60 |
Evidence for cGMP-dependent effects: Association of RBC HbF with intracellular [cGMP], indicating a role for sGC-mediated elevation of HbF; increase of plasma NO metabolites and cGMP levels in patients on HU61,77,83 |
Cytotoxic effects and long-term benefits for chronic organ damage still unclear70,71 |
||
|
Nitrite |
Improvement in SCD RBC deformability in vitro; reductions in RBC, leukocyte and platelet adhesion; reduction in hemolysis rate in vitro and in SCD mice93 |
Increase in regional blood flow in patients with SCD in steady state94 |
Reduced vasodilatory sensitivity in SCD individuals, compared with those without SCD94 |
||
| sGC stimulators | Elevation of cGMP by sGC in a heme-dependent manner. |
BAY 41-2272 |
Reduction of SCD neutrophil adhesion in vitro; improvement of cavernosal relaxation in SCD animal model95,96 |
No clinical studies for SCD. |
Unsuitable for clinical use: Low metabolic stability; low oral bioavailability97 |
|
Riociguat (BAY 63-2521) |
No pre-clinical studies for SCD. |
Safe and well tolerated, in SCD patients with chronic thromboembolic pulmonary hypertension: Significant improvements in exercise capacity, NT-proBNP, and RVSP in some of the treated patients98 |
Short half-life in humans and requires thrice-daily dosing;99 potential hypotensive effects should be monitored in SCD. |
||
|
Vericiguat (BAY 102-1189) |
No pre-clinical studies for SCD. |
No clinical studies for SCD. |
Not applicable. |
||
|
Olinciguat (IW-1701) |
Induces in vitro expression of γ-globin gene in erythroleukemic. Cells decreases leukocyte recruitment in C57BL/6 mice following an inflammatory stimulus100,101 |
Phase 2 double-blind, placebo-controlled multi-site trial for use in SCD currently undergoing (NCT03285178). Results not yet available. |
Not yet reported. |
||
|
sGC activators |
Production of cGMP by increasing oxidized sGC activity in a heme-independent manner. |
BAY 54-6544 |
Decreases cardiac remodeling and increases vaso-relaxation in SCD animal model102 |
No clinical studies for SCD. |
Not applicable. |
|
BAY 60-2770 |
Decreases adhesive properties of human SCD neutrophils in vitro; decreases leukocyte recruitment and vaso-occlusive processes in SCD animal model103 |
No clinical studies for SCD. |
Not applicable. |
||
| Phosphodiesterase inhibitors | Prevention of intracellular cGMP degradation. |
Sildenafil |
Beneficial effects on priapism in SCD animal models104 |
Prevention and resolution of recurrent ischemic episodes of priapism in SCD105,106A clinical trial (NCT00492531) to evaluate the effects of sildenafil in subjects with SCD with high TRV was terminated early due to increased hospitalizations for pain in patients on sildenafil; no favorable effects on the evaluated parameters were observed107 |
Risk of augmented pain processing107 |
|
BAY 73-6691 |
Decrease of in vitro adhesive properties of SCD neutrophils. Inhibition of leukocyte recruitment, vaso-occlusive processes and survival in SCD animal model95,108,109 |
No clinical studies in humans. |
Not applicable. |
||
|
|
Reduction of leukocyte-platelet aggregates and endothelial activation in SCD animal model110 |
A phase 1 clinical trial (NCT02114203) has been conducted to assess the safety, tolerability, pharmacokinetics and pharmacodynamics in subjects with SCD. Soluble E-selectin and heterocellular aggregates reportedly decreased in patients on PF-04447943.111 Apparent overall safety observed, although adverse events were observed in 3 out of 22 patients on PF-04447943 (vaso-occlusive crisis, biliary colic and pneumonia). |
Not yet reported. |
||
| IMR-687 | Inhibition of microvascular stasis following hypoxia; increase in RBC HbF content; decrease in RBC sickling and leukocytosis in SCD animal model112 | A randomized, placebo-controlled, multicenter study phase 2 trial (NCT03401112) to determine the safety, pharmacokinetics, and preliminary pharmacodynamics of escalating doses of IMR-687 in patients with SCD is underway. Results not yet available. | Not yet reported. |
HU; hydroxyurea: NO; nitric oxide; NT-proBNP; N-terminal pro-brain natriuretic peptide: RBC; red blood cell: RVSP; right ventricular systolic pressure: SCD; sickle cell disease: TRV; ricuspid regurgitant velocity.
Hydroxyurea also exerts acute and immediate anti-inflammatory effects via an NO-sGC dependent mechanism in animal models.55 The induction of acute inflammatory responses to hemolysis can be abolished by a single administration of hydroxyurea in a reaction that is dependent upon NO generation and sGC activity.55 As such, in addition to evidence that hydroxyurea may mediate its HbF-elevating effects via an NO-sGC dependent mechanism, data provide perspectives for the use of hydroxyurea as an acute treatment for SCD and other hemolytic diseases by mechanisms that are independent of HbF induction, and probably occur via generation of intravascular NO and sGC activation.
NO-based therapies in SCD
Having defined a critical role for reduced NO bioavailability in SCD, therapeutic strategies that improve NO bioavailability have been extensively studied (Table 1),40 with a view to enhancing smooth muscle relaxation, vasodilation, and increasing regional blood flow. Use of inhaled NO was first hailed as a prospective therapy for SCD, with potential for decreasing arterial NO consumption by oxidizing and nitrosylating cell-free Hb, and therefore restoring endogenous NO availability.12 In a mouse model of SCD, NO inhalation ameliorated survival and lung injury following exposure to hypoxia and hypoxia/reperfusion, respectively.84,85 Inhaled NO gas demonstrated safety and some therapeutic effects in small studies of SCD patients suffering from acute vaso-occlusive crisis (VOC), with improvements seen in pain scores.86,87 However, no effects of NO inhalation on the oxygen affinity of Hb have been found113 and, in a larger placebo-controlled study in 150 SCD patients hospitalized with VOC, the use of inhaled NO, compared with placebo, did not improve time to crisis resolution, length of hospitalization, visual analog pain scale scores, cumulative opioid usage, or the rate of ACS.114 While increased nitrate levels were observed following NO exposure in this study and indicated systemic effects of NO, levels of blood nitrite, which may exert important benefits at ischemic sites, were not significantly increased.114 Furthermore, when used for the treatment of ACS in SCD, inhaled NO did not ameliorate treatment failure rate.115
Low levels of L-arginine, the substrate for NO synthesis, are observed in SCD patients, particularly during VOC, which could reflect a state of depletion that results in decreased NO production.47,116 Supplementation with oral L-arginine has, therefore, been investigated for efficacy in SCD, as reduced L-Arginine levels appear to be rate-limiting for NO production during VOC.117 In mouse models of SCD, dietary arginine supplementation significantly increased NO metabolite levels, reduced lipid peroxidation and elevated antioxidant levels,88 supporting the rationale for use of L-arginine in human SCD. L-Argine therapy has shown benefits for pulmonary hypertension in SCD, with reduced estimated pulmonary artery systolic pressure reported after five days of therapy in 10 SCD patients with pulmonary hypertension.89 While L-arginine administration appears not to significantly augment NO metabolite production in SCD patients at steady state, data suggest that co-administration of L-arginine with hydroxyurea may amplify the NO response in SCD at steady state.90 Use of L-arginine for 12 weeks in a small number of SCD patients in steady-state on hydroxyurea, however, failed to demonstrate clinically detectable efficacy and actually induced alterations in redox potential in red cells,92 with data indicating that the L-arginine administered may be diverted to ornithine production due to the elevated levels of plasma L-arginase. L-arginine may, though, display greater benefits in SCD patients experiencing acute vaso-occlusive pain; in a double-blinded placebo-controlled trial, 38 children with SCD that were hospitalized for pain were randomized to receive L-arginine or placebo for five days or until discharge. L-arginine was found to be safe to use and, importantly, a very significant reduction in total parenteral opioid use and lower pain scores at discharge were observed in those that received L-arginine, compared to placebo, although there was no significant difference in the hospital length of stay.91
