Abstract
Previous studies showed that Livin, a member of inhibitors of apoptosis protein (IAP), played an important role in drug and radiation resistance. When the expression of Livin was blocked, the sensitivity to both chemotherapy and radiotherapy was improved in lung cancer cells. A total of 79 patients diagnosed with non-small cell lung cancer (NSCLC) were enrolled into the current study from Jan 2012 to Apr 2016. The Livin and MUC-1 groups received one-cycle autologous DCs/CIKs infusion on days 11 to 14 additionally. The clinical efficacy, immune index, KPS score and adverse events were compared among the three groups. Median progression-free survival (mPFS) in Livin and MUC-1 groups was significantly longer than that in Chemo group (195 and 211 vs 138 days, P < 0.05), and the objective response rate (ORR) in Livin and MUC-1 groups was significantly higher than that in Chemo group (23.1% and 22.2% vs 5.1%, P < 0.05). The Tetramer value after treatment in Livin group was significantly higher than that before treatment (4.07 ± 3.77 vs 3.16 ± 3.82, P < 0.05). The concentration of Livin antibody in patients’ peripheral blood before and after treatment in Livin group had no significant difference (P > 0.05). As for KPS score, scarce decrease was found in Livin and MUC-1 groups after chemotherapy treatment (0.77 ± 6.41 and 0.37 ± 5.18, respectively). However, obvious decrease of KPS score (P < 0.039) was recorded in Chemo group (3.85 ± 6.33). There was no significant difference in disease control rate (DCR), overall survival (OS), T cell subsets, cytokine levels (IFN-γ and IL-2) and adverse events between the three groups (P > 0.05). Livin peptide could be a novel substitute to trigger cell immunity by loading DCs in combination with chemotherapy in NSCLC.
Keywords: Dendritic cells, cytokine-induced killer cells, Livin peptide, non-small-cell lung cancer, cytotherapy
Introduction
Lung cancer accounts for the largest proportion of diagnosed cancers all around the world (1.8 million, 13.0% of the total), and remains a leading cause of cancer death (1.6 million, 19.4% of the total) [1]. Among all lung cancers, non-small cell lung cancer (NSCLC) constitutes approximately 85% and most of the NSCLC patients are diagnosed at advanced stages, being unsuitable for surgery [2]. Although the treatment of lung cancer has made great progress over the past decade, the overall 5-year survival rate is still less than 20% [3]. Therefore, developing new and more effective treatment for NSCLC patients, especially for those in late stages, has vital significance.
Developing rapidly at present, tumor immunotherapy is considered a very promising treatment. It takes advantage of the patient’s own immune system to combat cancer cells through mobilization or enhancement of immune function, thereby improving the Karnofsky performance score (KPS) and prolonging survival of patients with the advantages of low toxicity and high efficiency [4,5]. Dendritic cells (DCs) are known as one type of antigen-presenting cells (APCs), which have the strongest ability to present antigens. DCs facilitate the activation and proliferation of primary T cells by taking in, processing and presenting antigens [6,7]. By activating and culturing DCs loaded with tumor antigens in vitro, and then transfusing the cells into the patient’s body, a very strong anti-tumor immune response can be induced [8]. Cytokine-induced killer cells (CIKs) are a group of immune cells with both strong anti-tumor activity of T cells and non-MHC restricted killing feature of natural killer cells (NK), thus also known as NK cell-like T lymphocytes (NKT) [9]. DCs combined with CIKs can produce highly effective immune response, and enhance tumor killing activity and anti-tumor effects [10,11]. Moreover, our study has confirmed that DCs/CIKs combined with thoracic radiotherapy can improve the mPFS and ORR of patients with locally advanced or metastatic NSCLC compared with thoracic radiotherapy alone [12].
Previous studies have reported that inhibitors of apoptosis protein (IAP) are involved in an important mechanism of tumor resistance to chemotherapy and radiotherapy [13,14]. Livin is a member of IAP family, which can inhibit apoptosis through a variety of ways [15-17]. Livin is highly expressed in many malignant tumor tissues and lowly or not expressed in normal tissues of adults, which indicated that it may have important clinical significance [18-21]. Similarly, our previous studies have also demonstrated that livin is highly expressed in NSCLC tissues and closely related to the occurrence and development of malignant tumors [22]. When the expression of Livin was blocked, the sensitivity to chemotherapy and radiotherapy was improved in lung cancer cells. Our study confirmed that the sensitivity of SPC-A1 cells to many chemotherapeutic drugs (including cisplatin, carboplatin, cyclophosphamide and adriblastine) was markedly increased after silencing of the Livin gene [23]. Another work proved that after transfection with vectors expressing Livin, the positive cells, especially A549 cells expressing Livin, showed an ~20% increase in colony-forming ability, a shorter doubling time and lower sensitivity to chemotherapeutic drugs and radiation [24]. The application of HLA-A2 restricted Livin peptide (RLQEERTCKV) as a tumor antigen to cell immunotherapy has been gradually reported [25]. Based on the hypothesis that Livin peptide-loaded DCs/CIKs combined with chemotherapy could benefit the HLA-A2+ NSCLC patients, we therefore sponsored a phase II clinical trial from Jan 2012 to Apr 2016 to investigate the availability of Livin acting as a new tumor antigen for DCs/CIKs cytotherapy to activate cell immunity, enhance the sensitivity of NSCLC patients to chemotherapy, and then improve local control rate, reduce recurrence and metastasis and prolong patients’ survival.
