Abstract
CD103 is a transmembrane heterodimer complex that mediates cell adhesion, migration, and lymphocyte homing of cell through interaction with E-cadherin. Recently, CD103+ immune cells in human carcinoma has been investigated as a prognostic factor, however, the correlation between CD103+ immune cells and survival are still elusive. Therefore, a meta-analysis was performed to determine the prognostic value of CD103+ immune cells in solid tumor. Studies relevant to the subject was searched from PubMed, Embase, and Web of Science. Ten studies including 2,824 patients were eligible for the analysis. Tumors positive for CD103+ immune cells were associated with favorable overall survival, disease-free survival, and disease-specific survival. Subgroup analysis revealed that assessing CD103+ immune cells in epithelial and total (both epithelial and stromal) areas or using whole slide section were associated with good prognosis. Furthermore, stromal CD103+ immune cells or CD103+ immune cells evaluated by tissue microarrays were not always significantly prognostic. In conclusion, these results show that CD103+ immune cells are associated with prognosis in solid tumor. However, the region of assessment and selection of material for the evaluation could affect the value of CD103 as a prognostic biomarker.
Introduction
CD103, also known as integrin αEβ7 (ITGAE), is a transmembrane heterodimer complex that is involved in cell to cell or cell to matrix interaction1. CD103 mediates cell adhesion, migration, and lymphocyte homing of cell through interaction with E-cadherin, which is expressed in epithelial cells2. Therefore, CD103 is considered a hall mark of specific subset of immune cells that resides within the epithelium of mucosal organs including genital tract, stomach, lung, and skin3–6. The immune cells that express CD103 includes effector memory CD8+ T cells, regulatory CD8+ T cells (Tregs), and CD11c-high/MHC class II-high dendritic cells6–9.
In recent years, the importance of CD103+ immune cells in cancer has grown due to its intimate relationship with immune checkpoint and BRAF blockade. In ovarian cancer, intraepithelial CD8+ tumor-infiltrating lymphocytes (TILs) that expressed CD103 were suspected to cause exhausted phenotype by chronic stimulation that leads to resistance in immune therapy, and CD103 was widely co-expressed with PD-110. In rodent melanoma, expansion and activation of CD103+ dendritic cells were the only antigen presenting cells that transported intact antigen which led to enhanced response of PD-L1 and BRAF target therapies11. CD103+ immune cells were also a predictive marker of immunotherapy in lung cancer and increased expression was observed following anti-PD-1 therapy in ovarian cancer and melanoma12–14.
A number of studies suggests that CD103+ TIL in human malignancy is associated with prognosis15–18. However, this characteristic of CD103+ immune cells in cancer are still elusive with the exception of a meta-analysis of ovarian cancer19. Some studies define CD103 as predictive markers of intraepithelial CD8+ T cells or Tregs20,21, but other studies show that CD103+ immune cells are present within the stroma22 and could be considered to be associated with survival23. Moreover, recent studies have shown that the usage of tissue microarray (TMA) instead of whole tissue section slides could have issues when evaluating TIL within tumors24,25 but a comparison between TMA and whole section has not been addressed for CD103+ immune cells.
In this study, we conducted a meta-analysis to determine the prognostic significance of CD103+ immune cells in solid tumors. The aim of this study is to clarify the prognostic role of CD103 expression in immune cell as a biomarker across multiple tumors and to verify which region within tumor (epithelial, stromal, or both) and material (TMA or whole section) should be used in the assessment of the marker.
Results
Eligible studies and study characteristics
A total of 160 records were found from PubMed, Embase, and Web of Science after removing repeated records. Two records were added from references of a previous meta-analysis19. After the titles and abstracts were reviewed, 131 records were removed due to the irrelevance with prognosis. Full text of remaining 31 records were reviewed in detail. Among the remaining records, 3 were reviews, 9 had no CD103-related prognostic value, 8 were not clinical studies, and one used dual immunofluorescence staining to identify CD103+ CD8+ T cells. In the end, 10 articles with 2,824 patients were selected as eligible for the meta-analysis (Fig. 1). Most studies dichotomized the patients in to high (positive) and low (negative), but a single study22 used continuous value for evaluation. The median sample size of all studies was 257.5, with a close range from 98 to 485. Seven of the studies were tumors from the genitourinary tract, followed by two studies from lung, and a single study from breast (Table 1).