Given the disappointing lack of efficacy of the use of inhaled NO in SCD, use of nitrite as therapy has been suggested. The nitrite anion (NO2−) is a potent and fast vasodilator at near-physiological concentrations and acts as an endocrine reservoir of NO; furthermore, it does not induce tolerance, as observed with the organic nitrates.118 Importantly, nitrite can be reduced to NO by deoxygenated Hb,119,120 signifying that it can augment vasodilation under hypoxic conditions.121 Due to the constant processes of ischemia/reperfusion that occur in the vasculature2 and the fact that nitrites can be bioactivated in the presence of RBCs,122 these agents make very attractive candidates for use in SCD, although the rate of nitrite reduction by HbS in polymer form may be decreased, when compared to HbA and non-polymerized HbS.123
In vitro studies and in vivo studies with transgenic SCD mice have shown that nitrite may also improve RBC deformability as well as reduce RBC, leukocyte, and platelet adhesion, in addition to reducing hemolysis.93 When administered to a small number of individuals with SCD and in steady state, sodium nitrite infusions augmented plasma nitrite concentrations and augmented forearm blood flow without causing hypotension or clinically significant methemoglobinemia.94
Potential for sGC agonist therapy in SCD
While decreased NO signaling may play a major role in SCD pathophysiology, restoration of NO-dependent signaling more directly with NO inhalation and L-arginine supplementation in patients has shown limited efficacy, perhaps due to the fact that the mechanisms that limit NO production and bioavailability are sustained even during the therapy. Furthermore, the effects of NO donors such as nitrates in cardiovascular disease, for example, are limited by increased oxidative stress and tolerance.124 As such, approaches that aim to directly stimulate the molecular target of NO could provide a more efficient approach to increasing the downstream effects of NO signaling in SCD. The NO receptor, sGC, is the target of two novel classes of drugs that enhance cGMP production and signaling. These compounds, denominated as the sGC stimulators and sGC activators, boost the enzymatic activity of sGC to generate cGMP, independently of the presence of NO (Figure 2 and Table 1).125
sGC stimulators
The sGC stimulators (NO-independent heme-dependent sGC stimulators) were the first drugs to be developed to specifically modulate sGC activity; this class of drugs acts on the native conformation of the sGC enzyme in which the heme moiety is maintained. The sGC stimulators have a dual mode of action, as they synergize with endogenously available NO, and are also capable of directly binding and stimulating native sGC, to produce cGMP, independently of NO.125,126 YC-1 was the first heme-dependent sGC stimulator to be characterized and was found to inhibit platelet aggregation independently of the presence of NO by stimulation of cGMP synthesis127,128; however, while this compound presents low substrate specificity and a poor pharmacokinetic profile, it paved the way for the development of more potent and specific sGC modulators.125 Given the antiproliferative, antifibrotic, antiinflammatory, proapoptotic, and neuroprotective effects of cGMP, sGC stimulators potentially have a wide range of beneficial effects.97
With regard to effects specifically in SCD, the potent sGC stimulator, BAY 41–2272, has been shown to reduce the increased adhesive properties of neutrophils from SCD patients,95 while in mice with SCD, this compound improves cavernosal relaxation, indicating potential for this class of drugs for preventing leukocyte recruitment (and therefore vaso-occlusive processes) and priapism in SCD.96 BAY 41–2272, however, has low metabolic stability and oral bioavailability, making it unsuitable for clinical use.97 Riociguat (BAY 63–2521), a compound with enhanced pharmacokinetics and oral bioavailability, was the first sGC stimulator licensed for clinical use and was approved in 2013 for the treatment of pulmonary hypertension.129 Use of riociguat in a small case series of SCD patients with chronic thromboembolic pulmonary hypertension was recently reported; riociguat therapy was found to be safe overall and well tolerated, and showed clinical efficacy in some of the patients evaluated.98 Riociguat, however, has a short-half life in humans and requires thrice-daily dosing.99 Other sGC stimulators in clinical development, include vericiguat (BAY 102–1189), which has a longer half-life than riociguat and is in development for the treatment of chronic heart failure130 and olinciguat (IW-1701), which is currently in clinical development specifically for use in SCD, and also achalasia. Olinciguat is an orally available sGC stimulator, with potential anti-inflammatory and vasodilating properties that has been shown to elevate γ-globin mRNA expression in erythroleukemic K562 cells.100 In an in vivo study in C57BL/6 mice, prophylactic treatment with olinciguat decreased TNFα-stimulated adhesive interactions between leukocytes and endothelial cells in mice, and this effect was potentiated when olinciguat was combined with hydroxyurea.101 In a Phase 1b placebo-controlled, randomized, multiple-ascending-dose study in healthy subjects, olinciguat given once-daily for up to 14 consecutive days was well tolerated and no serious adverse events were observed. Olinciguat displayed rapid absorption, an adequate half-life for once-daily dosing and achieved plasma cGMP elevation.131 Olinciguat recently received orphan-drug status from the FDA as a potential treatment for SCD132 and a phase 2 double-blind, placebo-controlled multi-site trial (NCT03285178; STRONG-SCD) with a planned enrolment of 88 SCD patients is underway to evaluate the safety and tolerability, pharmacokinetics, and pharmacodynamics of three dose levels of olinciguat in steady-state individuals with SCD (aged 16–70 years), when administered for 12 weeks. Study completion is estimated for the first half of 2019. Crucially, based on their pharmacological mode of action, heme-dependent sGC stimulators may hold potential for use in combination therapy to amplify the NO-donating effects of hydroxyurea.
sGC activators
In contrast to the sGC stimulators, the sGC activators (NO-independent heme-independent sGC activators) increase the activity of sGC only in its oxidized Fe3+ or heme-free inactive (apo-) state.133,134 sGC activators, therefore, trigger sGC activity independently of NO, but their efficacy appears to be additive to the effects of endogenous NO.124,135 Since the sGC enzyme must be oxidized for these agents to have effect, they are particularly valuable for use in conditions of oxidative stress, making them extremely attractive for investigation in SCD, a disease characterized by oxidative stress.136
Cinaciguat (BAY 58–2667) is a potent sGC activator that has been shown to promote vasodilation and attenuate pulmonary hypertension, endothelial dysfunction, platelet aggregation and thrombosis in experimental models.134,137 Cinaciguat, however, has a short half-life and despite success in preclinical studies did not show significant efficacy in clinical studies of patients with acute decompensated heart failure, and in fact was associated with hypotension in some patients.138 Another NO- and heme-independent sGC activator, ataciguat (HMR1766), improves vasomotor function and reduces platelet activation in rats with congestive heart failure,139 but data regarding its clinical efficacy in clinical trials for aortic valve stenosis (NCT02049203), and neuropathic pain (NCT00799656) have not been published.
