Abstract
Maize is a sensitive crop to drought and heat stresses, particularly at the reproductive stages of development. The present study investigated the individual and interactive effects of drought (50% field capacity) and heat (38 °C/30 °C) stresses on morpho-physiological growth, yield, nutrient uptake and oxidative metabolism in two maize hybrids i.e., ‘Xida 889’ and ‘Xida 319’. The stress treatments were applied at tasseling stage for 15 days. Drought, heat and drought + heat stress caused oxidative stress by the over-production of ROS (O2−, H2O2, OH−) and enhanced malondialdehyde contents, which led to reduced photosynthetic components, nutrients uptake and yield attributes. The concurrent occurrence of drought and heat was more severe for maize growth than the single stress. However, both stresses induced the metabolites accumulation and enzymatic and non-enzymatic antioxidants to prevent the oxidative damage. The performance of Xida 899 was more prominent than the Xida 319. The greater tolerance of Xida 889 to heat and drought stresses was attributed to strong antioxidant defense system, higher osmolyte accumulation, and maintenance of photosynthetic pigments and nutrient balance compared with Xida 319.
Introduction
Under natural environments, crops are often subjected to different abiotic stresses simultaneously during their life cycle which adversely affect the growth and productivity of field crops1,2. Among abiotic stresses, extreme temperature and water deficit conditions are two of the most frequent environmental threats to crop growth and productivity, and ultimately the food security under changing climate3–8. These stresses negatively affect the yield of major staple food crops5,6,9, that account for 60% of global food energy supply10. Previous studies showed that the combined drought and heat stresses caused a disproportionate damage on plant growth and productivity compared with each of individual stress11–15. Temperature above 35 °C affects the vegetative and reproductive growth of maize, from germination to grain filling16. However, the reproductive stages of the crop plants are more sensitive to combined drought and heat stresses than the vegetative stages, although each stress affected reproductive traits differently12,15. These stresses noticeably reduced the photosynthetic activity, altered oxidative metabolism, caused membrane instability7,17, affected stomatal conductance, and decreased the leaf area and water-use efficiency in maize and other cereal crops5,6,18. Nonetheless, the effects of these stress factors might be different depending on the plant species2.
Decreased and uneven precipitation can lead to more severe drought events, and high air temperature coupled with drought results in higher plant tissue temperature19. Wahid et al.20 stated that the heat stress can be accompanied by drought because of rapid water loss from plant and soil surface under high temperature. While, Pie et al.21 reported that heat stress may occur in plants due to insufficient water supply to meet the evaporative demand. Both drought and heat stresses are well known to decrease the the nutrient uptake and photosynthetic efficiency of plants22. In wheat crop, heat stress during early as well as late season disturbed the growth period for various development stages (e.g. tillering, jointing, booting, anthesis, and grain filling) and reduced the organ size (tiller, leaf, and spikes)23. Recently, Zandalinas et al.2 and Lamaoui et al.22 concluded that drought and heat stresses generally trigger similar physiological responses in plants, however, plants were severely damaged by the combined action of both stresses than by the individual stress factor, indicating that conserved defense mechanisms exist among different plant species to deal with a combination of heat and drought stresses. In order to adapt stress conditions, plants possess several mechanisms including accumulation of compatible solutes (e.g. sugars, proline) and protective proteins (e.g. heat shock proteins), and activation of enzymatic (total superoxide dismutase; T-SOD, catalase; CAT, peroxidase; POD, ascorbate peroxidase; APX) and non-enzymatic (e.g. reduced glutathione GSH) antioxidant systems1,24,25. However, it is worthy mentioned that the stress sequence imposition of heat and drought do not alter the global response to combined stresses2.
Maize (Zea mays L.) is one of the most important and widely grown crops in the world26. However, in many parts of the world, it is mainly cultivated in semi-arid environments and thus often faces high temperature, water scarcity, and a combination of these factors in field conditions27,28. Maize is originated from the tropics but is still sensitive to heat and drought stresses, particularly after 8th leaf stage29. In China, 60% of crops in maize growing regions are often subjected to heat and drought spells, which may result in 30% yield losses per year28. Owing to changing climatic patterns worldwide, it is projected that these stresses will become major threats to maize yields and will decrease the world maize production by 15–20% each year30,31.
Despite the frequent existence of combined heat and drought stress episodes under field conditions, most of the previous studies have focused on independent drought or heat stress responses in plants15. Therefore, little is known on the morpho-physiological and biochemical responses of maize to combined drought and heat stresses. The present study was aimed at elucidating the mechanism of combined drought and heat stress tolerance in maize hybrids by exploring the physiological and biochemical responses of plants along with grain yield. The specific objectives of the study were (1) to investigate the individual and concurrent effects of heat and drought stresses on morpho-physiological growth, grain yield, osmolyte accumulation, nutrient uptake and oxidative status in maize, (2) to examine the basis of maize tolerance against drought and heat stresses, and (3) to assess the performance of two different maize hybrids under drought and/or heat stresses.
Results
Growth and yield
Drought and heat stress conditions alone or in combination hampered the morphological growth, grain yield and yield related attributes of both maize cultivars. Compared with control, combined drought + heat stress significantly reduced the plant height, shoot fresh weight, shoot dry weight, stem diameter, leaf area, kernels/ear, 100-kernel weight, grain yield/plant and harvest index (HI) of two maize hybrids (Tables 1 and 2). However, ears/plant and kernel rows/ear in both maize hybrids remained unaffected by all stress treatments (Table 2). At individual level, the effects of drought stress were more severe for plant height, stem diameter, shoot fresh weight, shoot dry weight, leaf area, 100-kernel weight, and grain yield/plant of both hybrids compared with those of heat stress. Under all stress treatments, the growth and yield performance of Xida 319 was lower than Xida 889 (Tables 1 and 2).
Table 1.
Effect of drought and heat stresses on maize agronomic traits.
| Maize cultivars | Treatments | Plant height (cm) | Stem diameter (mm) | Leaf area (cm2) | Shoot fresh weight/plant (g) | Shoot dry weight/plant (g) |
|---|---|---|---|---|---|---|
| Xida 319 | Ck | 181.67 ± 3.28 | 22.84 ± 0.70 | 455.87 ± 20.13 | 216.20 ± 7.63 | 51.43 ± 1.82 |
| H | 165.67 ± 2.96* | 22.59 ± 0.69 | 438.54 ± 20.88 | 209.10 ± 2.29 | 49.50 ± 3.50 | |
| D | 163.33 ± 2.85* | 20.48 ± 0.92 | 435.07 ± 22.52 | 190.43 ± 7.47* | 42.87 ± 1.95* | |
| H + D | 154.87 ± 6.26** | 19.06 ± 0.60** | 386.19 ± 9.13* | 173.30 ± 6.36** | 41.90 ± 1.63* | |
| Means | 166.38 | 21.24 | 428.92 | 197.26 | 46.43 | |
| Xida 889 | Ck | 185.67 ± 3.76 | 23.34 ± 0.22 | 542.73 ± 6.80 | 233.83 ± 10.08 | 53.67 ± 1.86 |
| H | 173.67 ± 2.19* | 22.63 ± 0.11 | 517.11 ± 15.21 | 214.93 ± 8.03 | 51.87 ± 0.85 | |
| D | 170.50 ± 4.80* | 20.92 ± 0.40* | 494.68 ± 4.22* | 191.33 ± 10.84* | 48.13 ± 1.82* | |
| H + D | 158.33 ± 2.85** | 20.25 ± 1.08** | 465.37 ± 15.31** | 178.90 ± 8.59** | 46.23 ± 1.13* | |
| Means | 172.04 | 21.79 | 504.98 | 204.75 | 49.98 |
Values are means of three replicates ±SE. For Duncan’s results, means with asterisks are considered as significant different compared with control. *P ≤ 0.05; **P ≤ 0.01.
