Abstract
Calcium carbonates (CaCO3) often accumulate in mangrove and seagrass sediments. As CaCO3 production emits CO2, there is concern that this may partially offset the role of Blue Carbon ecosystems as CO2 sinks through the burial of organic carbon (Corg). A global collection of data on inorganic carbon burial rates (Cinorg, 12% of CaCO3 mass) revealed global rates of 0.8 TgCinorg yr−1 and 15–62 TgCinorg yr−1 in mangrove and seagrass ecosystems, respectively. In seagrass, CaCO3 burial may correspond to an offset of 30% of the net CO2 sequestration. However, a mass balance assessment highlights that the Cinorg burial is mainly supported by inputs from adjacent ecosystems rather than by local calcification, and that Blue Carbon ecosystems are sites of net CaCO3 dissolution. Hence, CaCO3 burial in Blue Carbon ecosystems contribute to seabed elevation and therefore buffers sea-level rise, without undermining their role as CO2 sinks.
Calcium carbonates (CaCO3) often accumulate in mangrove and seagrass sediments. Here the authors conducted a meta-analysis of inorganic carbon burial rates in mangrove and seagrass sediments and found that CaCO3 burial contributes to Blue Carbon ecosystems’ capacity to offset sea-level rise without undermining the role as CO2 sinks.
Introduction
Mangrove forests and seagrass meadows have the capacity to elevate the seabed through the accretion of inorganic and organic particles1 at global rates of ~0.5 and ~0.2 cm yr−1, respectively1. Sediment accretion in mangrove forests and seagrass meadows leads to the sequestration of organic carbon (Corg)2,3 originating from within and outside of the vegetated ecosystem4. Although mangroves and seagrass ecosystems occupy only a small fraction of the total coastal area (< 2%), they contribute 10% and 25% to the yearly Corg sequestration in the coastal zone1,5, respectively. Recognition of mangrove and seagrass meadows, together with saltmarshes, as sites of intense Corg burial led to the formulation of Blue Carbon strategies to mitigate and adapt to climate change, through conservation and restoration of these ecosystems1,6–8. The focus on Blue Carbon has provided substantial impetus to assess sediment Corg concentrations and burial rates in vegetated coastal ecosystems, which recently have been widely reviewed9.
Corg generally represents a minor fraction (2–3%) of buried material within mangrove and seagrass sediments10,11 (although this is highly variable12), the rest being siliciclastic and carbonate particles. A global assessment of the concentration of inorganic carbon concluded that Cinorg can exceed Corg concentration in seagrass sediments13. Seagrass and mangrove plants do not calcify per se; however, they provide habitats for an abundant associated calcifying fauna and flora (e.g., crabs, sea stars, snails, bivalves, calcified algae, foraminifera), whose shells and skeletons may be deposited and buried in the sediment along with the plant litter, and the organic and inorganic particles imported from adjacent ecosystems.
Counterintuitively, CaCO3 production represents a source of CO2 to the atmosphere, as calcification produces CO2 with a ratio of ~0.6 mol of CO2 emitted per mol of CaCO3 precipitated14. This has led to the argument that high CaCO3 burial may partially offset CO2 sequestration associated with Corg burial in some seagrass meadows and mangrove forests15. However, there are several caveats that affect these arguments and render inferences on the role of Blue Carbon ecosystems as net CO2 sinks or sources inconclusive13,16, based on the comparison of Corg and Cinorg sediment burial rates. To date, very few articles report the burial rates of CaCO3 in mangrove and seagrass ecosystems15–17, and the role of CaCO3 burial in sediments and CO2 emissions depends on the balance between dissolution and production. If CaCO3 dissolution equals local calcification, then the burial of CaCO3 is supported exclusively by allochthonous inputs and is neutral in terms of CO2 emissions or sequestration. If dissolution exceeds local calcification, then CaCO3 dynamics add to the CO2 sink capacity of Blue Carbon ecosystems, even if CaCO3, which must be subsidized from allochthonous sources, is buried in the sediments. Only if CaCO3 dissolution is lower than local calcification does CaCO3 burial result in CO2 emissions.
Here we address the current gap in global estimates of Cinorg burial in seagrass and mangrove ecosystems by providing first estimates of contemporary (last century) Cinorg burial rates. We rely on a compilation and analysis of data on sediment chronologies (i.e., including radiometric dating of sediment cores with 210Pb) and Cinorg concentrations from around the world (Fig. 1). We compare burial, calcification and dissolution rates in three locations where most of the carbon mass balance terms were available. We then address the role of CaCO3 burial in CO2 emissions by resolving the source of the CaCO3 buried in seagrass meadows as either allochthonous or autochthonous (i.e., from associated flora and fauna). We conclude that the high amounts of CaCO3 found in Blue Carbon sediments can not be converted into CO2 emissions.
Fig. 1.
World map of sediment cores locations. Brown circles: mangrove cores locations; blue: seagrass cores locations; yellow: seagrass cores non-dated but with inorganic carbon content measured13
Results
Global disparities in Blue Carbon sediments
CaCO3 supports an important part of sediment accretion rates (SARs) in seagrass ecosystems, although with large geographical disparities and a non-normal distribution (Shapiro–Wilks test, p < 0.001). Indeed, in 40% of global locations, the CaCO3 concentration was under 10% dry weight (DW), whereas in 28% of locations the CaCO3 content exceeded 80 %DW (see Supplementary Figure 1a). Overall, the median (interquartile range: IQR) global concentration of CaCO3 in seagrass meadow sediments was 61 (56) %DW (mean ± SE of 54 ± 7).
In mangrove forests, we observe a large difference between the mean (± SE) and the median (IQR) CaCO3 concentration: 21 ± 11% and 3 (31)%, respectively. This is explained by the strong non-normal distribution between the eight study locations examined, including a group of five locations with < 5 %DW CaCO3 in their sediments and three locations with CaCO3 contents between 20 and 75 %DW (Shapiro–Wilks test, p < 0.001, see Supplementary Fig. 1b). Converted into Cinorg concentrations (after normalization for the sediment bulk density), we obtain median (IQR) Cinorg concentrations in seagrass and mangrove sediments of 59 (66) and 1 (21) mgCinorg cm−3, respectively (means ± SE of 63 ± 11 and 35 ± 17 mgCinorg cm−3) (Fig. 2a).
