Abstract
In India, the country with the world’s largest burden of tuberculosis (TB), most patients first seek care in the private healthcare sector, which is fragmented and unregulated. Ongoing initiatives are demonstrating effective approaches for engaging with this sector, and form a central part of India’s recent National Strategic Plan: here we aimed to address their potential impact on TB transmission in urban settings, when taken to scale. We developed a mathematical model of TB transmission dynamics, calibrated to urban populations in Mumbai and Patna, two major cities in India where pilot interventions are currently ongoing. We found that, when taken to sufficient scale to capture 75% of patient-provider interactions, the intervention could reduce incidence by upto 21.3% (95% Bayesian credible interval (CrI) 13.0–32.5%) and 15.8% (95% CrI 7.8–28.2%) in Mumbai and Patna respectively, between 2018 and 2025. There is a stronger impact on TB mortality, with a reduction of up to 38.1% (95% CrI 20.0–55.1%) in the example of Mumbai. The incidence impact of this intervention alone may be limited by the amount of transmission that has already occurred by the time a patient first presents for care: model estimates suggest an initial patient delay of 4–5 months before first seeking care, followed by a diagnostic delay of 1–2 months before ultimately initiating TB treatment. Our results suggest that the transmission impact of such interventions could be maximised by additional measures to encourage early uptake of TB services.
Introduction
India has the world’s largest burden of tuberculosis (TB)1. Over the past two decades India’s Revised National Tuberculosis Control Programme (RNTCP) has made notable progress in reducing TB deaths, through the provision of basic TB services via the public sector2–5. Nonetheless, major challenges remain: healthcare in India is dominated by the private sector, where the majority of patients first seek care6–9. Private healthcare providers often use inaccurate diagnostic tests for TB, or omit testing altogether, leading to diagnostic delays while patients cycle between different providers7,10,11. Even once patients are diagnosed, a general lack of treatment adherence monitoring and support is unfavourable for long-term treatment outcomes12. Moreover, although tuberculosis was made a notifiable disease in 201213, there remain major challenges in encouraging private providers to comply with these obligations14,15. For these reasons, in India’s recently-announced plan to eliminate TB, private sector engagement forms a key strategic priority16.
In a demonstration of private sector engagement in India, the ‘Public Private Support Agency’ (PPSA) model used a combination of patient subsidies and provider incentives to encourage higher standards of diagnosis and treatment amongst private providers17. Originally implemented in two Indian cities, Mumbai and Patna (respectively by the NGOs PATH and World Health Partners), these measures have yielded rapid increase in TB notification from the private sector3. However, their potential epidemiological impact remains unclear; measuring such impact empirically presents prohibitive challenges in the intervention coverage, population size and study duration that would be needed.
Here we take an alternative approach, using a dynamical model of TB transmission, developed to capture the complexity of careseeking in urban settings in India. The model is calibrated to detailed patient careseeking surveys in Mumbai and Patna, as well as data on TB epidemiology in these settings. While Patna is typical of an urban setting in India, Mumbai is exceptional in its high burden of MDR-TB18,19. We ask: What impact could such engagement have on TB transmission, in particular on TB incidence? What are the key drivers of this impact?
In what follows we present an overview of the model framework, with further details in the supporting information. We describe the pathway surveys, and the approach for incorporating this evidence in the model framework. We then present results for the potential epidemiological impact of private sector engagement in Mumbai and in Patna, followed by an examination of the drivers of this impact: in particular, we investigate specific types of patient and provider behaviour that matter most for TB transmission. Finally we discuss implications for controlling TB transmission in India, and important questions arising for future work.
Methods
Model overview
We developed a deterministic, compartmental model, whose overall structure is illustrated schematically in Fig. 1. The model divides the population into different states, reflecting their disease and careseeking states, with a set of coupled, differential equations capturing transmission dynamics, and the transitions between states (see appendix). We first give an overview of the essential dynamical processes captured by the model, before describing the evidence sources used to quantify these dynamics.
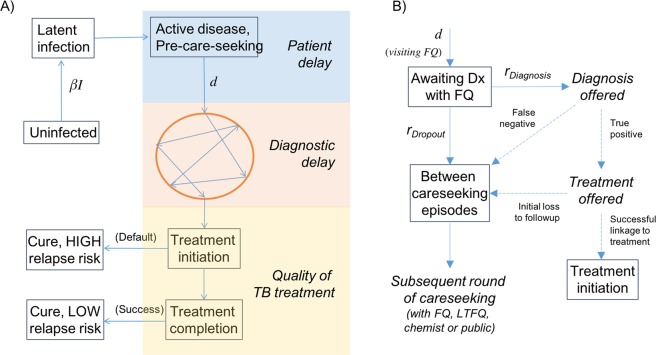
Figure 1.