Ck, control; H, heat stress; D, drought stress and H + D, heat + drought stress.
Table 2.
Effect of drought and heat stresses on maize yield and related characteristics.
| Maize cultivars | Treatments | Ears/plant | Kernel rows/ear | Kernels/ear | 100-kernel weight(g) | Grain yield/plant(g) | HI |
|---|---|---|---|---|---|---|---|
| Xida 319 | Ck | 2.02 ± 0.09 | 14.32 ± 0.36 | 281.33 ± 9.26 | 22.97 ± 0.37 | 107.33 ± 4.98 | 0.30 ± 0.01 |
| H | 1.93 ± 0.05 | 13.89 ± 0.23 | 248.70 ± 6.67* | 22.20 ± 0.44 | 89.50 ± 5.07* | 0.25 ± 0.01* | |
| D | 1.87 ± 0.11 | 13.95 ± 0.54 | 237.67 ± 11.35** | 21.97 ± 0.90 | 85.20 ± 7.16* | 0.24 ± 0.01* | |
| H + D | 1.82 ± 0.06 | 13.51 ± 0.51 | 202.67 ± 3.71** | 18.67 ± 0.33** | 72.70 ± 2.76** | 0.20 ± 0.02** | |
| Means | 1.91 | 13.92 | 242.59 | 21.45 | 88.69 | 0.25 | |
| Xida 889 | Ck | 2.11 ± 0.05 | 14.86 ± 0.55 | 308.00 ± 9.02 | 23.93 ± 0.62 | 117.07 ± 5.53 | 0.33 ± 0.03 |
| H | 1.94 ± 0.06 | 14.05 ± 0.53 | 266.00 ± 4.16* | 22.63 ± 1.16 | 100.03 ± 3.18* | 0.28 ± 0.01 | |
| D | 1.94 ± 0.03 | 13.67 ± 0.64 | 261.70 ± 4.44* | 21.50 ± 0.76 | 96.10 ± 4.80* | 0.26 ± 0.02* | |
| H + D | 1.96 ± 0.05 | 13.97 ± 0.09 | 221.43 ± 15.50** | 20.02 ± 0.58* | 90.97 ± 4.98** | 0.23 ± 0.01* | |
| Means | 1.99 | 14.14 | 264.28 | 22.02 | 101.04 | 0.27 |
Values are means of three replicates ±SE. For Duncan’s results, means with asterisks are considered as significant different compared with control. *P ≤ 0.05; **P ≤ 0.01.
Ck, control; H, heat stress; D, drought stress and H + D, heat + drought stress.
Photosynthetic characteristics
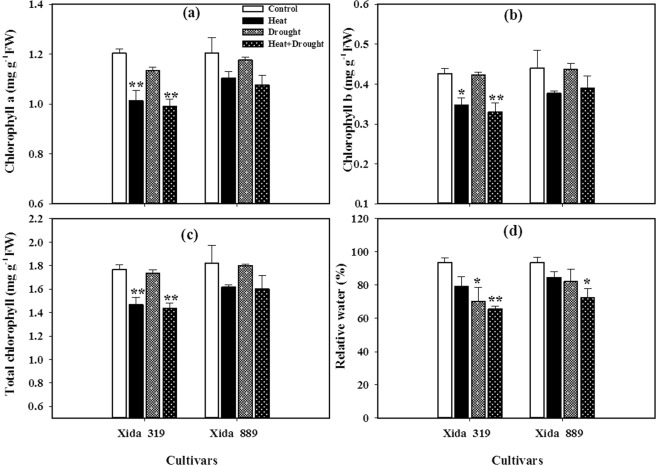
The leaf chlorophyll concentration of maize hybrids decreased under stress conditions as compared with control. However, the negative effects of heat were more severe for chlorophyll contents than the individual effects of drought stress (Fig. 1). Compared with control, heat stress alone or in combination with drought significantly declined the chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll content in Xida 319, which indicated its sensitivity under stress conditions (Fig. 1a–c).
Figure 1.
Influence of heat, drought, and heat + drought stresses on (a) chlorophyll a content, (b) chlorophyll b content, (c) total chlorophyll content and (d) relative water content in two maize hybrids. Capped bars above means represent ± SE of three replicates. Asterisks above columns means denote the significant differences compared with control treatment for a single maize hybrid. *P ≤ 0.05; **P ≤ 0.01.
Heat stress resulted in a slight decrease of relative water contents (RWC) in both maize hybrids, and these values were statistically similar with control. However, drought stress significantly reduced the RWC in Xida 319, which were further decreased under drought + heat stress. The reduction of RWC in Xida 319 was greater under drought + heat stress than that in Xida 889 (Fig. 1d).
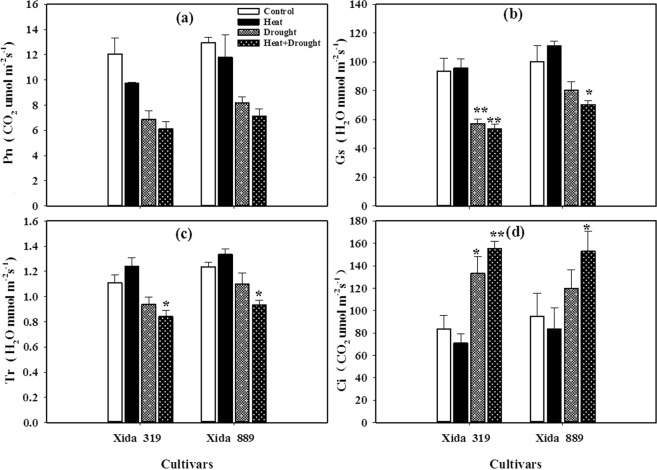
The photosynthetic rate (Pn), transpiration rate (Tr), and stomatal conductance (Gs) in both Xida 319 and Xida 889 hybrids were reduced by drought and drought + heat stresses. However, the reductions in these traits were more severe under combined drought + heat stresses than drought stress individually (Fig. 2a–c). As compared with control, the levels of Gs and Tr were slightly increased by heat stress in both hybrids, but these effects were statistically non-significant. The intercellular CO2 concentration (Ci) was increased under drought and significantly enhanced by drought + heat stresses, while it was decreased slightly under heat stress (Fig. 2d). The drought and heat stresses alone or in combination did not have the same effects on photosynthetic gas exchange parameters (Fig. 2). For instance, Tr was increased by heat stress, while drought stress decreased it, and Tr was further decreased when plants were subjected to combined drought + heat stresses. Overall, Xida 889 showed greater photosynthetic ability compared with Xida 319 (Fig. 2).
Figure 2.
Influence of heat, drought, and heat + drought stresses on (a) photosynthetic rate (Pn), (b) stomatal conductance (Gs), (c) transpiration rate (Tr) and (d) intercellular CO2 (Ci) in two maize hybrids. Capped bars above means represent ± SE of three replicates. Asterisks above columns means denote the significant differences compared with control treatment for a single maize hybrid. *P ≤ 0.05; **P ≤ 0.01.