Fig. 2.
Sediment cores data. a Inorganic carbon (Cinorg) concentration, b sediment accumulation rates (SAR), and c Cinorg burial rates. The x represents the mean. Bars are the first and last quartile
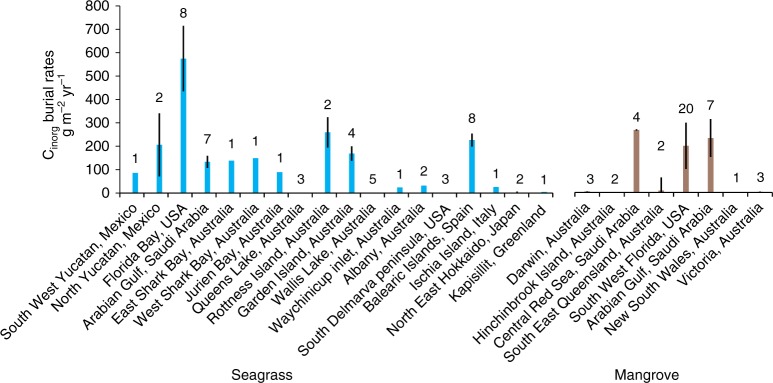
Using the median SARs in seagrass and mangrove ecosystems compiled in this study (0.22 and 0.23 cm yr−1, respectively; Fig. 2b), we estimate median (IQR) Cinorg burial rates in seagrass and mangrove ecosystems of 87 (154) and 6 (207) gCinorg m−2 yr−1, respectively (means ± SE of 182 ± 94 and 90 ± 43 gCinorg m−2 yr−1) (Fig. 2c, Fig. 3). These values correspond to vertical accretion rates of CaCO3 of the order of 0.1 and 0.001 cm yr−1 in seagrass and mangrove ecosystems, respectively. Our mean SAR values agree with previously reported global values1,3. However, our new estimates of burial rates are lower than the previous, indirect median estimate of Cinorg burial rate of 108 gCinorg m−2 yr−1 (mean ± SE of 126 ± 31 gCinorg m−2 yr−1) reported by Mazarrasa et al.13.
Fig. 3.
Inorganic carbon burial rates in all locations. Mean Cinorg burial rates in all locations in sediment cores for seagrass meadows and mangrove forests, organized from low to high latitudes. Bars are the SE. Labels are the number of cores per location
Global annual burial rates of Cinorg
Scaling up to the global seagrass coverage (150,000–600,000 km2)9, the annual burial rate of Cinorg ranged from 13 to 52 TgCinorg yr−1 for the twentieth century (Table 1). Partitioning between tropical and non-tropical seagrass meadows as in Mazarrasa et al.13 showed that 90% of the global Cinorg burial occurs in the tropics (Table 1). In seagrass meadows, our estimates of global burial of Cinorg are equivalent to 31–55% of the available estimates of contemporary Corg burial rates (48–112 TgCorg yr−1)1,7, depending on the estimated global seagrass coverage considered. If all buried CaCO3 is locally produced (i.e., of autochthonous origin), the burial rates of Cinorg in seagrass meadows would represent emissions of 8–37 TgC yr−1 to the atmosphere and thus would offset their role as CO2 sinks through the sequestration of Corg by ~17–33%.
Table 1.
Median (mean) global Cinorg burial rates for seagrass meadows and mangrove forests considering one, and, for seagrass, two world regions (tropical and higher latitudes)
| Burial rate, (TgCinorg yr−1) | |||||
|---|---|---|---|---|---|
| Global | Tropical | Higher lat. | Sum | ||
| Seagrass | This study | 13(27)–52(109) | 14(41)–57(163) | 1(3)–5(14) | 15(43)–62(177) |
| Mazarassa et al.13 | 19(28)–65(79) | ||||
| Mangrove | This study | 0.8(12) | |||
The extent of global mangrove coverage yields a median burial rate of 0.8 TgCinorg yr−1 (mean of 13 TgCinorg yr−1) (Table 1). This value should be considered as a first-order estimate because of the scarcity of data available on Cinorg burial rates in mangroves and because of the non-normal distribution between CaCO3-rich and CaCO3-poor mangrove sediments (Supplementary Figure 1b). When comparing with the global Corg burial rates estimate of 31 TgCorg yr−1 3, the median Cinorg burial rates would correspond to a negligible reduction of net atmospheric CO2 uptake. However, assuming that all sedimentary CaCO3 was produced in situ, the Cinorg burial rates can largely outweigh Corg burial in CaCO3-rich mangroves. For example, the Cinorg burial rate corresponds to 10–20 times the Corg burial rate in the Arabian Peninsula17–19.
Discussion
CaCO3 burial in Blue Carbon ecosystems is the balance between inputs (autochthonous and allochthonous) and losses (dissolution and export). Assessments of the mass balance of CaCO3 in seagrass meadows are few and none have been reported, to our knowledge, in mangrove forests. For seagrass ecosystems, we assessed the balance between calcification, dissolution and burial of CaCO3 in three locations: the Balearic Islands, Spain20,21, Shark Bay in Western Australia22 and Florida Bay, USA23,24 (Table 2).
Table 2.