Schematic illustration of the transmission model. (A) The figure shows two important parameters in the model, the annual infections per active TB case (β) and the mean, per-capita rate of careseeking once a patient develops active TB (d), which are calibrated to yield the correct ARTI and prevalence (see Table S2). The ‘bubble’ in orange denotes the sequence of providers that a patient visits before receiving a TB diagnosis. Here, we distinguish the associated ‘diagnostic delay’ with the initial ‘patient delay’. This model also includes the acquisition and transmission of multi-drug-resistant (MDR) TB, not shown here for clarity. (B) Detail of the diagnostic process depicted in the ‘bubble’ in panel (A), showing the case of a formally qualified (FQ) provider (this structure applies also to other provider types). Here and elsewhere, ‘Dx’ denotes ‘diagnosis’. Solid lines represent hazard rates in the model, while dashed lines represent proportions. Note the ‘competing hazards’ of diagnosis vs patient dropout. Terms in boxes represent compartments in the model, while terms in italics show intermediate stages, associated with the quality of TB care (accuracy of diagnosis, and treatment initiation).
We assumed that each active case of TB causes, on average, β infections per year. We further assumed that, upon development of active disease, there is a ‘patient delay’ before first seeking care. In the model equations (see supporting information), this delay is governed by the per-capita careseeking rate d. As described below, β and d are calibrated for consistency with the TB epidemiology in urban slums. Once patients enter the careseeking pathway (denoted by the circle in Fig. 1A), they visit a series of providers: the resulting ‘diagnostic delay’ is the interval from first careseeking to initiation of anti-TB treatment. This delay is governed by the timeliness with which these providers can offer an accurate TB diagnosis, and retain a patient for long enough to initiate appropriate treatment.
Upon initiating treatment, patients exit the diagnostic pathway illustrated in Fig. 1A, where the next hurdle is to complete high-quality (DOTS standard) treatment. Most patients in the private sector lack adherence support, and thus do not complete the 6-month, first-line regimen12,20: we assume that those defaulting from treatment, although immediately lacking infectiousness and being relieved of symptoms, face an increased risk of relapse in the long term, compared to patients successfully completing the 6-month regimen, with a parameter conservatively sourced from clinical trials of shorter durations of rifampicin treatment.
In this framework, a PPSA has two functions: (i) to subsidise high-quality diagnosis for patients in the private sector, increasing the probability of an accurate TB diagnosis, and thus reducing the overall diagnostic delay (depending on coverage, or the proportion of providers engaged), and (ii) providing adherence support to maximize treatment completion. In both cases, we assumed that private providers engaged by a PPSA are able to match the quality of TB care in the public sector, on these dimensions.
For simplicity we ignored HIV/TB coinfection, which is estimated to account for only 5% and 1% of notified TB cases in Maharashtra and Bihar, respectively3. However, we incorporated the acquisition and transmission of multi-drug-resistant (MDR) TB. In particular, we assumed that each infectious case of MDR-TB, not undergoing appropriate second-line treatment, causes βMDR infections per year, to be calibrated to the estimated burden of drug resistance (see below). We assumed that there is essentially no management of MDR-TB in the private sector, and populated parameters for second-line treatment outcomes in the public sector to match those reported by RNTCP3.
Epidemiological inputs
WHO estimates for incidence and prevalence, although often used to inform transmission models21–23, pose two important limitations for the present work. First, national incidence estimates for India are informed by expert opinion on the proportion of cases that are notified to RNTCP24, which itself is subject to change1. Second, WHO national estimates do not address subnational heterogeneity, and thus would not accurately reflect the epidemiological conditions in urban settings considered in our study.
Instead, to relate the model as closely as possible to the primary data available, we used the Annual Risk of TB Infection (ARTI, a measure of the intensity of transmission in a given setting), and the prevalence of TB, as estimated by subnational prevalence surveys in India. Unfortunately, neither Mumbai nor Patna has yet had a prevalence or infection survey (to inform prevalence or ARTI estimates, respectively). Nonetheless, infection surveys in Chennai and Delhi25 suggest that ARTI in urban settings is in the range of 2–3%. We adopted this range in modelling Mumbai and Patna populations as well. For prevalence, we borrowed from a recent prevalence survey in Chennai, which estimated urban prevalence at 388 cases per 100,000 population26. To accommodate the uncertainty in applying these estimates to settings outside Chennai, As both prevalence and ARTI estimates are being borrowed from other settings, we incorporated broad uncertainty in applying these estimates in the present study. For example, for prevalence estimates we adopted uncertainty intervals 25% wider than those published for Chennai (see Table S2, supporting information).
For the burden of drug resistance, we assumed that Patna is typical of the national average, with 3–5% of incident TB cases being MDR-TB. For Mumbai, we used program-reported data on routine surveillance for drug-resistant TB to populate a more extreme scenario for drug resistance, assuming that 8–16% of incident cases have MDR-TB. These inputs are summarized in Table S2, supporting information.
Patient pathways
We adopted four different categories of provider: (i) those in the public sector (DOTS facilities); (ii) private chemists; (iii) private, ‘fully qualified’ (FQ) providers with qualifications in allopathic medicine; (iv) and private, ‘less-than-fully-qualified’ (LTFQ) providers with other medical qualifications, or none at all.
We used data from community-based patient pathway surveys, recently conducted in Mumbai (76 TB patients and 196 patient-provider interactions) and Patna (64 TB patients and 121 patient-provider interactions), and described in detail elsewhere11,27. In brief, individuals in the community, who had been on TB treatment within the preceding 6 months, were administered an in-depth interview, to identify the sequence and types of providers that each patient visited before their TB diagnosis. Although subject to the usual limitations of patient recall28, this community-based survey has nonetheless cast unprecedented light on the careseeking patterns in these urban slum settings11.
