Abstract
Immunotherapy has made a significant impact on the survival of patients with different tumor. However, it has become clear that they are not sufficiently active durable responses for many tumor patients, but only in a fraction of tumor patients. In order to improve this, combination regimens revealed impressive synergistic effects by combination of doublet or triplet immune agents. In this article, we will summarize the cancer-immunity cycle (CIC) and propose a rationale for the design of synergistic antitumor combinations. In addition, key issues in the development of these strategies are further discussed. Overall, we wish to highlight the backbone principles of combination regimens design at different points of the CIC, with the ultimate goal to guide better designs for future cancer combination therapies.
Keywords: Immunotherapy, combination, antitumor immunity, checkpoint inhibitors, chemotherapy, radiotherapy
Introduction
Successful generation of an immune response to eliminate cancer cells includes following steps (cancer-immunity cycle, CIC): 1) oncogenesis release neoantigens, dendritic cells (DCs) capture and process these neoantigens; 2) DCs present the captured antigens on MHC molecules to T cells; 3) priming and activation of tumor-specific naive T cells to become effector T cells; 4) the activated effector T cells from lymphoid organs into peripheral blood and traffic to tumor tissues; 5) the activated effector T cells infiltrate the tumor bed; 6) tumor-antigen specifically recognition; 7) tumor lysis and release tumor-associated antigens, effector T cells death and tumor-specific memory T cells generation, subsequent cycle with deeper and wider response coming (Figure 1) [1,2].
Figure 1.
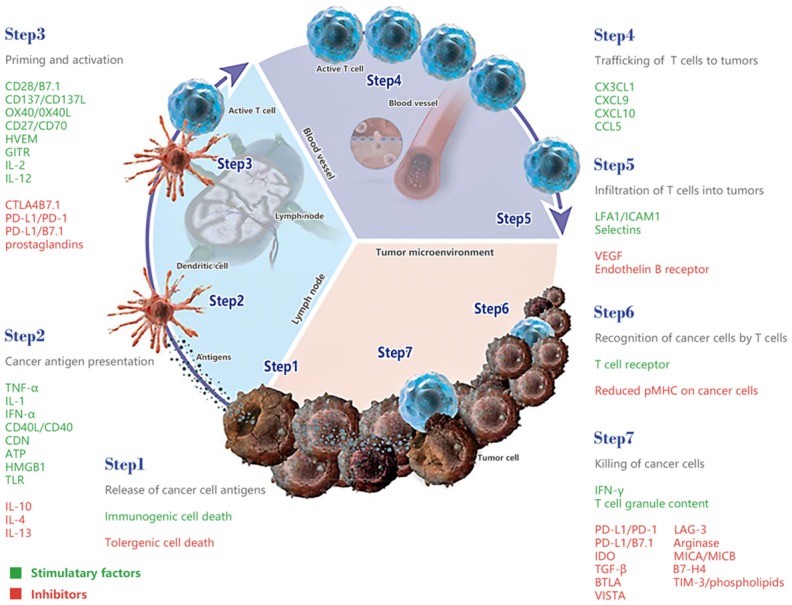
Stimulatory and inhibitory factors in each step of cancer-immunity cycle (CIC). Theoretical antitumor immune response is a self-motivated process, a series of stepwise events initiate, proceed and expand iteratively. This cycle can be subdivided into seven major steps. The neoantigens created by oncogenesis are released and captured by dendritic cells (DCs) for processing (step 1), DCs present the captured antigens on MHCI and MHCII molecules to T cells (step 2), resulting in the priming and activation of effector T cell responses against the tumor-specific antigens (step 3), the activated effector T cells traffic to (step 4) and infiltrate the tumor bed (step 5), specifically recognize and bind to tumor cells (step 6), and kill target tumor cells (step 7). Killing of the tumor cell releases additional tumor-associated antigens (step 1 again) to increase the breadth and depth of the response in subsequent revolutions of the cycle. The step 1-2-3, step 4-5 and step 6-7 development in the lymph node, blood vessel and tumor respectively. Each step of the CIC requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors are shown in green promote immunity, whereas inhibitors are shown in red help keep the process in check and reduce immune activity and/or prevent autoimmunity. Modified version from the reference 1.