Figure 1.
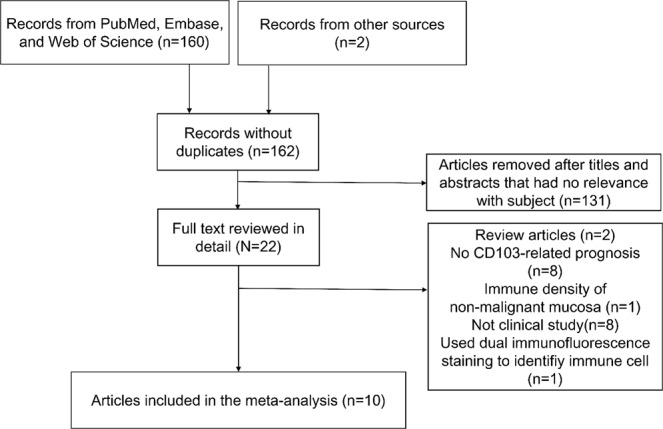
Flow chart of the selection process for meta-analysis.
Table 1.
Characteristics of articles included in the meta-analysis.
| Author | Year | Cancer | Material | Cut-off value | Region | Endpoint | Patient | CD103+ (%) |
|---|---|---|---|---|---|---|---|---|
| Djenidi22 | 2015 | Non-small cell lung cancer | WS | none (continuous) | all three | OS, DFS | 98 | — |
| Wang16 | 2016 | Breast cancer | TMA | 90th percentile | epithelium | OS | 424 | 43 (10.1) |
| Wang33 | 2015 | Urothelial cell carcinoma | WS | >9cells/HPF | epithelium | OS | 262 | 148 (56.5) |
| Bosmuller34 | 2016 | HGSC | TMA | >1 cell/HPF | epithelium | OS | 202 | 140 (69.3) |
| Santoiemma26 | 2016 | Ovarian cancer | TMA | ≥10 cells/core | epithelium | OS | 135 | 31 (23.0) |
| Koh23 | 2017 | Squamous cell lung cancer | TMA | mean | epithelium/stroma | OS, DFS | 378 | 108/111 (28.6/29.4)a |
| Workel20 | 2016 | Endometrial adenocarcinoma | TMA | >27.28 cells/mm2 | total | DFS, DSS | 253 | 84 (33.2) |
| Komdeur35 | 2017 | Cervical cancer | TMA | >29 cells/mm2 | total | DFS, DSS | 387 | 200 (51.7) |
| Zhou27 | 2018 | Renal cell carcinoma | WS | >1 cell/HPF | total | DSS | 200 | 117 (58.5) |
| Webb36 | 2013 | Ovarian cancer | TMA | ≥5(1) cells/coreb | TMA | DSS | 485 | 339 (69.9) |
*OS: overall survival, DFS: disese-free survival, DSS: disease-specific survival, WS: whole slide, TMA: tissue microarray, HPF: high power field, HGSC: high grade serous ovarian cancer.
aPositive for epithelial area/positive for stromal area.
b≥5 cells/core to evaluate prognosis, ≥1 cell/core to evaluate positivity.