Reactive oxygen species and lipid peroxidation
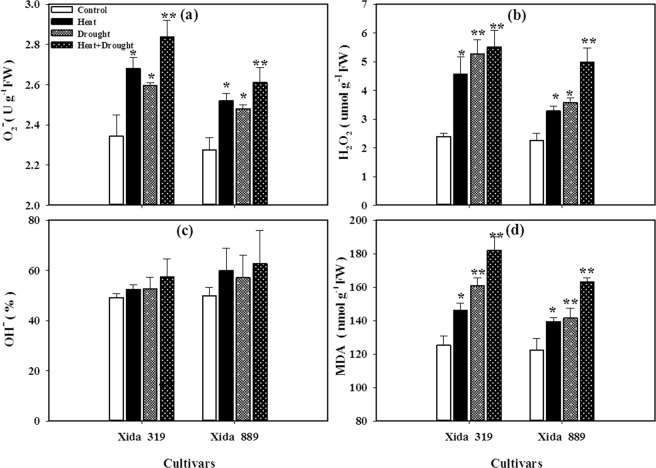
The reactive oxygen species (ROS) accumulation and the levels of membrane damage in two maize hybrids were increased under all stress conditions. However, the negative influence of drought + heat stress was more pronounced than those of individual stresses. The concentrations of hydroxyl free radical (OH−) in both hybrids under stress conditions were statistically similar with control. At individual stress level, the superoxide anion radical (O2−) contents were higher under heat stress, while hydrogen peroxide (H2O2) contents were higher under drought stress (Fig. 3). Compared with the control, drought, heat and drought + heat stress treatments significantly increased the levels of H2O2 and malondialdehyde (MDA) in both hybrids. Nevertheless, higher oxidative damage in Xida 319 indicated that this cultivar is less tolerant to heat and drought stresses than Xida 889 (Fig. 3).
Figure 3.
Influence of heat, drought, and heat + drought stresses on (a) superoxide anion (O2−), (b) hydrogen peroxide (H2O2), (c) hydroxyl radical (OH−) and (d) malonaldehyde (MDA) contents in two maize hybrids. Capped bars above means represent ± SE of three replicates. Asterisks above columns means denote the significant differences compared with control treatment for a single maize hybrid. *P ≤ 0.05; **P ≤ 0.01.
Activities/levels of antioxidants
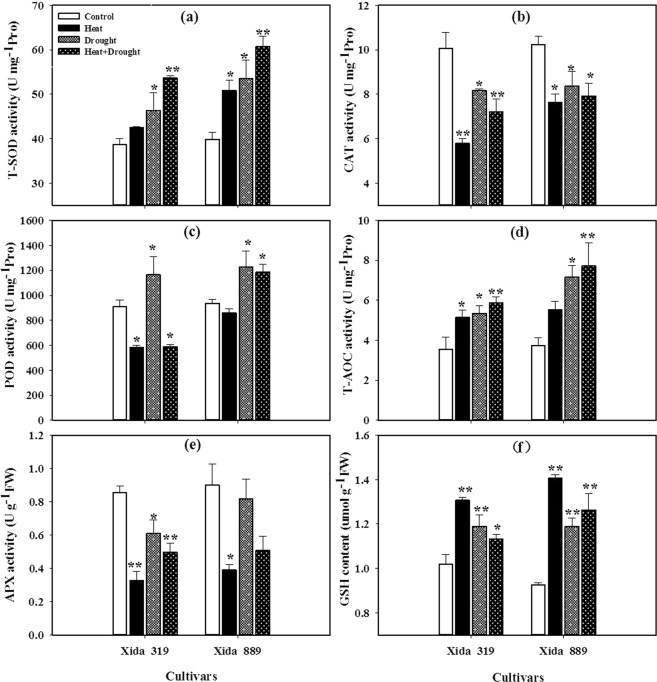
In both maize hybrids, T-SOD, GSH and total antioxidant capacity (T-AOC) were significantly increased under stress conditions as compared with control (Fig. 4a,d,f). The enhancement of T-SOD and T-AOC was more by combined drought + heat stress as compared with their individual effects. The activities of T-SOD and T-AOC in Xida 889 were significantly higher than Xida 319 under different stress conditions. Moreover, the levels of GSH varied between two maize hybrids. As compared with control, GSH contents were increased by 28, 17 and 11% in Xida 319 and 52, 28 and 36% in Xida 889 under heat, drought and drought + heat stress conditions, respectively (Fig. 4f). However, the activities of CAT and APX in Xida 319 and in Xida 889 were significantly decreased under stress conditions, compared to control condition (Fig. 4b,e). The severe decline of CAT, POD and APX occurred under heat stress, followed by drought and drought + heat combine stress. The responses of POD to heat stress and drought stress were variable. The maximum activities of POD were recorded at drought stress in two maize hybrids (Fig. 4c).
Figure 4.
Influence of heat, drought, and heat + drought stresses on the activities/levels of (a) total superoxide dismutase (T-SOD), (b) catalase (CAT), (c) peroxidase (POD), (d) total antioxidant capacity(T-AOC), (e) ascorbate peroxidase (APX) and (f) reduced glutathione (GSH) in two maize hybrids. Capped bars above means represent ± SE of three replicates. Asterisks above columns means denote the significant differences compared with control treatment for a single maize hybrid. *P ≤ 0.05; **P ≤ 0.01.
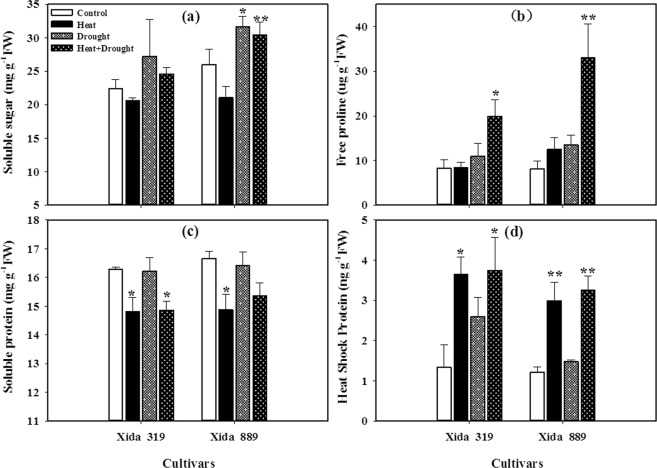
Osmolytes accumulation
Drought and heat stresses regulated the accumulation of soluble sugars, free proline, and heat shock protein in hybrid maize hybrids (Fig. 5). Exposure of drought, and drought + heat enhanced the soluble sugar, free proline and heat shock protein in both maize hybrids as compared with control. In contrast, the heat stress decreased the contents of soluble sugar as compared with control, but these effects were statistically non-significant. However, the concentration of soluble protein was decreased under drought, heat and drought + heat stress conditions, as compared with control. The response of heat shock protein to heat stress was greater than that to drought stress, and the maximum accumulation of heat shock protein was observed under combined drought + heat stress. Compared with Xida 319, Xida 889 showed slightly higher accumulation osmolytes, which indicated that Xida 889 may perform better under osmotic stress (Fig. 5a–d).
Figure 5.
Influence of heat, drought, and heat + drought stresses on the accumulations of (a) soluble sugar, (b) free proline, (c) soluble protein and (d) heat shock protein in two maize hybrids. Capped bars above means represent ± SE of three replicates. Asterisks above columns means denote the significant differences compared with control treatment for a single maize hybrid. *P ≤ 0.05; **P ≤ 0.01.
Nutrient uptake
Compared with control treatment, heat stress did not significantly affect nitrogen (N) concentrations in root, leaf, and stem of both maize hybrids, while drought stress alone was found to significantly decrease the leaf N concentrations in both hybrids (Table 3). The N concentrations in root and stem of both maize hybrids under drought stress were statistically similar with those in control treatment. Under these stress treatments, N concentrations in different plant parts of maize hybrids were in the order of root N > leaf N > stem N. Exposure of drought or heat stress individually did not significantly alter the phosphorus (P) and potassium (K) concentrations in the roots and leaves of both maize hybrids with respect to control. However, drought stress significantly reduced the stem P and stem K concentrations in Xida 889 (Table 3). The combined effect of drought + heat stress was more detrimental regarding the uptake of N, P, and K for both maize hybrids. Compared with control, root N, root K, stem P, stem K, and leaf K concentrations were significantly reduced in both maize hybrids under combined drought + heat stress (Table 3). Among different plant parts, the P and K concentrations in root were lower than those in stem and leaf for both maize cultivars. The concentrations of N, P and K in the root, stem and leaf of Xida 319 were lower than Xida 889 which indicates that Xida 319 accumulated less nutrients than Xida 889, under stress conditions (Table 3).
