Abstract
The aim of this study was to develop an interventional oncologic technique, “Image-guided intratumoral radiofrequency hyperthermia (RFH)-enhanced herpes simplex virus-thymidine kinase (HSV-TK) gene therapy of ovarian cancer. This study consisted of three portions: (1) serial in-vitro experiments to establish “proof-of-principle” of this novel technique using human ovarian cancer cells; (2) serial in-vivo experiments to validate technical feasibility using animal models with the same orthotopic ovarian cancers; and (3) serial investigations into the underlying bio-molecular mechanisms of this technique. We included four subject groups: (i) combination therapy with RFH+HSV-TK gene therapy; (ii) gene therapy-only; (iii) RFH-only; and (iv) Phosphate-buffered saline (PBS). For in-vitro experiments, confocal microscopy and MTS assays were performed to quantify HSV-TK gene expression and assess cell viability. For in-vivo experiments, bioluminescence optical and ultrasound imaging were used to assess therapeutic effectiveness. These results were correlated with subsequent pathologic/laboratory studies to further elucidate the biologic mechanisms of this technique. In in-vitro experiments, combination therapy resulted in the lowest cell proliferation and greatest increase in HSV-TK gene expression among four subject groups. In in-vivo experiments, combination therapy lead to significant decreases of bioluminescence signals and sizes of tumors in combination therapy by optical and ultrasound imaging. Pathology/laboratory examinations confirmed the significantly increased expression of Bax, Caspase-3, HSP70, IL-2, and CD94 in cancer tissues subjected to combination therapy. “Image-guided intratumoral RFH-enhanced direct gene therapy” is an effective interventional oncologic technique which functions through apoptotic/anti-tumor immunity pathways. This technical development may open new avenues for treating ovarian cancer.
Keywords: Radiofrequency hyperthermia, gene therapy, HSV-TK/GCV, ovarian cancer, molecular image
Introduction
Ovarian cancer is one of the deadliest known malignancies [1]. This is in large part because it is usually diagnosed at an advanced stage due to the absence of specific symptoms in most patients and lack of reliable screening modalities [2]. Although different chemotherapeutic agents are available for ovarian cancers, this malignancy is usually characterized by repeated remission and relapse. In most cases, the disease will eventually develop resistance to all chemotherapeutic agents and become incurable [3,4]. Thus, the development of new therapeutic modalities is highly desirable.
The herpes simplex virus-thymidine kinase (HSV-TK)/ganciclovir (GCV) suicide gene therapy system has been extensively investigated as a promising approach in the treatment for a variety of cancers [5-7]. Effective HSV-TK/GCV gene therapy depends on sufficient transfection of HSV-TK genes into tumor cells, where it converts nontoxic GCV to highly toxic phosphorylated GCV. Phosphorylated GCV disrupts DNA replication and induces cell death, which can be further amplified via “bystander effect” to induce death of neighboring untransfected cells [8,9]. Although effectiveness of this technique has been proven in cancer cells and rodent cancer models, clinical studies have not been widely applied, primarily due to the low gene transfection efficiency observe in the currently utilized systemic approach to administering HSV-TK genes. An image-guided, interventional approach for local delivery of high-dose genes to tumors could potentially solve this problem.
To date, percutaneous image-guided thermal ablation techniques, such as radiofrequency ablation (RFA) and microwave ablation (WMA), have emerged as an effective, minimally invasive, and reproducible method in the locoregional treatment of solid tumors. Previous studies have confirmed that radiofrequency hyperthermia (RFH) can significantly enhance the effectiveness of chemotherapy, gene therapies, and immunotherapies in treating various malignancies [10-12]. It is believed that RFH can increase permeability of cell membranes, allowing for more influx of therapeutic agents into the cancer cells [13]. Encouraged by these exciting preliminary results, we aimed to develop a new interventional oncology technique, namely “Image-guided intratumoral RFH-enhanced direct gene therapy”, for the treatment of ovarian cancer.
Materials and methods
Study design
This study included three components: (1) serial in-vitro experiments to establish “proof-of-principle” of our novel technique; (2) serial in-vivo experiments to validate the feasibility of the technique using living animal models afflicted with the same orthotopic ovarian cancers; and (3) serial laboratory examinations to determine underlying biomolecular mechanisms in-vivo and in-vitro, to further improve the technique.
In-vitro confirmation with ovarian cancer cells
Cells
Human epithelial ovarian cancer cell (SKOV 3) were cultured in McCoy’s 5A (Gibico-Life Technologies, Mulgrave, Australia) adjusted to contain 1.5 mM L-glutamine with 10% fetal bovine serum. They were incubated at 37°C in a humidified atmosphere with 5% CO2.