A patient’s contact with a given provider may last several days, sometimes weeks: this process ends either when the provider eventually suspects and confirms TB, or when the patient drops out to visit an alternative provider. Here, we model this combination of behaviours using independent, competing exponential hazards, taking both to be specific to the type of provider involved (public, FQ, LTFQ or chemist). Figure 1B shows the overall framework: for Mumbai and Patna separately, we used the pathway survey data to estimate the hazard rates rDiagnosis, rDropout in Fig. 1B, as well as the probabilities of accurate diagnosis per provider visit. We also used this data to estimate the role of different provider types in the careseeking pathway, in particular: the proportions of patients visiting each type of provider on the first careseeking attempt, and the corresponding proportions on subsequent visits, conditional on the type of provider last seen. We used the Expectation-Maximisation algorithm as a systematic approach for estimating rates and uncertainty (see Supporting Information for further details).
For parameters related to the treatment cascade (the proportion of TB diagnoses initiating and completing treatment), we draw from a recent systematic review for the public sector29. In the absence of systematic evidence for private providers, we incorporate plausible uncertainty distributions for these parameters (Table S2, supporting information).
Simulating impact
In both Mumbai and Patna, evidence suggests a marked heterogeneity amongst providers, with certain specialist providers handling a substantially higher TB caseload than others. While this suggests important opportunities for efficiency, by ‘targeting’ such providers, in the present work, for simplicity we chose instead to measure PPSA ‘coverage’ from a patient perspective: that is, the proportion of patient-provider interactions that involve a PPSA-engaged provider. Thus, for example, we present a 75% ‘coverage’ in the understanding that – in practice – this could be brought about by recruiting fewer than 75% of providers, in a targeted way.
For a given PPSA coverage, we simulated cumulative TB incidence and mortality between 2018 and 2025. We then estimated the TB cases and deaths averted, relative to a ‘no-PPSA’ baseline, with the standard of TB care in public and private sectors projected forward without change. We simulated two types of PPSA: an ‘accurate diagnostic’ scenario in which engaged providers have diagnostic accuracy equal to those of the public sector, and a ‘timely diagnostic’ scenario which, as well as accurate diagnosis, additionally encouraged private providers to conduct a diagnostic test as early as possible (whether for TB or not). Note that, in both cases, treatment outcomes were also assumed to be improved to the level of the public sector.
Uncertainty
We used a Bayesian melding procedure30 to capture uncertainty in the epidemiological and pathway inputs described above, as well as in other input parameters in the model (see uncertainty ranges in Tables S2 and S3, supporting information). In brief, this procedure yields 100,000 parameter sets that, in ensemble, capture simultaneously the uncertainty in the parameter inputs, and in the data. Projecting the epidemiological impact of a PPSA from each of these parameter sets, under given scenarios for PPSA coverage, we then calculated the central estimate and uncertainty in impact by calculating the 2.5th, 50th and 97.5th percentiles in the outcomes of interest (lives saved, percent cases averted). We refer to these uncertainty intervals as ‘credible intervals’ (CrI) to distinguish them from the ‘confidence intervals’ arising from frequentist statistical approaches. Further details are provided in the supporting information.
The model includes several different parameters (including epidemiological inputs). To identify those parameters that are most important for model findings, we performed a multivariate sensitivity analysis on the output of the Bayesian analysis described above. In particular, we examined which model inputs accounted for the greatest amount of uncertainty in model outputs: that is, the inputs that are most influential in the precision of the model output. To do this we selected, as a model output, the percent cases averted by a PPSA intervention at 75% coverage under the ‘timely diagnostic’ scenario described above, in both cities. We computed the partial rank correlation coefficient (PRCC) between this output and each of the model parameters: in brief, the PRCC quantifies the correlation between a given model input and the model output, when variance in all other parameters has been accounted for. Those model inputs expressing the greatest PRCC are those to which the model is most sensitive.
As well as this parameter uncertainty, we additionally tested the model sensitivity to two forms of structural uncertainty: (i) First, in the simulations described above we assumed that each TB case undergoes a constant infectiousness β through time. In practice, over time β may increase (for example if bacillary load rises with symptom severity), or decrease (for example if TB patients exhaust their closest contacts as opportunities for infection), with implications for the transmission that a PPSA could impact31. To capture these scenarios in a simple way, we assumed that infectivity during the patient delay in Fig. 1A is k times that during the diagnostic delay. We tested sensitivity of model findings to k. (ii) Second, the PPSA we have modelled is a combination of interventions, each involving different indicators for the quality of TB care in the private sector. To examine the most important, we simulated a ‘partial’ PPSA that could implement improvements in all but one of the indicators for quality of care. We recorded the resulting drop in impact (percent cases averted), relative to a ‘full’ PPSA, and repeated this analysis for each of the indicators involved.
