Abstract
Risk reducing mastectomy is the only surgical approach for the prevention of breast cancer in women with deleterious genetic mutations or in those deemed to be at extremely high risk. However, up to 10.5% of these women still developed breast cancer. Thus, developing new strategies for complete prevention of breast cancer is imperative. Mammary ducts were ablated by mammary-specific ablation of forkhead box protein A1 (Foxa1). Mammary tumorigenesis was induced in control and mammary-specific Foxa1 knockout mice using carcinogens. No mammary tumors were observed in these knockout mice compared to four types of breast tumors induced in control mice. We present a promising novel strategy for the prevention of breast cancer by genetic ablation of mammary ducts via targeting Foxa1.
Keywords: Foxa1, mammary ducts, carcinogenesis, mastectomy, breast cancer prevention
Introduction
Women with BRCA1 or BRCA2 mutations have a 50-80% lifetime risk of developing breast cancer [4,21]. Bilateral risk reducing mastectomy (RRM) is one strategy to significantly reduce breast cancer occurrence in unaffected BRCA mutation carriers. However, multiple studies suggest nearly 5% to 10.5% of these women undergoing preventive surgery still developed breast cancer arising possibly from residual breast cells after prophylactic surgery [6,9-11,14,19,20]. Among BRCA carriers presenting with an index ipsilateral breast cancer, nearly 60% will develop a contralateral breast cancer over their lifetime; and risk reducing contralateral mastectomy in this setting could also result in up to 94% reduction of contralateral breast cancer [5,15,16,18]. However, the rate of disease-free and overall survival after contralateral prophylactic mastectomy is under debate. Recent studies showed that there were no overall survival benefits after contralateral prophylactic mastectomy, especially for estrogen receptor-negative patients, whereas another study showed improved 10-year overall survival after contralateral prophylactic mastectomy [2,5,7,17,22]. These results indicate that it is difficult to completely remove every single mammary tumor cell or mammary progenitor cell with tumorigenic potential using surgery alone or even in combination with chemotherapy and radiotherapy because the rate of breast cancer recurrence after mastectomy could rise up to 40%, depending on breast cancer subtypes. Thus, completely ablating these residual mammary cells after mastectomy to prevent the breast cancer occurrence is of significant scientific importance.
Recently, we developed a mouse model with complete ablation of mammary ducts by mammary gland-specific ablation of Foxa1 [13]. Forkhead box protein A1 (Foxa1 in mice and FOXA1 in humans) is a pioneer transcription factor regulating organogenesis and tumorigenesis in many tissues, including breast tissues [12], but Foxa1 regulation in mammary gland development is poorly understood [3]. Thus, to better understand the role of Foxa1 in mammary development and tumorigenesis in vivo, a mouse model with mammary-specific ablation of Foxa1 is needed. We have recently shown that completely ablating Foxa1 in mammary glands using Foxa1loxP/loxP; Krt14-Cre mice led to the complete loss of mammary epithelial cells or mammary ducts, indicating that Foxa1 is essential for the formation of mammary ducts by controlling the growth of all types of mammary epithelial cells in the ducts [13]. More importantly, the complete loss of mammary epithelia in Foxa1-deficient mammary glands suggests that terminating mammary epithelial growth could be a new direction for the prevention of breast cancer. Mastectomy is currently the only surgical approach for eliminating breast tissue and thereby breast cancer prevention for women who carry deleterious genetic mutations with breast cancer susceptibility. Most breast cancers originate from mammary terminal ductal lobular epithelia. Thus, our genetic approach of ablating mammary ducts by suppressing Foxa1 is a genetic “mastectomy”, which is promising for complete, clean removal of mammary ducts.
Here, we present that this mammary duct-free mouse model is completely resistant to carcinogen-induced mammary tumorigenesis.
Materials and methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the Mayo Clinic. The experiment was carried out under controlled conditions with a 12-h light/dark cycle. Cages with filters were used along with sterile bedding, ad libitum diet, and water. Animals were maintained on a normal chow. The derivation of Foxa1loxP/loxP mice (from Dr. Klaus H. Kaestner, University of Pennsylvania) has been reported previously [8]. Foxa1loxP/loxP mice were mated with Krt14-Cre mice (from Jackson Laboratory) to obtain Foxa1loxP/loxP; Krt14-Cre mice. Foxa1loxP/loxP mice were used as control wild-type mice. Genotypes of Foxa1loxP/loxP and Cre were determined by PCR.