Burial rates of CaCO3 compared to calcification rates in seagrass ecosystems
| Community production rate of CaCO3 | Community net calcification rate | Sediment | |||||
|---|---|---|---|---|---|---|---|
| gCaCO3 m−2 yr−1 | gCinorg m−2 yr−1 | gCaCO3 m−2 yr−1 | gCinorg m−2 yr−1 | %CaCO3 | gCaCO3 m−2 yr−1 | gCinorg m−2 yr−1 | |
| Florida Bay, USA | 62623,24 | 75 | 1825 | 2.2 | 82 ± 2 | 4792 ± 756 | 756 ± 91 |
| Balearic Islands, Spain | 6820 | 8 | 5121 | 6 | 81 ± 3 | 1886 ± 214 | 226 ± 30 |
| West Shark Bay, Australia | 375 ± 6222 | 45 ± 7 | 29522 | 35 | 60 ± 5 | 1240 ± 232 | 149 ± 30 |
Comparison between seagrass-associated community production rate of carbonate (obtained from standing stock assessments and leaves or calcifiers turnover rates) and community net calcification rates (balance between calcification and dissolution, calculated from variations of total alkalinity) from the literature, and carbonate burial rate in three locations with carbonate-rich sediments
The most comprehensive assessment of seagrass carbon budgets is that reported for a Mediterranean Posidonia oceanica meadow at Magalluf (Mallorca Island, Spain)20,21,25,26. In this meadow, Barrón et al.21 estimated a net CO2 uptake by primary production of 8.4 gC m−2 yr−1. This estimate was corroborated by the Corg burial rate, estimated independently, at 9 ± 2 gCorg m−2 yr−1 27. Barrón et al.21 also estimated net calcification rates of 51 gCaCO3 m−2 yr−1, corresponding to 6 gCinorg m−2 yr−1. This amount of calcification would result in a CO2 emission of 3.6 gC m−2 yr−1 (0.6-fold the net calcification14). The CO2 emission by calcification therefore represents an offset of 40% of the CO2 uptake from net primary production (thereby yielding a total CO2 sequestration of 4.8 gC m−2 yr−1 21). However, the Cinorg burial rate in this meadow is estimated here at 226 gCinorg m−2 yr−1. This is two orders of magnitude greater than the net calcification rate of 6 gCinorg m−2 yr−1 21(Table 2). This implies that about 90% of the CaCO3 burial in this seagrass meadow must be supported by allochthonous inputs. Therefore, calculation of the CO2 sequestration by comparing Corg and Cinorg burial rates or stocks would have concluded that this meadow is a strong source of CO2, whereas estimates of calcification rates and net primary production concludes that it is a sink (as confirmed independently through air–sea flux measurements26).
Similarly, in Shark Bay, the burial of Cinorg is four times higher than the independently reported net calcification rate22 (Table 2). This again could require large allochthonous carbonate inputs.
In Florida Bay, the low ratio between Corg and Cinorg concentration in the sediment (g cm−3) implied that seagrass meadows may be a net source of CO2 to the atmosphere15. However, such assessment requires consideration of the full carbon mass balance, including accounting for allochthonous inorganic carbon inputs and the balance between calcification and dissolution in the meadows. The contemporary Cinorg burial rates in Florida Bay are approximately ninefold higher than the global median, whereas median SAR is about fourfold higher than estimated globally, in an area where 80% of the sediment dry mass is composed of CaCO3. However, attempts to assess the gross or net calcification rates in the area yielded values one and two orders of magnitude lower than the estimated CaCO3 burial rates (Table 2)23,24. In contrast, past geological work in the Bay has suggested that it is a net producer of CaCO328. It is likely to be that some areas within this large Bay act as sources of CaCO3 and some others as sinks, helping explain the discrepancy between reported production and burial estimates. Hence, internal redistribution of CaCO3 production within Florida Bay needs to be considered when drawing inferences on the role of seagrass meadows from sediment composition.
These three example locations are in areas close to coral reefs and/or terrestrial lithogenic sources of CaCO3. We could not find estimates of calcification rates (net or gross) in areas without external sources of CaCO3. The discrepancies between calcification rates and burial rates in the three example locations could indicate that an important fraction of CaCO3 burial (> 90%) is supported by CaCO3 produced elsewhere and trapped in the seagrass sediments. This conclusion is consistent with comparable Cinorg concentrations within and outside seagrass meadows, whereas, in contrast, Corg concentrations are higher in seagrass sediments13. A large role of Cinorg import is also consistent with the large CaCO3 export from coral reefs to adjacent waters, equivalent to 25–50% of the CaCO3 production, predominantly to reef lagoons27, where seagrass meadows and mangroves often grow. Mangroves, seagrass and saltmarsh ecosystems are likely to be sites of net carbonate dissolution. Roots of marine plants release organic compounds and oxygenate the sediments during the day, promoting microbial aerobic remineralization of organic matter, thereby increasing sedimentary respiratory CO229,30 and/or stimulating the re-oxidation of reduced metabolites. These processes result in the release of strong acids (e.g., H2SO4, HNO3)31–33, which leads to CaCO3 dissolution in the sediment34,35 (although re-precipitation can occur34).
Dissolution of CaCO3 might also be influenced by the CO2 system in the water column of Blue Carbon ecosystems. Respiration and photosynthesis of the flora and fauna, together with sediment redox processes in seagrass and mangrove ecosystems, strongly influence the chemistry of the water column, generating large diel amplitudes of the saturation state for CaCO3 (Ω) with a tendency towards dissolution or the reduction of calcification at nighttime, amplified at low tide36–40. The dissolution of allochthonous CaCO3 in carbonate platform areas, caused directly or indirectly by metabolism of the marine vegetation and associated biota, leads to a reduction in pCO2 through the release of fossilized total alkalinity. This sink of atmospheric CO2 should be incorporated into the Blue Carbon framework. A recent assessment considers alkalinity addition through the dissolution of allochthonous carbonate as a very effective geo-engineering approach to remove atmospheric CO2 and mitigate climate change41,42.
Similarly, saltmarshes are not known to host high levels of calcifying organisms but can accumulate CaCO3 from allochthonous sources. In arid tropical saltmarshes of the Western Arabian Gulf, dominated by succulent shrubs, a concentration of CaCO3 of 57 ± 8% in sediments and a contemporary burial rate of Cinorg of 100 ± 15 gCinorg m−2 yr−1 (mean ± SE) were found17. In a temperate saltmarsh of the Western Scheldt estuary in the Netherlands, a concentration of CaCO3 of 14 ± 1% and a high contemporary burial rate of 467 ± 99 gCinorg m−2 yr−1, mostly due to high SAR (1.1 ± 0.3 cm yr−1), were measured43. Yet, this does not imply that CaCO3 dynamics have a negligible role in saltmarsh carbon budgets, as they may still act as sites of net dissolution of CaCO3, adding to the removal of CO2 associated with Corg burial. A dissolution rate of 24–96 gCinorg m−2 yr−1 was estimated in the sediment of the saltmarshes of the Eastern Scheldt estuary, corresponding to ~85% of the Cinorg burial rate44.