Results
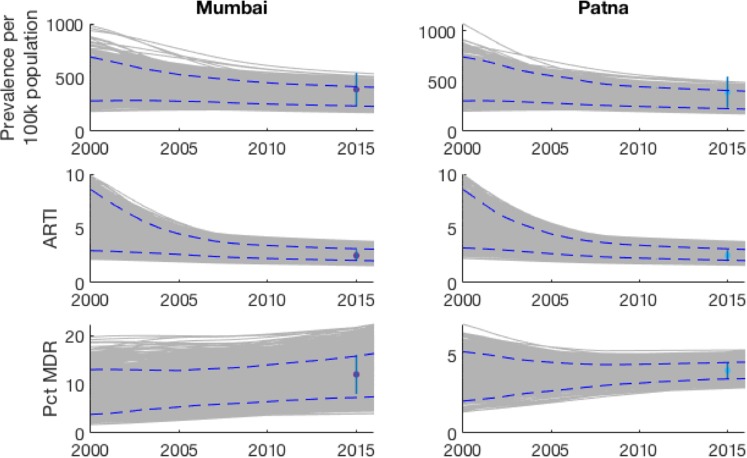
Figure 2 shows the model fits for prevalence, ARTI and percent MDR-TB, in both cities. The sampled parameters show agreement with the estimates in ARTI and prevalence data, while also accommodating the range of uncertainty in these inputs. Estimated parameter values are shown in Tables S3 and S4, supporting information. Mumbai and Patna show contrasting careseeking patterns, as illustrated by Fig. S1 in the appendix (see also Table S3). For example, chemists play a stronger role in first careseeking in Patna than in Mumbai, while for formally qualified providers the converse is the case. These differences underline the potential heterogeneity in healthcare settings across India.
Figure 2.
Illustration of the model fits to key epidemiological indicators (Prevalence, ARTI and proportion of TB cases being multi-drug resistant). Shown are the epidemic trajectories corresponding to each of the sample parameter sets (in grey); the simulated 95% credible intervals over time (blue dashed lines); and the calibration targets (points and vertical ranges, plotted in 2015).
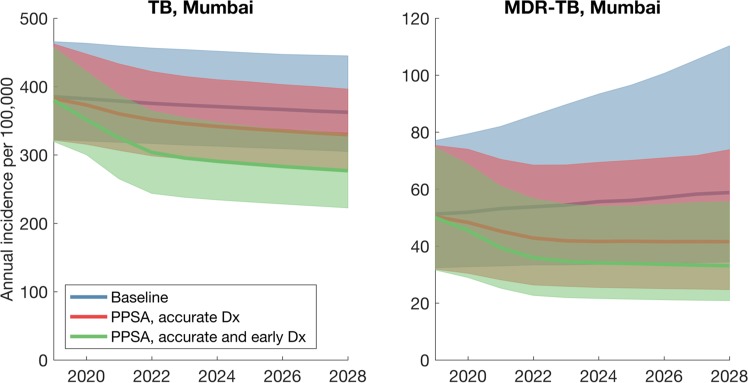
Figure 3 illustrates the potential epidemiological impact of a PPSA in Mumbai, assuming an intervention that scales up over 5 years from 2018 to cover 75% of patient visits to a provider. Such an intervention is focused on improving diagnostic accuracy and treatment outcomes in the private sector, without addressing the promptness with which a provider offers a diagnosis. A PPSA of this scale would reduce cumulative TB incidence by 8.5% (95% CrI 4.2–15.6%) over the next ten years. There is a stronger impact on MDR-TB, with a reduction of 21.2% (95% CrI 13.0–32.5%) in cumulative incidence. Further, a PPSA of this scale could have a substantial effect on TB mortality, reducing TB deaths by 21.7% (95% CrI 10.6–35.0%).
Figure 3.
Illustration of the TB dynamics under scale-up of a PPSA, in the example of Mumbai. These results capture the scenario of a PPSA being scaled up (over three years from 2018) to cover 75% of patient-provider interactions. Lines show central estimates, and shaded regions show 95% credible intervals.
If providers are additionally encouraged to order a diagnostic test as early as possible (i.e. a ‘timely diagnosis’ scenario to pre-empt patient dropout), PPSA impact increases substantially, to an incidence reduction of 21.4% (95% CrI 11.1–32.7%) and a mortality reduction of 38.1% (95% CrI 20.0–55.1%). Figure S2 (supporting information) shows similar, corresponding results for Patna. Figure S3 (supporting information) illustrates how these types of impact could vary with PPSA coverage.
To examine factors that may be limiting the impact shown in Fig. 3, we examined the model estimates for the patient and diagnostic delays illustrated in Fig. 1. As illustrated in Fig. 4, while the simulated diagnostic delay is consistent with the 1 month estimated in previous analysis8,11, results suggest that the initial patient delay could be still longer, at 4.4 months and 5.2 months in Mumbai and Patna, respectively, although with broad uncertainty around these estimates. Figure S4 in the appendix shows the potential epidemiological impact of a PPSA that is enhanced by measures to shorten the patient delay; below we discuss possible examples of such measures.
Figure 4.