Meta-analysis
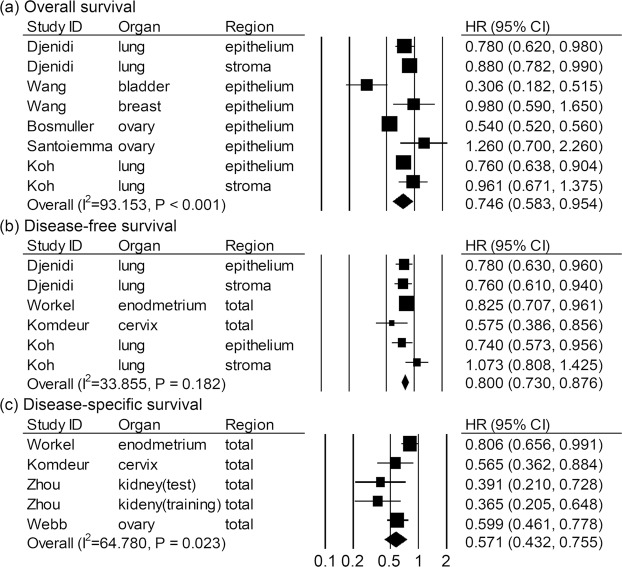
The meta-analysis between CD103+ immune cells and prognostic value of overall survival (OS) involved six articles with eight effect sizes (ES) (Fig. 2a). CD103+ immune cells were associated with favorable OS in solid tumor (HR = 0.746, 95% CI = 0.583–0.954, P value = 0.020). A random effect model was applied since heterogeneity test showed P value < 0.001 and I2 = 93.153. Fours studies with six ES included the correlation between CD103+ immune cells and disease-free survival (DFS) (Fig. 2b). In a fixed effect model (P value = 0.182 and I2 = 33.855), CD103+ immune cells were associated with prolonged DFS in solid tumor (HR = 0.800, 95% CI = 0.730–0.876, P value < 0.001). Survival outcomes involving smaller number of articles, disease-specific survival (DSS) (n = 4) had better prognosis in patients with CD103+ immune cells than those with CD103- immune cells in random effect model (HR = 0.571, 95% CI = 0.432–0.755, P value < 0.001) (Fig. 2c). In a subgroup analysis (Table 2), studies evaluating the immune cells of stromal area were showed significant correlation between CD103+ immune cells and OS (HR = 0.888, 95% CI = 0.794–0.993, P value = 0.037) but not DFS (HR = 0.892, 95% CI = 0.636–1.250, P value = 0.506). Studies using TMA had a significant correlation between CD103+ immune cells and DFS (HR = 0.805, 95% CI = 0.661–0.982, P value = 0.032) but not between CD103+ immune cells and OS (HR = 0.809, 95% CI = 0.595–1.100, P value = 0.176). However, studies using whole section or those that evaluate CD103+ immune cells of the epithelial or the total area always maintained correlation with survival regardless of the type of outcome.
Figure 2.
Correlation between CD103+ immune cells and survival in solid tumors. (a) Overall survival. (b) Disease-free survival. (c) Disease-specific survival.
Table 2.
Subgroup analysis of included studies.
| Outcome | Subgroup | Effect Model | N of studies | HR | 95%CI LL | 95%CI UL | P value |
|---|---|---|---|---|---|---|---|
| OS | Epithelium | Random | 6 | 0.688 | 0.528 | 0.896 | 0.006* |
| OS | Stroma | Fixed | 2 | 0.888 | 0.794 | 0.993 | 0.037* |
| OS | TMA | Random | 4 | 0.809 | 0.595 | 1.100 | 0.176 |
| OS | WS | Random | 2 | 0.654 | 0.443 | 0.968 | 0.034* |
| OS | Lung(epithelium) | Fixed | 2 | 0.767 | 0.668 | 0.881 | <0.001** |
| OS | Ovary | Random | 2 | 0.783 | 0.343 | 1.784 | 0.560 |
| DFS | Epithelium | Fixed | 2 | 0.764 | 0.649 | 0.898 | 0.001* |
| DFS | Stroma | Random | 2 | 0.892 | 0.636 | 1.250 | 0.506 |
| DFS | Total | Random | 3 | 0.802 | 0.701 | 0.918 | 0.001* |
| DFS | TMA | Random | 3 | 0.805 | 0.661 | 0.982 | 0.032* |
| DSS | TMA | Random | 3 | 0.571 | 0.432 | 0.755 | <0.001* |
*P value < 0.05, **P value < 0.001.
Publication bias and sensitivity analysis
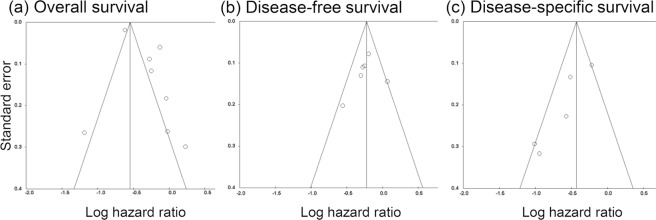
Begg’s funnel plot and Egg’s test were used to evaluate potential publication bias (Fig. 3). According to Egg’s test, OS and DFS did not show publication bias (P value = 0.090 and P value = 0.600, respectively) but DSS was significant for publication bias (P value = 0.029). Removing a single study from the meta-analysis did not have significant effect on the overall conclusion apart from a single study from Wang et al. that involved urothelial cell carcinoma (Fig. 4).
