Abstract
Black and brown-colored mucocutaneous lesions present a differential diagnostic challenge, with malignant melanoma being the primary clinical concern. The vast majority of pigmented lesions in the head and neck region are the result of benign, reactive factors such as post-inflammatory melanosis. However, it is not uncommon to discover a range of muco-cutaneous black and brown neoplasms in the oro-facial area. The majority of black/brown pigmented neoplasms are melanocytic in origin; these are neoplasms of neural crest derivation. Melanocytic nevi are a diverse group of benign neoplasms that are the result of specific oncogenic mutations. They are common on cutaneous surfaces but can manifest in mucosal sites. Currently, nevi are classified based on clinical and histological criteria. The most common cutaneous and oral mucosal nevus is the acquired melanocytic nevus; nevi do not pose an increased risk for the development of malignant melanoma. Emerging information on specific genetic differences supports the notion of biologically distinct nevi. This article will review the classic clinical and microscopic features of nevi commonly found in the head and neck region, and discuss emerging concepts in nevus pathogenesis and taxonomy. Melanoma is a malignant melanocytic neoplasm and is a result of cumulative genetic deregulation. The etiology of malignant melanoma (MM) is multifactorial and includes underlying genetic susceptibility, UV radiation, skin-type, and race. The majority of MM occurs on cutaneous surfaces and less commonly on mucosal and extra-cutaneous visceral organs. Regardless of location, MM exhibits clinical-pathological features that relate to horizontal or vertical tumor spread. Cutaneous and mucosal MM typically present as asymmetrical, irregularly bordered, large (> 0.5 cm), heterogeneous brown-black lesions with foci of erythema, atrophy or ulceration. As with melanocytic nevi, advances in melanomagenesis research have revealed primary oncogenic BRAF and NRAS mutations associated with cutaneous MM. Unlike their cutaneous counterparts, mucosal melanomas exhibit primary oncogenic alterations in c-KIT and other genes. This article will discuss the role of specific primary oncogenic and secondary/tertiary genetic defects in differential clinical presentation, anatomic distribution, future classification changes, and targeted therapy of melanoma. The clinical and microscopic features of mucosal melanomas and a summary of management guidelines will be discussed. Additionally, this article will cover the salient features of melanocytic neuroectodermal tumor of infancy, a neoplastic entity that can involve the oro-facial region, and the clinical-pathological features of selected, commonly occurring pigmented ectodermally-derived neoplasms that are often part of the clinical differential diagnosis of black–brown pigmented lesions.
Keywords: Melanocytic nevi, Melanoma, Pigmented neoplasm, Oral melanoma, Oral melanocytic nevus
Introduction
The discovery of black or brown-colored mucosal lesions in the orofacial region can present a diagnostic dilemma, and could potentially bring treatment plans to a grinding halt. Clinicians are often understandably concerned about malignant melanoma, the prototypical pigmented malignancy. While this concern is justified, the fact is that the vast majority of pigmented lesions that occur on mucosal surfaces in the head and neck region are benign and are associated with a slew of local factors. Regardless, as with any finding, clinicians should approach the diagnosis of pigmented lesions in a disciplined and logical manner. Clinicians should gather all relevant data regarding the finding, including history, progression, symptoms, location/distribution, clinical features, and should then proceed to build a differential diagnosis based on his/her understanding of pathological processes (e.g. reactive, infectious, neoplastic, manifestation of a systemic disease etc.). As discussed in the section on reactive pigmented lesions, post-inflammatory melanosis and amalgam tattoos are the most common brown, black, or grey pigmented oral mucosal lesions encountered in clinical practice. Mucosal neoplasms, benign or malignant, that are brown or black in color are uncommon and are almost always associated with endogenous melanin pigmentation. While primary mucosal black/brown neoplasms are uncommon, it is not uncommon to discover a range of cutaneous pigmented neoplasms on routine head and neck examination. They could either represent melanocytic neoplastic proliferation (e.g. melanotic nevi and melanoma) or ectodermal neoplasms with secondary melanin pigmentation (e.g. seborrheic keratosis and basal cell carcinoma). Therefore, in addition to focusing on brown and black oral mucosal neoplasms, this article will cover the clinical-pathological features of selected cutaneous neoplasms that are seen in the head and neck region.