The CIC perform optimally in healthy persons, however, does not in tumor patients. Immune dysfunction may be present at any step of CIC in tumor patients. Tumor antigens may not be released or not be detected even released; antigen presenting cells (APCs) and T cells may not treat antigens as non-self thereby creating T regulatory cell (Tregs) responses rather than effector responses; effector T cells may not properly traffic to tumors; effector T cells may not infiltrate the tumor bed; or effector T cells may not recognize or/and kill cancer cells suppressed by factors in the tumor microenvironment [3]. Specific to a tumor patient, may be single or a few steps in CIC have obstacles. However, we are very little knowledge about these obstacles. Thus far, most of the therapy strategies are not designed to correct or overcome an existing or known obstacle. It is critical to identify which obstacle is predominant in a tumor patient, since targeting inaccurate obstacle to a tumor patient will be inefficient, even overkilled, costly, and wasteful. For instance, targeting programmed death 1 (PD-1) pathway in a tumor patient that lacks immune activation may be pointless, even overkilled. Here, we discuss the steps toward the development of more effective immunotherapy programs for more tumor patients.
Principles of Compound-therapy based on CIC
Figure 1 list some known effector molecules at each step of the CIC (Figure 1), we can apply these effector molecules to directly attack tumor cells. Moreover, we can modulate the endogenous stimulatory and/or inhibitory factors of any step in the CIC to procedurally attack tumor cells (Figure 1). Unfortunately, above mentioned strategies are not designed to remove the existing antitumor immune obstacle in the CIC. Accurate immunotherapy is to reinitiate a self-sustaining CIC, enabling it returns to normal. Despite each step in the whole immune cycle has a certain killing or inhibiting effect on the tumor. Specific to a tumor patient, may be a step or a few steps in CIC have obstacles. If therapy at obstacle target, the whole CIC is reinitiate, tumor patient get round and round active immunity. If therapy at inaccurate obstacle target, there may be a certain level of active immunity at the therapy target, and may emerge a certain level of passive immunity at the inaccurate obstacle target. Since the accurate obstacle points have not been lifted, it is not possible to achieve repeated active immunity, which leads to poor therapeutic effect (Figure 2) [2]. The majority of tumor patients have obstacle at a step or a few steps in CIC, rather than have systemic immune obstacles. Thus Compound-therapy based on CIC must involve selectively targeting the immunity limiting step in tumor patient.
Figure 2.
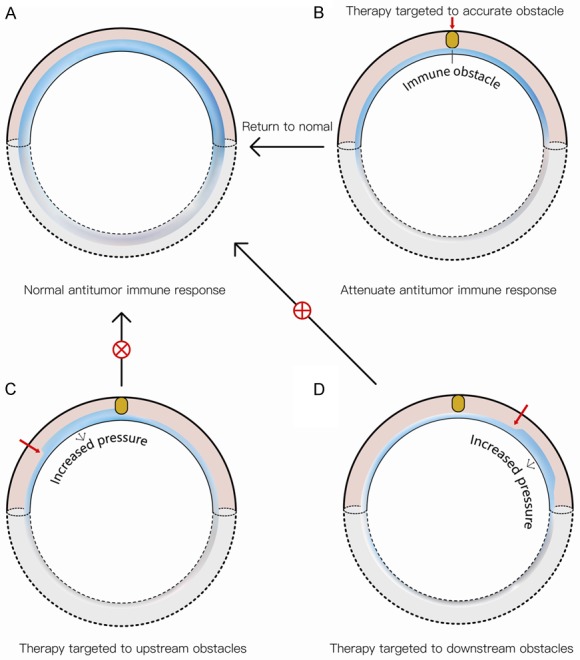
Illustration of the accurate immunotherapy versus the inaccurate immunotherapy. A. We speculate that the antitumor immune response in CIC (blue water flow) is appropriate in healthy persons. B. The antitumor immune response is insufficient when there is an immune obstacle in tumor patients. If the immune obstacle in tumor patients is removed precisely, the whole CIC returns to normal. C. If the immunotherapy is not target the obstacle precisely, instead treat at the upstream of the obstacle, the upstream (one step or a few steps) immune response is increase, even redundancy, but the whole immune response is still insufficient in CIC. D. If a treat at the downstream of the obstacle, the downstream (one step or a few steps) immune response is increased, but the whole immune response is still insufficient in CIC.