Figure 3.
Funnel plot for studies included in the meta-analysis. (a) Overall survival. (b) Disease-free survival. (c) Disease-specific survival.
Figure 4.
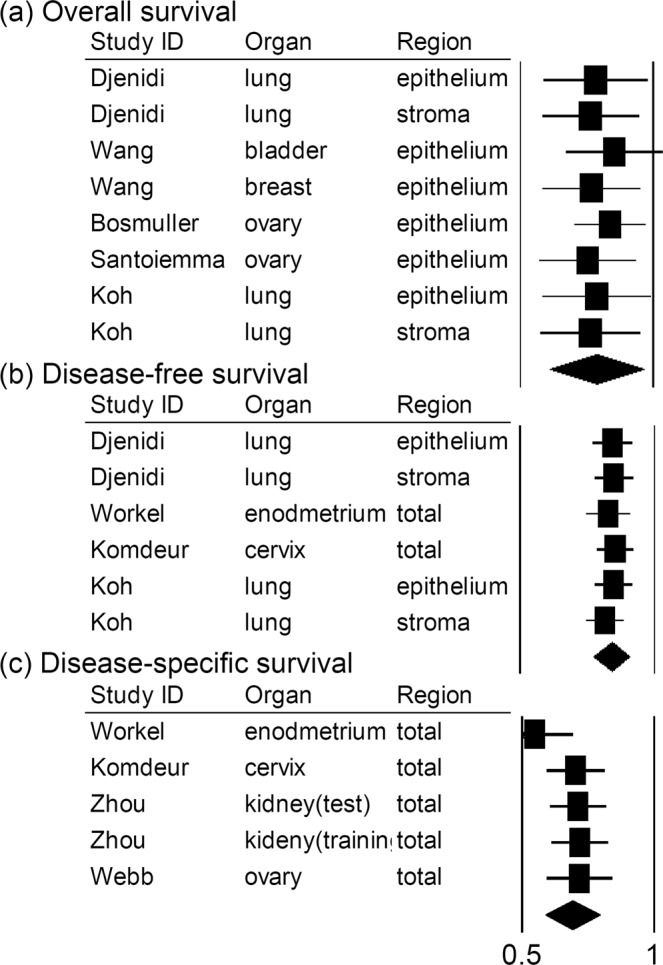
Sensitivity analysis of the meta-analysis. (a) Overall survival. (b) Disease-free survival. (c) Disease-specific survival.
Discussion
Expression of CD103 has been suggested as a marker of prolonged survival in solid tumors as well as a predictive marker of immunotherapy in recent years. However, the expression of CD103 has been reported in immune cells6–9 which limits the comprehensiveness of the subject of individual articles since most studies focus on a single material (TMA or whole slide) and a single region of interest (intraepithelial, stromal, or total areas) when evaluating CD103+ immune cells. In this meta-analysis, for the first time, the correlation between CD103+ immune cells and prognosis has been explored via pooled analysis of solid tumors.
A total of 2,824 patient from 10 different studies was included in the meta-analysis. The results showed that OS (HR = 0.746, 95% CI = 0.583–0.954, P value < 0.020), DFS (HR = 0.800, 95% CI = 0.730–0.876, P value < 0.001), and DSS (HR = 0.571, 95% CI = 0.432–0.755, P value < 0.001) are all indicative measurements of the prognostic value of CD103+ immune cells in solid tumors. Subgroup analysis revealed that CD103+ immune cells were not significantly correlated with OS when TMA studies were pooled and stromal CD103+ immune cells were not prognostic markers of DFS. On the contrary, pooled studies for whole slide or studies that investigated on intraepithelial or total CD103+ immune cells were determined prognostic whenever subgrouping was available.