To further examine the conclusion that Blue Carbon ecosystems are sites of substantial allochthonous CaCO3 deposition, based on existing mass balances for Blue Carbon sediments, we examined (qualitatively) the relationship between the CaCO3 %DW in sediments and the presence/absence of sources of CaCO3 adjacent to the coring locations, including coral reefs and terrestrial lithogenic sources of CaCO3 (Fig. 4, see dataset in Supplementary Information). Seagrass and mangrove ecosystems without potentially large adjacent allochthonous CaCO3 sources have a remarkably lower median (IQR) sediment CaCO3 content of 4 (15) and 1 (1) %DW (means ± SE of 11 ± 4 and 1.7 ± 0.8 %DW), respectively, compared with59 (51) and 61 (27) %DW (means ± SE of 56 ± 5 and 53 ± 16 %DW) when at least one allochthonous CaCO3 source was present (Fig. 3). For sediments in seagrass meadows, the presence of coral reefs (t-value = 4.68, df = 48.5, p < 0.0001) and lithogenic sources (t-value = 4.76, df = 57.3, p < 0.0001) increased the amount of CaCO3 in the sediment. However, there was a significant interaction between these factors (t-value = − 3.29, df = 53.2, p = 0.0018), because the CaCO3 %DW in the presence of both allochthonous sources was less than would be expected if these variables were additive. The presence/absence of coral reefs and lithogenic sources accounted for 36% of the variation in CaCO3, whereas the random variables (study, lithology grouping and marine province) accounted for 54% of the variation in CaCO3 (see Methods for model description). Mangrove sediment samples showed a similar pattern to the seagrass meadows and the presence of allochthonous sources had a marginally significant positive effect on the amount of CaCO3 in the sediment (t-value = 4.29, df = 1.81, p = 0.0596). The presence/absence of a CaCO3 source accounted for 71% of the variation of in CaCO3 within mangrove sediments, whereas the random variables accounted for 20% of the variation in CaCO3. In testing for biases of outlying cores and studies, we found that one study from Western Florida had an outlying data point that disproportionality skewed the results. The study from Western Florida had relatively low CaCO3 but did have an allochthonous source of CaCO3. When this study was removed from the analysis, the presence of an allochthonous source became significant (t-value = 7.92, df = 4.16, p = 0.0012). This highlights the need for more studies in mangrove sediments to determine the global influence of allochthonous sources on CaCO3 content.
Fig. 4.
Allochthonous sources and inorganic carbon in sediments. a %DW of carbonates (CaCO3) in seagrass sediments depending on the presence of potential allochthonous sources (lithogenic and/or coral reefs). All data distributions for locations with allochthonous sources are significantly different to the distributions for locations without allochthonous sources (Mann–Whitney U-tests, all p < 0.001). b %DW CaCO3 in mangrove sediments with and without allochthonous sources (coral reef and lithogenic source). Number on top of the box plots indicate the number of locations. The x represents the mean. Bars are the first and last quartile
In seagrass meadows, the median (IQR) Cinorg burial rate found in areas where no allochthonous sources were identified was 1 (13) gCinorg m−2 yr−1 (mean ± SE of 8 ± 4), only 1.1% of the global median. This contrast is consistent with our hypothesis that much of the Cinorg buried in seagrass and mangrove sediments is allochthonous. It explains the non-normal distribution of CaCO3 concentrations observed in sediments of seagrass and mangroves (Supplementary Figure 1), and indicates that the import of CaCO3 from carbonate-forming ecosystems or adjacent karstic areas is the norm27. The global burial rate of Cinorg in seagrass meadows is between a third to a half of their Corg burial rate1,7, whereas for mangroves our first estimate of global Cinorg burial rates is only 3% of the Corg burial rate3. If the buried CaCO3 and Corg in seagrass sediments were produced entirely in situ, Cinorg burial would offset up to a third of CO2 sequestration through Corg burial, particularly in tropical seagrass ecosystems where ~90% of the global Cinorg burial occurs. However, imbalances between production, dissolution and burial, and the observation of much higher CaCO3 concentrations in sediments near lithogenic formations and coral reefs, suggest that, where present, allochthonous CaCO3 inputs are substantial and support most of the net CaCO3 burial.
Locally, despite supporting significant CaCO3 burial, Blue Carbon ecosystems may be sites where imported CaCO3 dissolves, strengthening rather than weakening the capacity of these ecosystems to sequester CO2. Whereas there is emphasis on apportioning the sources of autochthonous and allochthonous Corg in Blue Carbon sediments (up to 50% of the buried Corg)4,9, determining the sources of CaCO3 in Blue Carbon sediments is just as important, to resolve the role of vegetated coastal ecosystems as CO2 sinks and, hence, their potential to support climate change mitigation. The current focus on Corg budgets in vegetated coastal ecosystems needs to be augmented with integrative assessments of organic and inorganic carbon fluxes and budgets, including both allochthonous and autochthonous inputs. Moreover, these assessments must consider the sources and fate of carbon exchanged between Blue Carbon and adjacent ecosystems, as Blue Carbon ecosystems export important amounts of their organic production45–47 but also import significant amounts of CaCO3 and organic matter from adjacent sources. A comparison of paired vegetated and unvegetated sediment CaCO3 %DW showed that vegetated and adjacent unvegetated sediments have similar carbonate concentrations, both using standard parametric statistics (general linear model (GLM), t-value = 1.32, df = 83.1, p = 0.191) and meta-analysis (z-value = 0.88, p = 0.379; Supplementary Fig. 2A,B), which also showed no evidence for reporting bias (all points within the 95% confidence lines of the funnel plot, Supplementary Fig. 2C. This provides further support to the hypothesis that much of the carbonate buried in vegetated coastal sediments derives from allochthonous sources rather than being produced within the habitat.
Inorganic carbon burial in Blue Carbon ecosystems has been overlooked, with the rates compiled here representing the first direct estimates reported in the literature. These estimates confirm that seagrass ecosystems, and to lesser extent mangrove ecosystems, are intense sites of CaCO3 burial, supporting sediment accretion. CaCO3 burial is a fundamental process supporting the role of Blue Carbon ecosystems in climate change adaptation, which is underpinned by their capacity to rapidly accrete sediments, reducing relative SLR by raising the seafloor1,17.