Therapy based on CIC
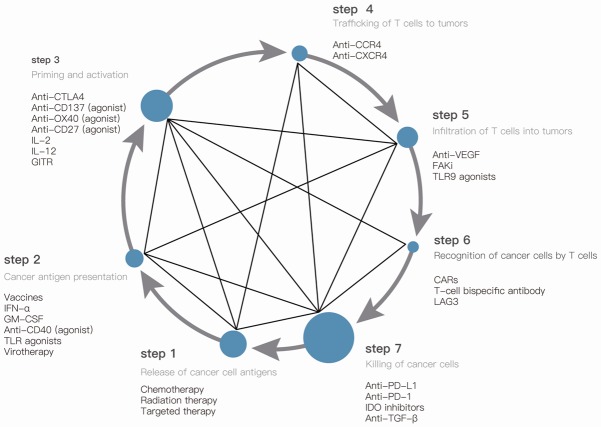
Factors that come into play in CIC provide the main idea to enhance antitumor immunity. We can 1) enhance antigen release to initiate and propagate antitumor immunity; 2) enhance neoantigen presentation by APCs; 3) enhance the priming and activation of T cells; 4) promotion of trafficking of effector T cells to access the tumor; 5) promotion of infiltration of effector T cells into tumor bed; 6) promotion of recognition of effector T cells to tumor cells; 7) enhance killing of T cells to tumor cells [2,4]. Here’s a brief review of therapies based on CIC under preclinical or clinical evaluation. Chemotherapy, radiation therapy, and targeted therapies can primarily promote step 1 of CIC; Vaccines can primarily promote step 2 of CIC; Anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA4) can primarily promote step 3 of CIC; Anti-CC-chemokine receptor 4 (anti-CCR4) and anti-chemokine (C-X-C motif) receptor 4 (anti-CXCR4) antibodies can primarily promote step 4 of CIC; Inhibitors of VEGF can potentially promote step 5 of CIC; T-cell specific antibody and chimeric antigen receptors (CARs) can primarily promote step 6 of CIC; and anti-programmed death-ligand 1 (anti-PD-L1) or anti-PD-1 antibodies can primarily promote step 7 of CIC.
Compound-therapy based on CIC
We refer to the ClinicalTrials.gov and the consensus statement on combination immunotherapy by Combination Immunotherapy Task Force of the Society for Immunotherapy of Cancer (SITC) [5,6] to draw the Figure 3. There is evidence that combinations within a step and across steps may be clinically beneficial. Chemotherapy-induced tumor cell death can promote tumor specific antigen release and presentation potentially leading to prime of specific T cells and reducing Tregs, macrophages or myeloid-derived suppressor cells [7]. Moreover, chemotherapy also induces PD-L1 expression on tumor cells [8]. Hence, chemotherapy have synergy with anti-PD-1/PD-L1 and anti-CTLA4 antibodies particularly in non-inflamed, chemotherapy sensitive tumors [9-11]. Radiotherapy can increase tumor antigens expression, promote soluble tumor antigens release [12], promote antigen presentation [13], prime and active T-cell [14], induce chemokine expression needed for T-cell trafficking [15,16], and mediate T-cell recognition and/or killing [17,18]. An effective antitumor vaccine will seek to co-administer antigens that can be targets for T cell recognition. Combined vaccines for inhibition of PD-1 or/and CTLA4 were synergistic in some tumor models [19,20]. The combination of CTLA-4 and PD-1/PD-L1 inhibition are backed by strong pre-clinical evidence [21,22]. Though no clinical efficacy data are reported from combination factors that involve trafficking and access of tumor with other therapy, a large number of early trials are ongoing. Combination of anti-CCR4 and anti-CXCR4 antibodies with anti-PD-1/PD-L1 antibodies (Nivolumab, durvalumab, pembrolizumab), anti-CTLA4 antibodies (ipilimumab, tremelimumab) and chemotherapy (lomustine, gemcitabine) are currently being tested [23]. Defactinib, an inhibitors of Focal adhesion kinase (FAKi) is currently being combined with pembrolizumab and gemcitabine in a phase I trial. IMO-2125, a TLR9 agonist involved in increasing T-cell infiltration is combining with ipilimumab [24,25]. Chimeric antigen receptor (CAR) T cell is promising partnering strategies with PD-1/PD-L1 blockade. Combined inhibition of IDO with CTLA-4, PD-1, or PD-L1 has shown T cell dependent synergy [26].