SKOV 3 cells were transfected with mCherry/Luciferase lentiviral particles to create mCherry/Luciferase-positive cells according to the manufacturer’s protocol (GeneCopoeia Inc., Rockville, MD). Recombinant green fluorescent protein (GFP)/HSV-TK lentiviral particles were produced by transient transfection of 293FT cells with GFP/HSV-TK lentiviral plasmid vectors and third-generation lenti-combo packing mix (Applied Biological Materials Inc., Richmond, BC, Canada). Since GFP and HSV-TK gene expressions were simultaneously driven by the same promoter, the detection of GFP by in-vitro fluorescent microscopy and in-vivo bioluminescence optical imaging allowed for assessment of HSV-TK expression efficiency.
2 × 104 cells/chamber mCherry/Luciferase-positive SKOV 3 cells were cultured in 4-chamber cell culture slides (Nalge Nunc International, Rochester, NY, USA). Slides were placed in a 37°C water bath. A 0.032-inch heating wire was attached under the bottom of chamber 4 of the four-chamber cell culture slide, and then connected to a 2450-MHz radiofrequency generator (GMP150, OPTHOS, Rockville, MD, USA) to induce RFH. A sterile 1.1 mm fiber optic temperature sensor was placed at the bottom of each chamber and connected to a temperature thermometer (PhotonControl, Burnaby BC, Canada) for real-time monitoring of temperature change during RFH. By adjusting RF output power to approximately 5-8 W, the temperature of chamber 4 was maintained at 42°C, while the temperature of chamber 1 was kept at 37°C.
For comparison of therapeutic effects across different treatments, mCharry/Luciferase-SKOV-3 cells were divided into four groups: (i) no treatment, as a control; (ii) RFH-only (42°C for 30 minutes); (iii) gene therapy-only with GFP/HSV-TK/lentivirus gene transduction followed by ganciclovir administration for 72 hours; and (iv) combination therapy of RFH +gene (GFP/HSV-TK/lentivirus gene transduction followed by RFH at 42°C for 30 minutes and ganciclovir administration for 72 hours).
Cell proliferation assay
Cell proliferation was evaluated by MTS assay 72 hours after the GCV treatment. Briefly, MTS [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] agent was added to the cell chamber and incubated for 4 hours. The absorbance was measured using a microplate reader at 490 nm. The relative cell proliferations of the different cell groups were evaluated using the equation Atreated-Ablank/Acontrol-Ablank, where “A” represents absorbance. Cells on slides were subsequently imaged with fluorescent microscopy to detect mCherry/Luciferase-positive cells. The experiment was repeated six times for each group.
In-vitro bioluminescent optical imaging (BOI) was performed for additional cell sets of four treatment groups. Cells in each group were treated with 5-μL Pierce D-Luciferin (Thermo Fisher Scientific, Rockford, IL). Then 50-μL cells was mixed with 50-μL 1% agarose (Invitrogen, Carlsbad, CA) in a transparent cylindrical glass tube for BOI (Bruker In-Vivo Xtreme, Billerica, MA). The bioluminescent signal intensity of each tube was calculated as the mean of all detected photon counts within a manually derived region of interest (ROI) using the Bruker MI software. Relative signal intensity (RSI) was determined by the following equation: RSI = SIT/SIC, where “SI” represents signal intensity, “T” represents the treatment group, and “C” represents the control group.
Confirmation of transfection efficiency of GFP/HSV-TK lentivirus to SKOV 3 cells
4 × 104 mCherry/Luciferase-positive SKOV 3 cells were seeded in a six-well cell culture plate (Becton Dickinson and Company, Franklin Lakes, NJ, USA). For comparison of transduction effects with or without hyperthermia, the SKOV 3 cells were divided into two groups: with RFH [RFH (+)] and without RFH [RFH (-)]. After 24-hour cell incubation, the culture medium was replaced with fresh medium containing lentiviral particles (106 IU/cell) and polybrene (8 μg/mL, Santa Cruz Biotechnology, Dallas, TX) according to protocol. Then, the RFH (+) group cells subjected to hyperthermia at 42°C for 30 minutes. Cells were collected at the time points of 72 hours after GFP/HSV-TK/lentiviral transduction for quantitative analysis of GFP expression by Western blot (WB). To quantify GFP expression by BOI, treated cells on plate were washed twice with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde, counterstained with 4’,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Bur-lingame, CA), and imaged with a laser confocal microscope (A1R; Nikon, Tokyo, Japan).