Methods
Study design and patients selection
This prospective single-center, open-label, phase II study was carried out at the Cancer Institute of PLA, Xinqiao Hospital, Army Medical University, Chongqing, China. The protocol was registered in Chinese Clinical Trial Registry (ChiCTR-TRC-12002369, http://www.chictr.org.cn) and approved by the Ethics Committee of General Logistics Department of PLA, China.
Qualified patients were histologically or cytologically (not including sputum cytology) diagnosed with unresectable stage III or IV advanced NSCLC (according to the 7th edition of the General Rule for Clinical and Pathological Record of Lung Cancer) [26]. Given their certain conditions, such as huge primary tumors, potential risk of heart failure, respiratory dysfunction and previous chemotherapy in other medical centers, etc., all enrolled stage III patients were reluctant to receive or unsuitable for concurrent chemoradiotherapy or radical radiotherapy. Other inclusion criteria were as follows: an age of 18 years or older at the time of signing the consent form; life expectancy of ≥3 months at registration; KPS score ≥70 at the time of first visit; adequate function of the liver, kidney, heart and hematopoietic system; and two or more cycles of previous platinum-based doublet chemotherapy without disease progression. No previous DCs/CIKs cytotherapy was allowed. One or more measurable lesions were necessary for therapeutic evaluation based on Response Evaluation Criteria in Solid Tumors (RECIST 1.1) [27]. All the patients were given a full explanation of the protocol and provided with informed consent before enrollment. Major exclusion criteria included an acute infection; any autoimmune disease; a history of severe allergic reaction; HIV infection; pregnancy or nursing; and other inappropriate conditions for enrollment as judged by clinicians.
The planned sample size was set as 20, 30 and 50 patients in Livin, MUC-1, and Chemo groups, respectively. When engaging a patient into cytotherapy groups (Livin and MUC-1 groups), we chose a counterpart who possessed a nearly identical characteristic to receive chemotherapy alone as a control. Eventually, from Jan 2012 to Apr 2016, 13 enrolled patients with HLA-A2+ NSCLC were assigned to Livin group, 27 patients receiving MUC-1-loaded DCs/CIKs combined with chemotherapy to MUC-1 group, and 39 patients receiving chemotherapy alone to Chemo group. The primary endpoint for this clinical trial was ORR, and the secondary endpoints were DCR, mPFS, median overall survival (mOS), KPS score change and adverse events. Immunologic effects were to be explored. After cytotherapy, enrolled patients would continue chemotherapy to reach a standard of 4 to 6 cycles in total.
Preparation of autologous DCs and CIKs
Apheresis blood and autologous DCs/CIKs were prepared according to the protocols described in our previous study [28]. Briefly, peripheral blood mononuclear cells (PBMCs) were collected from the patients in Livin and MUC-1 groups at the beginning, then isolated by Ficoll-Hypaque gradient density centrifugation, and cultured in X-VIVO medium for 2 h. The adherent cells were collected for preparing DCs in X-VIVO medium containing granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). Five days later, tumor necrosis factor-α (TNF-α) and HLA-A2-restricted Livin peptide (RLQEERTCKV) (GL Biochem, Shanghai, China) or MUC-1 peptide (SAPDTRPAPGSTAPPAHGVT) (GL Biochem, Shanghai, China) were added into DCs culture of Livin or MUC-1 groups respectively for another 2 days. For preparing CIKs, non-adherent cells were cultured in X-VIVO medium containing interferon γ (IFN-γ), CD3 monoclonal antibody, and interleukin-2 (IL-2) for 10 days. The immune phenotype markers CD80, CD83, CD86, and HLA-DR for DCs and CD3, CD56 for CIKs were analyzed by flow cytometry. Contamination of bacteria, fungi and endotoxin in all the cultured samples were detected during the course of cell culture.
DCs/CIKs cytotherapy
At the beginning of the study (day 0), we collected peripheral blood mononuclear cells (PBMCs) from the patients in Livin and MUC-1 groups for culturing DCs and CIKs respectively in vitro, and then all patients were treated according to the study design. The activity and safety of DCs and CIKs were tested 48 hours before the infusion. After confirmed the high activity and sterility of the vaccine, over 1×107 DCs were injected subcutaneously in the lymph node-rich regions (bilateral axillary or inguinal region) once a day for 4 successive days from day 11 to 14. Over 1×109 CIKs in 100 mL of normal saline (NS) (0.9%) were infused intravenously once a day for 4 successive days from day 11 to 14 (Figure 1).