Table 3.
Effect of drought and heat stresses on nutrient concentrations in root, stem, and leaf of maize hybrids.
| Maize cultivars | Treatments | Root | Stem | Leaf | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (mg/g) | P (mg/g) | K (mg/g) | N (mg/g) | P (mg/g) | K (mg/g) | N (mg/g) | P (mg/g) | K (mg/g) | ||
| Xida 319 | Ck | 19.52 ± 0.81 | 4.73 ± 0.34 | 10.14 ± 0.51 | 13.42 ± 1.42 | 6.29 ± 0.17 | 13.74 ± 0.25 | 17.66 ± 0.92 | 5.83 ± 0.50 | 11.60 ± 0.81 |
| H | 19.13 ± 0.27 | 4.46 ± 0.39 | 9.26 ± 0.48 | 12.76 ± 1.65 | 5.69 ± 0.41 | 13.26 ± 0.16 | 17.99 ± 0.86 | 6.28 ± 0.75 | 11.74 ± 0.99 | |
| D | 18.74 ± 0.21 | 3.97 ± 0.14 | 8.88 ± 0.38 | 10.73 ± 1.37 | 5.44 ± 0.25 | 11.20 ± 1.40 | 13.22 ± 0.15* | 4.96 ± 0.18 | 9.20 ± 1.29 | |
| D + H | 15.17 ± 1.07** | 3.55 ± 0.71 | 6.67 ± 0.33** | 10.52 ± 0.96 | 4.92 ± 0.13** | 8.81 ± 0.84** | 14.47 ± 2.30 | 5.50 ± 1.34 | 7.81 ± 1.03* | |
| Means | 18.14 | 4.18 | 8.74 | 11.86 | 5.58 | 11.75 | 15.83 | 5.64 | 10.09 | |
| Xida 889 | Ck | 21.62 ± 1.84 | 4.86 ± 0.21 | 10.20 ± 0.79 | 13.50 ± 1.34 | 7.16 ± 0.27 | 13.85 ± 0.30 | 17.85 ± 0.15 | 5.85 ± 0.22 | 11.24 ± 0.44 |
| H | 20.10 ± 0.54 | 4.64 ± 0.26 | 9.81 ± 0.46 | 12.74 ± 1.03 | 5.71 ± 0.28** | 13.24 ± 0.56 | 18.82 ± 0.68 | 5.98 ± 0.23 | 11.85 ± 0.87 | |
| D | 18.97 ± 0.62 | 3.92 ± 0.45 | 8.94 ± 0.42 | 12.53 ± 1.31 | 5.11 ± 0.35** | 12.60 ± 0.38* | 14.47 ± 1.18** | 4.98 ± 0.42 | 10.60 ± 0.62 | |
| D + H | 16.65 ± 0.78* | 3.63 ± 0.45 | 7.07 ± 0.58** | 10.71 ± 0.52 | 4.93 ± 0.28** | 10.81 ± 0.97* | 16.41 ± 0.54 | 5.57 ± 0.26 | 8.81 ± 0.82* | |
| Means | 19.33 | 4.26 | 8.98 | 12.37 | 5.73 | 12.62 | 16.89 | 5.59 | 10.62 | |
Values are means of three replicates ±SE. For Duncan’s results, means with asterisks are considered as significant different compared with control. *P ≤ 0.05; **P ≤ 0.01.
Ck, control; H, heat stress; D, drought stress and H + D, heat + drought stress.
Discussion
Among abiotic stresses, drought and heat stresses alone or combination are the major limiting factors for the growth and productivity of many field crops. Although drought and heat stresses may disturb the overall growth and development of crop plants, yet the reproductive growth of plants is more sensitive under these stress factors7,32,33. In the present experiment, drought and heat stress conditions reduced the plant height, leaf area and biomass accumulation that ultimately reduced the yield and related attributes of two maize hybrids, nevertheless, the negative effects of drought + heat stresses were more severe for both maize hybrids then their individual effects (Tables 1 and 2). These stress treatments had more serious effects on Xida 319 compared with Xida 889. Previously, reduced maize growth and severe yield losses were found when drought stress was imposed at pre-tasseling34 and high temperature near anthesis stage35. Furthermore, significant reductions in growth, biomass accumulation and yield of maize were observed under heat stress imposed at different reproductive stages35–37. In the past, several studies have reported the detrimental effects of a combined drought and heat stresses on the growth and yields of different cereal crops12,14,37–41, however, the extent of damage under these stresses varies with the severity of stress and crop growth stage7.
In the present study, Chl a, Chl b and total Chl contents were severely affected in maize by individual and concurrent heat and drought stresses (Fig. 1a–c). However, the impacts of heat and drought + heat stress on the Chl a, Chl b and total Chl contents were severe compared with drought stress. Chlorophyll is the main pigment for photosynthesis of plants, which is one of the physiological processes that are most sensitive to high temperature42. High-temperature stress promotes the degradation of chlorophyll43, thus reducing the acceptance of light quanta, avoiding excessive free radicals and causing damage to plants44. Drought stress could also reduce the leaf chlorophyll contents, which on the other hand may hamper the photosynthetic efficiency and plant growth45–47. Drought stress decreased the RWC more than heat stress and the significantly reduction were observed under combined drought and heat stresses (Fig. 1d), which is consistent with the results by Wang et al.48 who reported that heat and drought stresses decreased the RWC in wheat plants.
Water relations in plants are influenced by several factors including Tr, leaf water potential, leaf and canopy temperatures, and Gs, while, water losses under heat stress remain high during day time because of increased Tr, which ultimately impair certain key physiological processes in plants7. In the present study, responses of Pn, Tr, Gs, and Ci in both maize hybrids to heat, drought and the combination of these two stresses were different. The Pn, Tr, and Gs of maize leaves decreased greatly but Ci was increased under combined drought + heat stress than individual stresses (Fig. 2). On the other hand, under heat stress, Gs and Tr of maize leaves increased slightly but Ci was decreased (Fig. 2). Under drought conditions, closure of stomata decreases the availability of CO2 and thus limits the Pn in plants47,49. By contrast, heat stress hinders Pn mainly because of nonstomatal limitations, such as alterations in electron transport capacity and activity50,51. Therefore, in the present study, heat induced stomatal opening increased the Tr, but resulted in a decreased Pn (Fig. 2a–c), being consistent with other reports52–54. Moreover, Gs is not always linked with photosynthetic capacity of plants, particularly under severe and combined stresses49,55,56.
Drought and heat stress could lead to over production of ROS which deteriorate photosynthetic components in plant. Enhanced O2−, H2O2, OH− and MDA contents in both maize hybrids were observed under drought, heat and drought + heat stresses which indicate the occurrence of oxidative stress. The level of ROS and MDA was higher under drought + heat stress than their individual effects in maize seedlings (Fig. 4). Concentration of MDA contents is an indicator of extent of lipid peroxidation under stress conditions24,43,57. Under unfavorable environmental conditions, higher lipid peroxidation rates occur in plants because of over-production of ROS50,58. In general, when the rate of ROS production exceeds the anti-oxidant enzyme activities then caused damage to essential cellular components4,59. Plants use the complex antioxidant defense systems to overcome the uncontrolled production of ROS and protect the plants from oxidative damage50,57,58,60. However, the balance between ROS generation and antioxidant enzyme activities is vital to all plant species under stressful conditions61. Under the stress condition, SOD can disproportionate the O2− produced by plants to H2O2 and O2 to reduce the injury of plant62. At individual stress level, the O2− contents were higher under heat stress, while H2O2 contents were higher under drought stress (Fig. 3). The higher levels of O2− in both maize hybrids under heat stress (Fig. 3) were concomitant with lower activities of T-SOD (Fig. 4). Both CAT and POD have the ability to scavenging H2O2, and the activity of CAT enzyme is high, but the affinity for H2O2 is weak. POD has a high affinity for H2O2, but it can participate in the degradation of chlorophyll and cause membrane lipid peroxidation63. In the present study, the contents of T-SOD, T-AOC and GSH were increased (Fig. 5a,d,f), POD was variable (Fig. 5c) and CAT and APX were significantly decreased (Fig. 5b,e), in both maize hybrids under heat, drought and heat + drought stress conditions which were consistent with overproduction of ROS and poor growth performance of these hybrids.