Melanocytic Neoplasms
Melanocytes derive from the neural crest during embryogenesis. During development melanocytes migrate to the juxtabasal regions of ectodermal structures like the skin, hair follicles, retina, oral mucosal membranes, nasal and upper respiratory tract mucosae. Depending on the site, and independent of skin color, there are approximately 10–20 keratinocytes for every melanocyte within the epithelium. Residing within the basal epithelial region, melanocytes do not contact one another, nor do they form adhesion complexes with adjacent keratinocytes, nor with the underlying basement membranes. Melanocytes are characterized by dendritic cytoplasmic processes and are induced to deposit or “inject” melanosomes (melanin containing vesicles) into adjacent keratinocytes thus imparting brown-black pigmentation and protection. Melanocytes are highly specialized cells that serve several important functions: (i) absorption of UV light (ii) scavenger for cytotoxic free radicals from tissue injury (iii) development of neural structures. In addition to para-ectodermal distribution, melanocytes reside in internal organs and the CNS where they are referred to as autochthonous melanocytes. Somatic neoplastic genetic mutations, and selected germline mutations, that deregulate melanocyte homeostasis and proliferation can promote the formation of a range of benign and malignant melanocytic tumors [1]. As a rule, benign neoplasms of melanocytic lineage are termed melanocytic nevi and malignant ones are termed melanomas. In addition, secondary melanin pigmentation can be seen within certain ectodermally derived tumors like seborrheic keratosis and basal cell carcinoma. The above tumors present on cutaneous and mucosal sites as brown, black or grey neoplasms. This article will discuss the salient clinical and histopathological features of each of these muco-cutaneous entities, and focus on the progress and understanding of underlying pathogenetic mechanisms, especially in the age of targeted therapeutics and management.
Melanocytic Nevus
Melanocytic nevi are a diverse group of benign melanocytic neoplasms that are the result of specific oncogenic mutations [1–4]. Nevi are commonly seen on cutaneous surfaces and are classified based on clinical, morphological, and microscopic features (features of selected nevi presented in Table 1). Nevi that present at birth are known as congenital nevi and are uncommon; they are typically larger (small < 1.5 cm, medium 1.5 to 20 cm, large > 20 cm) and may present with surface alterations and appendageal structures (Fig. 1a). Acquired melanocytic nevi (banal nevi) typically present in childhood and progress into adulthood. They are the most commonly encountered cutaneous and mucosal nevus. Although the current clinico-pathological classification scheme is efficient and clinically functional, emerging information on genetic alterations in specific types of nevi strongly supports the notion of biologically distinct melanotic nevi; this potentially provides the framework for future changes in taxonomy and understanding of nevogenesis [2–5]. In the following section, the classic clinical and microscopic features of selected cutaneous and oral mucosal nevi that present in the head and neck region will be presented, followed by a discussion on nevus pathogenesis.
Table 1.