Figure 1.
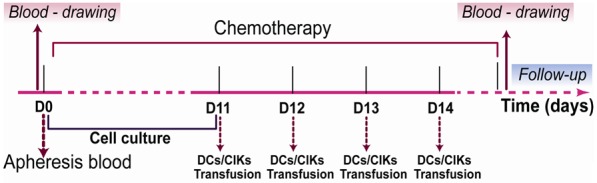
The study design of Livin, MUC-1, Chemo groups in the current clinical trial.
Assessment of clinical outcomes
The treatment efficacy was classified as complete response (CR), partial response (PR), stable disease (SD), and progression disease (PD) on the basis of RECIST 1.1 [27]. The ORR was defined as the percentage of patients with CR or PR, and DCR as the percentage of patients with CR, or PR, or SD. mPFS was defined as the median time scale from enrollment to disease progression, while mOS was the median time scale from first treatment to death. The follow-up was conducted in the 1st and 3rd month after chemotherapy, and then every 3 months for the first year, and every 6 months henceforth. Conventional follow-up assessments included physical examinations, vital signs, computed tomographic scans (CT), and laboratory tests.
Assessment of immunologic effects
Blood was drawn from participants on day 0 and within a week after chemotherapy (Figure 1). Direct detection of the number of specific CD8+ T cells in Livin group could be achieved by tetramer techniques (Beijing QuantoBio Biotechnology Co. Ltd, Beijing, China) according to the manufacturer’s instructions. Livin antibodies in the serum of Livin group’s patients were detected by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, MN, USA) following the manufacturer’s instructions. For assay of T cell subsets and NK cells, 100 μL of EDTA anticoagulant blood samples were stained with relevant antibodies (BD Bioscience), namely, anti-CD3+, CD4+ and CD8+ for T cells, anti-CD3+ and CD56+ for NK cells, in darkness for 20 min. Then, erythrocyte lysis buffer was added. After being vortexed for 15 s and incubated at room temperature for 5 min, the samples were centrifuged to remove the supernatant and washed with PBS. After being resuspended with staining buffer, the samples were analyzed on the BD Aria flow cytometer (BD Bioscience). And cytokine levels, including IFN-γ and IL-2, in the serum of three groups’ patients were detected by ELISA (R&D Systems, MN, USA) following the manufacturer’s instructions.
KPS score and adverse events
KPS score was assessed on day 0 of the study and within a week after chemotherapy. Adverse events, such as insomnia, anorexia, fever, skin rash, and joint pain, were monitored and observed once a week during the therapy and once a month during the follow-up.
Statistical analysis
SPSS 18.0 was used for data analysis. The measurement data were presented as the mean ± standard error (x̅ ± s). Statistical significance was determined using one way ANOVA in three groups. The enumeration data were analyzed using χ2 test. Differences in Livin antibodies and the Tetramer values of the patients in the Livin group were analyzed using the independent Student t test. mPFS and mOS were estimated by using Kaplan-Meier curves with the log-rank test. P < 0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
From Jan 2012 to Apr 2016, a total of 79 patients with locally advanced or metastatic NSCLC were enrolled and assigned to Livin, MUC-1, and Chemo groups. Clinicopathological characteristics such as age, gender, clinical stage of tumor, previous systemic chemotherapy, pathological type and KPS score in three groups were analyzed. None of them showed significant differences (P > 0.05, Table 1), suggesting a nearly identical baseline between the three groups.
Table 1.
Baseline characteristics of the patients in three groups
| Characteristic | Livin | MUC-1 | Chemo | P value |
|---|---|---|---|---|
| Cases | 13 | 27 | 39 | |
| Median age (yrs, Range) | 61 (35-74) | 56 (27-73) | 59 (36-76) | 0.246 |
| Gender | 0.631 | |||
| Male | 10 (76.9%) | 21 (77.8%) | 26 (66.7%) | |
| Female | 3 (23.1%) | 6 (22.2%) | 13 (33.3%) | |
| Histological type | ||||
| ADC | 9 (69.2%) | 16 (59.3%) | 27 (69.2%) | 0.731 |
| SCC | 4 (30.8%) | 11 (40.7%) | 12 (30.8%) | |
| Clinical stage | ||||
| IIIB | 5 (38.5%) | 13 (48.1%) | 15 (38.5%) | 0.748 |
| IV | 8 (61.5%) | 14 (51.9%) | 24 (61.5%) | |
| Cycles of previous chemotherapy | 2.92 ± 0.76 | 3.04 ± 0.76 | 3.10 ± 0.72 | 0.746 |
| KPS score | 83.85 ± 5.06 | 85.93 ± 5.72 | 84.10 ± 5.95 | 0.383 |
Clinical outcomes
The median follow-up time in Livin, MUC-1, and Chemo groups was 290, 400 and 410 days, respectively. For the therapeutic efficacy, 3 PR, 5 SD and 5 PD were found in Livin group, 6 PR, 10 SD and 11 PD in MUC-1 group, and 2 PR, 18 SD, 19 PD in Chemo group. The ORR of Livin and MUC-1 groups was both significantly higher than that of Chemo group (23.1% and 22.2% vs 5.1%, P < 0.05) (Figure 2). However, no obvious difference in DCR was observed between the three groups (61.5% and 59.3% vs 51.3%, P > 0.05).