Proline, soluble protein and soluble sugar are the main osmotic regulators in plants. Under stress conditions, proline can be used as a solute to regulate changes in the cell’s water environment, reducing the effects of high temperature and drought stress on plant water deficit. Proline accumulated under stress conditions can also serve as a repository for energy and ammonia sources, directly participating in plant metabolism after stress relief 64,65. In current study, concentrations of soluble sugar and proline were considerably higher in both maize hybrids under stress conditions as compared with control, however, soluble proteins was decreased under all stress conditions, being consistent with Wang et al.48 report. Moreover, Xida 889 accumulated more osmolytes than Xida 319 (Fig. 3a–c). In this study, response of heat shock protein to heat stress was greater than that to drought stress, and the greater heat shock protein was observed with exposure to the drought + heat stress. Heat shock proteins play vital role in abiotic stresses particularly under temperature stress66. Several studies regarding the effects of a combination of drought and heat stress on the heat shock protein of tobacco11, wheat67 and Arabidopsis53 have suggested that the effect of combined stresses on plants was different from that of heat or drought stress applied individually.
The absorption, accumulation and distribution of N, P and K in various organs of maize affect growth and productivity68. Changes in temperature may modify the plant-nutrient relationships; however, such effects are difficult to generalize because these effects vary with different physiological processes and plant organs69. In the present study, heat stress alone did not significantly affect the N, P, and K concentrations in all plant parts of both maize hybrids, only stem P concentration in Xida 889 was significantly reduced compared with control (Table 3). Previously, Tingey et al.70 reported that high temperature did not change the total plant N absorption of plants in Douglas-fir (Pseudotsuga menziesii), however, the concentrations of N in roots and woody tissues were decreased with respect to control. Drought stress alone was found to significantly decrease the leaf N concentrations in both hybrids and stem P and stem K concentrations in Xida 889, compared with control (Table 3). In the past, several authors have documented the drought-evoked decreases in uptake and transfer of macro nutrients (N, P, and K) in various plant species71–74. The detrimental effects of combined drought and heat stresses were more severe regarding nutrient absorption than the individual effects of these stresses. Compared with control, combined drought + heat stress significantly reduced root N, root K, stem P, stem K, and leaf K concentrations in both maize hybrids (Table 3), which might be attributed to reduced availability, uptake, and transfer of these nutrient elements under stressful conditions. Recently, Fahad et al.7 concluded that both drought and heat stresses, particularly in combination, negatively affect the nutrient cycling, uptake, availability, and utilization in plants by disrupting different physiological functions.
Materials and Methods
Growth conditions and plant material
The experiment was carried out in controlled green house at the College of Agronomy and Biotechnology, Southwest University, Chongqing, China (longitude 106°26′02″E, latitude 29°49′32″N, and altitude 220 m) during spring 2017. The seeds of two maize hybrids i.e., Xida 889 and Xida 319 were obtained from Maize Research Institute, College of Agronomy and Biotechnology, Southwest University, Chongqing, China. Seeds were planted in plastic pots (24 cm in depth, 34 cm in diameter) filled with sandy-loam soil and farmyard manure in a proportion of 7:3. The total weight of each filled pot was 11 kg. In each pot, five seeds were initially sown and after emergence, two plants were maintained in each pot. Fertilizer containing urea, KCl and P2O5 (10:6:6 respectively), was applied 5 g per pot prior to sowing.
Stress treatments
The maize plants were maintained up to tasseling stage under normal conditions. At the tasseling stage, plants were subjected to heat, drought, and combined stress treatments for a period of 15 days. For well-watered/control (Ck), and drought (D) stress conditions, the temperature was 28 °C/20 °C (day/night); while for heat (H) and drought + heat stress (D + H) treatments, temperature was set at 38 °C/30 °C (day/night). The relative humidity was in the range of 55–70%. The drought stress was imposed at 50% field capacity (FC). The drought stress treatments were regularly checked and maintained using a moisture meter TRIME-EZ/-IT (IMKO Micromodultechnik GmbH, Germany). The experimental treatments were arranged in completely randomized design (CRD) under factorial arrangement. Each treatment was replicated three times, and there were five pots in each replicate.
Biochemical assays
Fully expanded, healthy, and undamaged plant leaves (fourth from the top) from each replicate were sampled at 15 days after imposition of stress treatments. After washing with pure distilled water, plant leaves were frozen in the liquid N2 and stored at −80 °C for measuring different biochemical analysis.
Measurement of growth and yield parameters
Leaf area (LA) of maize plants was assessed following the method of Montgomery et al. (1911): LA = L × W × 0.75, where L indicates leaf length, W represents the maximum leaf width, and 0.75 is the factor used for determination of leaf area in maize. Plant height was measured using a meter scale, while electronic weighing balance was used to assess the shoot fresh and dry weights. Digimatic caliper (500-197-30, Mitutoyo group, Japan) was used to measure stem diameter. For the analysis of yield parameters, six plants from each replication were randomly sampled and harvested at maturity. After sun-drying, the ears were manually shelled and yield constituents such as number of ears per plant, number of kernels rows per ear and grain yield per plant were recorded. The HI was calculated as the percent ratio of grain yield and biological yield.
Photosynthetic components and water relations
Photosynthetic components were recorded on intact leaves from the forth branch from the top, at 15 days after imposition of stress treatments. The Chlorophyll concentrations (Chl a, Chl b and total Chl) were determined according to Peng and Liu75. Extraction of 250 mg leaf without vein (leaf blade) was done with 10 ml ethanol-acetone (vol. 1:2), and the extract was transferred to 15 ml tube. The tubes were placed in dark to avoid light for 24 hours. The absorbance was measured at 645 nm, 663 nm, and 652 nm. The chlorophyll content was computed by the following formulae:
where, D663, D645 and D652 respectively are the corresponding wavelengths of the light density value, V is extracting liquid volume and W is leaf fresh weight.
Relative water content of maize leaves was measured following the method of Barrs and Weatherley76. The fresh leaves from the uppermost branch were sampled from plants and were cut into small segments (1.5 cm length), weighed (FW: fresh weight), floated on distilled water for 3 h under low light, and weighed again for turgid weight (TW), after surface moisture was removed. Afterward, the leaf samples were oven dried at 80 °C for 24 h and dry weight (DW) was recorded. The RWC was calculated as follows:
The photosynthetic gas exchange attributes including net photosynthesis rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) were recorded using a portable infrared gas exchange analyzer (Li-6400, Li-Cor, Lincoln, Nebraska, USA).
Lipid peroxidation rate and ROS accumulation
Lipid peroxidation in maize leaves was determined as MDA content; measured by thiobarbituric (TBA) method using ‘MDA Kit (A003-3)’ obtained from Suzhou Comin Biotechnology Co., Ltd., China. Lipid hydroperoxide decomposition products can condense with thibabituric acid (TBA) to produce red compounds. The absorbance for MDA was measured at 532 nm and represented as nmol/g fresh weight.