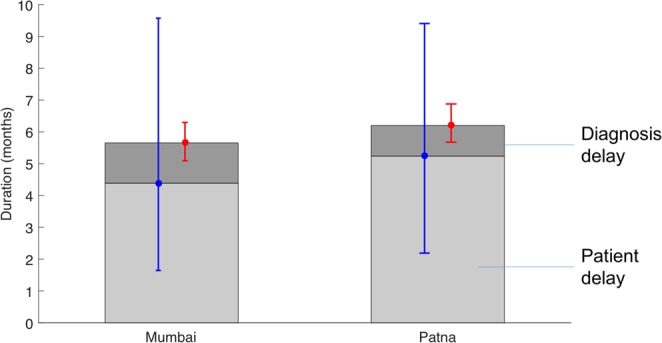
Components of the mean infectious period, i.e. the duration from the start of active disease to treatment initiation, death or self cure. Simulated in the absence of any PPSA intervention. The light grey region shows the simulated patient delay, while the dark grey region shows the delay in diagnosis (i.e. from first provider visit). Error bars in blue and red show the uncertainty in these estimates, respectively. The patient delay estimate is driven by prevalence and ARTI, while the diagnostic delay estimate is driven by the process illustrated in Fig. 1B. A PPSA addressing only patient behaviour would impact only the dark grey regions.
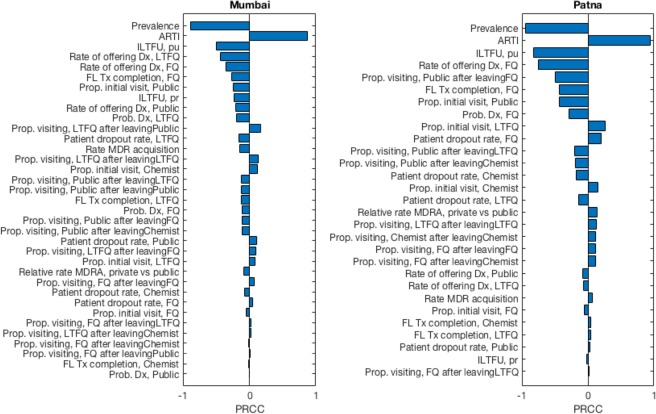
Figure 5 shows the results of parameter sensitivity analysis, in which we quantified the influence of each model input against ‘simulated impact’, the latter measured as the percent cases averted by a PPSA at 75% coverage in both Mumbai and Patna (corresponding to the green shaded region in Fig. 3). Figure 5 illustrates the importance of epidemiological inputs, for this output. In both cities, the assumed prevalence and ARTI are the model inputs accounting for the greatest amount of output uncertainty. Where the true value of prevalence in either city lies towards the lower end of the assumed range, the percent cases averted approaches the upper end of the uncertainty illustrated in Fig. 3, and vice versa for ARTI. In both settings the levels initial loss to followup in the public sector (i.e. those diagnosed who do not initiate treatment) is also a leading factor; remaining parameters, to which the model is less sensitive, depend on the local conditions in both of these settings.
Figure 5.
Multivariate sensitivity analysis of model inputs (parameters and data). Bars show the partial rank correlation coefficient (PRCC) between each model input and a selected output: ‘simulated impact’, or the percent cases averted by a PPSA acting at 75% coverage, with accurate and early diagnosis. Figures show that in both Mumbai and Patna, the two model inputs to which simulated impact is most sensitive are the prevalence and ARTI. Prevalence has a negative partial rank correlation with impact: that is, lower values of prevalence are associated with higher levels of impact, and vice versa for ARTI. Note that the combined effect of uncertainty in all of these parameters corresponds to the full uncertainty range illustrated in Fig. 3A, green shaded region. This range indicates the maximum extent to which model outputs could diverge from central estimates, subject to the assumed uncertainty ranges in model inputs.
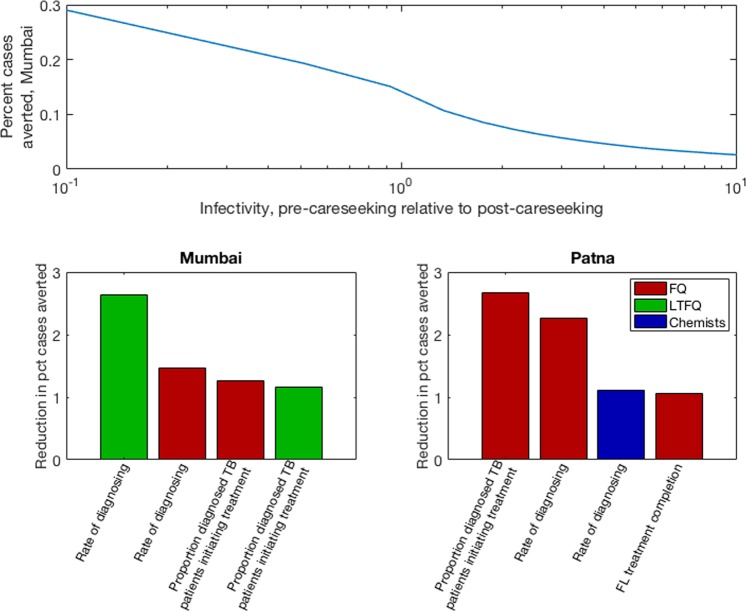
In addition to addressing parameter uncertainty we finally conducted sensitivity analysis to some underlying assumptions. First, as described above, we allowed for differential infectiousness in the two stages of delay shown in Fig. 4. Figure 6A shows results for the percent cases averted, as a function of the longitudinal variation in infectiousness. As expected, scenarios with increasing infectivity over a patient’s clinical course (decreasing k in the figure) yield greater predicted impact of a PPSA.