Figure 2.
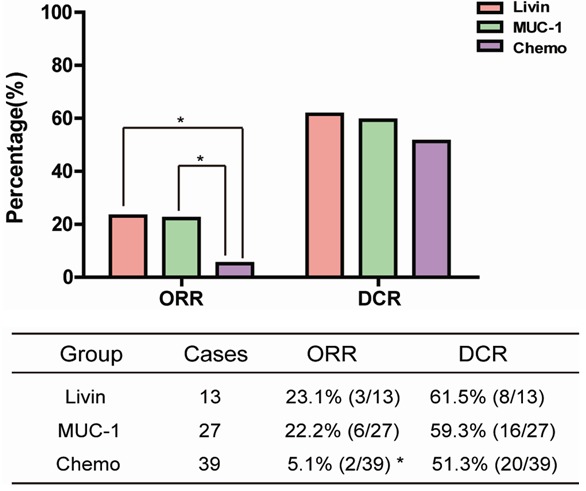
Short-term clinical effects. *ORR was significantly higher in Livin and MUC-1 groups than in Chemo group (P < 0.05), but there was no significant ORR difference between Livin and MUC-1 groups. No obvious difference in DCR was observed between the three groups.
As for long-term evaluation, the mPFS of Livin group (195 days) and MUC-1 group (211 days) was significantly longer than that of Chemo group (138 days) (P < 0.05). However, there was no significant difference in mOS between the three groups (290 and 400 days vs 410 days, respectively; P > 0.05) (Figure 3).
Figure 3.
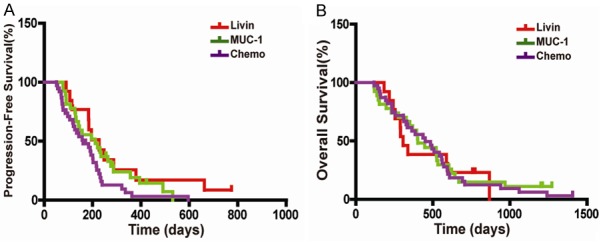
Kaplan-Meier curves of mPFS and mOS. A. Compared with Chemo group, mPFS in Livin and MUC-1 groups was significantly longer (P < 0.05), but there was no significant mPFS difference between Livin and MUC-1 groups. B. No significant mOS difference between the three groups (P > 0.05).
Immunologic response
Among the 39 patients in Chemo group, complete immunologic results were obtained in only 19 cases before and after chemotherapy. There was a lack of some medical materials in the rest patients because of their refusal to draw blood and the delayed follow-up, and some other reasons. The results of T cell subsets and NK cells were analyzed. There were no obvious changes in the percentage of CD3+, CD4+, CD8+ T cells and CD3-CD56+ NK cells before and after chemotherapy in three groups (P > 0.05, Table 2). However, we found that the changing trends of T cell subsets in the Livin and MUC-1 groups were similar, as there was an increasing trend of CD8+ T cells in Livin and MUC-1 groups after the treatment (P = 0.063 and P = 0.057, respectively; Table 2) and the CD4+/CD8+ ratio decreased significantly in three groups after treatment (P < 0.05, Table 2). Besides, we detected the expression levels of IL-2 and IFN-γ in the serum of three groups’ patients. The results showed no statistical difference before and after treatment (P > 0.05, Table 3). Meanwhile, we also detected Livin antibodies in the serum of patients. Although the level of antibodies after treatment was not significantly changed compared with that before treatment (0.80 ± 0.19 vs 0.88 ± 0.28), the Tetramer results in Livin group indicated that the Tetramer values of the patients were significantly higher than those before treatment (4.07 ± 3.77 vs 3.16 ± 3.82, P < 0.05) (Figure 4).
Table 2.