In-vivo validation with living animal models of orthotopic ovarian cancers
Animal models
The animal protocol was approved by our Institutional Animal Care and Use Committee. All animals underwent general anesthesia with 1%-3% isoflurane (Piramal Health care, Andhra Pradesh, India) delivered in 100% oxygen. Nude rat models (Charles River Laboratories, Wilmington, MA) with orthotopic human ovarian tumors were created by inoculating mCherry/luciferase-SKOV 3 into the right ovaries by a sub-capsular injection approach during laparotomy. The nude rat is deficient of some T-cell subtypes, but has normal complement and B-cell function [14,15]. Once the size of the tumor reached 8-10 mm in diameter, we started the experimental procedures.
RFH-enhanced HSV-TK gene therapy of orthotopic ovarian tumors
Twenty-four rats with orthotopic ovarian cancers were allocated into four groups to receive the different treatments of (i) intratumoral injection of 50-µL PBS as a control; (ii) RFH-only with 20 minutes of intratumoral RFH; (iii) gene therapy-only with intratumoral injection of 50-µL (1 × 108) HSV-TK/lentivirus, followed by 7 days of intraperitoneal administration of 5-mg/kg ganciclovir; and (iv) combination therapy (RFH+Gene) by intratumoral injection of 50-µL (1 × 108) HSV-TK/lentivirus with simultaneous RFH at 42°C for 30 minutes, followed by 7 days of intraperitoneal injection of 5 mg/kg ganciclovir. GFP/HSV-TK/lentiviral particles were directly injected into the tumor through the agent perfusion needle of a single polar perfusion-thermal RF electrode (Welfaremedic, Beijing, China), which was initially inserted into the tumor center under real-time sonographic imaging guidance (Sonosite Inc., Bothell, WA). Im mediately after the intratumoral delivery of genes, RFH was generated by connecting the electrode to an RF generator. The temperature in the tumor was monitored by the thermal sensor integrated into the electrode, which was set at 42°C±0.5°C for 30 minutes.
Bioluminescence optical imaging and ultrasound imaging were used to follow up the changes in bioluminescence emitting from the tumors and tumor sizes at the time points of 7 and 14 days post-treatment. Bioluminescent signal intensity of each tumor was quantified by summing detected photon counts using Bruker MI software (Brucker, Billerica, MA). Data were normalized to relative signal intensity (RSI) by using the following equation: RSI = SIDn/SID0, where “SI” is signal intensity, “Dn” represents days after treatment and “D0” is the day before treatment.
Pathology/laboratory correlation and confirmation
After achieving satisfactory images at day 14 after the treatments, the tumors were harvested. The volume of each harvested tumor was calculated according to the formula: volume = (W2 × L)/2, where “W” is the tumor width and “L” is the tumor length, which were measured by caliper [16].
TUNEL assay
Apoptosis of treated tumors was analyzed by immunohistochemical (IHC) staining using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) apoptosis detection kit (R&D Systems, Minneapolis, MN). TUNEL-positive cells were imaged using a microscope (Olympus, Japan). The apoptotic index (AI) was defined as the percentage of positive cells counted per total number of cells in ten randomly-selected fields under a light microscope. Cells with brown nuclei were interpreted as apoptotic positive.
Investigation of underlying bio-molecular mechanisms
Immunohistochemistry (IHC)
Briefly, tumors were fixed in PBS-buffered 10% formalin for 24 hours, and then embedded in paraffin. The samples were sectioned using a slicing machine (Leica, Germany) and mounted in Poly-L-Lysine-coated slides for IHC examination. For examining the proteins/molecules expressed through apoptosis and anti-tumor immunity pathways, the sections were incubated with the following primary antibodies (1:200): (i) caspase-3 and (ii) Bax for activating apoptosis; (iii) Bcl-2 for overcoming apoptosis resistance; and (iv) a group of C-type lectin receptors related to immune-regulatory, including heat shock protein (HSP)-70 for immunoreaction, IL-2 for inflammation, and CD94 for macrophages. After incubating with horseradish peroxidase-conjugated secondary antibodies, whole section digital histological scans were acquired with the Olympus digital camera using its image processing software (Image-pro plus 6.0), and quantitative data were counted randomly from six fields of each sample slice.