The contents of H2O2 and OH− in the leaves of maize were measured using the commercial ‘H2O2 Kit (H2O2-Y)’ and ‘OH− Kit (QZQ-G)’, respectively, obtained from Suzhou Comin Biotechnology Co., Ltd., China. H2O2 content was measured at 415 nm and expressed as μmol/g fresh weight. The ability to remove hydroxyl radicals measured at 536 nm and expressed as percent content. The contents of O2− in the leaves of maize were noted using the commercial ‘O2− kit (A052)’, obtained from Nanjing Jiancheng Bioengineering Institute, China. The O2− content was demonstrated as unit’s g−1 fresh weight and one unit was equivalent to superoxide anion radical inhibition by 1 mg of Vc for 40 minutes at 37 °C reaction24.
Estimation of enzymatic and non-enzymatic antioxidants
The activities of antioxidant enzymes were recorded using commercial kits in accordance with the instructions of manufacturer. The kits for total superoxide dismutase (T-SOD, A001), catalase (CAT, A007) and ascorbate peroxidase (APX, A123) were procured from Nanjing Jiancheng Bioengineering Institute, China. The absorbance readings of T-SOD, CAT and APX were detected at 550 nm, 405 nm and 290 nm, respectively. The T-SOD and CAT activities in maize leaves were articulated as U/mg protein, while APX activity was demonstrated as U/g protein. One unit (U) of T-SOD was the amount of enzyme required for 1 mg tissue proteins in 1 ml of a reaction mixture to raise SOD inhibition rates to 50% at 550 nm. One unit of CAT activity was estimated as the amount of enzyme that decomposes 1μmol H2O2 at 405 nm sec−1 in 1 mg tissue proteins. One unit of APX was defined as the amount of enzyme required for catalyzing 1μmol ASA at 290 nm min−1 of 1 mg tissue proteins in 1 ml of a reaction mixture.
The POD activity, GSH content and T-AOC in the leaves of maize were determined using the commercial kit ‘POD (POD-Y)’, ‘GSH (GSH-W)’ and ‘T-AOC (TAOC-G)’, respectively, obtained from Suzhou Comin Biotechnology Co., Ltd., China. One unit of POD activity was defined as the absorbance change of 0.005 at 470 nm min−1 for 1 mg tissue proteins in 1 ml of a reaction mixture. The absorbance for GSH was measured at 412 nm and expressed as μmol/g fresh weight. The absorbance for T-AOC was measured at 593 nm and expressed as U/mg protein. One unit of T-AOC was expressed as the amount that enhanced the absorbance by 0.01 at the 37 °C.
Osmolyte accumulation profiles
Free proline contents were assessed by following the acid ninhydrin method77. Fresh leaf material (0.5 g) was extracted using 5 ml of 3% sulphosalicylic acid for 10 min with shaking at 100 °C. The 2 ml of filtered aqueous extract was mixed with glacial acetic acid (2 ml) and acid ninhydrin reagent (2 ml), and heated (100 °C) for 30 min. The reaction mixture after cooling was segregated against toluene (4 ml) and the absorbance of the organic phase was recorded at 520 nm. The resulting values were related with a standard curve plotted using known amounts of proline (Sigma, St Louis, MO, USA). Total soluble sugar was estimated by anthracene ketone method as described by Zong and Wang78. The fresh leaf sample (0.2 g) was homogenized with 25 ml distilled water and centrifuged (4000 rpm) for 20 minutes. Anthracene (0.1 g) was dissolved in 100 ml diluted sulfuric acid to prepare anthracene sulfuric acid reagent. One ml extract and 5 ml anthracene sulfuric acid reagent were taken in a tube, shaken and put in boiling bath for 10 minutes. After 2 h stability, the sample was transferred in cuvette and the absorbance was read at 620 nm. The total protein content in the leaves of maize seedling was determined by Coomassie brilliant blue method using ‘Protein Quantification Kit (A045-2)’ obtained from Suzhou Comin Biotechnology Co., Ltd., China. The absorbance for protein was measured at 595 nm and unit of protein was quantified as mg/ml sample solution. The level of heat shock protein (HSP) in the leaves of maize was determined using the kit ‘HSP (YX-081916P)’ obtained from Sino Best Bio, Shanghai. The absorbance for HSP was measured at OD 450 nm and expressed as pg/ml sample solution.
Determination of nutrient uptake
Roots, stems and leaves were thoroughly washed with distilled water and were oven-dried at 105 °C for 1 hour and later at 80 °C until constant weight. Dry samples of 300 mg were digested with 8 ml sulfuric acid (H2SO4) in a sealed chamber79. Total N was determined using the Kjeldahl method80. The K level was analyzed using an Elemental Analyzer, while P concentration was determined by vanadate molybdate method using a UV/visible spectrophotometer as suggested by Chapman and Pratt81.
Statistical analysis
The collected data were statistically analyzed following analysis of variance (ANOVA) technique using SPSS 22.0 software (SPSS, Chicago, IL, USA) whereas the differences between control and stress treatments were separated according to Duncan’s multiple range test at a 1% and 5% level of significance. SigmaPlot 13.0 software (Systat Software Inc., San Jose, CA, USA) was used for the graphical presentation of data.
Conclusions
In summary, exposure of drought, heat and drought + heat stress at tasseling stage brought severe negative effects on growth and the yield attributes of the maize hybrids. Concurrent effects of drought + heat stress on photosynthetic components, osmolyte accumulation, enzymatic and non-enzymatic antioxidants and nutrients uptake of both maize hybrids were more severe than their individual effects. Under drought and heat stress conditions, higher ROS accumulation and rate of lipid peroxidation inhibited the photosynthetic efficiency and restricted the growth of maize. The levels of ROS scavengers were variable in both maize hybrids. Overall, the morpho-physiological growth, and yield performance of Xida 889 cultivar was better than the Xida 319 cultivar. The greater tolerance of Xida 889 to heat and drought stresses was attributed to strong antioxidant defense system, higher osmolyte accumulation, and maintenance of photosynthetic pigments and nutrient balance compared with Xida 319.
Acknowledgements
This work was financially supported by Special Fund for Agro-scientific Research in the Public Interest (No. 201503127) and the National Science Foundation (No. 31271673).
Author Contributions
H.H., S.H. and L.W. conceived and designed the research. H.H. and S.M. performed the experiment and wrote the initial draft of manuscript. Z.S., K.Z., Y.L., Q.X. and C.L. contributed for reagents, materials, and analysis tools. S.H., Y.C., S.A. and L.W. revised subsequent versions of the manuscript, prepared the final version and provided the technical guidance.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hafiz Athar Hussain and Shengnan Men contributed equally.
Contributor Information
Saddam Hussain, Email: sadamhussainuaf@gmail.com.
Longchang Wang, Email: wanglc@swu.edu.cn.
References
- 1.Hussain, H. A. et al. Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front. Plant. Sci., 10.3389/fpls.2018.00393 (2018). [DOI] [PMC free article] [PubMed]
- 2.Zandalinas SI, Mittler R, Balfagón D, Arbona V, Gómezcadenas A. Plant adaptations to the combination of drought and high temperatures. Physiol Plant. 2018;162:1. doi: 10.1111/ppl.12624. [DOI] [PubMed] [Google Scholar]
- 3.Zandalinas, S. I., Balfagón, D., Arbona, V. & Gómezcadenas, A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front. Plant Sci., 10.3389/fpls.2017.00953 (2017). [DOI] [PMC free article] [PubMed]
- 4.Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006;1:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Alghabari F, Ihsan MZ, Hussain S, Aishia G, Daur I. Effect of rht alleles on wheat grain yield and quality under high temperature and drought stress during booting and anthesis. Environ. Sci. Pollut. Res. 2015;22:15506–15515. doi: 10.1007/s11356-015-4724-z. [DOI] [PubMed] [Google Scholar]
- 6.Alghabari F, et al. Gibberellin-sensitive rht alleles confer tolerance to heat and drought stresses in wheat at booting stage. J. Cereal Sci. 2016;70:72–78. doi: 10.1016/j.jcs.2016.05.016. [DOI] [Google Scholar]
- 7.Fahad, S. et al. Crop production under drought and heat stress: plant responses and management options. Front. Plant. Sci., 10.3389/fpls.2017.01147 (2017). [DOI] [PMC free article] [PubMed]
- 8.Zhou R, et al. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017;17:24. doi: 10.1186/s12870-017-0974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray DK, Gerber JS, Macdonald GK, West PC. Climate variation explains a third of global crop yield variability. Nat Commun. 2015;6:5989. doi: 10.1038/ncomms6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FAO: FAO Statistical Pocketbook World Food and Agriculture. Rome: Food and Agriculture Organization of the United Nations (2015).