Carcinogenesis of breast cancer
A group of eight female Foxa1loxP/loxP; Krt14-Cre mice (six-week old) received a single dose of 20 mg progestin medroxyprogesterone acetate (MPA) pellets (Innovative Research of America) subcutaneously implanted into the interscapular area. 7,12-dimethylbenz[a]anthracene (DMBA) at a concentration of 1 mg/dose was administered intragastrically to these mice at the age of weeks 9, 10, 11, 12, and 13 [1]. Another group of 22 female Foxa1loxP/loxP mice received the same carcinogenesis treatments as controls. At the age of 20 weeks old, all mammary glands in these mice from each group were collected for tumor evaluation.
Immunohistochemical staining
Excised mammary gland tissues with and without tumors were fixed by immersion in 10% buffered formalin overnight and then transferred to 70% ethanol for long-term fixation. Representative sections of fixed tissue were trimmed and embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E), cytokeratin 19 (CK19) antibody (clone, TROMA-3), Foxa1 antibody (C-20, Santa Cruz), ERα antibody (MC-20, Santa Cruz), PR antibody (C-19, Santa Cruz), or HER2 antibody (C-18, Santa Cruz) for histological examination. The TROMA-3 antibody was purchased from The University of Iowa, Iowa City, IA. All of the stained sections were imaged using Aperio ScanScope XT (Vista, CA, USA).
Results
DMBA initiation with MPA promotion or DMBA/MPA-induced mammary tumorigenesis is a classical model of mammary carcinogenesis and about 70% of mammary tumors in this model are estrogen receptor alpha-positive (ERα+) and about 30% are triple-negative and other breast tumors [1]. When we induced mammary tumorigenesis in our control Foxa1loxP/loxP mice using the DMBA/MPA method, we were able to recapitulate the carcinogenesis of multiple mammary tumors in all control mice (Figure 1A). Moreover, by immunostaining of molecular markers, including ERα, progesterone receptor (PR), and human epidermal growth factor receptor 2 or erythroblastic oncogene B2 (HER2 or ERBB2), we were able to identify all four types of breast cancers in this DMBA/MPA model, including the luminal A (ERα+; PR+; HER2-, 34.3%), luminal B (ERα+; PR+; HER2low, 38.1%) with low HER2 expression, HER2+ (ERα-; PR-; HER2high, 6.1%) with high HER2 expression, and triple-negative (ERα-; PR-; HER2-, 21.5%) breast cancers (Figure 1A and 1B). And the ERα+ breast cancers were still the dominant ones. However, when we induced mammary tumors in our mutant Foxa1loxP/loxP; Krt14-Cre mice using the DMBA/MPA method, in addition to observing complete ablation of mammary ducts by ablating Foxa1, no tumors of any types were observed in these mice (Figure 2), though little blood cell infiltration was observed near the lymph node in the mammary fat pads (Figure 2), indicating that mammary ductal epithelial cells are essential for mammary tumorigenesis and complete ablation of mammary ducts prevents the occurrence of any types of breast cancers.
Figure 1.