The meta-analysis unveils novel characteristics of CD103+ immune cells within solid tumors. Although expression of CD103 was also observed in the stromal area, prognostic impact was only significant when CD103+ immune cells of epithelial or those of both epithelial and stromal (total) areas were accounted for. A pooled survival analysis of studies using TMA where not significantly correlated with OS but still maintained prognostic for DFS and DSS. This might be considered as a short side for TMA, but when reviewing the counting strategies of each article, studies using TMA counted multiple cores with 0.6 mm diameter or a single core with 2.0 mm diameter16,23,26, whereas studies that used whole section counted not the entire slide but 3 to 5 microscopic fields within a given slide22,27. Moreover, studies that used TMA had a larger average number of patients compared with that of studies with whole section (323.4 vs 186.7). Another caveat is the fact that not enough studies were available to compare the effect of TMA and whole section according to each tumor. Therefore, for better prognostic resolution of CD103, assessing intraepithelial or total CD103+ immune cells and using multiple TMA cores per sample with large diameter could be suggested in a large scale study.
Human malignancy and its microenvironment shows diversity according to genetic background and etiology, and a pooled analysis could undermine unique characteristics of each tumor type. In our current study, subgroup analysis according to tumor type was available in two out of seven different types included in the meta-analysis, and not all tumor types were significantly associated with prognosis. Therefore, although the current study provides meaningful findings in survival-related features of CD103+ immune cells across solid tumors and various materials used for assessment, it should be noted that the correlation between CD103+ immune cells and prognosis could vary among different tumor types. Further investigation is required to fully elucidate clinical value of CD103+ immune cells in prognostic predictions.
Other limitations of this study are as follows. In our study, the subtype of CD103+ immune cells that correlates with prognosis is still poorly determined because none of the included studies used double staining immunohistochemistry or multiplex immunohistochemistry to identify if the immune cells are TIL although most studies defined CD103+ immune cells as CD103+ TIL. Furthermore, relatively small number of studies were available for the pooled analysis, which lead to exclusion of solid tumors of major systems, such as gastrointestinal tract. Since colorectal cancer has shown different prognostic meaning for FOXP3+ Treg compared with other organs in previous studies28–30, and CD103+ immune cells are widely expressed in Tregs, the inclusion of some organs has the potential to shift the result. Finally, Egg’s test determined that the result between CD103+ immune cells and DSS has a publication bias.
In conclusion, CD103+ immune cells are markers of favorable prognosis when effect sizes for survival analysis of solid tumors from various organs where pooled and meta-analyzed, although prognostic feature between tumor types may vary. For further research, to find out the full spectrum of prognostic value and therapeutic potential of CD103, we suggest that immune cell subtyping, optimal tumor region selection, and proper material selection for evaluating CD103+ immune cells that could suffice in producing data for large sample size are necessary.
Materials and Methods
Publication search strategy
Articles relevant with the subject were retrieved from electronic databases, PubMed, Embase, and Web of Science until June 1st of 2018. The search term were ‘CD103’, ‘cancer, carcinoma or tumor’, and ‘prognosis, prognostic or survival’. Additional articles were retrieved from reference cited in the eligible studies and a meta-analysis article of the ovarian cancer19. The results were limited to English articles based on human malignancies. Each source and collecting period of tumor samples were carefully recorded to avoid studies with identical patient population. When duplicate data was found, the most recent iteration of the cohort was included.
Inclusion and exclusion criteria
Studies eligible for the inclusion were: (1) studies that measured expression of CD103 by immunohistochemical (IHC) staining in immune cells within any human tumor samples, (2) studies that report the correlation between CD103 expression within the primary tumor and survival, (3) studies that offer hazard ratio (HR) and 95% confidence intervals (CI) directly from the main article or supplementary materials or provide alternative values that could estimate HR and 95% CIs. Exclusion criteria of this study were: (1) studies that were from abstracts of conferences, reviews, and comments, (2) studies that were based on xenograft models or human hematopoietic or lymphoid malignancies, (3) studies that had insufficient pathological or survival data which led to unavailability to extract HR and 95% CIs.
Data extraction and quality assessment
Two reviewers (YK and GK) independently collected data from eligible articles according to the criteria. Newcastle-Ottawa Scale (NOS) was used to evaluate quality of all studies. Scores equal to or higher than 6 out of 9 was defined as qualified. Parameters that were extracted from each study were as follows: surname of first author, year of publication, primary organ and type of cancer, material and regions of assessment for CD103, cut-off value for CD103+ immune cells, type of outcome, and number of analyzed patients. Meta-analysis of CD103+ immune cells were measured by four different outcome endpoints including OS, DFS, and DSS. Survival data were extracted as HR and 95% CIs from Cox univariate analysis. For articles with only Kaplan-Meiere curves, HR and 95% CIs were estimated by using Engauge Digitizer V9.8 (http://digitizer.sourceforge.net) and spreadsheets provided by Tierney et al.31 and Altman et al.32. However, this estimate was only available when number of patients from the research arm and control arm were reported. For this reason, we contacted the authors of articles that missed on this information, which one of the authors responded and provided patient numbers for each arm23.