Methods
Calculation of the Cinorg accretion rate
We searched the peer-reviewed literature for data on sediment cores dated with 210Pb, including CaCO3 or Cinorg concentration in seagrass and mangrove sediments. Search terms on Google Scholar were seagrass OR mangrove AND 210Pb OR SAR OR sediment accretion rate. We then searched returned articles that contained data on SAR and CaCO3 or Cinorg data. We found only one study presenting CaCO3 content in a dated sediment core. However, we found 15 and 22 studies with SAR for seagrass and mangrove sediments, respectively. To obtain the CaCO3 or Cinorg concentrations needed to calculate Cinorg burial rates, we used the database of Mazarrasa et al. 13, which was the most recent exhaustive compilation of sediment cores from Blue Carbon habitats, for data on CaCO3 in seagrass sediments. We also contacted experts in Blue Carbon studies (published studies using cores from Blue Carbon habitats) for unpublished CaCO3 sediment concentration data (see data and references in Supplementary Data 1). In total, we compiled 42 and 53 210Pb dated cores with CaCO3 content in mangrove and seagrass ecosystems, respectively (see PRISMA checklist and flow diagram48 in Supplementary Note 1).
The SARs (cm yr−1) from the literature were re-calculated according to the constant flux–constant sedimentation model49, to have a coherent and comparable dating system between all cores. The CaCO3 concentration (% sediment DW) was calculated as the mean between all slices younger than 1900, for cores with the contemporary 210Pb chronologies. The Cinorg concentration in sediment (gCinorg m−3) was calculated from the dry bulk density (g m−3) and the percentage of CaCO3 content (using sediment DW), considering a mass ratio of 12% carbon in CaCO3. The Cinorg burial rate (gCinorg m−2 yr−1) was then calculated as the product of the SAR and the Cinorg concentration for each sediment core. Cores with negligible content of CaCO3 were also included in the calculation (see Supplementary Figure 1).
All cores from the same site or area and with similar presence or absence of allochthonous sources of CaCO3 (see below) were treated as replicates for a global location and averaged for the analysis (geologic grouping). For seagrass, the 51 cores dated with 210Pb were grouped into 17 locations (Figs. 2, 3). For mangroves, we compiled a total of 42 cores dated with 210Pb in 8 locations (Figs. 2, 3). Seagrass locations ranged from tropical to sub-arctic locations, with 50% of estimates derived from tropical and subtropical locations and 50% from higher latitudes. Mangrove sediment derived mostly from subtropical locations (seven out of eight locations), particularly in Australia and the Arabian Peninsula (Supplementary Figure 2).
Determination of the influence of allochthonous sources of CaCO3
We analysed the influence of the presence/absence of proximity of coral reefs and continental surface lithology (qualitative data), as potential allochthonous sources of CaCO3 in seagrass and mangrove sediments (in %DW) (see dataset in Supplementary Data 1). For seagrass, we expanded our dataset by including CaCO3 concentrations from 264 cores compiled by Mazarrasa et al.13, reaching a total of 341 cores with measured CaCO3 %DW.
We estimated the presence/absence of coral reefs using the map of the global distribution of warm-water coral reefs compiled by the UNEP-WCMC50 and the presence/absence of nearby lithogenic sources using the global lithology map of Hartmann and Moosdorf51 and the world soil map of the FAO/UNESCO (http://www.fao.org/soils-portal/en/). The coring locations were associated with climate regions following the Köppen–Geiger classification system52.
Statistical analysis
All data distributions were tested for normality to determine the most reliable central tendency measured with Shapiro–Wilks normality test (Statistica, Dell Software). None of the datasets of SAR, Cinorg concentration, Cinorg burial rate or CaCO3 %DW were normally distributed (all p < 0.05). We therefore chose to use the median (IQR) as the most appropriate description of central tendency. Traditional meta-analysis tools, which calculate effect sizes to standardize the difference between control and experimental treatments, thereby allowing comparison among disparate response variables and weighting to account for unequal variance among studies, could not be used for this analysis for multiple reasons. These reasons include that the question posed and the studies available did not include experimental designs with paired control and experimental plots required for effect size calculations, that there was a single response variable facilitating direct comparison and data integration, and, most importantly, that we used the raw data for each core. Instead, we ran a statistical test using a mixed effect GLM to determine the effect of coral reefs and lithogenic sources on the CaCO3 %DW of the sediment. For sediments within seagrass meadows, the GLM included two fixed factors (presence/absence of coral reefs and of lithogenic sources), as well as the interaction between the two factors. For sediment within mangrove forests, the GLM included one fixed factor (presence/absence of allochthonous sources), because replication did not exist for all combinations of the two factors. The data had unequal samples among studies and studies were not evenly distributed around the globe (Fig. 1), which could result in pseudo-replication and biased results. To account for the data structure and minimize non-independence, we included three separate random variables, which included study, lithology grouping and marine province. The marine province was determined for each sample location using the marine provinces of the world as defined by Spalding et al.53. Separate models where run for seagrass and mangrove sites. The statistical model was produced using the lmer function within the lme4 package54 and p-values were calculated with the lmerTest package55. The R2 was calculated for the fixed and random effects using the r.squared GLMM function in the MuMIn package56. The response variable was log transformed, which improved the model fit compared with raw data. The model fit was assessed by plotting the Q–Q plot (linear relationship) and the fitted values compared with the residuals (random distribution). To test whether individual cores or studies were biased and having a disproportionate influence on findings, we systematically removed any studies that contained outlying samples as determined from being outside the 95% confidence interval for the fitted values vs. residuals comparison using the plot model function from sjPlot package57. This analysis was conducted in R version 3.4.2.