Classification of selected cutaneous and mucosal melanotic nevi
| Melanocytic nevus—clinical features | Microscopic diagnostic criteria | Molecular genetic changes | |
|---|---|---|---|
| Nevi that originate from melanocytes within epithelium | |||
| Acquired melanocytic nevus (originating in epithelium) Discrete < 0.5 cm diameter pigmented lesion with symmetrical borders and uniform coloration |
Nevocytes originate from overlying epithelium and exhibit nesting and maturation | BRAF mutations (70–80%)a higher frequency in sun-exposed areas NRAS mutations (7–18%) |
|
| (a) Junctional | Young patient. Discrete < 0.5 cm macule with uniform brown-grey pigmentation | Nevocyte proliferation restricted to basal epithelium. Benign cytology | |
| (b) Compound | Discrete < 0.5 cm uniformly pigmented macules with raised dome-shaped elevation | Nevus theques both within the epithelium and in the lamina propria. Benign cytology | |
| (c) Intradermal/intramucosala | Discrete < 0.5–1 cm uniformly pigmented or non-pigmented dome-shaped/cerebriform nodules | Nevus theques found only within the dermis/submucosa. Type A, B, C cell neurotization and maturation | |
| Dysplastic nevusb | Adults. Irregular outlines, color variegation, diameter > 0.5 cm (exhibit 2–3 ABCDE factors) | Melanocytic proliferation. Cytological atypia—nuclear pleomorphism, cytological clearing, prominent nucleoli; junctional/compound activity. Lentiginous hyperplasia | BRAFV600E (61%) CDKN2A mutations (p16,p14) (Familial) |
| Spitz nevusb | Children to juveniles. Uncommon solitary discrete, rapidly growing pink to reddish brown dome shaped papule or nodule | Architecture of a compound nevus with zonal differentiation—superficial to deep. Spindle shaped or epithelioid nevus cells, multinucleated | HRAS BRAF |
| Nevi that originate from melanocytes not associated with epithelium | |||
| Blue nevusa (not originating in epithelium) | Discrete < 0.5 cm macule or dome shaped nodule with a blue-black pigmentation | Collection of spindle-shaped melanocytes deep within the stromal tissue arranged parallel to the surface—pigmented | GNAQ somatic mutations (83%) GNA11 somatic mutations (4–7%) |
| Congenital nevusb (not originating within epithelium) | Present at birth. Discrete, often large 1.5 cm or greater macules, nodules or textured lesions. Hyperpigmentation, appendageal structures | Variable microscopy. Changes in epithelial architecture, nevocytes in theques, sheets widely distributed—junctional, compound, among appendageal structures | NRAS gene on Chr 1 (70–88%) BRAF mutations (rare) |
aCommon on oral mucosal surfaces
bRare on oral mucosal surfaces
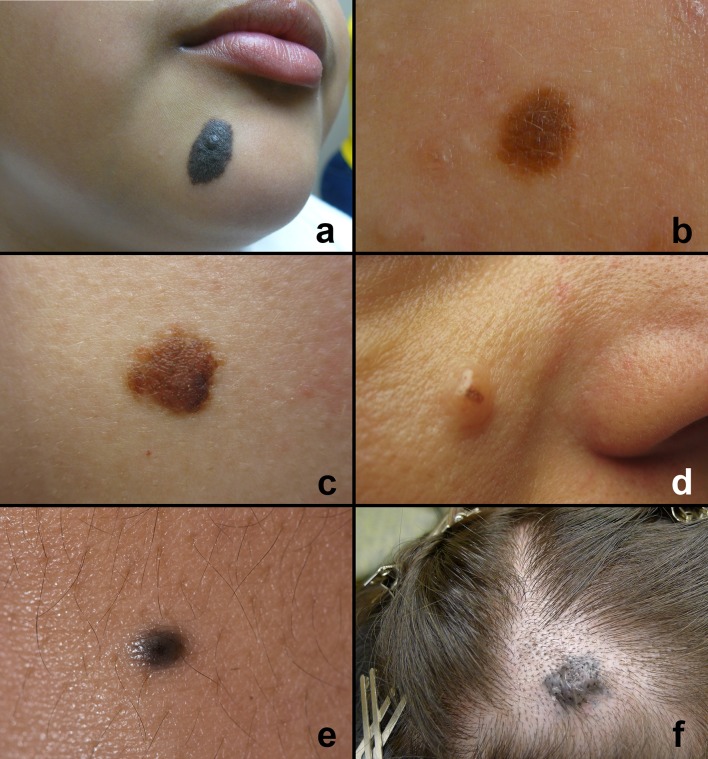
Fig. 1.
