Abstract
Sexual dimorphism in the incidence of human esophageal cancer, including both esophageal adenocarcinoma and squamous cell carcinoma, shows male dominancy. However, the mechanisms that underlie sexual dimorphism of esophageal cancer have been understudied in vivo due to the lack of sex-dimorphic mouse models. Here, we developed a sex-dimorphic mouse model of esophageal squamous cell carcinoma (ESCC) using a lower amount of 4-nitroquinoline-1-oxide (4-NQO) and a shorter latency of tumorigenesis compared to the traditional carcinogenesis procedures. In this model, we found that male mice were highly sensitive to the tumorigenesis of ESCC whereas female mice were resistant to it. This model provided us an opportunity for investigating the mechanisms underlying sexual dimorphism of ESCC in vivo and for better understanding the sex-dimorphic incidence of ESCC in humans.
Keywords: Sexual dimorphism, mouse model, esophageal squamous cell carcinoma
Introduction
Sex dimorphism is an important feature of human cancers but has been under-investigated and mostly neglected in clinical diagnosis and therapy. More importantly, the regulatory mechanisms underlying sexual dimorphism of each cancer have been barely addressed in vivo. Cancer of the esophagus or esophageal cancer arises from the food pipe between throat and stomach. Cigarette smoking, alcohol consumption, and poor oral health are risk factors for esophageal cancer [1,2]. Esophageal cancer is highly male-dominant in the United States and worldwide as the average incidence ratio of esophageal cancer from men to women was about 3.66 in 1975-2014 from the SEER data and 2.90 in 2012 from the World Cancer Report data. Sex hormone signaling was found to be involved in the regulation of sex disparities in esophageal cancer with mixed results and all of these studies were completed in human cancer cell lines [3-13]. There is barely any study on sexual dimorphism of esophageal cancer in vivo. A key reason is due to the lack of in vivo mouse models with sexual dimorphism of esophageal cancer. Esophageal cancer has two major subtypes: esophageal adenocarcinoma and esophageal squamous cell carcinoma (ESCC) and both subtypes show male dominancy. In this study, we will mainly focus on ESCC.
Here, we developed a sex-dimorphic mouse model of ESCC using a lower amount of 4-nitroquinoline-1-oxide (4-NQO) and a shorter latency of tumorigenesis compared to traditional 4-NQO approaches. In this model, we found that male mice were highly sensitive to the tumorigenesis of ESCC whereas female mice were resistant to it. This model provided us an opportunity to investigate the mechanism underlying sexual dimorphism of ESCC in vivo in the future.
Methods
Reagents
4-NQO and propylene glycol were purchased from Sigma.
Induction of sexual dimorphism of ESCC in mice
All animal procedures were performed in compliance with ethical regulations and approved by the Institutional Animal Care and Use Committee (IACUC) at the Mayo Clinic. 129J/C57B6L mice were maintained on a normal chow diet. 4-NQO was dissolved in propylene glycol at 5 mg/ml as the stock solution and then diluted in drinking water. Both male and female mice fed with just propylene glycol in drinking water did not induce ESCC as controls [14-17]. Thus, mice (8 males and 8 females) at age of 12 weeks were treated with 18 μg/ml 4-NQO in drinking water for 8 weeks and mice were then maintained in regular drinking water for 10 weeks. At the end of 18 weeks of tumorigenesis, the entire esophagus was collected for tumor evaluation.
Tumor evaluation
The entire esophagus was opened laterally for tumor evaluation. Tumor volumes and incidences were measured using a small animal ultrasonography (VisualSonic). H&E staining of paraformaldehyde-fixed sections of esophagi were also used for the evaluation of ESCC. Immunohistochemical staining of Ki67 using anti-Ki67 antibodies (Abcam) was used for the evaluation of cell proliferation of ESCC tumors.
Statistical analysis
Data are expressed as mean ± SEM. One-way ANOVA was used for the analysis of body weight changes between groups during carcinogenesis, tests were 2-tailed, and values of P < 0.05 were considered as statistically significant. Fisher’s exact text was used for the analysis of tumor incidence and volumes between groups and values of P < 0.05 were considered as significant changes.