T cell subsets in PBMCs (x̅ ± s)
| Group | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | CD3-CD56+ (%) |
|---|---|---|---|---|---|
| Livin | |||||
| Pre-treatment | 67.44 ± 11.03 | 34.90 ± 7.15 | 24.37 ± 7.09 | 1.54 ± 0.49 | 13.50 ± 6.9 |
| Post-treatment | 73.29 ± 11.28 | 33.80 ± 5.28 | 31.76 ± 14.39 | 1.24 ± 0.48 | 11.73 ± 6.71 |
| P value | 0.071 | 0.596 | 0.063 | 0.019* | 0.378 |
| MUC-1 | |||||
| Pre-treatment | 64.31 ± 12.51 | 34.20 ± 10.30 | 28.38 ± 8.82 | 1.33 ± 0.66 | 22.12 ± 12.99 |
| Post-treatment | 66.86 ± 11.75 | 33.16 ± 10.10 | 29.58 ± 8.61 | 1.12 ± 0.52 | 21.24 ± 12.56 |
| P value | 0.199 | 0.571 | 0.057 | 0.011* | 0.407 |
| Chemo | |||||
| Pre-treatment | 64.86 ± 16.16 | 32.77 ± 10.00 | 30.88 ± 11.61 | 1.25 ± 0.67 | 20.02 ± 8.26 |
| Post-treatment | 67.11 ± 15.20 | 28.37 ± 10.87 | 35.75 ± 13.31 | 0.91 ± 0.54 | 20.36 ± 11.10 |
| P value | 0.655 | 0.145 | 0.186 | 0.015* | 0.890 |
CD4+/CD8+ ratio decreased significantly in three groups after treatment (P < 0.05).
Table 3.
Cytokines detection in serum (x̅ ± s)
| Group | IL-2 (ng/L) | IFN-r (pg/mL) |
|---|---|---|
| Livin | ||
| Pre-treatment | 358.37 ± 49.00 | 491.19 ± 60.00 |
| Post-treatment | 376.09 ± 44.44 | 507.32 ± 59.87 |
| P value | 0.19 | 0.48 |
| MUC-1 | ||
| Pre-treatment | 330.42 ± 79.25 | 575.85 ± 179.85 |
| Post-treatment | 330.94 ± 66.12 | 567.12 ± 151.64 |
| P value | 0.98 | 0.83 |
| Chemo | ||
| Pre-treatment | 346.65 ± 53.03 | 541.05 ± 70.73 |
| Post-treatment | 335.74 ± 61.92 | 534.62 ± 66.35 |
| P value | 0.56 | 0.77 |
Figure 4.
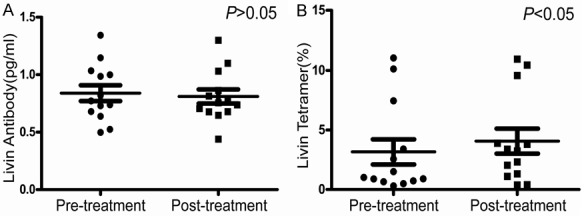
Livin antibodies detection and Tetramer assay in Livin group. A. Livin antibody in patients’ peripheral blood had no significant difference before and after treatment (P > 0.05). B. The Tetramer values of the patients in the Livin group were significantly higher than those before treatment (P < 0.05).
KPS score and adverse events
At the beginning of the study, the KPS score was 83.85 ± 5.06, 85.93 ± 5.72 and 84.10 ± 5.95 in Livin, MUC-1 and Chemo groups, respectively (Table 1). At the end of chemotherapy, the KPS score was 83.08 ± 4.80, 85.56 ± 6.40 and 80.26 ± 6.28 in the three groups, respectively (Table 4). Scarce decrease of KPS score was found in Livin and MUC-1 groups after chemotherapy (0.77 ± 6.41 and 0.37 ± 5.18, respectively). However, obvious decrease of KPS score was recorded in Chemo group (3.85 ± 6.33). The decrease of KPS score in Livin and MUC-1 groups was significantly smaller than that in Chemo group (P = 0.039, Table 4).
Table 4.
Adverse events
| Event | Livin | MUC-1 | Chemo | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Fever | 3 (23.1) | 0 | 5 (18.5) | 0 | 11 (28.2) | 0 |
| Anorexia | 2 (15.4) | 0 | 7 (25.9) | 0 | 13 (33.3) | 0 |
| Allergy | 1 (7.7) | 0 | 1 (3.7) | 0 | 11 (28.2) | 0 |
| Nausea, vomiting | 2 (15.4) | 0 | 6 (22.2) | 0 | 17 (43.6) | 0 |
| Heart function | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver function | 0 | 0 | 0 | 0 | 0 | 0 |
| Renal function | 0 | 0 | 0 | 0 | 0 | 0 |
| Myelosuppression | 2 (15.4) | 0 | 5 (18.5) | 0 | 9 (23.1) | 3 (7.7) |
| KPS score | 83.08 ± 4.80* | 85.56 ± 6.40* | 80.26 ± 6.28 | |||
KPS change was significantly smaller than in Chemo group (P < 0.05).