Clinical features of cutaneous melanotic nevi—head and neck region. a Congenital nevus—typically presents at birth. Note the size, surface texture and presence of appendageal structures. b–d Acquired melanocytic nevi—present as discrete, symmetrical < 0.5 cm uniformly colored pigmented or non-pigmented lesions. b Junctional nevus—discrete macule. c Compound nevus—macule with a papular elevation on the right lower aspect corresponding to area of junctional nevocyte. d Intradermal nevus—nodule with focus of pigmentation. e Common blue nevus—discrete black-blue pigmented elevated lesion. f Cellular blue nevus—elevated blue-black pigmented lesion on the scalp
Acquired Melanocytic Nevus: Cutaneous
Acquired melanocytic nevi (AMN) are the most common type of nevi. They emerge during adolescence. The average adult develops 15–30 nevi over their lifetime, reaching a maximum number in the third to fourth decade before the vast majority undergo regression. Although poorly understood, AMN involution is a well-known phenomenon, the frequency of which increases with advancing age [6, 7]. The head and neck, trunk and limbs are sites of predilection. All AMN exhibit stages of evolution; their clinical presentation depends upon their stage of evolution. Junctional nevi typically start off as small symmetrical macules less than 0.5 cm in diameter with uniform coloration (Fig. 1b). The lesions are generally solitary. Compound nevi present as discrete macules, slightly raised, dome shaped with smooth or warty surfaces with uniform coloration and size < 0.5 cm. Hair follicles may be seen projecting from these lesions (Fig. 1c). Intradermal nevi present as discrete, pigmented or non-pigmented, dome-shaped or pedunculated nodules measuring 0.5–1.0 cm with smooth or cerebriform surfaces (Fig. 1d). The clinical presentation of AMN correlates with evolution and progression of nevus cell proliferation at the microscopic level (Table 1). AMNs are more common in individuals with lighter skin color. Individuals with darker pigmentation (i.e. Asians, South Americans, Africans etc.) tend to develop AMNs on acral surfaces. Distinction between banal nevi, melanoma and other pigmented lesions in the majority of cases is straightforward and depends on evaluation of symmetry, borders, coloration, size and evolution of discovered pigmented lesions on naked-eye examination or routine dermoscopy (epiluminescence microscopes) [8].
Blue Nevus: Cutaneous
The common blue nevus (BN) is a relatively frequently encountered lesion on cutaneous surfaces. Although classified along with other benign melanocytic neoplasms (nevi), this entity represents a distinct pathogenetic entity with specific clinical and pathological features. This nevus has a predilection for the hands, feet, scalp and face. The blue nevus is typically solitary, well-demarcated, dome-shaped and blue-black in color measuring < 1.0 cm in diameter (Fig. 1e). BN appear blue as a result of a Tyndall effect; light reflects of pigmented nevocytes that are deep within the stroma and parallel to the surface. Although typically a nevus of the integumentary system, blue nevi have been reported in a variety of extracutaneous sites including the oral mucosa, maxillary sinus, conjunctiva, orbit, lymph nodes, vagina, breast, prostate, spermatic and the pulmonary hilum. Several clinical subtypes of blue nevus have been described: patch-like BN, plaque-like BN, targetoid BN, compound BN, hypopigmented BN, epithelioid BN and cellular BN. The cellular BN (Fig. 1f) variant is exceedingly rare but it is important as it can be confused clinically and histologically with melanoma. Careful clinical, microscopic correlation and potential genetic evaluation may be necessary to differentiate this benign neoplasm from invasive melanoma.