BAY60–2770 is another sGC activator with cardioprotective effects.140 Preliminary data indicate that this sGC activator can abrogate the adhesive properties of neutrophils from SCD individuals and significantly decrease leukocyte recruitment and vaso-occlusive-like processes, in a mouse model of inflammatory SCD vaso-occlusion.103 Interestingly, BAY 60-2770 is also able to provide renal protection in a mouse model of albuminuria,141 a property that could be important for protecting against the nephropathy that can occur in individuals with SCD.142 Like cinaciguat, BAY60-2770 is a heme-mimicking protein, and can stably insert into the sGC enzyme during protein biosynthesis and maturation.143 Insertion of BAY60-2770 displaces heme in sGC, independently of its redox state, possibly altering the pharmacodynamic profile of the drug and may explain why sGC activators can induce hypotension when long infusion times are used.143
With regard to the potential use of sGC activators in SCD, the chronic oral administration of the sGC activator, BAY 54–6544, was found to decrease cardiac remodeling in mice with SCD more efficiently than the sGC stimulator, BAY 41–8543, without altering systemic blood pressure.102 Furthermore, the BAY 54–6544 sGC activator improved ex vivo endothelium-dependent and -independent relaxation of the pulmonary artery of SCD mice, while the sGC stimulator was unable to augment vasorelaxation.102
Data suggest that sGC is oxidized in the pulmonary arteries of SCD mice, hindering NO-induced responses144; sGC activation may therefore represent a potential therapy that bypasses the need for NO-induced responses to improve vasorelaxation in SCD and potentially provide therapy for pulmonary arterial hypertension and cardiac remodeling in these patients. While clinical findings for cinaciguat were disappointing for treating acute decompensated heart failure, the compound did exert some clinical benefits, such as a rapid and sustained reduction in pulmonary capillary wedge pressure.138 Therefore, investigations continue to identify sGC activators with improved pharmacokinetics that do not significantly alter systemic blood pressure, particularly for those pathologies in which oxidative stress is associated.
Phosphodiesterase inhibitor therapy in SCD
Phosphodiesterases (PDEs) are a class of enzymes that hydrolyze cGMP and cAMP, in turn diminishing intracellular cGMP and/or cAMP activity.145 The PDE enzymes are divided into 11 families, each containing different isoforms. As each PDE has a different and specific tissue/cellular distribution and expression, they make ideal targets for drug development due to the potential for fewer adverse effects and greater specificity; furthermore, the low intracellular concentrations of PDEs make them good substrates for competitive inhibitors and their inhibition provides a very rapid manner of effectively amplifying intracellular cGMP levels.146
PDE5 specifically hydrolyzes cGMP and is an important regulator of vascular smooth muscle contraction, especially in the penis and in the lung, where it is reportedly up-regulated in conditions of pulmonary hypertension.147,148 Sildenafil is a selective PDE5 inhibitor that improves pulmonary hemodynamics and functional capacity in pulmonary hypertension149 and preliminary investigations indicated that this compound could be beneficial in SCD pulmonary hypertension (Table 1).150 However, a clinical trial (NCT00492531) conducted to evaluate the effects of sildenafil in subjects with SCD with high tricuspid regurgitant velocity (TRV) and decreased exercise capacity had to be terminated early due to an increased rate of hospitalizations for severe pain episodes in patients on sildenafil therapy compared with placebo,107 and no favorable effects on the evaluated parameters were observed. In contrast, sildenafil has shown benefits for preventing and resolving recurrent ischemic episodes of priapism in SCD.104–106
The PDE9A (PDE9) enzyme has the highest affinity of all the PDEs for cGMP.151 In contrast to PDE5, the expression of PDE9A is largely restricted to the brain,151 although it is also very highly expressed in hematopoietic cells, with even higher expression in the neutrophils and reticulocytes of patients with SCD.108 PDE9 inhibitors have been largely developed for use in Alzheimer’s disease; however, there is evidence that PDE9 inhibition could be of benefit in SCD. BAY73-6691 is a potent and selective PDE9 inhibitor (PDE9i)152 that has been demonstrated to decrease the in vitro adhesive properties of SCD neutrophils.95,108 Moreover, BAY73-6691 was found to elevate gene expression of γ-globin in erythroleukemic cells in vitro.108 In vivo studies, in an inflammatory SCD mouse model, later found that inhibition of PDE9 with BAY73-6691 significantly inhibited leukocyte recruitment and vaso-occlusive processes in the cremaster microcirculation when given in a single intravenous administration. Furthermore, this PDE9i presented synergistic effects when administered intravenously together with hydroxyurea, reducing endothelial activation and augmenting animal survival in a cGMP-dependent manner.109 Due to its high expression in hematopoietic cells, PDE9, therefore, represents a tissue-specific target to rapidly enhance cGMP activity, amplifying the NO-mediated acute beneficial effects of hydroxyurea in the leukocytes and endothelium, in turn inhibiting SCD vaso-occlusive processes.109 Thus, the use of PDE9 inhibitors represents an extremely attractive approach in SCD, due not only to their immediate and acute anti-inflammatory effects in the vasculature and their relative hematopoietic tissue selectivity, but also their potential for elevating HbF synthesis when used chronically (Table 1).108
PF-04447943 is a selective PDE9i that was developed for use in Alzheimer’s disease, but has also been investigated in SCD. Chronic administration of PF-04447943 reduces leukocyte–platelet aggregates and markers of endothelial activation in mice with SCD110 and a phase 1 clinical trial (NCT02114203) has been conducted to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of PF-04447943 in subjects with SCD. Improvements in the inflammatory markers, soluble E-selectin and heterocellular aggregates, were reported in patients on PF-04447943111; however, although apparent safety was observed, some adverse events were also registered in patients in the PF-04447943 arms (vaso-occlusive crisis, biliary colic and pneumonia in 3 out of 22 patients). Another PDE9i, IMR-687, developed specifically for the treatment of SCD, displays a very high specificity for PDE9A, with a low brain penetration. Treatment of SCD mice with IMR-687 for 30 days inhibited microvascular stasis following hypoxia, decreased RBC sickling, elevated HbF-positive RBC, and reduced leukocytosis.112 IMR-687 was granted Rare Pediatric Disease designation by the FDA and is currently being evaluated in a randomized, placebo-controlled, multicenter study phase 2 trial (NCT03401112) to determine the safety, pharmacokinetics, and preliminary pharmacodynamics of escalating doses of IMR-687 in patients with SCD.
Potential limitation of cGMP modulation therapy in SCD
One potential disadvantage of employing cGMP modulation therapy in SCD could be the role that NO and cGMP signaling may play in pain sensing. NO and cGMP participate in inflammatory and neuropathic pain processing. NO can act as a neurotransmitter, mediating peripheral and central nociception,153 while neuronal or inducible NO synthase-derived NO and consequent cGMP-dependent neuronal signaling play a role in central sensitization, inducing pain hypersensitivity and contributing to inflammatory and neutropathic pain154 Conversely, there is some evidence that NO may also have analgesic effects, mediating the analgesic effects of opioids and other substances.153
Given that pain is a major complication of SCD, use of these therapies could be potentially problematic in this disease. Indeed, as previously mentioned, use of sildenafil in patients with SCD was unsuccessful due to an unexpectedly higher frequency of pain episodes in those patients in the sildenafil arm, compared with the placebo arm.107 However, while myalgia and back pain have been associated with chronic PDE5 inhibitor administration,107 such side effects have not yet been associated with guanylate cyclase activator/stimulator therapy for the treatment of heart failure,124 although reports of musculoskeletal disorders for riociguat, compared with sildenafil and tadalafil, are higher during their use for pulmonary hypertension.155 As such, pain evaluations will need to be carefully made when carrying out clinical trials of cGMP modulators in patients with SCD.
Conclusion
SCD is associated with a reduced life expectancy and lifetime morbidity. Hydroxyurea, the current mainstay of SCD pharmacotherapy, reduces mortality, transfusion requirement, VOE frequency and incidence of ACS via HbF elevation, and probably, via its immediate anti-inflammatory effects, all of which may be mediated by the ability of hydroxyurea to upregulate intracellular cGMP-dependent signaling. Despite the success of hydroxyurea therapy in SCD, investigations continue to identify compounds that can be used in combination with hydroxyurea, or alone, with a view to further reducing the frequency of painful VOEs and also for use following the onset of VOC and hospitalization of patients, as current therapeutic options for treating VOC are limited to pain medication and hydration. Pharmacological grade L-glutamine supplementation was recently approved by the FDA156 and biological drugs such as crizanlizumab,157 which demonstrated promising results in a recent clinical trial, show potential for use in SCD, due to their ability to reduce oxidative stress and abrogate P-selectin mediated cellular interactions, respectively. However, classes of drugs that upregulate cGMP-dependent signaling by providing NO under hypoxic conditions or by bypassing the need for NO delivery and amplifying intracellular cGMP concentrations may also constitute a major approach for use in SCD (see Table 1), in combination, or not, with hydroxyurea.