Reporting bias and its effect on findings is an important consideration for meta-analyses58 and when the result from a meta-analysis is not the same as it would have been if data from all correctly conducted studies were included in the analysis59. A main cause of reporting bias is not publishing research because of a lack of merit as determined by the researcher, reviewer or editor60. As indicated by the data inclusion flow diagram (Supplementary Fig. 3), researchers often measured but did not publish data on soil CaCO3 content and authors needed to be directly contacted for these results. In addition, the researchers not only provided information from published studies but also unpublished data on CaCO3 content (10 of 51 seagrass studies included in the analysis were not published). For these reasons, it is unlikely to be that our findings were affected by reporting bias. A subset of data collected for this study included the appropriate information to run both a GLM and a traditional meta-analysis (effect size could be calculated between paired data). The data included information from nine studies that measured the CaCO3 content of sediment from both vegetated and unvegetated habitats. There were 92 core samples with 32 from unvegetated and 60 from vegetated habitats (Supplementary Fig. 2A). The GLM followed the same procedures as detailed in the main text, except it had only two random factors, study and marine province, because study and lithology grouping differed in only one instance. For the meta-analysis, the data were paired for each study and the mean CaCO3 %DW, number of samples and SD were calculated for vegetated and unvegetated cores for each study. Two studies included in the GLM were removed for the meta-analysis, because they only had one core for an unvegetated habitat and SD could not be calculated, leaving seven comparisons for this analysis. Hedges’ g was calculated for the effect size following Borenstein et al.60 (Eqs. 1–3) and a variance for each effect size was also calculated (Vg)59 (Eq. 4), as indicated by equations:
| 1 |
| 2 |
| 3 |
| 4 |
XE and XC are the mean (n is sample size) of vegetated and unvegetated sediments, along with SDpooled and J, which accounts for biases associated with different sample sizes. The meta-analysis included the same two random variables as the GLM and was conducted using the rma.mv function from the metafor package61.
Calculation of global yearly burial rates of Cinorg
The global annual burial of inorganic carbon (TgCinorg yr−1) in seagrass meadows was calculated as the product of the global median Cinorg burial rates and the estimated global seagrass area, which ranges from 150,000 to 600,000 km29. We also calculated the global annual burial of Cinorg as the sum of separate calculations for tropical and arid climates and meadows at higher latitude climates. Median Cinorg burial rates were calculated for tropical (core locations with tropical and hot desert climates) and non-tropical areas (temperate, continental and polar climates) and multiplied by the global seagrass cover range under the assumption that 2/3 of the seagrass area is in the tropical and subtropical zone13. The global annual burial of inorganic carbon (TgCinorg yr−1) in mangroves was calculated as the product of the global median Cinorg burial rates and the estimated global mangrove cover of 137,760 km262.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This research was supported by King Abdullah University of Science and Technology (KAUST) through baseline funding and workshop funding to C.M.D. Support from the Australian Research Council through grants LIEF Project LE170100219, DE160100443, DE170101524, DP150103286, DP150102092, DP160100248, DE130101084, LP160100242 and LE140100083 is acknowledged. J.J.M. was supported by the Netherlands Earth System Science Center. J.W.F. was supported by the US National Science Foundation through the Florida Coastal Everglades Long-Term Ecological Research programme under Grant Number DEB-1237517. D.K.-J. received financial support from the Independent Research Fund Denmark (8021-00222B, CARMA) and the COCOA project under the BONUS programme, funded by the EU 7th Framework Programme and the Danish Research Council. A.A.-O. was supported by an “Obra Social la Caixa” fellowship (LCF/BQ/ES14/10320004). A.A.-O. and P.M. acknowledge the support by the Generalitat de Catalunya (grant 2017 SGR-1588). This work is contributing to the ICTA ‘Unit of Excellence’ (MinECo, MDM2015-0552). H.K.’s input is a contribution to the CESEA project (NE/L001535/1) funded by NERC. T.K., K.W. and T.T. were supported by JSPS KAKENHI (18H04156) and the Environment Research and Technology Development Fund (S-14) of the Ministry of the Environment, Japan. J.M.S. was supported by the National Science Foundation under South Florida Water, Sustainability and Climate grant EAR-1204079. I.M. was supported by a Juan de la Cierva Formación post-doctoral fellowship from the Spanish Ministry of Science, Innovation and Universities.
Author contributions
C.M.D. and V.S. conceived and designed this work. V.S. wrote the manuscript with support from N.R.G., P.I.M., D.T.M., J.J.M., O.S. and C.M.D. V.S., N.R.G., P.I.M., D.T.M., J.J.M., O.S., H.A., A.A.-O., M.C., B.D.E., J.W.F., H.K., D.K.-J., T.K., P.S.L., C.E.L., N.M., P.M., M.A.M., I.M., K.J.M.G., M.P.J.O., C.J.S., I.R.S., J.M.S., T.T., K.W. and C.M.D. contributed data and quality check was done by A.A.-O. and P.M. Analysis of data was performed by V.S. and N.R.G. V.S., N.R.G., P.I.M., D.T.M., J.J.M., O.S., H.A., A.A.-O., M.C., B.D.E., J.W.F., H.K., D.K.-J., T.K., P.S.L., C.E.L., N.M., P.M., M.A.M., I.M., K.J.M.G., M.P.J.O., C.J.S., I.R.S., J.M.S., T.T., K.W. and C.M.D. discussed and commented on the manuscript.
Data availability
The dataset is available as Supplementary Data 1.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-08842-6.
References
- 1.Duarte CM, Losada IJ, Hendriks IE, Mazarrasa I, Marbà N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013;3:961–968. doi: 10.1038/nclimate1970. [DOI] [Google Scholar]
- 2.Duarte CM, Middelburg J, Caraco N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences. 2005;1:1–8. doi: 10.5194/bg-2-1-2005. [DOI] [Google Scholar]
- 3.Rosentreter, J. A., Maher, D. T., Erler, D. V., Murray, R., & Eyre, B. D. CH4 emissions partially offset ‘blue carbon’ burial in mangroves. Sci. Adv.4, eaao4985 (2018) [DOI] [PMC free article] [PubMed]
- 4.Kennedy H, et al. Seagrass sediments as a global carbon sink: Isotopic constraints. Glob. Biogeochem. Cycles. 2010;24:1–8. doi: 10.1029/2010GB003848. [DOI] [Google Scholar]
- 5.Alongi DM. Carbon cycling and storage in mangrove forests. Ann. Rev. Mar. Sci. 2014;6:195–219. doi: 10.1146/annurev-marine-010213-135020. [DOI] [PubMed] [Google Scholar]
- 6.Nellemann, C. et al. (eds.) Blue Carbon: A rapid response assessment. (United Nations Environment Programme, GRID-Arendal, 2009).