Western blot analysis
Cells and tumor tissues of rats were processed by homogenation. 40-80 μg proteins were subjected to western blotting (WB) analysis (Thermo Scientific). Briefly, 50 μg of lysates were separated by 10% SDS-PAGE and then electro-transferred to a nitrocellulose membrane. After being blocked in blocking buffer, each nitrocellulose membrane was incubated with various primary antibodies overnight at 4°C. Then, antigen-antibody complexes were visualized using enhanced chemo-luminescence reagents. The level of protein expression was quantified by measuring the density of the immune-reactive bands and subsequently normalizing to Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH).
Statistical analysis
Results are expressed as the mean ± SDs of six independent experiments. The statistical significance of differences between groups was obtained by the student’s t-test or ANOVA multiple comparisons using GraphPad Pro6.0 (Graphpad, San Diego, CA). Statistical significance was assume for P < 0.05.
Results
In-vitro confirmation
RFH-enhanced killing of HSV-TK/GCV in ovarian cancer cells
Fluorescence microscopy demonstrated a significant attenuation of cells treated with combination therapy, compared with the other three treatments (Figure 1A). MTS assay demonstrated the lowest cell viability in combination therapy, compared to treatments of gene, RFH, and control (35.97±3.68 vs. 75.34±2.39 vs. 97.23±4.64 vs. 100%, P < 0.05) (Figure 1B). In addition, the quantitative analysis of cell bioluminescence signal revealed a significant decrease in photon signal and relative photon signal intensity in combination therapy, compared to the other treatments (0.27±0.15 vs. 0.45±0.21, 0.87±0.68, and 1, P < 0.05) (Figure 1C, 1D).
Figure 1.
In-vitro experiment on RFH-enhanced killing of HSV-TK/GCV in human ovarian cancer (SKOV 3) cells. A. Fluorescence microscopy demonstrates decreased numbers of cells in RFH+Gene combination therapy, compared with other treatments. Scale bar = 50 μm. B. Results of MTS assay showing the lowest cell proliferation in combination therapy. C, D. Results of in-vitro quantitative bioluminescence analysis demonstrating the lowest photon signal in cells treated with combination therapy, compared with the other three treatments. **P < 0.01, ***P < 0.001.
RFH-enhanced GFP/HSV-TK lentivirus transduction in ovarian cancer cells
Confocal microscopy demonstrated a significantly increased number of GFP/TK-positive cells in the RFH (+) group compared with the RFH (-) group (Figure 2A), which was quantitatively confirmed by WB assay (69±5.9 vs. 36.4±3.4, P < 0.05) (Figure 2B, 2C).
Figure 2.

In-vitro experiments on RFH-enhanced GFP/HSV-TK gene expression in SKOV 3 cells. A. Confocal microscopy demonstrates a significantly increased number of GFP-positive cells in RFH (+) group, compared with RFH (-) group. Blue fluorescence indicates nuclei and green fluorescence indicates GFP-positive cells. Scale bar = 100 μm. B, C. Western blotting further confirms a significantly increased level of GFP protein expression in the RFH (+) group, compared to the RFH (-) group. *P < 0.05.
In-vivo validation
Intratumoral RFH-enhanced direct HSV-TK gene therapy for orthotopic ovarian cancer
Bioluminescence optical imaging of treated tumors exhibited significantly lower photon signal of tumors in combination therapy, compared with RFH alone, gene therapy alone, and PBS (Figure 3A, 3B). This result was confirmed by follow-up ultrasound imaging and ultimately gross pathology of the harvested ovarian cancers, with the smallest tumor volume observed in combination therapy, compared to the other three treatment groups (Figure 3A, 3C). Furthermore, the highest apoptic index by TUNEL staining was observed in combination therapy (Figure 3A, 3D).
Figure 3.
In-vivo experiments on RFH-enhanced HSV-TK gene therapy of rat models with orthrotopic SKOV 3 tumors. (A) Both optical and ultrasound imaging show the lowest relative photons (B) and smallest tumor volume (C) in cells treated by combination therapy with RFH+Gene compared with other three treatments. These results are confirmed by gross pathology. (D) TUNEL stain further confirms the highest quantity of apoptotic cells in combination therapy, compared with other treatments. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar = 50 μm.
Investigations on underlying bio-molecular mechanisms
Combination of RFH and HSV-TK/GCV therapy inducing anti-tumor immunity
In both in-vitro and in-vivo experiments, both IHC staining and WB assay showed significantly increased expression of HSP70, IL-2, and CD94 in combination therapy, compared with RFH alone, HSV-TK gene therapy alone, and PBS treatment (Figures 4, 5).
Figure 4.