Authors’ contributions
NC and LT wrote the Review together. Both authors have read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS
NC has received research funding from Bayer AG and participates in technology registered with the INPI, Brazil, for use of a pharmaceutical composition of hydroxyurea and a PDE9 inhibitor for the control of vaso-occlusive processes.
FUNDING
The authors acknowledge grant support from FAPESP Brazil (Grant numbers 2018/08010–9, 2017/14594–0 and 2014/00984–3).
References
- 1.Steinberg MH. Overview of sickle cell anemia pathophysiology In: FF Costa, Conran N. (eds) Sickle cell anemia: from basic science to clinical practice. Switzerland: Springer International, 2016, pp.49–75 [Google Scholar]
- 2.Kato GJ, Piel FB, Reid CD.Gaston MH, Ohene-Frempong K, Krishnamurti L, Smith WR, Panepinto JA, Weatherall DJ, Costa FF, Vichinsky EP. Sickle cell disease. Nat Rev Dis Primers 2018; 4:18010. [DOI] [PubMed] [Google Scholar]
- 3.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med 2017; 376:1561–73 [DOI] [PubMed] [Google Scholar]
- 4.Brittenham GM, Schechter AN, Noguchi CT. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood 1985; 65:183–9 [PubMed] [Google Scholar]
- 5.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010; 376:2018–31 [DOI] [PubMed] [Google Scholar]
- 6.Conran N, Belcher JD. Inflammation in sickle cell disease. Clin Hemorheol Microcirc 2018; 68:263–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballas SK, Lieff S, Benjamin LJ, Dampier CD, Heeney MM, Hoppe C, Johnson CS, Rogers ZR, Smith-Whitley K, Wang WC, Telen MJ. Definitions of the phenotypic manifestations of sickle cell disease. Am J Hematol 2010; 85:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hebbel RP. Ischemia-reperfusion injury in sickle cell anemia: relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol Oncol Clin North Am 2014; 28:181–98 [DOI] [PubMed] [Google Scholar]
- 9.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 2017; 127:750–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nottage K, Estepp J, Hankins JS. Future perspectives for the treatment of sickle cell anemia In: Costa FF, Conran N. (eds) Sickle cell anemia: from basic science to clinical practice. Switzerland: Springer International, 2016, pp.399–429 [Google Scholar]
- 11.Hebbel RP. Reconstructing sickle cell disease: a data-based analysis of the “hyperhemolysis paradigm” for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol 2011; 86:123–54 [DOI] [PubMed] [Google Scholar]
- 12.Reiter CD, Wang X, Tanus-Santos JE.Hogg N, Cannon RO, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 2002; 8:1383–9 [DOI] [PubMed] [Google Scholar]
- 13.Figueiredo RT, Fernandez PL, Mourao-Sa DS.Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graça-Souza AV, Bozza MT. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem 2007; 282:20221–9 [DOI] [PubMed] [Google Scholar]
- 14.Dutra FF, Alves LS, Rodrigues D.Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci U S A 2014; 111:E4110–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merle NS, Grunenwald A, Rajaratnam H.Gnemmi V, Frimat M, Figueres ML, Knockaert S, Bouzekri S, Charue D, Noe R, Robe-Rybkine T, Le-Hoang M, Brinkman N, Gentinetta T, Edler M, Petrillo S, Tolosano E, Miescher S, Le Jeune S, Houillier P, Chauvet S, Rabant M, Dimitrov JD, Fremeaux-Bacchi V, Blanc-Brude OP, Roumenina LT. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 2018; 3:pii:96910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, Frenette PS. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 2014; 123:3818–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh S, Adisa OA, Chappa P.Tan F, Jackson KA, Archer DR, Ofori-Acquah SF. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest 2013; 123:4809–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallelian F, Schaer CA, Deuel JW.Ingoglia G, Humar R, Buehler PW, Schaer DJ. Revisiting the putative role of heme as a trigger of inflammation. Pharmacol Res Perspect 2018; 6:e00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vendrame F, Olops L, Saad STO, Costa FF, Fertrin KY. Differences in heme and hemopexin content in lipoproteins from patients with sickle cell disease. J Clin Lipidol 2018;12:1532-8 [DOI] [PubMed] [Google Scholar]
- 20.Santiago RP, Guarda CC, Figueiredo CVB.Fiuza LM, Aleluia MM, Adanho CSA, Carvalho MOS, Pitanga TN, Zanette DL, Lyra IM, Nascimento VML, Vercellotti GM, Belcher JD, Gonçalves MS. Serum haptoglobin and hemopexin levels are depleted in pediatric sickle cell disease patients. Blood Cells Mol Dis 2018; 72:34–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solovey A, Somani A, Belcher JD.Milbauer L, Vicent L, Pawlinski R, Nath KA, Kelm RJ Jr, Mackman N, O'Sullivan MG, Gupta K, Vercellotti GM, Hebbel RP. A monocyte-TNF-endothelial activation axis in sickle transgenic mice: therapeutic benefit from TNF blockade. Am J Hematol 2017; 92:1119–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turhan A, Jenab P, Bruhns P, Ravetch JV, Coller BS, Frenette PS. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood 2004; 103:2397–400 [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood 2016; 127:801–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med 1980; 302:992–5 [DOI] [PubMed] [Google Scholar]
- 25.Hebbel RP, Yamada O, Moldow CF, Jacob HS, White JG, Eaton JW. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest 1980; 65:154–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennewitz MF, Jimenez MA, Vats R.Tutuncunoglu E, Jonassaint J, Kato GJ, Gladwin MT, Sundd PP. Lung vaso-occlusion in sickle cell disease mediated by arteriolar neutrophil-platelet microemboli. JCI Insight 2017; 2:e89761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald LJ, Murad F. Nitric oxide and cyclic GMP signaling. Proc Soc Exp Biol Med 1996; 211:1–6 [DOI] [PubMed] [Google Scholar]
- 28.Monica FZ, Bian K, Murad F. The endothelium-dependent nitric oxide-cGMP pathway. Adv Pharmacol 2016; 77:1–27 [DOI] [PubMed] [Google Scholar]
- 29.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A 1977; 74:3203–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao X, Huang Y. From nitric oxide to endothelial cytosolic Ca2+: a negative feedback control. Trends Pharmacol Sci 2003; 24:263–6 [DOI] [PubMed] [Google Scholar]
- 31.Roberts W, Riba R, Homer-Vanniasinkam S, Farndale RW, Naseem KM. Nitric oxide specifically inhibits integrin-mediated platelet adhesion and spreading on collagen. J Thromb Haemost 2008; 6:2175–85 [DOI] [PubMed] [Google Scholar]
- 32.Salvemini D, Masini E, Anggard E, Mannaioni PF, Vane J. Synthesis of a nitric oxide-like factor from L-arginine by rat serosal mast cells: stimulation of guanylate cyclase and inhibition of platelet aggregation. Biochem Biophys Res Commun 1990; 169:596–601 [DOI] [PubMed] [Google Scholar]
- 33.Marcondes S, Cardoso MH, Morganti RP.Thomazzi SM, Lilla S, Murad F, De Nucci G, Antunes E. Cyclic GMP-independent mechanisms contribute to the inhibition of platelet adhesion by nitric oxide donor: a role for alpha-actinin nitration. Proc Natl Acad Sci U S A 2006; 103:3434–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ignarro LJ. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol Toxicol 1990; 67:1–7 [DOI] [PubMed] [Google Scholar]
- 35.Niu XF, Smith CW, Kubes P. Intracellular oxidative stress induced by nitric oxide synthesis inhibition increases endothelial cell adhesion to neutrophils. Circ Res 1994; 74:1133–40 [DOI] [PubMed] [Google Scholar]