- 7.McLeod E, et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011;9:552–560. doi: 10.1890/110004. [DOI] [Google Scholar]
- 8.Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles. 2003;17:1111. doi: 10.1029/2002GB001917. [DOI] [Google Scholar]
- 9.Duarte CM. Reviews and syntheses: hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences. 2017;14:301–310. doi: 10.5194/bg-14-301-2017. [DOI] [Google Scholar]
- 10.Kristensen E, Bouillon S, Dittmar T, Marchand C. Organic carbon dynamics in mangrove ecosystems: a review. Aquat. Bot. 2008;89:201–219. doi: 10.1016/j.aquabot.2007.12.005. [DOI] [Google Scholar]
- 11.Fourqurean JW, et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012;5:505–509. doi: 10.1038/ngeo1477. [DOI] [Google Scholar]
- 12.Sanderman J, et al. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 2018;13:055002. doi: 10.1088/1748-9326/aabe1c. [DOI] [Google Scholar]
- 13.Mazarrasa I, et al. Seagrass meadows as a globally significant carbonate reservoir. Biogeosciences. 2015;12:4993–5003. doi: 10.5194/bg-12-4993-2015. [DOI] [Google Scholar]
- 14.Ware JR, Smith SV, Reaka-kudla ML. Coral reefs: sources or sinks of atmospheric CO2? Coral Reefs. 1992;11:127–130. doi: 10.1007/BF00255465. [DOI] [Google Scholar]
- 15.Howard JL, Creed JC, Aguiar MVP, Fouqurean JW. CO2 released by carbonate sediment production in some coastal areas may offset the benefits of seagrass ‘Blue Carbon’ storage. Limnol. Oceanogr. 2017;63:160–172. doi: 10.1002/lno.10621. [DOI] [Google Scholar]
- 16.Macreadie PI, Serrano O, Maher DT, Duarte CM, Beardall J. Addressing calcium carbonate cycling in blue carbon accounting. Limnol. Oceanogr. Lett. 2017;2:195–201. doi: 10.1002/lol2.10052. [DOI] [Google Scholar]
- 17.Saderne V, et al. Accumulation of carbonates contributes to coastal vegetated ecosystems keeping pace with sea level rise in an arid region (Arabian Peninsula) J. Geophys. Res. Biogeosci. 2018;2050:1–13. [Google Scholar]
- 18.Almahasheer H, et al. Low carbon sink capacity of Red Sea mangroves. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-10424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cusack M, et al. Organic carbon sequestration and storage in vegetated coastal habitats along the western coast of the Arabian Gulf. Environ. Res. Lett. 2018;13:074007. doi: 10.1088/1748-9326/aac899. [DOI] [Google Scholar]
- 20.Canals M, Ballesteros E. Production of carbonate by phytobenthic comunities on the Mallorca -Menorca shelf, northwestern Mediterranean sea. Deep Sea Res. Part II. 1997;44:611–629. doi: 10.1016/S0967-0645(96)00095-1. [DOI] [Google Scholar]
- 21.Barrón C, Duarte CM, Frankignoulle M, Borges AV. Organic carbon metabolism and carbonate dynamics in a Mediterranean seagrass (Posidonia oceanica), meadow. Estuaries Coasts. 2006;29:417–426. doi: 10.1007/BF02784990. [DOI] [Google Scholar]
- 22.Walker D, Woelkerling W. Quantitative study of sediment contribution by epiphytic coralline red algae in seagrass meadows in Shark Bay, Western Australia. Mar. Ecol. Prog. Ser. 1988;43:71–77. doi: 10.3354/meps043071. [DOI] [Google Scholar]
- 23.Bosence DWJ. Biogenic carbonate prodction in Florida Bay. Bull. Mar. Sci. 1989;44:419–433. [Google Scholar]
- 24.Yates KK, Halley RB. Diurnal variation in rates of calcification and carbonate sediment dissolution in Florida Bay. Estuaries Coasts. 2006;29:24–39. doi: 10.1007/BF02784696. [DOI] [Google Scholar]
- 25.Mazarrasa I, et al. Effect of environmental factors (wave exposure and depth) and anthropogenic pressure in the C sink capacity of Posidonia oceanica meadows. Limnol. Oceanogr. 2017;62:1436–1450. doi: 10.1002/lno.10510. [DOI] [Google Scholar]
- 26.Gazeau F, et al. Whole-system metabolism and CO2 fluxes in a Mediterranean Bay dominated by seagrass beds (Palma Bay, NW Mediterranean) Biogeosciences. 2005;2:43–60. doi: 10.5194/bg-2-43-2005. [DOI] [Google Scholar]
- 27.Milliman JD. Production and accumulation of calcium carbonate in the ocean: Budget of a nonsteady state. Glob. Biogeochem. Cycles. 1993;7:927–957. doi: 10.1029/93GB02524. [DOI] [Google Scholar]
- 28.Stockman KW, Ginsburg RN, Shinn EA. The production of lime mud by algae in South Florida. J. Sed. Petrol. 1967;37:633–648. [Google Scholar]
- 29.Mucha AP, Almeida CMR, Bordalo AA, Vasconcelos MTSD. Exudation of organic acids by a marsh plant and implications on trace metal availability in the rhizosphere of estuarine sediments. Estuar. Coast. Shelf Sci. 2005;65:191–198. doi: 10.1016/j.ecss.2005.06.007. [DOI] [Google Scholar]
- 30.Haoliang L, Chongling Y, Jingchun L. Low-molecular-weight organic acids exuded by Mangrove (Kandelia candel (L.) Druce) roots and their effect on cadmium species change in the rhizosphere. Environ. Exp. Bot. 2007;61:159–166. doi: 10.1016/j.envexpbot.2007.05.007. [DOI] [Google Scholar]
- 31.Middelburg J, Nieuwenhuize J, Slim F, Ohowa B. Sediment biogeochemistry in an East African mangrove forest (Gazi Bay, Kenya) Biogeochemistry. 1996;34:133–155. doi: 10.1007/BF00000899. [DOI] [Google Scholar]
- 32.Ku TCW, Walter LM, Coleman ML, Blake RE, Martini AM. Coupling between sulfur recycling and syndepositional carbonate dissolution: evidence from oxygen and sulfur isotope composition of pore water sulfate, South Florida Platform, U.S.A. Geochim. Cosmochim. Acta. 1999;63:2529–2546. doi: 10.1016/S0016-7037(99)00115-5. [DOI] [Google Scholar]
- 33.Burdige, D. J. & Zimmerman, R. C. Impact of sea grass density on carbonate dissolution in Bahamian sediments. Limnol. Oceanogr. 47, 1751–1763 (2002).