Figure 6.
Sensitivity analysis for key assumptions in the model. (A) Effect of assumptions for how TB infectivity varies during the clinical course. Shown is the impact of a PPSA at 75% coverage in Mumbai (percent cases averted over ten years). The x-axis shows a range of scenarios for the infectivity during the patient delay, relative to that during the diagnostic delay. (B,C) Identifying key elements of private provider behaviour. The figures show the drop in overall impact that results, when a PPSA that fails to improve the provider behaviour shown (while addressing all remaining provider behaviours). For clarity, plots show only the four most important factors in each setting. Bar colours denote different provider types, as shown in the right-hand legend. Panel (B) shows results for Mumbai, while panel (C) shows results for Patna.
Second, we examined the sensitivity of projected impact to the assumption that all PPSA activities are performed effectively. We aimed to identify which activities accounted for most of the impact shown in Fig. 3. Results, shown in Fig. 6B,C, suggest that in Mumbai, the quality of diagnosis and treatment amongst LTFQ providers is key. In Patna, by contrast, the quality of care amongst FQ providers is most important. Echoing the contrasting pathways illustrated in Fig. S1, these results highlight how intervention priorities in different cities may need to be tailored to the local conditions.
Discussion
Engaging with India’s vast, fragmented private healthcare sector is a key step in enhancing TB control in India. Our work adds to other modelling studies capturing the role of the private sector in TB care in India, including a multi-model comparison examining packages of interventions in the context of the End TB goals23, and the potential impact of implementing molecular diagnostics in the private sector21. A strength of the current work is that it is informed by unique, detailed patient pathway data from Mumbai and Patna. This data enables us to analyse the relative importance of the different delays illustrated in Fig. 1, to a greater extent than in previous work.
Our findings illustrate that a PPSA taken to scale in urban settings, such as Mumbai and Patna, could have a meaningful impact on TB burden (Figs 3 and S3). Improved diagnosis and treatment adherence could strongly reduce TB mortality. Moreover, the use of rapid molecular tests in the private sector could have strong implications for MDR-TB: by facilitating the early recognition of drug sensitivity status, such measures could turn a growing drug resistance epidemic into a diminishing one (Fig. 3B, blue vs green curves).
Nonetheless, in terms of overall TB burden, our results suggest that engaging the private sector alone will not be enough to meet the country’s aspirations for TB elimination. Rather, such measures lay the foundations for TB control by maximising the quality and coordination of basic TB services, across India’s vast and fragmented healthcare system16. To explain limitations on PPSA transmission impact, our work highlights the complexity of the delay from symptoms to treatment initiation, showing how it arises from a combination of factors. For example, while the importance of diagnosis accuracy is well-recognised8,11,32, pre-empting patient dropout, through offering a rapid diagnosis, can be as impactful for the diagnostic delay (Figs 3 and 6B,C). Second, our results suggest that the ‘patient delay’ in Fig. 1A may play a larger role than previously recognized (Fig. 4).
We note that this latter result is not directly measured, but inferred through reconciling ARTI and prevalence in the model. Previous studies have approached patient and diagnostic delays through retrospective patient interviews in various settings in India: a recent meta-analysis of these studies8 found a median patient delay of around 18 days. To our knowledge there is no other independent, direct evidence for the ‘true’ patient delay. Nonetheless, there are some notable comparisons in a recent TB prevalence survey in Gujarat state. Of the bacteriologically positive TB cases, only 28% had sought care for their symptoms, including 11% that were on TB treatment33. Although cross-sectional, these survey findings appear consistent with the picture of substantial transmission occurring independently of the ‘diagnostic delay’.
There are several possible reasons for these discrepancies between model and prevalence survey findings on the one hand, and patient interviews on the other. For example, in urban areas with poor air quality, prolonged cough is a common symptom: TB patients may tend to visit a provider when their symptoms become more advanced (e.g. fever), ultimately reporting only the duration of these more developed symptoms. Alternatively, the patient delay may truly be as short as 18 days, but only amongst those patients who seek care: there may remain a patient population who never contact the healthcare system, for example due to the opportunity costs of doing so. These factors may differ by region in India, as well as by gender and urban/rural setting. As illustrated by Fig. S4, mitigating these factors could maximize the impact of a PPSA.
Approaches towards mitigating these factors could involve active case-finding (ACF)34. India’s recent National Strategic Plan underlines the potential importance of ACF in risk groups such as urban slums16, while recent work in Viet Nam has also demonstrated the potential value of screening close contacts of diagnosed TB cases, together with longitudinal followup of these contacts35. However, it is also possible for the patient delay to be impacted by measures to improve the demand for TB services; for example, social protection mechanisms36 could have the secondary effects of encouraging TB symptomatics to come forward for care37. Such effects are currently hypothetical, and present an important evidence gap for future studies to address.