Results
High concentrations of 4-NQO, such as 100 μg/ml and 5 mg/ml, have been broadly used for the induction of ESCC in rodents with 24 to 66 weeks of tumorigenesis periods; however, at these conditions, both male and female rodents developed ESCC, though females developed less tumors than males [14-17]. To identify the cutting point when only males do but females do not develop tumors, we decided to reduce the concentration of 4-NQO and also shorten the latency of carcinogenesis. To develop a clear sex-dimorphic mouse model of ESCC, we used a lower concentration (18 μg/ml) of 4-NQO and a shorter tumorigenesis period of 8 weeks of initiation and 10 weeks of tumor growth. We found that body weights of both male and female mice did not show any clear changes during 18 weeks of carcinogenesis except for a decrease around 2 weeks after the initial carcinogen treatment, but both male and female mice with carcinogen treatment did show significant reduction in body weights compared to male and female controls without carcinogen treatment, respectively (Figure 1A). We found that male mice grew large and/or multiple tumors of ESCC whereas no tumors were observed in female mice (Figure 1A), indicating that male mice are sensitive to the tumorigenesis of ESCC whereas female mice are resistant to it. All male mice had multiple tumors with the volumes of 0.9-316.6 mm3 (Figure 1B and 1C). Next, we performed H&E and Ki67 staining to trace tumor growth and cell proliferation in the esophagi (Figure 2A and 2B). Although we did not observe clear tumors in female mice (Figure 1B and 1C), certain regions of female esophagi showed pre-tumorigenic features, such as increased cell proliferation as indicated by increased nuclei and increased Ki67 staining of epithelial cells (Figure 2A and 2B). Interestingly, esophageal basal epithelial cells were highly positive for Ki67 staining in both male and female mice regardless of carcinogen treatment (Figure 2B), indicating potential stem cell-like features of these cells. The 4-NQO treatment induced massive proliferation of ESCC tumor cells in male mice and caused increased proliferation and the loss of lining of epithelial cells in female esophagi (Figure 2B). In all, we developed a sex-dimorphic mouse model of ESCC in vivo resembling the similar sex-dimorphic incidence of ESCC in humans.
Figure 1.
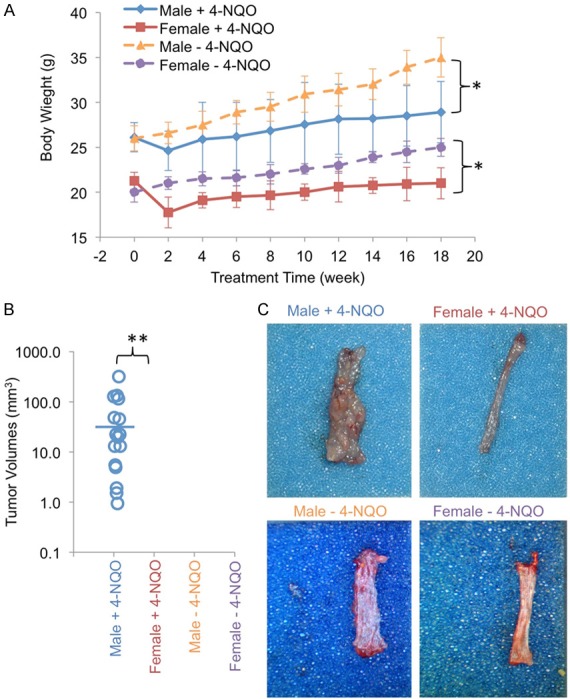
Sex-dimorphic tumorigenesis of ESCC in mice. A. Body weight of male and female mice with (+) and without (-) 4-NQO treatments. *, P < 0.05 were found in the comparison between carcinogen-treated and non-treated male or female mice, respectively. B. Tumor volumes of ESCC were measured by small animal ultrasonography. Blue line, mean volume. C. ESCC tumors were induced in male but not in female mice by 4-NQO. No tumors were observed in control mice without 4-NQO treatments. n = 8 for each group. **, P < 0.0001 was found in the comparison between male and female mice with carcinogen treatment.
Figure 2.