- 11.Rizhsky L, Liang H, Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002;130:1143–1151. doi: 10.1104/pp.006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnabás B, Jager K, Feher A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008;31:11–38. doi: 10.1111/j.1365-3040.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 13.De Boeck HJ, Bassin S, Verlinden M, Zeiter M, Hiltbrunner E. Simulatedheat waves affected alpine grassland only in combination with drought. New Phytol. 2015;209:531–541. doi: 10.1111/nph.13601. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, et al. Phosphoproteomic analysis of the response of maize leaves to drought, heat and their combination stress. Front. Plant. Sci. 2015;6:298. doi: 10.3389/fpls.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. Abiotic and biotic stress combinations. New Phytol. 2014;203:32–43. doi: 10.1111/nph.12797. [DOI] [PubMed] [Google Scholar]
- 16.Hatfield JL, et al. Climate impacts on agriculture: implications for crop production. Agron. J. 2011;103:351–370. doi: 10.2134/agronj2010.0303. [DOI] [Google Scholar]
- 17.Zheng M, et al. Seed priming in dry direct-seeded rice: consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul. 2016;78:167–178. doi: 10.1007/s10725-015-0083-5. [DOI] [Google Scholar]
- 18.Shah NH, Paulsen GM. Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant. Soil. 2003;257(219):226. [Google Scholar]
- 19.Lawas, L., Zuther, E., Jagadish, S. K. & Hincha, D. K. Molecular mechanisms of combined heat and drought stress resilience in cereals. Curr. Opin. Plant Biol., 10.1016/j.pbi.2018.04.002 (2018). [DOI] [PubMed]
- 20.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an over view. Environ. Exp. Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 21.Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science. 1998;282:287–290. doi: 10.1126/science.282.5387.287. [DOI] [PubMed] [Google Scholar]
- 22.Lamaoui M, Jemo M, Datla R, Bekkaoui F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018;6:26. doi: 10.3389/fchem.2018.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hossain A, et al. Evaluation of growth, yield, relative performance and heat susceptibility of eight wheat (Triticum aestivum L.) genotypes grown under heat stress. Int. J. Plant Prod. 2013;7:615–636. [Google Scholar]
- 24.Hussain S, Khan F, Cao W, Wu L, Geng M. Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front. Plant Sci. 2016;7:16. doi: 10.3389/fpls.2016.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anjum SA, et al. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ. Sci. Pollut. Res. 2016;23:17132–17141. doi: 10.1007/s11356-016-6894-8. [DOI] [PubMed] [Google Scholar]
- 26.Tesfaye K, et al. Potential benefits of drought and heat tolerance for adapting maize to climate change in tropical environments. Clim. Risk Manag. 2017;19:106–119. doi: 10.1016/j.crm.2017.10.001. [DOI] [Google Scholar]
- 27.Cairns JE, et al. Maize production in a changing climate: impacts, adaptation, and mitigation strategies. Adv. Agron. 2012;114:1–58. doi: 10.1016/B978-0-12-394275-3.00006-7. [DOI] [Google Scholar]
- 28.Zhao F, et al. The difference of physiological and proteomic changes in maize leaves adaptation to drought, heat, and combined both stresses. Front. Plant Sci. 2016;7:1471. doi: 10.3389/fpls.2016.01471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JP, Xu WW, Burke JJ, Xin ZG. Role of phosphatidic acid in high temperature tolerance in maize. Crop Sci. 2010;50:2506–2515. doi: 10.2135/cropsci2009.12.0716. [DOI] [Google Scholar]
- 30.Chen J, Xu W, Velten J, Xin Z, Stout J. Characterization of maize inbred lines for drought and heat tolerance. J. Soil Water Conserv. 2012;67:354–364. doi: 10.2489/jswc.67.5.354. [DOI] [Google Scholar]
- 31.Lobell DB, Bänziger M, Magorokosho C, Vivek C. Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat. Clim. Chang. 2011;1:42–45. doi: 10.1038/nclimate1043. [DOI] [Google Scholar]
- 32.Zhang B, Li W, Chang X, Li R, Jing R. Effects of favorable alleles for water-soluble carbohydrates at grain filling on grain weight under drought and heat stresses in wheat. Plos One. 2014;9(7):e102917. doi: 10.1371/journal.pone.0102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edreira JIR, Mayer LI, Otegui ME. Heat stress in temperate and tropical maize hybrids: Kernel growth, water relations and assimilate availability for grain filling. Field Crops Res. 2014;166:162–172. doi: 10.1016/j.fcr.2014.06.018. [DOI] [Google Scholar]
- 34.Anjum SA, et al. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017;8:69. doi: 10.3389/fpls.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabaldón-Leal C, et al. Modelling the impact of heat stress on maize yield formation. Field Crops Res. 2016;198:226–237. doi: 10.1016/j.fcr.2016.08.013. [DOI] [Google Scholar]
- 36.Slafer GA, Savin R. Can N management affect the magnitude of yield loss due to heat waves in wheat and maize? Curr. Opin. Plant Biol. 2018;45:276–283. doi: 10.1016/j.pbi.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Elazab A, Ordóñez RA, Savin R, Slafer GA, Araus JL. Detecting interactive effects of N fertilization and heat stress on maize productivity by remote sensing techniques. Eur. J. Agron. 2016;73:11–24. doi: 10.1016/j.eja.2015.11.010. [DOI] [Google Scholar]
- 38.Gooding MJ, Ellis RH, Shewry PR, Schofield JD. Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J. Cereal Sci. 2003;37:295–309. doi: 10.1006/jcrs.2002.0501. [DOI] [Google Scholar]
- 39.Lipiec J, Doussan C, Nosalewicz A, Kondracka K. Effect of drought and heat stresses on plant growth and yield: a review. Int. Agrophys. 2013;27:463–477. doi: 10.2478/intag-2013-0017. [DOI] [Google Scholar]
- 40.Dreesen PE, De Boeck HJ, Janssens IA, Nijs I. Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ. Exp. Bot. 2012;79:21–30. doi: 10.1016/j.envexpbot.2012.01.005. [DOI] [Google Scholar]
- 41.Rollins JA, et al. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.) J. Exp. Bot. 2013;64:3201–3212. doi: 10.1093/jxb/ert158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berry J, Bjorkman O. Photosynthetic response and adaptation to temperature in higher plants. Annual Rev. Pl. Physiol. 1980;31:491–543. doi: 10.1146/annurev.pp.31.060180.002423. [DOI] [Google Scholar]
- 43.Zafar SA, Hameed A, Khan AS, Ashraf M. Heat shock induced morpho-physiological response in indica rice (Oryza sativa L.) at early seedling stage. Pak. J. Bot. 2017;49:453–463. [Google Scholar]
- 44.Havaux M, Tardy F. Loss of chlorophyll with limited reduction of photosynthesis as an adaptive response of syrian barley landraces to high-light and heat stress. Funct. Plant Biol. 1999;26:569–578. doi: 10.1071/PP99046. [DOI] [Google Scholar]
- 45.Ommen OE, Donnelly A, Vanhoutvin S, Mvan O, Manderscheid R. Chlorophyll content of spring wheat flag leaves grown under elevated CO2 concentrations and other environmental stresses within the ‘espace-wheat’ project. Eur. J. Agron. 1999;10:197–203. doi: 10.1016/S1161-0301(99)00011-8. [DOI] [Google Scholar]