Statistical analysis
The correlation between CD103+ immune cells and prognosis of patients with solid tumor were measured via meta-analysis. Pooled HR and 95% CIs for OS, DFS, and DSS were calculated by using Comprehensive meta-analysis V3.3 (Biostat, Englewood, NJ, USA). Cochran’s Q and I2 were used to determine statistical heterogeneity. Subgroup analysis was performed when multiple studies were in each subgroup. P value <0.05 or I2> 50 were considered to have presence of heterogeneity and random effect model was used instead of fixed effect model. Sensitivity analysis was conducted to find out the effect of a single study to the pooled analysis. A graphical funnel plot and Egg’s test were used to evaluate potential publication bias. All tests were two-sided and P value < 0.05 was considered as statistically significant, except for tests for heterogeneity.
Acknowledgements
This work is supported by grant from the National Research Foundation of Korea grants funded by the Korea government (Ministry of Science and ICT) (2016M3A9B6026921), and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Korea government (Ministry of Health and Welfare) (HI14C1277).
Author Contributions
Designing the study: Y.K. Preparing the manuscript: Y.K. Concept of study: G.H.K. Edit manuscript: G.H.K. and Y.S. Prepare figures: Y.K. and Y.S. Data analysis: Y.K. and G.H.K. Statistics: Y.K.
Data Availability
All Relevant data are within the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 2010;234:268–281. doi: 10.1111/j.0105-2896.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 2.Fousteri G, Dave A, Juntti T, von Herrath M. CD103 is dispensable for anti-viral immunity and autoimmunity in a mouse model of virally-induced autoimmune diabetes. J Autoimmun. 2009;32:70–77. doi: 10.1016/j.jaut.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erle DJ, Brown T, Christian D, Aris R. Lung epithelial lining fluid T cell subsets defined by distinct patterns of beta 7 and beta 1 integrin expression. Am J Respir Cell Mol Biol. 1994;10:237–244. doi: 10.1165/ajrcmb.10.3.7509610. [DOI] [PubMed] [Google Scholar]
- 4.Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr Opin Cell Biol. 2000;12:563–568. doi: 10.1016/S0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 5.de Vries IJ, et al. Nonspecific T-cell homing during inflammation in atopic dermatitis: expression of cutaneous lymphocyte-associated antigen and integrin alphaE beta7 on skin-infiltrating T cells. J Allergy Clin Immunol. 1997;100:694–701. doi: 10.1016/S0091-6749(97)70175-1. [DOI] [PubMed] [Google Scholar]
- 6.Shin H, Iwasaki A. Tissue-resident memory T cells. Immunol Rev. 2013;255:165–181. doi: 10.1111/imr.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anz D, et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells. International journal of cancer. 2011;129:2417–2426. doi: 10.1002/ijc.25902. [DOI] [PubMed] [Google Scholar]
- 8.Jaensson E, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. The Journal of experimental medicine. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung SS, et al. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. Journal of immunology. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 10.Webb JR, Milne K, Nelson BH. PD-1 and CD103 Are Widely Coexpressed on Prognostically Favorable Intraepithelial CD8 T Cells in Human Ovarian. Cancer. Cancer immunology research. 2015;3:926–935. doi: 10.1158/2326-6066.Cir-14-0239. [DOI] [PubMed] [Google Scholar]
- 11.Salmon H, et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, et al. CD103(+)CD8(+) T lymphocytes in non-small cell lung cancer are phenotypically and functionally primed to respond to PD-1 blockade. Cellular immunology. 2018;325:48–55. doi: 10.1016/j.cellimm.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Edwards, J. et al. CD103(+) Tumor-Resident CD8(+) T Cells Are Associated with Improved Survival in Immunotherapy-Naive Melanoma Patients and Expand Significantly During Anti-PD-1 Treatment. Clinical cancer research: an official journal of the American Association for Cancer Research, 10.1158/1078-0432.Ccr-17-2257 (2018). [DOI] [PubMed]