- 36.Hossain M, Qadri SM, Liu L. Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature. J Inflamm 2012; 9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conran N, Ferreira HH, Lorand-Metze I, Thomazzi SM, Antunes E, de Nucci G. Nitric oxide regulates human eosinophil adhesion mechanisms in vitro by changing integrin expression and activity on the eosinophil cell surface. Br J Pharmacol 2001; 134:632–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davenpeck KL, Gauthier TW, Lefer AM. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology 1994; 107:1050–8 [DOI] [PubMed] [Google Scholar]
- 39.De Caterina R, Libby P, Peng HB.Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 1995; 96:60–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim-Shapiro DB, Gladwin MT. Nitric oxide pathology and therapeutics in sickle cell disease. Clin Hemorheol Microcirc 2018; 68:223–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris CR, Kato GJ, Poljakovic M.Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 2005; 294:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aslan M, Ryan TM, Adler B.Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Harbele I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A 2001; 98:15215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Usmani A, Machado RF. Vascular complications of sickle cell disease. Clin Hemorheol Microcirc 2018; 68:205–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood KC, Granger DN. Sickle cell disease: role of reactive oxygen and nitrogen metabolites. Clin Exp Pharmacol Physiol 2007; 34:926–32 [DOI] [PubMed] [Google Scholar]
- 45.Rees DC Cervi P, Grimwade DO'Driscoll A, Hamilton M, Parker NE, Porter JB. The metabolites of nitric oxide in sickle-cell disease. Br J Haematol 1995; 91:834–7 [DOI] [PubMed] [Google Scholar]
- 46.French JA, 2nd, Kenny D, Scott JP.Hoffman RG, Wood JD, Hudetz AG, Hillery CA. Mechanisms of stroke in sickle cell disease: sickle erythrocytes decrease cerebral blood flow in rats after nitric oxide synthase inhibition. Blood 1997; 89:4591–9. [PubMed] [Google Scholar]
- 47.Lopez BL, Kreshak AA, Morris CR, Davis-Moon L, Ballas SK, Ma XL. L-arginine levels are diminished in adult acute vaso-occlusive sickle cell crisis in the emergency department. Br J Haematol 2003; 120:532–4 [DOI] [PubMed] [Google Scholar]
- 48.Minneci PC, Deans KJ, Zhi H.Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest 2005; 115:3409–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikuta T, Thatte HS, Tang JX.Mukerji I, Knee K, Bridges KR, Wang S, Montero-Huerta P, Joshi RM, Head CA. Nitric oxide reduces sickle hemoglobin polymerization: potential role of nitric oxide-induced charge alteration in depolymerization. Arch Biochem Biophys 2011; 510:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pritchard KA, Jr., Ou J, Ou Z.Shi Y, Franciosi JP, Signorino P, Kaul S, Ackland-Berglund C, Witte K, Holzhauer S, Mohandas N, Guice KS, Oldham KT, Hillery CA. Hypoxia-induced acute lung injury in murine models of sickle cell disease. Am J Physiol Lung Cell Mol Physiol 2004; 286:L705–14. [DOI] [PubMed] [Google Scholar]
- 51.Mozar A, Connes P, Collins B.Hardy-Dessources MD, Romana M, Lemonne N, Bloch W, Grau M. Red blood cell nitric oxide synthase modulates red blood cell deformability in sickle cell anemia. Clin Hemorheol Microcirc 2016; 64:47–53 [DOI] [PubMed] [Google Scholar]
- 52.Space SL, Lane PA, Pickett CK, Weil JV. Nitric oxide attenuates normal and sickle red blood cell adherence to pulmonary endothelium. Am J Hematol 2000; 63:200–4 [DOI] [PubMed] [Google Scholar]
- 53.Gutsaeva DR, Montero-Huerta P, Parkerson JB, Yerigenahally SD, Ikuta T, Head CA. Molecular mechanisms underlying synergistic adhesion of sickle red blood cells by hypoxia and low nitric oxide bioavailability. Blood 2014; 123:1917–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canalli AA, Franco-Penteado CF, Saad ST, Conran N, Costa FF. Increased adhesive properties of neutrophils in sickle cell disease may be reversed by pharmacological nitric oxide donation. Haematologica 2008; 93:605–9 [DOI] [PubMed] [Google Scholar]
- 55.Almeida CB, Souza LE, Leonardo FC.Costa FT, Werneck CC, Covas DT, Costa FF, Conran N. Acute hemolytic vascular inflammatory processes are prevented by nitric oxide replacement or a single dose of hydroxyurea. Blood 2015; 126:711-20 [DOI] [PubMed] [Google Scholar]
- 56.Vinjamur DS, Bauer DE, Orkin SH. Recent progress in understanding and manipulating haemoglobin switching for the haemoglobinopathies. Br J Haematol 2018; 180:630–43 [DOI] [PubMed] [Google Scholar]
- 57.Habara AH, Shaikho EM, Steinberg MH. Fetal hemoglobin in sickle cell anemia: the Arab-Indian haplotype and new therapeutic agents. Am J Hematol 2017; 92:1233–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bauer DE, Orkin SH. Hemoglobin switching's surprise: the versatile transcription factor BCL11A is a master repressor of fetal hemoglobin. Curr Opin Genet Dev 2015; 33:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikuta T, Ausenda S, Cappellini MD. Mechanism for fetal globin gene expression: role of the soluble guanylate cyclase-cGMP-dependent protein kinase pathway. Proc Natl Acad Sci U S A 2001; 98:1847–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cokic VP, Smith RD, Beleslin-Cokic BB.Njoroge JM, Miller JL, Gladwin MT, Schechter AN. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J Clin Invest 2003; 111:231–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conran N, Oresco-Santos C, Acosta HC, Fattori A, Saad ST, Costa FF. Increased soluble guanylate cyclase activity in the red blood cells of sickle cell patients. Br J Haematol 2004; 124:547–54 [DOI] [PubMed] [Google Scholar]
- 62.Cortese-Krott MM, Mergia E, Kramer CM.Lückstädt W, Yang J, Wolff G, Panknin C, Bracht T, Sitek B, Pernow J, Stasch JP, Feelisch M, Koesling D, Kelm M. Identification of a soluble guanylate cyclase in RBCs: preserved activity in patients with coronary artery disease. Redox Biol 2018; 14:328–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keefer JR, Schneidereith TA, Mays A, Purvis SH, Dover GJ, Smith KD. Role of cyclic nucleotides in fetal hemoglobin induction in cultured CD34+ cells. Exp Hematol 2006; 34:1151–61 [DOI] [PubMed] [Google Scholar]
- 64.Ikuta T, Kuroyanagi Y, Odo N, Liu S. A common signaling pathway is activated in erythroid cells expressing high levels of fetal hemoglobin: a potential role for cAMP-elevating agents in beta-globin disorders. J Blood Med 2013; 4:149–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Charache S, Barton FB, Moore RD, Terrin ML, Steinberg MH, Dover GJ, Ballas SK, McMahon RP, Castro O, Orringer EP. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The multicenter study of hydroxyurea in sickle cell anemia. Medicine 1996; 75:300–26 [DOI] [PubMed] [Google Scholar]
- 66.Charache S, Terrin ML, Moore RD. Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the multicenter study of hydroxyurea in sickle cell anemia. N Engl J Med 1995; 332:1317–22 [DOI] [PubMed] [Google Scholar]
- 67.Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, Nathan DG. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest 1984; 74:652–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinberg MH, Barton F, Castro O.Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Echman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges S, Waclawiw M, Bonds D, Terrin M. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA 2003; 289:1645–51 [DOI] [PubMed] [Google Scholar]