- 34.Hu X, Burdige DJ. Enriched stable carbon isotopes in the pore waters of carbonate sediments dominated by seagrasses: evidence for coupled carbonate dissolution and reprecipitation. Geochim. Cosmochim. Acta. 2007;71:129–144. doi: 10.1016/j.gca.2006.08.043. [DOI] [Google Scholar]
- 35.Linto N, et al. Carbon dioxide and methane emissions from mangrove-associated waters of the Andaman Islands, Bay of Bengal. Estuaries Coasts. 2014;37:381–398. doi: 10.1007/s12237-013-9674-4. [DOI] [Google Scholar]
- 36.Saderne V, Fietzek P, Herman PMJ. Extreme variations of pCO2 and pH in a Macrophyte Meadow of the Baltic Sea in summer: evidence of the effect of photosynthesis and local upwelling. PLoS ONE. 2013;8:e62689. doi: 10.1371/journal.pone.0062689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sippo JZ, Maher DT, Tait DR, Holloway C, Isaac R. Are mangroves drivers or buffers of coastal acidification? Insights from alkalinity and dissolved inorganic carbon export estimates across a latitudinal transect. Glob. Biogeochem. Cycles. 2016;30:753–766. doi: 10.1002/2015GB005324. [DOI] [Google Scholar]
- 38.Challener RC, Robbins LL, McClintock JB. Variability of the carbonate chemistry in a shallow, seagrass-dominated ecosystem: implications for ocean acidification experiments. Mar. Freshw. Res. 2016;67:163–172. doi: 10.1071/MF14219. [DOI] [Google Scholar]
- 39.Ray R, Baum A, Rixen T, Gleixner G, Jana TK. Exportation of dissolved (inorganic and organic) and particulate carbon from mangroves and its implication to the carbon budget in the Indian Sundarbans. Sci. Total Environ. 2018;621:535–547. doi: 10.1016/j.scitotenv.2017.11.225. [DOI] [PubMed] [Google Scholar]
- 40.Maher DT, Santos IR, Golsby-Smith L, Gleeson J, Eyre BD. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnol. Oceanogr. 2013;58:475–488. doi: 10.4319/lo.2013.58.2.0475. [DOI] [Google Scholar]
- 41.Gattuso JP, et al. Ocean solutions to address climate change and its effects on marine ecosystems. Front. Mar. Sci. 2018;5:337. doi: 10.3389/fmars.2018.00337. [DOI] [Google Scholar]
- 42.Maher, D. T., Call, M., Santos, I. R., & Sanders, C. J. Beyond burial: Lateral exchange is a significant atmospheric carbon sink in mangrove forests. Biol. Lett. 14, 20180200 (2018). [DOI] [PMC free article] [PubMed]
- 43.Zwolsman JJG, Berger GW, Van Eck GTM. Sediment accumulation rates, historical input, postdepostional mbility and rentention of major elements and trace metals in salt marsh sediments of the Scheldt estuary, SW Netherlands. Mar. Chem. 1993;44:73–94. doi: 10.1016/0304-4203(93)90007-B. [DOI] [Google Scholar]
- 44.Vranken M, Oenema O, Mulder J. Effects of tide range alterations on salt marsh sediments in the Eastern Scheldt, S. W. Netherlands. Hydrobiologia. 1990;195:13–20. doi: 10.1007/BF00026810. [DOI] [Google Scholar]
- 45.Duarte CM, Cebrián J. The fate of marine autotrophic production. Limnol. Oceanogr. 1996;41:1758–1766. doi: 10.4319/lo.1996.41.8.1758. [DOI] [Google Scholar]
- 46.Krause-Jensen D, Duarte CM. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016;9:737–742. doi: 10.1038/ngeo2790. [DOI] [Google Scholar]
- 47.Duarte, C. M. & Krause-Jensen, D. Export from seagrass meadows contributes to marine carbon sequestration. Front. Mar. Sci. 4, 13 (2017).
- 48.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnaswamy S, Lal D, Martin JM, Meybeck M. Geochronology of lake sediments. Earth Planet. Sci. Lett. 1971;11:407–414. doi: 10.1016/0012-821X(71)90202-0. [DOI] [Google Scholar]
- 50.UNEP-WCMC. Global distribution of warm-water coral reefs, compiled from multiple sources including the Millennium Coral Reef Mapping Project, Version 1.3. (UNEP World Conservation Monitoring Centre, 2010).
- 51.Hartmann, J. & Moosdorf, N. The new global lithological map database GLiM: A representation of rock properties at the Earth surface. Geochem. Geophys. Geosyst.13, Q12004 (2012).
- 52.Rubel, F., Brugger, K., Haslinger, K. & Auer, I. The climate of the European Alps: Shift of very high resolution Köppen-Geiger climate zones 1800–2100. Meteorol. Zeitschrift26, 115–125 (2016).
- 53.Spalding MD, et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience. 2007;57:573–583. doi: 10.1641/B570707. [DOI] [Google Scholar]
- 54.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 55.Kuznetsova A, Brockhoff PB, Christensen RHB. “lmerTest Package: tests in Linear Mixed Effects Models.”. J. Stat. Softw. 2017;82:1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 56.Bartoń, K. MuMIn: Multi-Model Inference. R package version 1.42.1. https://CRAN.R-project.org/package=MuMIn (2018).
- 57.Lüdecke, D. sjPlot: Data Visualization for Statistics in Social Science. R package version 2.6.0. https://CRAN.R-project.org/package=sjPlot (2018).
- 58.Gurevitch J, Koricheva J, Nakagawa S, Stewart G. Meta-analysis and the science of research synthesis. Nature. 2018;555:175–182. doi: 10.1038/nature25753. [DOI] [PubMed] [Google Scholar]
- 59.Koricheva, J., Gurevitch, J. & Mengersen, K. Handbook of Meta-analysis in Ecology and Evolution (Princeton Univ. Press, 2013)
- 60.Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. Introduction to meta-analysis. (John Wiley & Sons, 2011).
- 61.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 62.Giri C, et al. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011;20:154–159. doi: 10.1111/j.1466-8238.2010.00584.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The dataset is available as Supplementary Data 1.