Oral Melanocytic Nevi
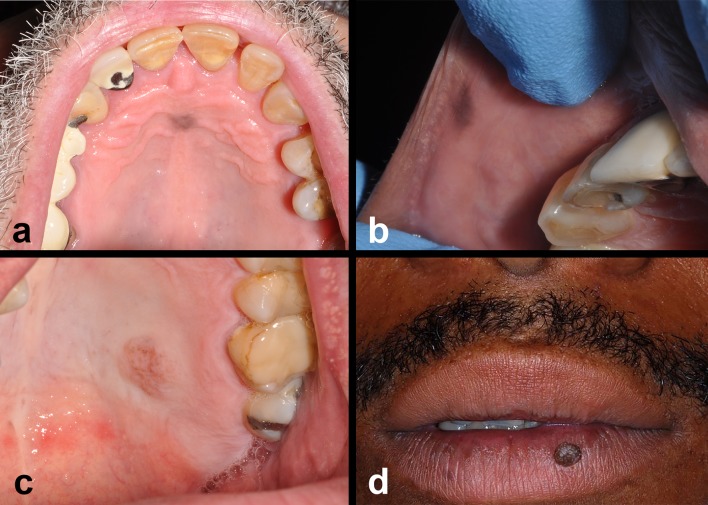
Oral mucosal melanocytic nevi (OMN) are relatively rare with a prevalence of 0.1% in the general population [1, 9–12]. Among these, the intramucosal nevus is the most frequently observed oral nevus; the blue nevus is the second most common [1, 9–12]. Spitz nevi, dysplastic nevi, and other subtypes are rare on oral mucosal surfaces. Several series of OMN demonstrate similar results. Buchner and Hansen in 1987 [10] analyzed a total of 191 OMN and discovered that intramucosal AMN (55%) were the most common type of OMN followed by common BN (32%). Similar series of OMN from other authors [12, 13] revealed comparable results: intramucosal AMN were the most common oral AMN (80.6%, 61%) [12, 13] followed by common blue nevus (8.3%, 23%). The most common location was the hard palate (41, 32, 33%) followed by the mucobuccal fold, vermilion of the lip, buccal mucosa and gingiva. As with cutaneous nevi, OMN present as well-circumscribed, round to ovoid macules or slightly elevated papules, or pedunculated nodules (Fig. 2a–d). The average OMN measures 0.9 cm or less in diameter. The mean age of patients with OMN is 35 years and similar across multiple reports [9–13].
Fig. 2.
Clinical features of acquired melanocytic nevi—oral mucosal and vermilion. a–c Acquired melanocytic nevi (junctional/compound/intramucosal) oral mucosal lesions present as discrete, symmetrical < 0.5–1 cm uniformly pigmented or non-pigmented mucosal lesions typically on the hard palate or gingival surfaces. d Intradermal nevus—discrete, mamillated nevus on the lower vermilion of the lip
Microscopic Features of Melanocytic Nevi
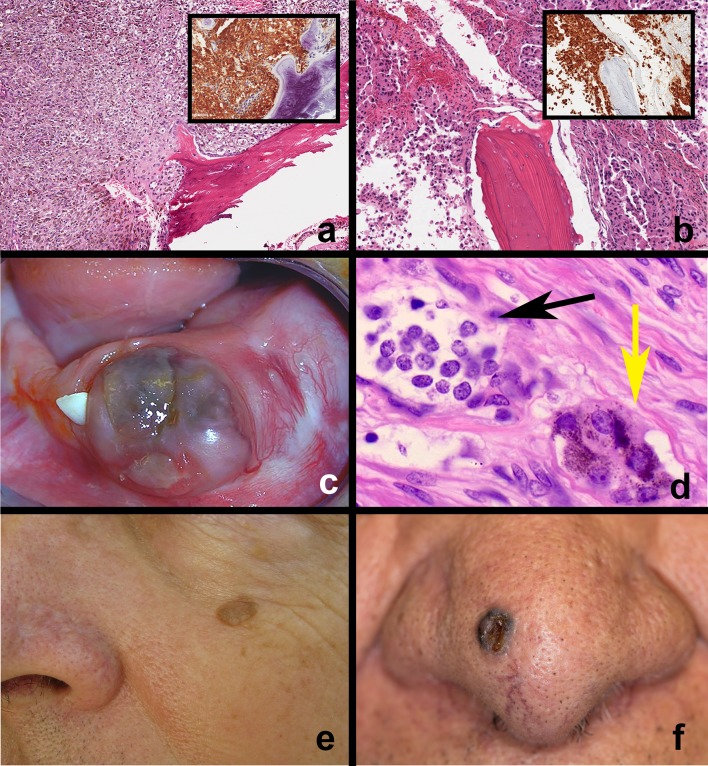
The microscopic features of the commonly occurring nevi, AMN and BN are different (Table 1) due to differences in origin, underlying oncogenic mutations and progression. Acquired melanocytic nevi originate from melanocytes that reside within the epithelium that undergo oncogenic proliferation (nevocytes). Microscopically, AMN of skin and mucosa exhibit similar features. AMN are well circumscribed, symmetric and composed of nevocyte proliferation that has a monotonous, banal cytology. Two cardinal features of nevi are nesting and maturation. Nesting refers to the tendency of nevocytes to form clusters of cells, theques (Fig. 3c). Maturation is a feature of nevi with a stromal (dermal or intramucosal) component and refers to the gradual change in nest architecture and nevocyte cytology going from round nests, to smaller clusters, to taking on a more fusiform/spindled morphology (neurotization) (Fig. 3a–d). In the earliest stage of AMN development (junctional nevus), junctional nests of melanocytes appear in the basal aspect of epithelium usually within the rete pegs and occasionally broadening or blunting them; lentiginous change may be observed in some lesions. Nevocytes are typically rounded or polygonal with clear to pale staining eosinophilic cytoplasm. The nuclei are round to ovoid with prominent