In-vitro investigation of underlying bio-molecular mechanisms with SKOV 3 cells. (A) Western blot assay shows more expression (as wide and darken bands) for HSP70, Bax, and Caspase-3, and less expression of Bcl-2 in combination treatment compare with other treatments, as further confirmed by quantitative Western blot analysis (B). **P < 0.01, ***P < 0.001. GAPDH = internal control.
Figure 5.
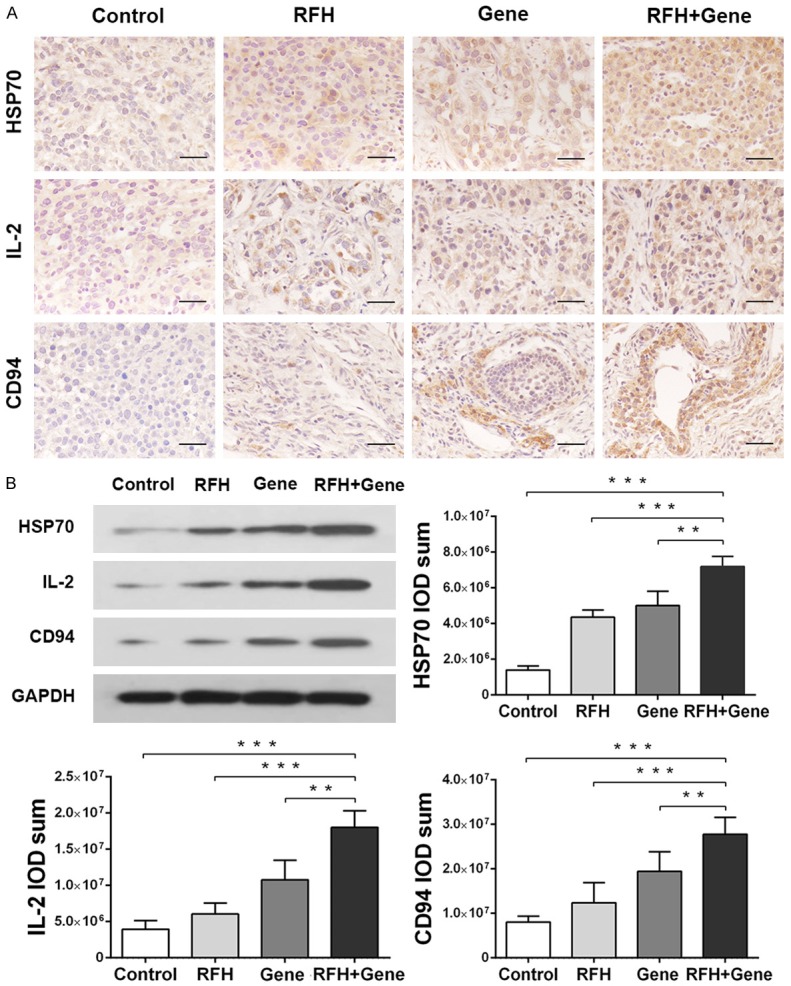
In-vivo investigation of underlying bio-molecular mechanisms with ovarian cancer tissues, confirming the synergetic effect of RFH plus gene therapy via the anti-tumor immunity pathway. A. Immunohistochemistry stain of HSP70, IL-2, and CD94 proteins, demonstrating more blown-colored stains in combination therapy than other treatments. Scale bar = 50 μm. B. Quantitative western blot analysis further shows significantly increased expressions of HSP70, IL-2, and CD94 in combination therapy. **P < 0.01, ***P < 0.001. GAPDH = internal control.
Combination of RFH and HSV-TK/GCV therapy induced apoptosis
In both in-vitro and in-vivo experiments, both IHC staining and WB assay further demonstrated the significantly decreased expression of the anti-apoptotic protein Bcl-2 and significantly increased expression of the activating apoptotic proteins Bax and caspase-3 in combination therapy, compared to the other three groups (Figures 4, 6).
Figure 6.

In-vivo investigation of underlying bio-molecular mechanisms with ovarian cancer tissues, confirming the synergetic effects of RFH plus gene therapy via the apoptotic pathway. (A) Immunohistochemistry stain of Bcl-2, Bax, and Caspase-3 proteins, demonstrating more brown-color stains for Bax and Caspase-3 with less brown-color stain for Bcl-2 in combination therapy compared to other treatments, which are further confirmed by quantitative western blot analysis (B). **P < 0.01, ***P < 0.001. Scale bar = 50 μm. GAPDH = internal control.