- 69.Ware RE, Aygun B. Advances in the use of hydroxyurea. Hematology Am Soc Hematol Educ Program 2009;1:62–9 [DOI] [PubMed] [Google Scholar]
- 70.Berthaut I, Bachir D, Kotti S.Chalas C, Stankovic K, Eustache F, Ravel C, Habibi A, Brailly-Tabard S, Lévy-Dutel L, Bleibtreu A, Simon T, Galactéros F, Lionnet F, Mandelbaum J. Adverse effect of hydroxyurea on spermatogenesis in patients with sickle cell anemia after 6 months of treatment. Blood 2017; 130:2354–6 [DOI] [PubMed] [Google Scholar]
- 71.Nevitt SJ, Jones AP, Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst Rev 2017; 4:CD002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fraser DI, Liu KT, Reid BJ.Hawkins E, Sevier A, Pyle M, Robinson JW, Ouellette PH, Ballantyne JS. Widespread natural occurrence of hydroxyurea in animals. PLoS One 2015; 10:e0142890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kettani T, Cotton F, Gulbis B, Ferster A, Kumps A. Plasma hydroxyurea determined by gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877:446–50 [DOI] [PubMed] [Google Scholar]
- 74.Singh A, Xu YJ. The cell killing mechanisms of hydroxyurea. Genes 2016; 7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim-Shapiro DB King SB Shields H Kolibash CP Gravatt WL, Ballas SK.. The reaction of deoxy-sickle cell hemoglobin with hydroxyurea. Biochim Biophys Acta 1999; 1428:381–7 [DOI] [PubMed] [Google Scholar]
- 76.Pacelli R, Taira J, Cook JA, Wink DA, Krishna MC. Hydroxyurea reacts with heme proteins to generate nitric oxide. Lancet 1996; 347:900. [DOI] [PubMed] [Google Scholar]
- 77.Gladwin MT, Shelhamer JH, Ognibene FP.Pease-Fye ME, Nichols JS, Link B, Patel DB, Jankowski MA, Pannell LK, Schechter AN, Rodgers GP. Nitric oxide donor properties of hydroxyurea in patients with sickle cell disease. Br J Haematol 2002; 116:436–44 [DOI] [PubMed] [Google Scholar]
- 78.King SB. The nitric oxide producing reactions of hydroxyurea. Curr Med Chem 2003; 10:437–52 [DOI] [PubMed] [Google Scholar]
- 79.Cokic VP, Beleslin-Cokic BB, Tomic M, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea induces the eNOS-cGMP pathway in endothelial cells. Blood 2006; 108:184–91 [DOI] [PubMed] [Google Scholar]
- 80.Huang J, Kim-Shapiro DB, King SB. Catalase-mediated nitric oxide formation from hydroxyurea. J Med Chem 2004; 47:3495–501 [DOI] [PubMed] [Google Scholar]
- 81.Huang J, Sommers EM, Kim-Shapiro DB, King SB. Horseradish peroxidase catalyzed nitric oxide formation from hydroxyurea. J Am Chem Soc 2002; 124:3473–80 [DOI] [PubMed] [Google Scholar]
- 82.Cokic VP, Andric SA, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood 2008; 111:1117–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nahavandi M, Tavakkoli F, Wyche MQ, Perlin E, Winter WP, Castro O. Nitric oxide and cyclic GMP levels in sickle cell patients receiving hydroxyurea. Br J Haematol 2002; 119:855–7 [DOI] [PubMed] [Google Scholar]
- 84.Martinez-Ruiz R, Montero-Huerta P, Hromi J, Head CA. Inhaled nitric oxide improves survival rates during hypoxia in a sickle cell (SAD) mouse model. Anesthesiology 2001; 94:1113–8 [DOI] [PubMed] [Google Scholar]
- 85.de Franceschi L, Baron A, Scarpa A.Adrie C, Janin A, Barbi S, Kister J, Rouyer-Fessard P, Corrocher R, Leboulch P, Beuzard Y. Inhaled nitric oxide protects transgenic SAD mice from sickle cell disease-specific lung injury induced by hypoxia/reoxygenation. Blood 2003; 102:1087–96 [DOI] [PubMed] [Google Scholar]
- 86.Weiner DL, Hibberd PL, Betit P, Cooper AB, Botelho CA, Brugnara C. Preliminary assessment of inhaled nitric oxide for acute vaso-occlusive crisis in pediatric patients with sickle cell disease. JAMA 2003; 289:1136–42 [DOI] [PubMed] [Google Scholar]
- 87.Head CA, Swerdlow P, McDade WA.Joshi RM, Ikuta T, Cooper ML, Eckman JR. Beneficial effects of nitric oxide breathing in adult patients with sickle cell crisis. Am J Hematol 2010; 85:800–2 [DOI] [PubMed] [Google Scholar]
- 88.Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med 2006; 41:1771–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morris CR, Morris SM, Jr., Hagar W.Van Warmerdam J, Claster S, Kepka-Lenhart D, Machado L, Kuypers FA, Vichinsky EP. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med 2003; 168:63–9. [DOI] [PubMed] [Google Scholar]
- 90.Morris CR, Vichinsky EP, van Warmerdam J.Machado L, Kepka-Lenhart D, Morris SM Jr, Kuypers FA. Hydroxyurea and arginine therapy: impact on nitric oxide production in sickle cell disease. J Pediatr Hematol Oncol 2003; 25:629–34 [DOI] [PubMed] [Google Scholar]
- 91.Morris CR, Kuypers FA, Lavrisha L.Ansari M, Sweeters N, Stewart M, Gildengorin G, Neumayr L, Vichinsky EP. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 2013; 98:1375–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Little JA, Hauser KP, Martyr SE.Harris A, Maric I, Morris CR, Suh JH, Taylor J, Castro O, Machado R, Kato G, Gladwin MT. Hematologic, biochemical, and cardiopulmonary effects of L-arginine supplementation or phosphodiesterase 5 inhibition in patients with sickle cell disease who are on hydroxyurea therapy. Eur J Haematol 2009; 82:315–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wajih N, Basu S, Jailwala A.Kim HW, Ostrowski D, Perlegas A, Bolden CA, Buechler NL, Gladwin MT, Caudell DL, Rahbar E, Alexander-Miller MA, Vachharajani V, Kim-Shapiro DB. Potential therapeutic action of nitrite in sickle cell disease. Redox Biol 2017; 12:1026–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mack AK, McGowan Ii VR, Tremonti CK.Ackah D, Barnett C, Machado RF, Gladwin MT, Kato GJ. Sodium nitrite promotes regional blood flow in patients with sickle cell disease: a phase I/II study. Br J Haematol 2008; 142:971–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miguel LI, Almeida CB, Traina F.Canalli AA, Dominical VM, Saad ST, Costa FF, Conran N. Inhibition of phosphodiesterase 9A reduces cytokine-stimulated in vitro adhesion of neutrophils from sickle cell anemia individuals. Inflam Res 2011; 60:633–42 [DOI] [PubMed] [Google Scholar]
- 96.Claudino MA, Franco-Penteado CF, Corat MA.Gimenes AP, Passos LA, Antunes E, Costa FF. Increased cavernosal relaxations in sickle cell mice priapism are associated with alterations in the NO-cGMP signaling pathway. J Sex Med 2009; 6:2187–96 [DOI] [PubMed] [Google Scholar]
- 97.Sandner P. From molecules to patients: exploring the therapeutic role of soluble guanylate cyclase stimulators. Biol Chem 2018; 399:679–90 [DOI] [PubMed] [Google Scholar]
- 98.Weir NA, Conrey A, Lewis D, Mehari A. Riociguat use in sickle cell related chronic thromboembolic pulmonary hypertension: a case series. Pulm Circ 2018; 8:1–7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hill NS, Rahaghi FF, Sood N, Frey R, Ghofrani HA. Individual dose adjustment of riociguat in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respir Med 2017; 129:124–9 [DOI] [PubMed] [Google Scholar]
- 100.Miyashiro J, Pant P, Tchernychev B.Milne TG, Currie MG, Graul RM, Masferrer J. The effect of the soluble guanylyl cyclase stimulator olinciguat on ƴ-globin gene induction in K562 cells. Blood 2018; 132:1078 [Google Scholar]
- 101.Tchernychev B, Feil S, Germano P.Warren W, Lonie E, Feil R, Milne TG, Hadcock J, Chien Y, Currie MG, Graul RM. The clinical-stage sgc stimulator IW-1701 prevents increase of plasma biomarkers of intravascular inflammation and suppresses leukocyte-endothelial interactions in tnfα-treated mice. Blood 2017; 130:447 [Google Scholar]