- 14.Flies DB, et al. Immune checkpoint blockade reveals the stimulatory capacity of tumor-associated CD103(+) dendritic cells in late-stage ovarian cancer. Oncoimmunology. 2016;5:e1185583. doi: 10.1080/2162402x.2016.1185583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massi D, et al. Baseline beta-catenin, programmed death-ligand 1 expression and tumour-infiltrating lymphocytes predict response and poor prognosis in BRAF inhibitor-treated melanoma patients. European journal of cancer. 2017;78:70–81. doi: 10.1016/j.ejca.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZQ, et al. CD103 and Intratumoral Immune Response in Breast Cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:6290–6297. doi: 10.1158/1078-0432.Ccr-16-0732. [DOI] [PubMed] [Google Scholar]
- 17.Komdeur FL, et al. CD103+ intraepithelial T cells in high-grade serous ovarian cancer are phenotypically diverse TCRalphabeta+ CD8alphabeta+ T cells that can be targeted for cancer immunotherapy. Oncotarget. 2016;7:75130–75144. doi: 10.18632/oncotarget.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohneis P, et al. Cytotoxic tumour-infiltrating T lymphocytes influence outcome in resected pancreatic ductal adenocarcinoma. European journal of cancer. 2017;83:290–301. doi: 10.1016/j.ejca.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Wang J, Chen R, Bai Y, Lu X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8:15621–15631. doi: 10.18632/oncotarget.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Workel HH, et al. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. European journal of cancer. 2016;60:1–11. doi: 10.1016/j.ejca.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Webb JR, et al. Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (alphaE/beta7 Integrin) in high-grade serous ovarian cancer. Gynecologic oncology. 2010;118:228–236. doi: 10.1016/j.ygyno.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Djenidi F, et al. CD8+ CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. Journal of immunology. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 23.Koh J, et al. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget. 2017;8:13762–13769. doi: 10.18632/oncotarget.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, Y., Wen, X., Cho, N. Y. & Kang, G. H. Intratumoral immune cells expressing PD-1/PD-L1 and their prognostic implications in cancer: a meta-analysis. Int J Biol Markers, 1724600818770941, 10.1177/1724600818770941 (2018). [DOI] [PubMed]
- 25.Zhao T, Li C, Wu Y, Li B, Zhang B. Prognostic value of PD-L1 expression in tumor infiltrating immune cells in cancers: A meta-analysis. PloS one. 2017;12:e0176822. doi: 10.1371/journal.pone.0176822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santoiemma PP, et al. Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecologic oncology. 2016;143:120–127. doi: 10.1016/j.ygyno.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 27.Zhou J, Liu L, Yang T, Lu B. Prognostic and therapeutic value of CD103(+) cells in renal cell carcinoma. Experimental and therapeutic medicine. 2018;15:4979–4986. doi: 10.3892/etm.2018.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, et al. Prognostic value of tumor-infiltrating Foxp3+ regulatory T cells in patients with breast cancer: a meta-analysis. J Cancer. 2017;8:4098–4105. doi: 10.7150/jca.21030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y, Bae JM, Li G, Cho NY, Kang GH. Image Analyzer-Based Assessment of Tumor-Infiltrating T Cell Subsets and Their Prognostic Values in Colorectal Carcinomas. PloS one. 2015;10:e0122183. doi: 10.1371/journal.pone.0122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, et al. Prognostic value of tumor-infiltrating FoxP3+ T cells in gastrointestinal cancers: a meta analysis. PloS one. 2014;9:e94376. doi: 10.1371/journal.pone.0094376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman DG, Bland JM. How to obtain the P value from a confidence interval. Bmj. 2011;343:d2304. doi: 10.1136/bmj.d2304. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, et al. CD103+ Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. The Journal of urology. 2015;194:556–562. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 34.Bosmuller HC, et al. Combined Immunoscore of CD103 and CD3 Identifies Long-Term Survivors in High-Grade Serous Ovarian Cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2016;26:671–679. doi: 10.1097/igc.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 35.Komdeur FL, et al. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology. 2017;6:e1338230. doi: 10.1080/2162402x.2017.1338230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clinical cancer research. 2014;20:434–444. doi: 10.1158/1078-0432.Ccr-13-1877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All Relevant data are within the paper.