Adverse events were assessed in all patients. The functions of the liver, kidneys and heart of all the participants remained normal at the end of the chemotherapy. The most common adverse events were fever, anorexia, nausea, vomiting, and myelosuppression (Table 4). Most of them were at grade I~II. All patients recovered after suitable treatment within 2 months. There were no cases with treatment-related deaths.
Discussion
Our preliminary results have shown that Livin as a member of the IAP family plays an important role in the resistance of lung cancer cells to chemotherapy and radiotherapy [27]. The expression of Livin was up-regulated in NSCLC patients after chemotherapy and radiotherapy, and blocking Livin expression could enhance the chemosensitivity and radiosensitivity of lung cancer cells [29]. Livin is highly expressed in a variety of malignant tumors, and Livin antibodies can be also detected in the serum of patients, suggesting that Livin may be the main tumor-associated antigen [30-32]. Therefore, some studies have speculated that Livin can be a new immunotherapy target for patients with NSCLC [33]. Adoption of anti-Livin immunotherapy has been gradually reported, and achieved desirable results [30,33,34]. This study was designed to explore the efficacy of Livin peptide-loaded DCs/CIKs combined with chemotherapy for patients with HLA-A2+ NSCLC.
According to the present results, Livin group had a similar clinical efficacy to MUC-1 group, and they both had an advantage over the Chemo group. A longer mPFS was observed in Livin and MUC-1 groups than in Chemo group (195 and 211 days vs 138 days, P < 0.05), and ORR was higher in Livin and MUC-1 groups (23.1% and 22.2% vs 5.1%; P < 0.05). Although there was no significant difference in DCR and mOS between the three groups (P > 0.05), the positive results in mPFS and ORR were still encouraging.
For safety analysis, during the combination therapy of DCs/CIKs and chemotherapy, a majority of adverse events were mild, tolerant and similar to those caused by chemotherapy alone. No new safety signals were identified, and no treatment-related deaths occurred. In addition, we found a significant KPS score decrease after chemotherapy in Chemo group (P < 0.05). However, there was a minor KPS score decrease in Livin and MUC-1 groups (P > 0.05). These results suggest that combined cytotherapy improves the KPS score for advanced patients receiving chemotherapy. Thus, DCs/CIKs in combination with chemotherapy showed a good safety profile.
As for T cell subsets statistics, we found that the changing trends of T cell subsets in the Livin and MUC-1 groups were similar, as there was an increasing trend of CD8+ T cells in Livin and MUC-1 groups after the treatment (P = 0.063 and P = 0.057, respectively) and the CD4+/CD8+ ratio decreased significantly in the three groups after treatment (P < 0.05). However, there was no significant difference in each index of T cell subsets between the three groups (P > 0.05). Similarly, there was also no significant difference in the expression levels of IL-2 and IFN-γ in the serum of three groups’ patients (P > 0.05). Meanwhile, we detected Livin antibodies in the serum of patients in Livin group. Although the level of antibodies after treatment was not significantly changed compared with before, the Tetramer values of the patients after treatment were significantly higher than those before treatment (P < 0.05).
The MHC-peptide tetramer technique was established by Altmen et al. [35] in 1996. Its principle is that when the MHC-peptide tetramer binds to the TCR of specific T cells, it can directly detect the number of specific CD8+ T cells by flow cytometry, truly reflecting the status of specific CD8+ T cells. This technique has the advantage of being rapid, direct, sensitive and highly specific [36]. Now it is an important immune technology, which is widely used in immunological research, clinical diagnosis and treatment of some diseases. In the current study, the Tetramer values of Livin group were significantly increased after treatment (4.07 ± 3.77 vs 3.16 ± 3.82, P < 0.05). This result shows that Livin antigen has induced specific cytotoxic T lymphocyte (CTL) response in vivo, which results an increasing number of CTL. However, we measured the concentration of Livin antibody in serum and found no significant change before and after treatment. Therefore, the current results demonstrate that Livin peptide-loaded DCs treatment mainly stimulates patient’s cellular immunity, rather than humoral immunity, providing a basis for Livin as a new tumor antigen for cellular immunotherapy.
Restricted to national policy which prohibits DCs/CIKs cytotherapy as routine anti-cancer treatment in China from Apr. 2016, we have to close the enrollment in advance. Therefore, the current study did not reach the predicted sample size. Nevertheless, we found that the efficacy of Livin group was not inferior to MUC-1 group regardless of clinical efficacy or outcomes of immune index. Also, our current findings supported that Livin-loaded DCs could elicit patient-specific CTL responses. However, there were some limitations in our study. The recruitment of patients in the Chemo group was non-random selection. Cytotherapy in the Livin and MUC-1 groups was performed for only one cycle because of the high cost of multicycle cytotherapy. Whether DCs/CIKs cytotherapy taking Livin peptide as a new tumor antigen can improve chemosensitivity of lung cancer needs further higher level clinical trial. Further conclusions would be acquired from study of chemosensitivity by expanding the sample size, and conducting randomization design, thus to provide more and more favorable evidences that Livin could be used as a tumor antigen to stimulate the body’s cellular immune function and benefit the patients.