- 102.Potoka KP, Wood KC, Baust JJ.Bueno M, Hahn SA, Vanderpool RR, Bachman T, Mallampalli GM, Osei-Hwedieh DO, Schrott V, Sun B, Bullock GC, Becker-Pelster EM, Wittwer M, Stampfuss J, Mathar I, Stasch JP, Truebel H, Sandner P, Mora AL, Straub AC, Gladwin MT. Nitric oxide-independent soluble guanylate cyclase activation improves vascular function and cardiac remodeling in sickle cell disease. Am J Respir Cell Mol Biol 2018; 58:636–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferreira WA, Jr, Chweih H, Brito PL.Almeida CB, Penteado CF, Saad STO, Costa FF, Frenette PS, Brockschnieder D, Stasch JP, Sander P, Conran N. The soluble guanylyl cyclase activator, BAY60-2770, abrogates leukocyte adhesion and recruitment in sickle cell disease: in vitro and in vivo studies. BMC Pharmacol Toxicol 2017; 64:A70 [Google Scholar]
- 104.Bivalacqua TJ, Musicki B, Hsu LL, Berkowitz DE, Champion HC, Burnett AL. Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. PLoS One 2013; 8:e68028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bialecki ES, Bridges KR. Sildenafil relieves priapism in patients with sickle cell disease. Am J Med 2002; 113:252. [DOI] [PubMed] [Google Scholar]
- 106.Burnett AL, Anele UA, Trueheart IN, Strouse JJ, Casella JF. Randomized controlled trial of sildenafil for preventing recurrent ischemic priapism in sickle cell disease. Am J Med 2014; 127:664–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Machado RF, Barst RJ, Yovetich NA.Hassell KL, Kato GJ, Gordeuk VR, Gibbs JD, Little JA, Schraufnagel DE, Krishnamurti L, Girgis RE, Morris CR, Rosenzweig EB, Badesch DB, Lanzkron S, Onyekwere O, Castro OL, Sachdev V, Waclawiw MA, Woolson R, Goldsmith JC, Gladwin MT, walk-PHaSST Investigators and Patients. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood 2011; 118:855–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Almeida CB, Traina F, Lanaro C.Canalli AA, Saad ST, Costa FF, Conran N. High expression of the cGMP-specific phosphodiesterase, PDE9A, in sickle cell disease (SCD) and the effects of its inhibition in erythroid cells and SCD neutrophils. Br J Haematol 2008; 142:836–44 [DOI] [PubMed] [Google Scholar]
- 109.Almeida CB, Scheiermann C, Jang JE.Prophete C, Costa FF, Conran N, Frenette PS. Hydroxyurea and a cGMP-amplifying agent have immediate benefits on acute vaso-occlusive events in sickle cell disease mice. Blood 2012; 120:2879–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jasuja R, Parks E, Murphy JE, Pittman DD. Chronic administration of the PDE9 inhibitor PF-04447943 reduces leukocyte-platelet aggregates and markers of endothelial activation in a mouse model of sickle cell disease. Blood 2016; 128:1293 [Google Scholar]
- 111.Charnigo RJ, Howard J, Beidler D.Rybin D, Tan B, Michelson AD, Frelinger III AL, Clarke N. A phase 1b, randomized, double-blind, placebo-controlled study of PF-04447943 in patients with stable sickle cell disease: changes in exploratory biomarkers. Blood 2017; 130:97428637662 [Google Scholar]
- 112.McArthur JG, Maciel T, Chen C. A novel, highly potent and selective pde9 inhibitor for the treatment of sickle cell disease. Blood 2016; 128:268 [Mismatch] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gladwin MT, Schechter AN, Shelhamer JH.Pannell LK, Conway DA, Hrinczenko BW, Nichols JS, Pease-Fye ME, Noguchi CT, Rodgers GP, Ognibene FP. Inhaled nitric oxide augments nitric oxide transport on sickle cell hemoglobin without affecting oxygen affinity. J Clin Invest 1999; 104:937–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gladwin MT, Kato GJ, Weiner D.Onyekwere OC, Dampier C, Hsu L, Hagar RW, Howard T, Nuss R, Okam MM, Tremonti CK, Berman B, Villella A, Krishnamurti L, Laznkron S, Castro O, Gordeuk VR, Coles WA, Peters-Larwrence M, Nichols J, Hall MK, Hildesheim M, Blackwelder WC, Baldassare J, Casella JF. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA 2011; 305:893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maitre B, Djibre M, Katsahian S.Habibi A, Stankovic Stojanovic K, Khelaff M, Bourgeon I, Lionnet F, Charles-Nelson A, Brochard L, Lemaire F, Galacteros F, Brun-Buisson C, Fartoukh M, Mekontso Dessap A. Inhaled nitric oxide for acute chest syndrome in adult sickle cell patients: a randomized controlled study. Intensive Care Med 2015; 41:2121–9 [DOI] [PubMed] [Google Scholar]
- 116.Morris CR, Kuypers FA, Larkin S, Vichinsky EP, Styles LA. Patterns of arginine and nitric oxide in patients with sickle cell disease with vaso-occlusive crisis and acute chest syndrome. J Pediatr Hematol Oncol 2000; 22:515–20 [DOI] [PubMed] [Google Scholar]
- 117.Morris CR, Kuypers FA, Larkin S.Sweeters N, Simon J, Vichinski EP, Styles LA. Arginine therapy: a novel strategy to induce nitric oxide production in sickle cell disease. Br J Haematol 2000; 111:498–500 [DOI] [PubMed] [Google Scholar]
- 118.Dejam A, Hunter CJ, Tremonti C.Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation 2007; 116:1821–31 [DOI] [PubMed] [Google Scholar]
- 119.Kim-Shapiro DB, Gladwin MT, Patel RP, Hogg N. The reaction between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation. J Inorg Biochem 2005; 99:237–46 [DOI] [PubMed] [Google Scholar]
- 120.Gladwin MT, Grubina R, Doyle MP. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc Chem Res 2009; 42:157–67 [DOI] [PubMed] [Google Scholar]
- 121.Munzel T, Daiber A. Inorganic nitrite and nitrate in cardiovascular therapy: a better alternative to organic nitrates as nitric oxide donors? Vascul Pharmacol 2018; 102:1–10 [DOI] [PubMed] [Google Scholar]
- 122.Chen K, Piknova B, Pittman RN, Schechter AN, Popel AS. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide 2008; 18:47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grubina R, Basu S, Tiso M, Kim-Shapiro DB, Gladwin MT. Nitrite reductase activity of hemoglobin S (sickle) provides insight into contributions of heme redox potential versus ligand affinity. J Biol Chem 2008; 283:3628–38 [DOI] [PubMed] [Google Scholar]
- 124.Breitenstein S, Roessig L, Sandner P, Lewis KS. Novel sGC stimulators and sGC activators for the treatment of heart failure. Handbook Exp Pharmacol 2017; 243:225–47 [DOI] [PubMed] [Google Scholar]
- 125.Follmann M, Griebenow N, Hahn MG.Hartung I, Mais FJ, Mittendorf J, Schäter M, Schirok H, Stasch JP, Stoll F, Straub A. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl 2013; 52:9442–62 [DOI] [PubMed] [Google Scholar]
- 126.Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol 2009;191:277–308 [DOI] [PubMed] [Google Scholar]
- 127.Wu CC, Ko FN, Kuo SC, Lee FY, Teng CM. YC-1 inhibited human platelet aggregation through NO-independent activation of soluble guanylate cyclase. Br J Pharmacol 1995; 116:1973–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1, a novel activator of platelet guanylate cyclase. Blood 1994; 84:4226–33 [PubMed] [Google Scholar]
- 129.Makowski CT, Rissmiller RW, Bullington WM. Riociguat: a novel new drug for treatment of pulmonary hypertension. Pharmacotherapy 2015; 35:502–19 [DOI] [PubMed] [Google Scholar]
- 130.Armstrong PW, Roessig L, Patel MJ.Anstrom KJ, Butler J, Voors AA, Lam CSP, Ponikowski P, Temple T, Pieske B, Ezekowitz J, Hernandez AF, Koglin J, O'Connor CM. A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail 2018; 6:96–104 [DOI] [PubMed] [Google Scholar]
- 131.Mittleman RS, Wilson P, Sykes K.Mihova M, Chickering JG, Ruff D, Hall M, Milne TG, Currie MG, Chien Y. Multiple-ascending-dose study of the soluble guanylate cyclase stimulator, IW-1701, in healthy subjects. Blood 2017; 130:3533 [Google Scholar]
- 132.Henriques C. SIckle Cell Anemia News, https://sicklecellanemianews.com/2018/06/13/fda-designates-olinciguat-orphan-drug-as-potential-sickle-cell-disease-treatment/. (accessed 26 September2018).