nucleoli (type A nevocyte). The cytoplasm contains sparse, evenly distributed melanin granules. By definition, junctional nevi are intraepithelial and should not demonstrate superficial pagetoid spread (sign of malignant transformation). Compound AMN exhibit junctional activity (Fig. 4a, b) and nevocytes within the lamina propria and superficial submucosal tissues; papillary and reticular dermis respectively on the skin. The superficial tumor cells retain junctional characteristics, whereas the deeper nests are much smaller with less cytoplasm and dark staining nuclei resembling lymphocytes (Type B nevocyte) (Fig. 3b); mitotic activity is rare but may be seen in younger patients. Compound AMN may exhibit surface corrugation, hyperkeratosis and stromal fibrosis correlating with the clinically raised component of compound nevi. The intramucosal/intradermal AMN represents the “end stage” of nevocyte maturation. Typically, there is no evidence of junctional nevocyte activity. Nevocyte theques are well demarcated and are surrounded by dense fibrosis, correlating with the nodular/papular clinical morphology (Fig. 4c, d). Occasionally, focal calcification may be seen in regions of fibrosis and destroyed hair follicles (Fig. 3a; arrow head). The deeper nevocyte cells develop a fusiform/spindle-celled morphology and resemble Schwann cells with pale cytoplasm and wavy nuclei (Type C nevocyte) (Figs. 3d, 4d). They are often seen enveloping underlying nerves (Fig. 3d) as well as surrounding appendageal structures. In oral intramucosal AMN, salivary gland ducts and lobules can be enveloped by nevocytes. Regardless of differences in location and arrangement, it is essential to note that nevocytes are similar to melanocytes in that they have similar nuclear morphology, banal cytology, identical organelles, and enzyme systems. The most significant differences are that nevocytes lack dendritic processes (cannot transfer melanosomes to keratinocytes) and they have lost contact inhibition; clusters of nevocytes proliferate while still in close contact.
Fig. 3.
Microscopic features of acquired melanocytic nevus maturation—cutaneous. a Intradermal nevus (low magnification)—nevi exhibit cardinal signs of maturation and nesting. Nevocytes migrate from the epidermis to dermis in a regulated manner over their lifetime (black arrow). Nevus cells surround normal appendageal structures; ruptured pilosebaceous units may undergo dystrophic calcification (tip of black arrow). b Intradermal nevus—no evidence of junctional activity at epidermo-dermal junction. Note that nevocytes exhibit round theques/nests close to the epidermis and transform into spindle-shaped (neurotized) cells deeper in the dermis. c Nevus cells close to the epidermal surface arranged in round theques/nests. Cell exhibit banal cytology and intracytoplasmic melanin pigment. d Nevocytes in the deep dermis exhibit spindle-shaped nuclear features and surround normal dermal structures. Note nevus cells around nerve fiber and adipose tissue
Fig. 4.