Discussion
The treatment of ovarian cancer has proven to be a worldwide critical medical challenge, with a paucity of effective non-invasive and minimally invasive treatments to date. In this study, we developed a novel interventional oncologic technology for the treatment of ovarian cancers, namely “image-guided intratumoral RFH-enhanced direct gene therapy”. The present study has yielded a number of promising findings. First, we confirmed that RFH can increase HSV-TK gene expression, which in turn enhances the killing efficacy of HSV-TK/GCV on human ovarian cancer cell lines-manifested as significantly decreased cell viability and bioluminescent signal intensity in our serial in-vitro confirmation experiments. In our in-vivo validation experiments, we found that intratumoral RFH can enhance the direct anti-tumor effect of HSV-TK/GCV on orthotopic ovarian cancers, which we observed in decreased optical signal intensities and volumes of treated tumors. We determined that the enhanced therapeutic effect of RFH and HSV-TK/GCV synergies occurs via two basic biomolecular pathways: (1) activation of anti-tumor immunity, evidenced by significantly increased expression levels of HSP70, IL-2, and CD94; and (2) activation of apoptosis, seen in significantly increased expressions of apoptosis-inducing proteins Bax and caspase-3, with accordantly decreased expression of the apoptosis-resistance protein Bcl-2.
One of the key steps promoting successful gene therapies primarily involves selecting an efficient method to boost gene transfection and expression in the target tumors. In this study, we attempted to fully apply the unique advantage of image-guided interventions to locally and directly deliver high-dose therapeutic genes, HSV-TK, into the target tumors. We were able to further enhance the therapeutic effective of these delivered genes via simultaneous RFH within the tumors [9,17]. One potential mechanism for RFH-enhanced gene transduction may be increased cell membrane permeability and cell metabolism induced by hyperthermia, facilitating both gene entry and gene expression in the tumor cells [11,18]. In addition, in this study we used a unique multi-modal perfusion-thermal electrode, which could be precisely inserted into the tumor center under real-time ultrasound imaging guidance and then used to deliver sufficient genes into the tumors. This image-guided local delivery approach overcomes the disadvantages of systemic gene administration while minimizing widely circulating gene/lentivirus toxicity and immunogenicity to other vital organs. This solution may address the safety issue of lentiviral vector-mediated systemic gene therapy in patients-one of the most important factors impeding the clinical application of tumor gene therapy.
Hyperthermia is associated with several additional cellular and molecular effects involved in eliciting complex immunological alterations in host cells [19]. Notably, hyperthermia up-regulates the expression of heat shock proteins in tumor cells; these heat shock proteins (HSP) are associated with antigen presentation and anti-tumor immunity [20]. HSP70, in particular, is a multi-functional protein which protects individual cells from environmental stressors, such as aging, metabolic challenge, oxidative stress, and hyperthermia [21]. Additionally, HSP70 is responsible for the generation of innate adaptive immune responses against tumor cells [20,22,23]. In our study, both immunohistochemistry staining and Western blot assay confirmed the significantly increased expressions of HSP70, as well as IL-2 and CD94, in tumor cells treated with RFH. This indicates that the synergetic anti-tumor effect of RFH and HSV-TK/GCV gene therapy on ovarian cancer is achieved through the anti-tumor immunity pathway.
Apoptosis-related proteins/molecules play critical roles in the regulation of apoptosis by either activating pro-apoptotic proteins such as Bax and caspase-3 or deactivating anti-apoptotic proteins such as Bcl-2 [24-27]. In ovarian cancer especially, over-expression of Bcl-2 can protect cancer cells from death and promote resistance to avariety of anti-tumor agents [28]. Caspase-3 is known to act downstream of Bcl-2/Bax control and plays an essential role in inducing apoptosis [29,30]. In the present study, both RFH or gene therapy alone significantly increased expression of pro-apoptotic proteins, Bax and caspase-3 while decreasing expression of the anti-apoptotic protein, Bcl-2, as confirmed by WB and IHC examinations. These results indicate that RFH-enhanced HSV-TK/GCV gene therapy of ovarian cancer functions through the apoptosis pathway.
Since this study primarily focused on the development of a novel technique, we did not compare treatment efficacy of our locoregional (intratumoral) approach versus traditional systemic administration approaches, or that of different anti-tumor agents. In addition, in our in-vivo experiments, the follow-up time was limited to two weeks post-treatment. This is because longer follow-up periods would result in orthotopic tumor masses in the control group greater than ten percent of test subject body weight, which was not approved by our institute’s animal care and use committee.