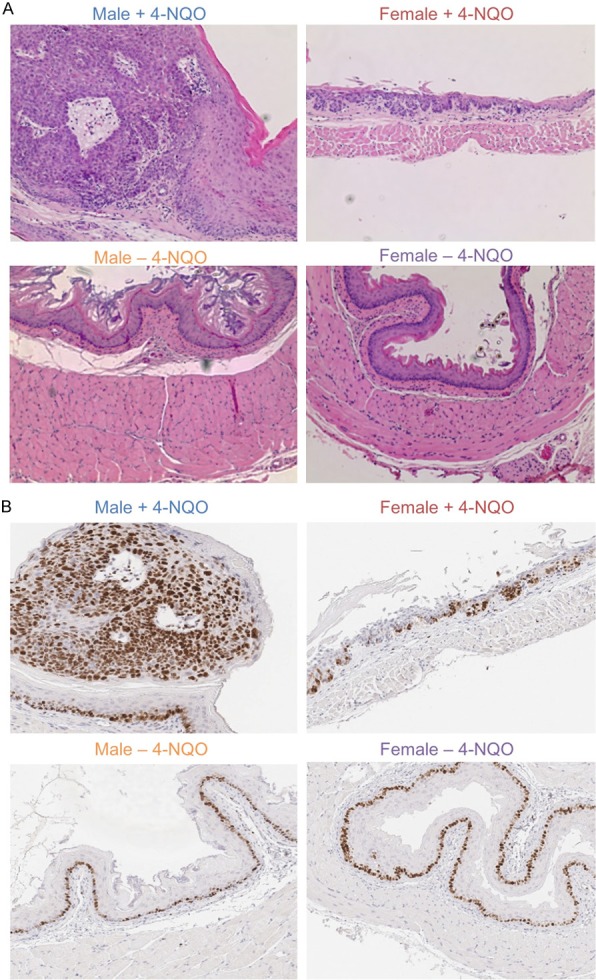
Histological analysis of normal esophagi and ESCC tumors in male and female mice with (+) and without (-) 4-NQO treatments. A. H&E staining (200 x) of male and female esophagi with and without ESCC tumors. B. Immunohistochemical staining (200 x) of Ki67 as the indicator of cell proliferation in male and female esophagi.
Discussion
Our mouse model with a lower concentration of 4-NQO and a shorter latency of tumorigenesis provides a unique model for investigating sexual dimorphism of ESCC in vivo. Addressing the mechanism underlying sexual dimorphism in ESCC or esophageal cancer in vivo will help us to fully understand how sexes play the roles in the pathological processes of ESCC tumorigenesis. Our study of developing the sex-dimorphic mouse model of ESCC is prerequisite for better understanding sex-dimorphic incidence of ESCC in humans. Further studies using this model in combinations with esophagus-specific ablation of sex hormone receptors will provide a clear view on regulatory mechanisms of sex hormone receptors in the sexual dimorphism of ESCC. Given successfully revealing the mechanism of sexual dimorphism in ESCC using our in vivo sex-dimorphic model, developing the sex-specific treatments for ESCC patients will be a critical step towards individualized precision medicine. Moreover, future development of sex-dimorphic mouse model for another type of esophageal cancer, esophageal adenocarcinoma, is also imperative for revealing its mechanism of sexual dimorphism. In conclusion, we developed a novel mouse model of esophageal squamous cell carcinoma with clear sexual dimorphism. This model will be beneficial for future in vivo studies on sexual dimorphism of esophageal squamous cell carcinoma.
Acknowledgements
We thank Dr. Anil Rustgi’s help on the characterizations of ESCC tumors from the University of Pennsylvania. We also thank great technical support from Brandy Edenfield in the histology core. We thank Xiao Wang’s initial work on this study. This study was partially supported by the Eveleigh Family Career Development Award to Z.L.
All authors read the manuscript and consent for publication.
Disclosure of conflict of interest
None.