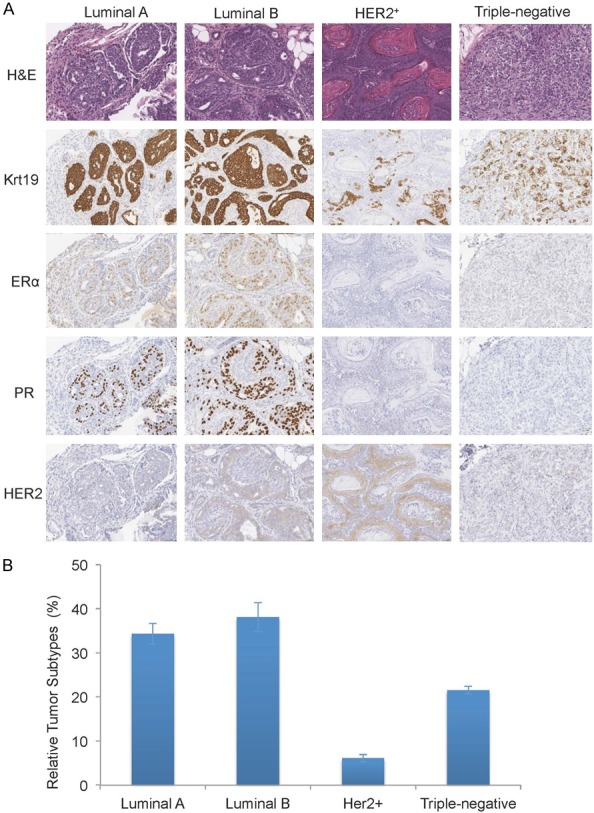
Mammary tumorigenesis was induced in mice using DMBA/MPA. A. Four types of breast tumors were observed in control Foxa1loxP/loxP mice after DMBA/MPA administration. B. Relative percentages of each tumor type in each mouse were calculated from total 22 mice after DMBA/MPA administration.
Figure 2.
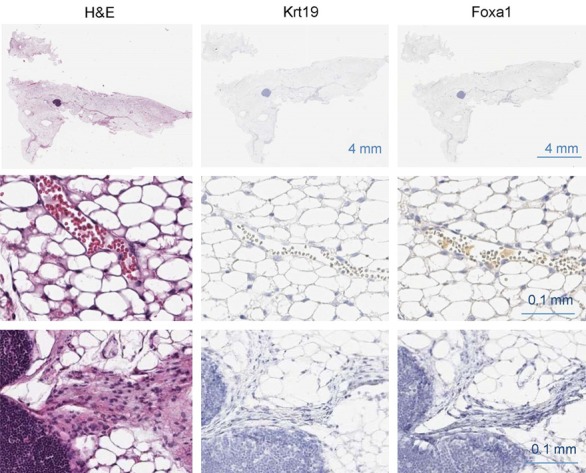
Mammary duct-free mice are resistant to mammary tumorigenesis. No tumor was observed in mutant Foxa1loxP/loxP; Krt14-Cre mice after DMBA/MPA administration (n = 8).
Discussion
Our study provides the first in vivo evidence that genetic ablation of mammary ducts is sufficient to completely prevent breast cancer occurrence. Thus, this strategy alone or in combination with mastectomy together as an adjuvant therapy could be promising for completely preventing the breast cancer occurrence in those women at the highest risk of breast cancer and in those breast cancer patients after mastectomy. Moreover, this strategy could also be useful for preventing breast cancer recurrence. In this direction, targeting FOXA1 or other pathways to eliminate mammary epithelia or ducts in humans could be a novel and promising approach for breast cancer prevention.
Acknowledgements
This study was partially supported by the grant # R00CA168983 from NCI to Z.L. We thank Brandy Edenfield for immunohistochemical staining.
Disclosure of conflict of interest
None.
References
- 1.Aldaz CM, Liao QY, LaBate M, Johnston DA. Medroxyprogesterone acetate accelerates the development and increases the incidence of mouse mammary tumors induced by dimethylbenzanthracene. Carcinogenesis. 1996;17:2069–2072. doi: 10.1093/carcin/17.9.2069. [DOI] [PubMed] [Google Scholar]
- 2.Bedrosian I, Hu CY, Chang GJ. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst. 2010;102:401–9. doi: 10.1093/jnci/djq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, Godwin AK, Korach KS, Visvader JE, Kaestner KH, Abdul-Karim FW, Montano MM, Keri RA. FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development. 2010;137:2045–2054. doi: 10.1242/dev.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comen E, Davids M, Kirchhoff T, Hudis C, Offit K, Robson M. Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi women. Breast Cancer Res Treat. 2011;129:185–190. doi: 10.1007/s10549-011-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies KR, Cantor SB, Brewster AM. Better contralateral breast cancer risk estimation and alternative options to contralateral prophylactic mastectomy. Int J Womens Health. 2015;7:181–187. doi: 10.2147/IJWH.S52380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles R, Pichert G, Van t’veer L, Tung N, Weitzel JN, Couch FJ, Rubinstein WS, Ganz PA, Daly MB, Olopade OI, Tomlinson G, Schildkraut J, Blum JL, Rebbeck TR. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fayanju OM, Stoll CR, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg. 2014;260:1000–1010. doi: 10.1097/SLA.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartmann LC, Schaid DJ, Woods JE, Crotty TP, Myers JL, Arnold PG, Petty PM, Sellers TA, Johnson JL, McDonnell SK, Frost MH, Jenkins RB. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 10.Heemskerk-Gerritsen BA, Menke-Pluijmers MB, Jager A, Tilanus-Linthorst MM, Koppert LB, Obdeijn IM, van Deurzen CH, Collée JM, Seynaeve C, Hooning MJ. Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: a prospective analysis. Ann Oncol. 2013;24:2029–35. doi: 10.1093/annonc/mdt134. [DOI] [PubMed] [Google Scholar]