In conclusion, we introduce a new interventional oncology technique, “image-guided intratumoral RFH-enhanced direct gene therapy”, which functions through the biomolecular mechanisms of inducing anti-tumor immunity and apoptotic pathways. Our technique incorporates the advantages of three advanced scientific fields: image-guided interventional oncology, RF technology, and direct gene therapy. We believe this technique may lead to new clinical therapies for effectively treating ovarian cancer.
Acknowledgements
This study was partially supported by NIH RO1EBO12467 grant and Innovation Funding of Renji Hospital (PYXJS16010).
Disclosure of conflict of interest
None.
References
- 1.Henderson JT, Webber EM, Sawaya GF. Screening for Ovarian Cancer: An Updated Evidence Review for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018. U.S. Preventive services task force evidence syntheses, formerly systematic evidence reviews. [PubMed] [Google Scholar]
- 2.Prat J FIGO Committee on Gynecologic Oncology. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol. 2015;26:87–89. doi: 10.3802/jgo.2015.26.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin-Camean M, Delgado-Sanchez E, Pinera A, Diestro MD, De Santiago J, Zapardiel I. The role of surgery in advanced epithelial ovarian cancer. Ecancermedicalscience. 2016;10:666. doi: 10.3332/ecancer.2016.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes D. Ovarian cancer: beyond resistance. Nature. 2015;527:S217. doi: 10.1038/527S217a. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JF, Wei F, Wang HP, Li HM, Qiu W, Ren PK, Chen XF, Huang Q. Potent anti-tumor activity of telomerase-dependent and HSV-TK armed oncolytic adenovirus for non-small cell lung cancer in vitro and in vivo. J Exp Clin Cancer Res. 2010;29:52. doi: 10.1186/1756-9966-29-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sangro B, Mazzolini G, Ruiz M, Ruiz J, Quiroga J, Herrero I, Qian C, Benito A, Larrache J, Olague C, Boan J, Penuelas I, Sadaba B, Prieto J. A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010;17:837–843. doi: 10.1038/cgt.2010.40. [DOI] [PubMed] [Google Scholar]
- 7.Hung CF, Chiang AJ, Tsai HH, Pomper MG, Kang TH, Roden RR, Wu TC. Ovarian cancer gene therapy using HPV-16 pseudovirion carrying the HSV-tk gene. PLoS One. 2012;7:e40983. doi: 10.1371/journal.pone.0040983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dillen IJ, Mulder NH, Sluiter WJ, Meijer C, de Jong S, Loncarek J, Mesnil M, de Vries EF, Vaalburg W, Hospers GA. Influence of p53 status on the HSV-Tk/GCV-induced bystander effect in a panel of human ovarian carcinoma cell lines. Oncol Res. 2005;15:151–159. doi: 10.3727/096504005776367942. [DOI] [PubMed] [Google Scholar]
- 9.Yin PT, Shah S, Pasquale NJ, Garbuzenko OB, Minko T, Lee KB. Stem cell-based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer. Biomaterials. 2016;81:46–57. doi: 10.1016/j.biomaterials.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, Zhang F, Bai Z, Wang J, Qiu L, Li Y, Meng Y, Valji K, Yang X. Orthotopic esophageal cancers: intraesophageal hyperthermia-enhanced direct chemotherapy in rats. Radiology. 2017;282:103–112. doi: 10.1148/radiol.2016152281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Shi Y, Bai Z, Li Y, Qiu L, Johnson G, Zhang F, Yang X. Radiofrequency hyperthermia-enhanced herpes simplex virus-thymidine kinase/ganciclovir direct intratumoral gene therapy of hepatocellular carcinoma. Int J Hyperthermia. 2016:1–8. doi: 10.1080/02656736.2016.1229045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu L, Zhang F, Shi Y, Bai Z, Wang J, Li Y, Lee D, Ingraham C, Feng X, Yang X. Gliomas: motexafin gadolinium-enhanced molecular mr imaging and optical imaging for potential intraoperative delineation of tumor margins. Radiology. 2016;279:400–409. doi: 10.1148/radiol.2015150895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taratula O, Dani RK, Schumann C, Xu H, Wang A, Song H, Dhagat P, Taratula O. Multifunctional nanomedicine platform for concurrent delivery of chemotherapeutic drugs and mild hyperthermia to ovarian cancer cells. Int J Pharm. 2013;458:169–180. doi: 10.1016/j.ijpharm.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Festing MF, May D, Connors TA, Lovell D, Sparrow S. An athymic nude mutation in the rat. Nature. 1978;274:365–366. doi: 10.1038/274365a0. [DOI] [PubMed] [Google Scholar]