Abbreviations
- ESCC
Esophageal squamous cell carcinoma
- 4-NQO
4-nitroquinoline-1-oxide
References
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Mao WM, Zheng WH, Ling ZQ. Epidemiologic risk factors for esophageal cancer development. Asian Pac J Cancer Prev. 2011;12:2461–2466. [PubMed] [Google Scholar]
- 3.Palethorpe HM, Drew PA, Smith E. Androgen signaling in esophageal adenocarcinoma cell lines in vitro. Dig Dis Sci. 2017;62:3402–3414. doi: 10.1007/s10620-017-4794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Due SL, Watson DI, Bastian I, Ding GQ, Sukocheva OA, Astill DS, Vat L, Hussey DJ. Tamoxifen enhances the cytotoxicity of conventional chemotherapy in esophageal adenocarcinoma cells. Surg Oncol. 2016;25:269–277. doi: 10.1016/j.suronc.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Sukocheva OA, Wee C, Ansar A, Hussey DJ, Watson DI. Effect of estrogen on growth and apoptosis in esophageal adenocarcinoma cells. Dis Esophagus. 2013;26:628–35. doi: 10.1111/dote.12000. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Xu J, Li W, Gan J, Lin W, Ke J, Jiang J, Du L, Chen Y, Zhong X, Zhang D, Yeung SJ, Li X, Zhang H. Reciprocal androgen receptor/interleukin-6 crosstalk drives oesophageal carcinoma progression and contributes to patient prognosis. J Pathol. 2017;241:448–462. doi: 10.1002/path.4839. [DOI] [PubMed] [Google Scholar]
- 7.Matsuoka H, Sugimachi K, Ueo H, Kuwano H, Nakano S, Nakayama M. Sex hormone response of a newly established squamous cell line derived from clinical esophageal carcinoma. Cancer Res. 1987;47:4134–40. [PubMed] [Google Scholar]
- 8.Zhang Y, Pan T, Zhong X, Cheng C. Androgen receptor promotes esophageal cancer cell migration and proliferation via matrix metalloproteinase 2. Tumour Biol. 2015;36:5859–64. doi: 10.1007/s13277-015-3257-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, He Q, Fu S, Zheng Z. Estrogen receptors in regulating cell proliferation of esophageal squamous cell carcinoma: involvement of intracellular Ca(2+) signaling. Pathol Oncol Res. 2017;23:329–334. doi: 10.1007/s12253-016-0105-2. [DOI] [PubMed] [Google Scholar]
- 10.Zuguchi M, Miki Y, Onodera Y, Fujishima F, Takeyama D, Okamoto H, Miyata G, Sato A, Satomi S, Sasano H. Estrogen receptor alpha and beta in esophageal squamous cell carcinoma. Cancer Sci. 2012;103:1348–55. doi: 10.1111/j.1349-7006.2012.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Utsumi Y, Nakamura T, Nagasue N, Kubota H, Harada T, Morikawa S. Effect of 17 beta-estradiol on the growth of an estrogen receptor-positive human esophageal carcinoma cell line. Cancer. 1991;67:2284–2289. doi: 10.1002/1097-0142(19910501)67:9<2284::aid-cncr2820670913>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Ueo H, Matsuoka H, Sugimachi K, Kuwano H, Mori M, Akiyoshi T. Inhibitory effects of estrogen on the growth of a human esophageal carcinoma cell line. Cancer Res. 1990;50:7212–7215. [PubMed] [Google Scholar]
- 13.Utsumi Y, Nakamura T, Nagasue N, Kubota H, Morikawa S. Role of estrogen receptors in the growth of human esophageal carcinoma. Cancer. 1989;64:88–93. doi: 10.1002/1097-0142(19890701)64:1<88::aid-cncr2820640116>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301–13. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 15.Vitale-Cross L, Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Chemical carcinogenesis models for evaluating molecular-targeted prevention and treatment of oral cancer. Cancer Prev Res (Phila) 2009;2:419–22. doi: 10.1158/1940-6207.CAPR-09-0058. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins BL, Heniford BW, Ackermann DM, Leonberger M, Martinez SA, Hendler FJ. 4NQO carcinogenesis: a mouse model of oral cavity squamous cell carcinoma. Head Neck. 1994;16:424–32. doi: 10.1002/hed.2880160506. [DOI] [PubMed] [Google Scholar]
- 17.Steidler NE, Reade PC. Experimental induction of oral squamous cell carcinomas in mice with 4-nitroquinolone-1-oxide. Oral Surg Oral Med Oral Pathol. 1984;57:524–531. doi: 10.1016/0030-4220(84)90312-8. [DOI] [PubMed] [Google Scholar]