- 11.Ingham SL, Sperrin M, Baildam A, Ross GL, Clayton R, Lalloo F, Buchan I, Howell A, Evans DG. Risk-reducing surgery increases survival in BRCA1/2 mutation carriers unaffected at time of family referral. Breast Cancer Res Treat. 2013;142:611–8. doi: 10.1007/s10549-013-2765-x. [DOI] [PubMed] [Google Scholar]
- 12.Kaestner KH. The FoxA factors in organogenesis and differentiation. Curr Opin Genet Dev. 2010;20:527–532. doi: 10.1016/j.gde.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Zhao Y, Skerry B, Wang X, Colin-Cassin C, Radisky DC, Kaestner KH, Li Z. Foxa1 is essential for mammary duct formation. Genesis. 2016;54:277–85. doi: 10.1002/dvg.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig KK, Neuner J, Butler A, Geurts JL, Kong AL. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am J Surg. 2016;212:660–669. doi: 10.1016/j.amjsurg.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 15.McDonnell SK, Schaid DJ, Myers JL, Grant CS, Donohue JH, Woods JE, Frost MH, Johnson JL, Sitta DL, Slezak JM, Crotty TB, Jenkins RB, Sellers TA, Hartmann LC. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. J. Clin. Oncol. 2001;19:3938–43. doi: 10.1200/JCO.2001.19.19.3938. [DOI] [PubMed] [Google Scholar]
- 16.Peralta EA, Ellenhorn JD, Wagman LD, Dagis A, Andersen JS, Chu DZ. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. Am J Surg. 2000;180:439–45. doi: 10.1016/s0002-9610(00)00505-5. [DOI] [PubMed] [Google Scholar]
- 17.Pesce C, Liederbach E, Wang C, Lapin B, Winchester DJ, Yao K. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann Surg Oncol. 2014;21:3231–9. doi: 10.1245/s10434-014-3956-3. [DOI] [PubMed] [Google Scholar]
- 18.Quan G, Pommier SJ, Pommier RF. Incidence and outcomes of contralateral breast cancers. Am J Surg. 2008;195:645–50. doi: 10.1016/j.amjsurg.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van ‘t Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E, Daly MB, Olopade OI, Weber BL. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J. Clin. Oncol. 2004;22:1055–62. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 20.Skytte AB, Cruger D, Gerster M, Laenkholm AV, Lang C, Brondum-Nielsen K, Andersen MK, Sunde L, Kølvraa S, Gerdes AM. Breast cancer after bilateral risk-reducing mastectomy. Clin Genet. 2011;79:431–7. doi: 10.1111/j.1399-0004.2010.01604.x. [DOI] [PubMed] [Google Scholar]
- 21.Tirkkonen M, Johannsson O, Agnarsson BA, Olsson H, Ingvarsson S, Karhu R, Tanner M, Isola J, Barkardottir RB, Borg A, Kallioniemi OP. Distinct somatic genetic changes associated with tumor progression in carriers of BRCA1 and BRCA2 germ-line mutations. Cancer Res. 1997;57:1222–7. [PubMed] [Google Scholar]
- 22.Zeichner SB, Zeichner SB, Ruiz AL, Markward NJ, Rodriguez E. Improved long-term survival with contralateral prophylactic mastectomy among young women. Asian Pac J Cancer Prev. 2014;15:1155–62. doi: 10.7314/apjcp.2014.15.3.1155. [DOI] [PubMed] [Google Scholar]