- 15.Rygh CB, Wang J, Thuen M, Gras Navarro A, Huuse EM, Thorsen F, Poli A, Zimmer J, Haraldseth O, Lie SA, Enger PO, Chekenya M. Dynamic contrast enhanced MRI detects early response to adoptive NK cellular immunotherapy targeting the NG2 proteoglycan in a rat model of glioblastoma. PLoS One. 2014;9:e108414. doi: 10.1371/journal.pone.0108414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faustino-Rocha A, Oliveira PA, Pinho-Oliveira J, Teixeira-Guedes C, Soares-Maia R, da Costa RG, Colaco B, Pires MJ, Colaco J, Ferreira R, Ginja M. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab Anim (NY) 2013;42:217–224. doi: 10.1038/laban.254. [DOI] [PubMed] [Google Scholar]
- 17.Walther W, Stein U. Heat-responsive gene expression for gene therapy. Adv Drug Deliv Rev. 2009;61:641–649. doi: 10.1016/j.addr.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Doukas AG, Flotte TJ. Physical characteristics and biological effects of laser-induced stress waves. Ultrasound Med Biol. 1996;22:151–164. doi: 10.1016/0301-5629(95)02026-8. [DOI] [PubMed] [Google Scholar]
- 19.Hietanen T, Kapanen M, Kellokumpu-Lehtinen PL. Natural killer cell viability after hyperthermia alone or combined with radiotherapy with or without cytokines. Anticancer Res. 2018;38:655–663. doi: 10.21873/anticanres.12269. [DOI] [PubMed] [Google Scholar]
- 20.Guzhova IV, Margulis BA. HSP70-based anti-cancer immunotherapy. Hum Vaccin Immunother. 2016;12:2529–2535. doi: 10.1080/21645515.2016.1190057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzhova IV, Shevtsov MA, Abkin SV, Pankratova KM, Margulis BA. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int J Hyperthermia. 2013;29:399–408. doi: 10.3109/02656736.2013.807439. [DOI] [PubMed] [Google Scholar]
- 22.Jolesch A, Elmer K, Bendz H, Issels RD, Noessner E. Hsp70, a messenger from hyperthermia for the immune system. Eur J Cell Biol. 2012;91:48–52. doi: 10.1016/j.ejcb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Zorzi E, Bonvini P. Inducible hsp70 in the regulation of cancer cell survival: analysis of chaperone induction, expression and activity. Cancers (Basel) 2011;3:3921–3956. doi: 10.3390/cancers3043921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muenyi CS, Trivedi AP, Helm CW, States JC. Cisplatin plus sodium arsenite and hyperthermia induces pseudo-G1 associated apoptotic cell death in ovarian cancer cells. Toxicol Sci. 2014;139:74–82. doi: 10.1093/toxsci/kfu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin X, Yu B, Tang Z, He B, Ren J, Xiao X, Tang W. Bifidobacterium infantis-mediated HSV-TK/GCV suicide gene therapy induces both extrinsic and intrinsic apoptosis in a rat model of bladder cancer. Cancer Gene Ther. 2013;20:77–81. doi: 10.1038/cgt.2012.86. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed K, Tabuchi Y, Kondo T. Hyperthermia: an effective strategy to induce apoptosis in cancer cells. Apoptosis. 2015;20:1411–1419. doi: 10.1007/s10495-015-1168-3. [DOI] [PubMed] [Google Scholar]
- 27.Zakki SA, Cui ZG, Sun L, Feng QW, Li ML, Inadera H. Baicalin augments hyperthermia-induced apoptosis in U937 cells and modulates the MAPK pathway via ROS generation. Cell Physiol Biochem. 2018;45:2444–2460. doi: 10.1159/000488263. [DOI] [PubMed] [Google Scholar]
- 28.Haldar S, Jena N, DuBois GC, Takayama S, Reed JC, Fu SS, Croce CM. Purification and characterization of the bcl-2 protein. Arch Biochem Biophys. 1994;315:483–488. doi: 10.1006/abbi.1994.1529. [DOI] [PubMed] [Google Scholar]
- 29.Salakou S, Kardamakis D, Tsamandas AC, Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos DS, Papapetropoulos T, Petsas T, Dougenis D. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo. 2007;21:123–132. [PubMed] [Google Scholar]
- 30.Choi YJ, Gurunathan S, Kim JH. Graphene oxide-silver nanocomposite enhances cytotoxic and apoptotic potential of salinomycin in human ovarian cancer stem cells (OvCSCs): a novel approach for cancer therapy. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19030710. [DOI] [PMC free article] [PubMed] [Google Scholar]