Conclusions
Livin peptide-loaded DCs/CIKs, combined with chemotherapy, was similar to MUC-1 peptide-loaded DCs/CIKs in clinical efficacy and immunological parameters, and they are both superior to chemotherapy alone. Livin peptide could be used as a new antigen to load DCs/CIKs, induce cellular immunity, and stimulate specific CTL response.
Acknowledgements
We appreciate the English editing by Department of Medical English, Third military medical university. This work was supported by the National Natural Science Foundation of China (No. 81773245, 81272496), Chongqing Natural Science Foundation (No. cstc2012jjB10003, cstc2012jjA10096), and Clinical Research Fund of Third Military Medical University (No. 2011XLC38).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Wang Z. Efficacy and safety of dendritic cells co-cultured with cytokine-induced killer cells immunotherapy for non-small-cell lung cancer. Int Immunopharmacol. 2015;28:22–8. doi: 10.1016/j.intimp.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo e Silva G, Chen WQ, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP CONCORD Working Group. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker ZC, Laguna BA, Moon E, Singhal S. Adjuvant immunotherapy for non-small cell lung cancer. Cancer Treat Rev. 2012;38:650–61. doi: 10.1016/j.ctrv.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 7.Frankenberger B, Schendel DJ. Third generation dendritic cell vaccines for tumor immunotherapy. Eur J Cell Biol. 2012;91:53–8. doi: 10.1016/j.ejcb.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 8.van Broekhoven CL, Parish CR, Demangel C, Britton WJ, Altin JG. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 2004;64:4357–65. doi: 10.1158/0008-5472.CAN-04-0138. [DOI] [PubMed] [Google Scholar]
- 9.Gutgemann S, Frank S, Strehl J, Schmidt-Wolf IG. Cytokine-induced killer cells are type II natural killer T cells. Ger Med Sci. 2007;5:Doc07. [PMC free article] [PubMed] [Google Scholar]
- 10.Su X, Zhang L, Jin L, Ye J, Guan Z, Chen R. Coculturing dendritic cells with zoledronate acid efficiently enhance the anti-tumor effects of cytokine-induced killer cells. J Clin Immunol. 2010;30:766–74. doi: 10.1007/s10875-010-9434-1. [DOI] [PubMed] [Google Scholar]
- 11.Chan JK, Hamilton CA, Cheung MK, Karimi M, Baker J, Gall JM, Schulz S, Thorne SH, Teng NN, Contag CH, Lum LG, Negrin RS. Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: a preclinical study. Clin Cancer Res. 2006;12:1859–67. doi: 10.1158/1078-0432.CCR-05-2019. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Xu Y, Shen J, He F, Zhang D, Chen Z, Duan Y, Sun J. Feasibility study of DCs/CIKs combined with thoracic radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer. Radiat Oncol. 2016;11:60. doi: 10.1186/s13014-016-0635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang H, Schimmer AD. Livin/melanoma inhibitor of apoptosis protein as a potential therapeutic target for the treatment of malignancy. Mol Cancer Ther. 2007;6:24–30. doi: 10.1158/1535-7163.MCT-06-0443. [DOI] [PubMed] [Google Scholar]
- 14.Yan B. Research progress on livin protein: an inhibitor of apoptosis. Mol Cell Biochem. 2011;357:39–45. doi: 10.1007/s11010-011-0873-7. [DOI] [PubMed] [Google Scholar]
- 15.Chen YS, Li HR, Lin M, Chen G, Xie BS, Xu NL, Lin LF. Livin abrogates apoptosis of SPC-A1 cell by regulating JNKI signaling pathway. Mol Biol Rep. 2010;37:2241–7. doi: 10.1007/s11033-009-9711-3. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Han M, Wen JK, Wang L. Livin/ML-IAP as a new target for cancer treatment. Cancer Lett. 2007;250:168–76. doi: 10.1016/j.canlet.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Yuan D, Liu L, Gu D. Transcriptional regulation of livin by beta-catenin/TCF signaling in human lung cancer cell lines. Mol Cell Biochem. 2007;306:171–8. doi: 10.1007/s11010-007-9567-6. [DOI] [PubMed] [Google Scholar]
- 18.Xie J, Xiong L, Tao X, Li X, Su Y, Hou X, Shi H. Antitumor effects of murine bone marrow-derived dendritic cells infected with xenogeneic livin alpha recombinant adenoviral vectors against lewis lung carcinoma. Lung Cancer. 2010;68:338–45. doi: 10.1016/j.lungcan.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Gazzaniga P, Gradilone A, Giuliani L, Gandini O, Silvestri I, Nofroni I, Saccani G, Frati L, Agliano AM. Expression and prognostic significance of LIVIN, SURVIVIN and other apoptosis-related genes in the progression of superficial bladder cancer. Ann Oncol. 2003;14:85–90. doi: 10.1093/annonc/mdg002. [DOI] [PubMed] [Google Scholar]