As with any modelling approach, our model has several limitations to note. First, it takes a simplified view of the host population, essentially averaging over variations by gender and age. In future, better data on careseeking and the quality of care with respect to these factors would support a more refined approach incorporating these factors. Second, our work concentrates on two major cities in India, informed by the available, community-based studies on careseeking pathways. Further work, deploying such surveys more broadly, should explore to what extent these findings may be generalized to other cities India; one potentially important factor is the greater HIV burden in states like Andhra Pradesh3. Moreover, this work does not address potential impact in rural settings. Indeed, recent work has highlighted the phenomenon of TB prevalence being higher in rural areas than urban38, suggesting even longer delays before initiation of appropriate TB treatment: there is therefore a pressing need for a better understanding of healthcare utilisation in these settings. Third, we have made several simplifying assumptions on provider behaviour, namely that ‘engaged’ providers would show the same standard of care as in the public sector. As noted above, it is promising that the PPSA pilots have shown a dramatic increase in the number of TB cases being notified1,3: ongoing data collection during the pilots will cast light on the extent to which the quality of TB care has been improved. Lastly, these results are quite sensitive to underlying assumptions about prevalence and ARTI, as well as to transmission over the course of illness. If more transmission is occurring at later stages of illness, then private provider engagement could more effectively interrupt transmission and avert twice as many cases as our baseline uninformed assumption of uniform infectivity. Objective data on the ‘transmission curve’ would be useful to clarify the appropriate baseline for these and most TB models.
In summary, private sector engagement is a key foundation for managing TB in India. In addition to its direct benefit to TB patients, an engaged private sector will also enable the maximum deployment of future interventions against TB in India. While building such favourable conditions for TB control, there is an urgent need to identify where TB transmission is occurring: only by addressing this transmission will it truly be possible to accelerate declines in India’s vast TB epidemic.
Supplementary information
Author Contributions
N.A., P.D. conceived the study. N.A., S.D., S.S. performed the analysis; S.K., R.R., B.V., N.K., D.G., K.R., S.A.N. and P.D. contributed to the interpretation and validation of model results; N.A. wrote a first draft of the manuscript, and all authors contributed to the final draft.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-39799-7.
References
- 1.World Health Organization. Global tuberculosis report 2017. WHO (2017). Available at, http://www.who.int/tb/publications/global_report/en/. (Accessed: 2nd September 2018).
- 2.World Health Organization. A brief history of tuberculosis control in India, ISBN 978 92 4 150015 9 (2010).
- 3.Revised National Tuberculosis Control Programme. RNTCP Annual Status Report. Available at, https://tbcindia.gov.in/showfile.php?lid=3314 (2018).
- 4.Mandal, S., Chadha, V. K., Laxminarayan, R. & Arinaminpathy, N. Counting the lives saved by DOTS in India: A model-based approach. BMC Med. 15 (2017). [DOI] [PMC free article] [PubMed]
- 5.Goodchild M, et al. A cost-benefit analysis of scaling up tuberculosis control in India. Int. J. Tuberc. Lung Dis. 2011;15:358–62. [PubMed] [Google Scholar]
- 6.Satyanarayana S, et al. From where are tuberculosis patients accessing treatment in India? Results from a cross-sectional community based survey of 30 districts. Plos One. 2011;6:e24160. doi: 10.1371/journal.pone.0024160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapoor SK, Raman AV, Sachdeva KS, Satyanarayana S. How Did the TB Patients Reach DOTS Services in Delhi? A Study of Patient Treatment Seeking Behavior. PLoS One. 2012;7:e42458. doi: 10.1371/journal.pone.0042458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sreeramareddy CT, Qin ZZ, Satyanarayana S, Subbaraman R, Pai M. Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int. J. Tuberc. Lung Dis. 2014;18:255–266. doi: 10.5588/ijtld.13.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arinaminpathy, N, et al. The number of privately treated tuberculosis cases in India: an estimation from drug sales data. Lancet Infect. Dis. 16 (2016). [DOI] [PMC free article] [PubMed]
- 10.Das J, et al. Use of standardised patients to assess quality of tuberculosis care: a pilot, cross-sectional study. Lancet Infect. Dis. 2015;15:1305–1313. doi: 10.1016/S1473-3099(15)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mistry N, et al. Durations and Delays in Care Seeking, Diagnosis and Treatment Initiation in Uncomplicated Pulmonary Tuberculosis Patients in Mumbai, India. PLos One. 2016;11:e0152287. doi: 10.1371/journal.pone.0152287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Udwadia ZF, Pinto LM, Uplekar MW. Tuberculosis Management by Private Practitioners in Mumbai, India: Has Anything Changed in Two Decades? Plos One. 2010;5:e12023. doi: 10.1371/journal.pone.0012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhaumik S, Biswas T. India makes tuberculosis a notifiable disease. CMAJ. 2012;184:E519–20. doi: 10.1503/cmaj.109-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas BE, et al. Perceptions of Private Medical Practitioners on Tuberculosis Notification: A Study from Chennai, South India. Plos One. 2016;11:e0147579. doi: 10.1371/journal.pone.0147579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philip S, et al. ‘They know, they agree, but they don’t do’ - the paradox of tuberculosis case notification by private practitioners in Alappuzha district, Kerala, India. Plos One. 2015;10:e0123286. doi: 10.1371/journal.pone.0123286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revised National Tuberculosis Control Programme. National Strategic Plan for Tuberculosis Elimination. Available at, https://tbcindia.gov.in/WriteReadData/NSP.Draft 20.02.2017 1.pdf. (Accessed: 2nd September 2018) (2017).