Figure 3.
Connection diagram of ongoing clinical trials investigating the effect of combination strategies in CIC. The therapies are given in each step of CIC. Promising prospects of combination strategies are connected with solid line [4-6]. The larger the dot is, the more combination therapy recorded at the ClinicalTrials.gov.
Challenges in developing Compound-therapy based on CIC
The goal of Compound-therapy based on CIC is to expand the broadness and depth of antitumor immune response and to improve the quality of clinical response. Concatenated regimens of synergistic steps in CIC should be investigated in a variety of tumor settings. However, there are still quite a few hurdles that hinder the development Compound-therapy based on CIC. Several challenges need to be considered in enabling future progress with Compound-therapy based on CIC includes, but it is not limited to the follows: 1) scientific evidence of underlying tumor cell and immune system biology. 2) identification of the dominant immune obstacle in the CIC of a given tumor patient. Identification of the dominant obstacles in the CIC by experience is not a long-term normalizing solution. Humanized animal models or explant 3D culture models may provide support to characterize the dominant obstacles in the given patient [27-30]. 3) technological challenges to study immune obstacles in the CIC. Another major challenge is sequential tumor tissue collection and analyses. The sequential biopsies in tumor patients are difficult. Small sample analysis technique (single-cell sequencing, mass cytometry) may help uncover the immunosuppressive mechanisms that favor tumor progression occurs in a specific patient [31,32]. 4) challenges of design trials that enable efficient testing of the safety and clinical activity of combination immunotherapy regimens. 5) rigorous assessment of the optimal dose, sequence, and schedule of combination immunotherapy regimens in both preclinical models and the clinical setting.
Perspectives and conclusion
Once activated, immune response is rapid, durable, adaptable, and self-propagating [33,34]. The immunotherapy can completely and safely eradicate viral infections. We believe that immunotherapy remains the complete and safe eradication of tumor. For the majority of tumor patients, a single therapeutic agent is unlikely to be effective. Future cancer immunotherapy should aim to identify the specific obstacles in CIC and remove them to recover the local antitumor response, meanwhile combining with other therapies to properly induce systemic immune response without the risk of increased immune-related adverse events. Rational combination regimens should extend to multiple not limited to doublet or triplet steps in CIC. Hence, rigorous investigate the dose and schedule of each component in combination thoroughly and with added flexibility to optimize the dose, schedule, and configuration of each agent. The potential development of Compound-therapy based on CIC for antitumor depend on further discussion, transformation and integration among academic, industry, and regulatory partners.
Acknowledgements
This work was supported by grants from the Basic Research Program of Shenzhen (JCYJ20130326110139687, JSGG20160226161357949); the China Postdoctoral Science Foundation (2014M552234).
Disclosure of conflict of interest
None.
References
- 1.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Miguel-Luken MJ, Mansinho A, Boni V, Calvo E. Immunotherapy-based combinations: current status and perspectives. Curr Opin Oncol. 2017;29:382–394. doi: 10.1097/CCO.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 5.Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. 2017;5:16. doi: 10.1186/s40425-017-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol. 2015;26:1813–1823. doi: 10.1093/annonc/mdv209. [DOI] [PubMed] [Google Scholar]
- 7.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 8.Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, Konishi I, Mandai M. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-kB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–5045. doi: 10.1158/0008-5472.CAN-14-3098. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 10.Ariyan CE, Brady MS, Siegelbaum RH, Hu J, Bello DM, Rand J, Fisher C, Lefkowitz RA, Panageas KS, Pulitzer M, Vignali M, Emerson R, Tipton C, Robins H, Merghoub T, Yuan J, Jungbluth A, Blando J, Sharma P, Rudensky AY, Wolchok JD, Allison JP. Robust antitumor responses result from local chemotherapy and CTLA-4 blockade. Cancer Immunol Res. 2018;6:189–200. doi: 10.1158/2326-6066.CIR-17-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamazaki N, Uhara H, Fukushima S, Uchi H, Shibagaki N, Kiyohara Y, Tsutsumida A, Namikawa K, Okuyama R, Otsuka Y, Tokudome T. Phase II study of the immune-checkpoint inhibitor ipilimumab plus dacarbazine in Japanese patients with previously untreated, unresectable or metastatic melanoma. Cancer Chemother Pharmacol. 