- 133.Zhou Z, Pyriochou A, Kotanidou A.Dalkas G, van Eickels M, Spyroulias G, Roussos C, Papapetropoulos A. Soluble guanylyl cyclase activation by HMR-1766 (ataciguat) in cells exposed to oxidative stress. Am J Physiol Heart Circ Physiol 2008; 295:H1763–71 [DOI] [PubMed] [Google Scholar]
- 134.Stasch JP, Schmidt P, Alonso-Alija C.Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, Schramm M, Schroeder W, Schröder H, Stahl E, Steinke W, Wunder F. NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol 2002; 136:773–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stasch JP, Schlossmann J, Hocher B. Renal effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Curr Opin Pharmacol 2015; 21:95–104 [DOI] [PubMed] [Google Scholar]
- 136.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 2006; 5:755–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dumitrascu R, Weissmann N, Ghofrani HA.Dony E, Beuerlein K, Schmidt H, Stasch JP, Gnoth MJ, Seeger W, Grimminger F, Schermuly RT. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation 2006; 113:286–95 [DOI] [PubMed] [Google Scholar]
- 138.Erdmann E, Semigran MJ, Nieminen MS.Gheorghiade M, Agrawal R, Mitrovic V, Mebazaa A. Cinaciguat, a soluble guanylate cyclase activator, unloads the heart but also causes hypotension in acute decompensated heart failure. Eur Heart J 2013; 34:57–67 [DOI] [PubMed] [Google Scholar]
- 139.Schafer A, Fraccarollo D, Werner L, Bauersachs J. Guanylyl cyclase activator ataciguat improves vascular function and reduces platelet activation in heart failure. Pharmacol Res 2010; 62:432–8 [DOI] [PubMed] [Google Scholar]
- 140.Lee KH, Lee SR, Cho H.Woo JS, Kang JH, Jeong YM, Cheng XW, Kim WS, Kim W. Cardioprotective effects of PKG activation by soluble GC activator, BAY 60-2770, in ischemia-reperfusion-injured rat hearts. PLoS One 2017; 12:e0180207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Romoli S, Bauch T, Dröbner K, Eitner F. Evaluation of kidney protection of soluble guanylate cyclase (sGC) modulators in a novel, fast mouse model. BMC Pharmacol Toxicol 2017; 18:A55 [Google Scholar]
- 142.Hariri E, Mansour A, El Alam A, Daaboul Y, Korjian S, Aoun Bahous S. Sickle cell nephropathy: an update on pathophysiology, diagnosis, and treatment. Int Urol Nephrol 2018; 50:1075–83 [DOI] [PubMed] [Google Scholar]
- 143.Sommer A, Sandner P, Behrends S. BAY 60-2770 activates two isoforms of nitric oxide sensitive guanylyl cyclase: evidence for stable insertion of activator drugs. Biochem Pharmacol 2018; 147:10–20 [DOI] [PubMed] [Google Scholar]
- 144.Potoka KP, Gladwin MT. Vasculopathy and pulmonary hypertension in sickle cell disease. Am J Physiol Lung Cell Mol Physiol 2015; 308:L314–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peng T, Gong J, Jin Y.Zhou Y, Tong R, Wei X, Bai L, Shi J. Inhibitors of phosphodiesterase as cancer therapeutics. Eur J Med Chem 2018; 150:742–56 [DOI] [PubMed] [Google Scholar]
- 146.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 2006; 58:488–520 [DOI] [PubMed] [Google Scholar]
- 147.Lin CS, Lau A, Tu R, Lue TF. Expression of three isoforms of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in human penile cavernosum. Biochem Biophys Res Commun 2000; 268:628–35 [DOI] [PubMed] [Google Scholar]
- 148.Murray F, MacLean MR, Pyne NJ. Increased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in models of pulmonary hypertension. Br J Pharmacol 2002; 137:1187–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee AJ, Chiao TB, Tsang MP. Sildenafil for pulmonary hypertension. Ann Pharmacother 2005; 39:869–84 [DOI] [PubMed] [Google Scholar]
- 150.Machado RF, Martyr S, Kato GJ.Barst RJ, Anthi A, Robinson MR, Hunter L, Coles W, Nicols J, Hunter C, Sachdev V, Castro O, Gladwin MT. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol 2005; 130:445–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singh N, Patra S. Phosphodiesterase 9: insights from protein structure and role in therapeutics. Life Sci 2014; 106:1–11 [DOI] [PubMed] [Google Scholar]
- 152.Wunder F, Tersteegen A, Rebmann A, Erb C, Fahrig T, Hendrix M. Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol Pharmacol 2005; 68:1775–81 [DOI] [PubMed] [Google Scholar]
- 153.Cury Y, Picolo G, Gutierrez VP, Ferreira SH. Pain and analgesia: the dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011; 25:243–54 [DOI] [PubMed] [Google Scholar]
- 154.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10:895–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khouri C, Lepelley M, Roustit M, Montastruc F, Humbert M, Cracowski JL. Comparative safety of drugs targeting the nitric oxide pathway in pulmonary hypertension: a mixed approach combining a meta-analysis of clinical trials and a disproportionality analysis from the world health organization pharmacovigilance database. Chest 2018; 154:136–47 [DOI] [PubMed] [Google Scholar]
- 156.Niihara Y, Miller ST, Kanter J.Lanzkron S, Smith WR, Hsu LL, Gordeuk VR, Viswanathan K, Sarnaik S, Osunkwo I, Guillaume E, Sadanandan S, Sieger L, Lasky JL, Panosyan EH, Blake OA, New TN, Bellevue R, Tran LT, Razon RL, Stark CW, Neumayr LD, Vichinsky EP, Investigators of the Phase 3 Trial of I-Glutamine in Sickle Cell Disease. A phase 3 trial of l-glutamine in sickle cell disease. N Engl J Med 2018; 379:226–35 [DOI] [PubMed] [Google Scholar]
- 157.Ataga KI, Kutlar A, Kanter J.Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, Gualandro S, Colella MP, Smith WR, Rollins SA, Stocker JW, Rother RP. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med 2017; 376:429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]