- 17.Pai M, Dewan P. Testing and treating the missing millions with tuberculosis. Plos Med. 2015;12:e1001805. doi: 10.1371/journal.pmed.1001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaakidis P, et al. Alarming Levels of Drug-Resistant Tuberculosis in HIV-Infected Patients in Metropolitan Mumbai, India. PLos One. 2014;9:e110461. doi: 10.1371/journal.pone.0110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair, S. A. et al. Factors associated with tuberculosis and rifampicin-resistant tuberculosis amongst symptomatic patients in India: A retrospective analysis. PLos One11 (2016). [DOI] [PMC free article] [PubMed]
- 20.Uplekar M, Juvekar S, Morankar S, Rangan S, Nunn P. Tuberculosis patients and practitioners in private clinics in India. Int. J. Tuberc. Lung Dis. 1998;2:324–9. [PubMed] [Google Scholar]
- 21.Salje H, et al. The Importance of Implementation Strategy in Scaling Up Xpert MTB/RIF for Diagnosis of Tuberculosis in the Indian Health-Care System: A Transmission Model. PLos Med. 2014;11:e1001674. doi: 10.1371/journal.pmed.1001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachdeva, K. S. et al. The potential impact of up-front drug sensitivity testing on India’s epidemic of multi-drug resistant tuberculosis. PLos One10 (2015). [DOI] [PMC free article] [PubMed]
- 23.Houben, R. M. G. J. et al. Feasibility of achieving the 2025 WHO global tuberculosis targets in South Africa, China, and India: a combined analysis of 11 mathematical models. Lancet Glob. Heal. 4 (2016). [DOI] [PMC free article] [PubMed]
- 24.Cowling K, Dandona R, Dandona L. Improving the estimation of the tuberculosis burden in India. Bull. World Health Organ. 2014;92:817–25. doi: 10.2471/BLT.13.129775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopi PG, et al. Annual risk of tuberculosis infection in Chennai city. Indian J. Tuberc. 2008;55:157–61. [PubMed] [Google Scholar]
- 26.Dhanaraj B, et al. Prevalence and risk factors for adult pulmonary tuberculosis in a metropolitan city of South India. PLos One. 2015;10:e0124260. doi: 10.1371/journal.pone.0124260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mistry N, Lobo E, Shah S, Rangan S, Dholakia Y. Pulmonary tuberculosis in Patna, India: Durations, delays, and health care seeking behaviour among patients identified through household surveys. J. Epidemiol. Glob. Health. 2017;7:241–248. doi: 10.1016/j.jegh.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmier JK, Halpern MT. Patient recall and recall bias of health state and health status. Expert Rev. Pharmacoecon. Outcomes Res. 2004;4:159–163. doi: 10.1586/14737167.4.2.159. [DOI] [PubMed] [Google Scholar]
- 29.Subbaraman R, et al. The Tuberculosis Cascade of Care in India’s Public Sector: A Systematic Review and Meta-analysis. Plos Med. 2016;13:e1002149. doi: 10.1371/journal.pmed.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alkema L, Raftery AE, Brown T. Bayesian melding for estimating uncertainty in national HIV prevalence estimates. Sex. Transm. Infect. 2008;84:i11–i16. doi: 10.1136/sti.2008.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arinaminpathy, N. & Dowdy, D. Understanding the incremental value of novel diagnostic tests for tuberculosis. Nature528 (2015). [DOI] [PubMed]
- 32.Sreeramareddy CT, Panduru KV, Menten J, Van den Ende J. Time delays in diagnosis of pulmonary tuberculosis: a systematic review of literature. BMC Infect. Dis. 2009;9:91. doi: 10.1186/1471-2334-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiran Rade, Gujarat Prevalence Survey: personal communication (2018).
- 34.Lönnroth K, et al. Systematic screening for active tuberculosis: rationale, definitions and key considerations [State of the art series. Active case finding/screening. Number 1 in the series] Int. J. Tuberc. Lung Dis. 2013;17:289–298. doi: 10.5588/ijtld.12.0797. [DOI] [PubMed] [Google Scholar]
- 35.Fox GJ, et al. Household-Contact Investigation for Detection of Tuberculosis in Vietnam. N. Engl. J. Med. 2018;378:221–229. doi: 10.1056/NEJMoa1700209. [DOI] [PubMed] [Google Scholar]
- 36.Revised National Tuberculosis Control Programme. Direct Benefit Transfer - Central TB Division. Available at, https://tbcindia.gov.in/index1.php?lang=1&level=1&sublinkid=4807&lid=3316. (Accessed: 2nd September 2018).
- 37.Boccia D, et al. Modelling the impact of social protection on tuberculosis: the S-PROTECT project. BMC Public Health. 2018;18:786. doi: 10.1186/s12889-018-5539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandey, S., Chadha, V. K., Laxminarayan, R. & Arinaminpathy, N. Estimating tuberculosis incidence from primary survey data: A mathematical modeling approach. Int. J. Tuberc. Lung Dis. 21 (2017). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.