2015;76:969–975. doi: 10.1007/s00280-015-2870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland HI, Puig M, Grajkowska LT, Tsuji K, Lee JP, Mason KP, Verthelyi D, Rosenberg AS. Regulatory T cells in γ irradiation-induced immune suppression. PLoS One. 2012;7:e39092. doi: 10.1371/journal.pone.0039092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, André F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 14.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kershaw MH, Devaud C, John LB, Westwood JA, Darcy PK. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. Oncoimmunology. 2013;2:e25962. doi: 10.4161/onci.25962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellevik T, Martinez-Zubiaurre I. Radiotherapy and the tumor stroma: the importance of dose and fractionation. Front Oncol. 2014;4:1. doi: 10.3389/fonc.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara TA, Hodge JW, Gulley JL. Combining radiation and immunotherapy for synergistic antitumor therapy. Curr Opin Mol Ther. 2009;11:37–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir GM, Hrytsenko O, Quinton T, Berinstein NL, Stanford MM, Mansour M. Anti-PD-1 increases the clonality and activity of tumor infiltrating antigen specific T cells induced by a potent immune therapy consisting of vaccine and metronomic cyclophosphamide. J Immunother Cancer. 2016;4:68. doi: 10.1186/s40425-016-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homicsko K, Duraiswamy J, Doucey MA, Coukos G. Combine and conquer: double CTLA-4 and PD-1 blockade combined with whole tumor antigen vaccine cooperate to eradicate tumors. Cancer Res. 2016;76:6765–6767. doi: 10.1158/0008-5472.CAN-16-2868. [DOI] [PubMed] [Google Scholar]
- 21.Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, Giles F. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC) J Immunother Cancer. 2018;6:39. doi: 10.1186/s40425-018-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loo K, Daud AI. Inhibitors of cytotoxic t lymphocyte antigen 4 and programmed death 1/programmed death 1 ligand for metastatic melanoma, dual versus monotherapy-summary of advances and future directions for studying these drugs. Cancer J. 2017;23:3–9. doi: 10.1097/PPO.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 23.Harris SJ, Brown J, Lopez J, Yap TA. Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med. 2016;13:171–193. doi: 10.20892/j.issn.2095-3941.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, Varner JA, Pu M, Messer KS, Guiducci C, Coffman RL, Kitaura K, Matsutani T, Suzuki R, Carson DA, Hayashi T, Cohen EE. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight. 2017;21:2. doi: 10.1172/jci.insight.93397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eggermont AMM, Crittenden M, Wargo J. Combination immunotherapy development in melanoma. Am Soc Clin Oncol Educ Book. 2018;23:197–207. doi: 10.1200/EDBK_201131. [DOI] [PubMed] [Google Scholar]
- 27.Zitvogel L, Pitt JM, Daillère R, Smyth MJ, Kroemer G. Mouse models in oncoimmunology. Nat Rev Cancer. 2016;16:759–773. doi: 10.1038/nrc.2016.91. [DOI] [PubMed] [Google Scholar]
- 28.Sanmamed MF, Chester C, Melero I, Kohrt H. Defining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies. Ann Oncol. 2016;27:1190–1198. doi: 10.1093/annonc/mdw041. [DOI] [PubMed] [Google Scholar]
- 29.Verma B, Ritchie M, Mancini M. Development and applications of patient-derived xenograft models in humanized mice for oncology and immune-oncology drug discovery. Curr Protoc Pharmacol. 2017;78:14.41.1–14.41.12. doi: 10.1002/cpph.26. [DOI] [PubMed] [Google Scholar]
- 30.Wege AK. Humanized mouse models for the preclinical assessment of cancer immunotherapy. BioDrugs. 2018;32:245–266. doi: 10.1007/s40259-018-0275-4. [DOI] [PubMed] [Google Scholar]
- 31.Burel JG, Apte SH, Doolan DL. Systems approaches towards molecular profiling of human immunity. Trends Immunol. 2016;37:53–67. doi: 10.1016/j.it.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Neu KE, Tang Q, Wilson PC, Khan AA. Single-cell genomics: approaches and utility in immunology. Trends Immunol. 2017;38:140–149. doi: 10.1016/j.it.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med. 2016;5:1481–1491. doi: 10.1002/cam4.732. [DOI] [PMC free article] [PubMed] [Google Scholar]