- 46.Upadhyaya H, Panda SK, Dutta BK. Variation of physiological and antioxidative responses in tea cultivars subjected to elevated water stress followed by rehydration recovery. Acta. Physiol. Plant. 2008;30:457–468. doi: 10.1007/s11738-008-0143-9. [DOI] [Google Scholar]
- 47.Oneto CD, et al. Water deficit stress tolerance in maize conferred by expression of an isopentenyltransferase (IPT) gene driven by a stress-and maturation-induced promoter. J. Biotechnol. 2016;220:66–77. doi: 10.1016/j.jbiotec.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Wang GP, et al. Improvement of heat and drought photosynthetic tolerance in wheat by overaccumulation of glycinebetaine. Plant Biotechnol. Rep. 2010;4:213–222. doi: 10.1007/s11816-010-0139-y. [DOI] [Google Scholar]
- 49.Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought from genes to the whole plant. Funct. Plant Biol. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- 50.Zafar SA, et al. Mechanisms and molecular approaches for heat tolerance in rice (Oryza sativa L.) under climate change scenario. J. Integr. Agric. 2018;17:726–738. doi: 10.1016/S2095-3119(17)61718-0. [DOI] [Google Scholar]
- 51.Way DA, Oren R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol. 2010;30:669–688. doi: 10.1093/treephys/tpq015. [DOI] [PubMed] [Google Scholar]
- 52.Hamerlynck EP, Huxman TE, Loik ME, Smith SD. Effects of extreme high temperature, drought and elevated co2 on photosynthesis of the mojave desert evergreen shrub, larreatridentata. Plant Ecol. 2000;148:183–193. doi: 10.1023/A:1009896111405. [DOI] [Google Scholar]
- 53.Rizhsky L, et al. When defense pathways collide. the response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004;134:1683. doi: 10.1104/pp.103.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wen X, Qiu N, Lu Q, Lu C. Enhanced thermotolerance of photosystem ii in salt-adapted plants of the halophyte Artemisia anethifolia. Planta. 2005;220:486–497. doi: 10.1007/s00425-004-1382-7. [DOI] [PubMed] [Google Scholar]
- 55.Caemmerer SV, et al. Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J. Exp. Bot. 2004;55:1157–1166. doi: 10.1093/jxb/erh128. [DOI] [PubMed] [Google Scholar]
- 56.Xu ZZ, Zhou GS. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta. 2006;224:1080–1090. doi: 10.1007/s00425-006-0281-5. [DOI] [PubMed] [Google Scholar]
- 57.Hussain S, Khan F, Hussain HA, Nie L. Physiological and biochemical mechanisms of seed priming-induced chilling tolerance in rice cultivars. Front. Plant Sci. 2016;7:116. doi: 10.3389/fpls.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 59.Okamoto OK, Pinto E, Latorre LR, Bechara EJH, Colepicolo P. Antioxidant modulation in response to metal induced oxidative stress in algal chloroplast, Arch. Environ. Contamin. Toxicol. 2001;40:18–24. doi: 10.1007/s002440010144. [DOI] [PubMed] [Google Scholar]
- 60.Sharma P, Jha AB, Dubey RS, Pessarakli M. Reactive oxygen species, oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012;2012:26. [Google Scholar]
- 61.Kocsy G, et al. Increasing the glutathione content in a chilling-sensitive maize genotype using safeners increased protection against chilling-induced injury. Plant Physiol. 2001;127:1147–1156. doi: 10.1104/pp.010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xia Q, He B, Liu Y, Xu J. Effects of high temperature stress on the morphological and physiological characteristics in Scaevola albida cutting seedlings. Acta Ecol. Sin. 2010;30:5217–5224. [Google Scholar]
- 63.Jiang, C., Yin, Y., Liu, X. & Wang, Z. Response of flag leaf lipid peroxidation and protective enzyme activity of wheat cultivars with different heat tolerance to high temperature stress after anthesis. Acta Agron. Sin. 143–148 (2007).
- 64.Paleg, L. G. & Aspinall, D. The physiology and biochemistry of drought resistance in plants. Physiol. Academic Press: Sydney. 178–179 (1981).
- 65.Tang, Z. C., Wang, Y. Q., Wu, Y. H. & Wang, H. C. The difference in proline accumulation between the seedlings of two varieties of sorghum with different drought resistance. Acta Photophysiol. Sin. 2 (1986).
- 66.Zafar SA, et al. Genome wide analysis of heat shock transcription factor (HSF) family in chickpea and its comparison with Arabidopsis. Plant Omics. 2016;9:136–139. doi: 10.21475/poj.160902.p7644x. [DOI] [Google Scholar]
- 67.Rampino P, et al. Novel durum wheat genes up-regulated in response to a combination of heat and drought stress. Plant Physiol. Biochem. 2012;56:72–78. doi: 10.1016/j.plaphy.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Zhao YL, Yang CS, Wang Q, Liu TX, Li CH. Effects of phosphorus placement depth on yield and nutrient uptake of summer maize. Scientia Agricultura Sinica. 2010;43:4805–4813. [Google Scholar]
- 69.Yan Q, Duan Z, Jingdong M, Xun L, Fei D. Effects of rootzone temperature and N, P, and K supplies on nutrient uptake of cucumber (Cucumis sativus L.) seedlings in hydroponics. Soil Sci. Plant Nutr. 2012;58:707–717. doi: 10.1080/00380768.2012.733925. [DOI] [Google Scholar]
- 70.Tingey DT, et al. Elevated CO2 and temperature alter nitrogen allocation in douglas‐fir. Glob. Chang. Biol. 2003;9:1038–1050. doi: 10.1046/j.1365-2486.2003.00646.x. [DOI] [Google Scholar]
- 71.Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 2009;29:185–12. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- 72.Asrar AWA, Elhindi KM. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. S. J. Biol. Sci. 2011;18:93–98. doi: 10.1016/j.sjbs.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Subramanian KS, Santhanakrishnan P, Balasubramanian P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 2006;107:245–253. doi: 10.1016/j.scienta.2005.07.006. [DOI] [Google Scholar]
- 74.Suriyagoda L, De Costa WAJM, Lambers H. Growth and phosphorus nutrition of rice when inorganic fertilizer application is partly replaced by straw under varying moisture availability in sandy and clay soils. Plant Soil. 2014;384:53–68. doi: 10.1007/s11104-014-2049-1. [DOI] [Google Scholar]
- 75.Peng YS, Liu E. A. comparative study of methods of extracting chlorophyll. Acta Agriculturae Universitatis Pekinensis. 1992;18:247–250. [Google Scholar]
- 76.Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- 77.Shan DP, et al. Cotton ghdreb1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol. 2007;176:70–81. doi: 10.1111/j.1469-8137.2007.02160.x. [DOI] [PubMed] [Google Scholar]
- 78.Zong X. F. & Wang S. G. Plant Pysiology Research Techniques. Southwest Normal University Pess (2011).
- 79.Anderson DL, Henderson LJ. Comparing sealed chamber digestion with other digestion methods used for plant tissue analysis. Agron. J. 1988;80:549–552. doi: 10.2134/agronj1988.00021962008000030031x. [DOI] [Google Scholar]
- 80.Sivasankar S, Oaks A. Regulation of nitrate reductase during early seedling growth (a role for asparagine and glutamine) Plant Physiol. 1995;107:1225–1231. doi: 10.1104/pp.107.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chapman HD, Parker PF. Methods of analysis for soils, plants and waters. Soil Sci. 1962;93:68. doi: 10.1097/00010694-196201000-00015. [DOI] [Google Scholar]