- 20.Tanabe H, Yagihashi A, Tsuji N, Shijubo Y, Abe S, Watanabe N. Expression of survivin mRNA and livin mRNA in non-small-cell lung cancer. Lung Cancer. 2004;46:299–304. doi: 10.1016/j.lungcan.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Xiang Y, Yao H, Wang S, Hong M, He J, Cao S, Min H, Song E, Guo X. Prognostic value of Survivin and Livin in nasopharyngeal carcinoma. Laryngoscope. 2006;116:126–30. doi: 10.1097/01.mlg.0000187392.87904.35. [DOI] [PubMed] [Google Scholar]
- 22.Sun JG, Liao RX, Zhang SX, Duan YZ, Zhuo WL, Wang XX, Wang ZX, Li DZ, Chen ZT. Role of inhibitor of apoptosis protein Livin in radiation resistance in nonsmall cell lung cancer. Cancer Biother Radiopharm. 2011;26:585–92. doi: 10.1089/cbr.2011.0962. [DOI] [PubMed] [Google Scholar]
- 23.Sun J, Liao R, Chen Z, Wang Z, Zhang Q, Hu Y. Study on enhancing sensitivity of SPC-A1 cells to chemotherapy by livin isoform-specific gene silencing. Zhongguo Fei Ai Za Zhi. 2007;10:461–5. doi: 10.3779/j.issn.1009-3419.2007.06.03. [DOI] [PubMed] [Google Scholar]
- 24.Sun JG, Liao RX, Chen ZT, Wang ZX, Zhang Q, Hu YD, Wang DL. Gene transfection of Livin isoforms into A549 cell line and its effect on cell growth and sensitivity to chemotherapy and radiotherapy. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28:836–40. [PubMed] [Google Scholar]
- 25.Andersen MH, Becker JC, Straten P. Identification of an HLA-A3-restricted cytotoxic T lymphocyte (CTL) epitope from ML-IAP. J Invest Dermatol. 2004;122:1336–7. doi: 10.1111/j.0022-202X.2004.22508.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao M, Li H, Li L, Zhang Y. Effects of a gemcitabine plus platinum regimen combined with a dendritic cell-cytokine induced killer immunotherapy on recurrence and survival rate of non-small cell lung cancer patients. Exp Ther Med. 2014;7:1403–7. doi: 10.3892/etm.2014.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Xu Y, Shen J, He F, Zhang D, Chen Z, Duan Y, Sun J. Feasibility study of DCs/CIKs combined with thoracic radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer. Radiat Oncol. 2016;11:60. doi: 10.1186/s13014-016-0635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun JG, Liao RX, Zhang SX, Duan YZ, Zhuo WL, Wang XX, Wang ZX, Li DZ, Chen ZT. Role of inhibitor of apoptosis protein livin in radiation resistance in nonsmall cell lung cancer. Cancer Biother Radiopharm. 2011;26:585–92. doi: 10.1089/cbr.2011.0962. [DOI] [PubMed] [Google Scholar]
- 30.Schmollinger JC, Vonderheide RH, Hoar KM, Maecker B, Schultze JL, Hodi FS, Soiffer RJ, Jung K, Kuroda MJ, Letvin NL, Greenfield EA, Mihm M, Kutok JL, Dranoff G. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc Natl Acad Sci U S A. 2003;100:3398–403. doi: 10.1073/pnas.0530311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuruma T, Hata F, Torigoe T, Furuhata T, Idenoue S, Kurotaki T, Yamamoto M, Yagihashi A, Ohmura T, Yamaguchi K, Katsuramaki T, Yasoshima T, Sasaki K, Mizushima Y, Minamida H, Kimura H, Akiyama M, Hirohashi Y, Asanuma H, Tamura Y, Shimozawa K, Sato N, Hirata K. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19. doi: 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nachmias B, Lazar I, Elmalech M, Abed-El-Rahaman I, Asshab Y, Mandelboim O, Perlman R, Ben-Yehuda D. Subcellular localization determines the delicate balance between the anti- and pro-apoptotic activity of livin. Apoptosis. 2007;12:1129–42. doi: 10.1007/s10495-006-0049-1. [DOI] [PubMed] [Google Scholar]
- 33.Schmollinger JC, Dranoff G. Targeting melanoma inhibitor of apoptosis protein with cancer immunotherapy. Apoptosis. 2004;9:309–13. doi: 10.1023/b:appt.0000025807.59668.5e. [DOI] [PubMed] [Google Scholar]
- 34.Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol. 2004;14:231–43. doi: 10.1016/j.semcancer.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 36.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]