Microscopic features of oral mucosal nevi. a, b Compound nevus. Note nevocyte nests at the junction of the epithelium and lamina propria with intracytoplasmic melanin pigmentation. Nevocytes arranged in theques exhibit banal nuclear features. c, d Intramucosal nevus. This is a discrete nodule with no evidence of junctional nevocyte activity. Nevocytes exhibit maturation from the surface taking on a neurotized morphology deeper in the submucosa. Nevocytes exhibit S-100 expression (inset). e, f Common blue nevus. Nevocytes within the connective tissue (arrow); note the absence of junctional activity and theque formation. Nevus cells are spindle shaped, exhibit banal cytology, contain melanin pigment, and often arranged parallel to the surface epithelium; this distribution and the Tyndall effect corresponds to the blue-black appearance of these lesions
Blue nevi, like congenital nevi, originate from oncogenic proliferation of melanocytes that are not associated with epithelium (Table 1). Microscopically, BN are characterized by proliferation of dendritic, spindled, ovoid or epithelioid melanocytes within stromal tissues. Nevocyte proliferation is typically seen within the dermis or submucosal tissue. The typical, common BN is composed of a discrete proliferation of small spindle-shaped nevocytes with elongated nuclei (Fig. 4e, f). Prominent perinevocyte stromal fibrosis is evident. A junctional epithelial component and cytological atypia is absent. Brown-black pigment is typically seen within the nevocyte cytoplasm. The epithelioid BN variant exhibits larger nevocytes, while the cellular BN variant exhibits ovoid melanocytes with clear cytoplasm. As described above, BN may be seen involving other organs such as the lung, gastrointestinal tract and genitourinary regions.
Melanotic nevocytes do not induce an immune-response and are typically uninflamed (exception—halo nevus).
Pathogenesis of Melanocytic Nevi
Melanocytic nevi are benign neoplasms arising from genetically altered melanocytes that undergo clonal expansion but ultimately cease dividing, explaining clinical regression [4]. This phenomenon of cessation of nevocyte proliferation is indicative of a homeostatic regulatory and safeguard mechanism that restricts the proliferation of genetically altered cells. So-called “oncogene induced senescence” is likely modulated variably by tumor suppressor gene activity, telomerase associated regulation, DNA damage responses (p53), and other regulatory mechanisms; these play a critical role in maintaining the benignity of melanotic nevi [2, 3, 14]. The primary oncogenic events in melanocytic nevi progression affect signaling molecules within the mitogen-activated kinase (MAP-kinase) pathways and phophatidyl-inositol-3-phosphate kinase (PI3-kinase pathways) [2, 3, 14]. The majority of melanocytic nevocytes originate from within the epithelium (Table 1). These nevi are clinically, microscopically, and genetically distinct from those nevi that originate from melanocytes that do not originate in epithelium. Nevi that originate within the epithelium are acquired MN, dysplastic MN and Spitz MN. They exhibit point mutations or kinase fusions in BRAF, NRAS, HRAS and CDKN2A (dysplastic nevi) (Table 1) [1–5]. Melanotic nevi that lack epithelial involvement share mutations in two G-protein alpha subunits GNAQ and GNA11 which gives rise to a distinct clade of nevi (congenital and blue nevi) (Table 1) [1–5]. It is essential to point out that the somatic oncogenic mutations noted in nevi are by themselves not sufficient for transformation to malignant melanoma; melanoma transformation requires additional, cumulative genetic alterations. As concerning as melanotic nevi may be to patients, transformation to melanoma is rare. The likelihood of nevus-to-melanoma transformation is 1/100,000 nevi, and mortality from this potential malignant transformation is 1/500,000 nevi. Therefore, widespread prophylactic removal or aggressive management of commonly occurring cutaneous or oral mucosal nevi is not indicated [14].
Malignant Melanoma
Cutaneous Malignant Melanoma
The incidence of cutaneous malignant melanoma has been on the rise over the past 30 years [14, 15]. An estimated 91,270 cases of melanoma will be diagnosed and an estimated 9400 people will die of this disease in the U.S. in 2018 (American Cancer Society). Malignant melanoma (MM) is a malignant neoplasm of melanocytes and is the result of a series of cumulative genetic alterations that takes years/decades to develop. Therefore, the risk of melanoma increases as people age: the average age at diagnosis is 63 years. Although melanoma is seen predominantly in adults, it can present in children. The latter often have an associated risk factor such as familial dysplastic nevus syndrome, genetic susceptibility due to defects in the CDKN2A locus (p16, p14 tumor suppressor deregulation), xeroderma pigmentosum, familial melanoma, or large congenital nevi [15–18]. While the etiology of MM is multifactorial, including genetic and racial factors, cumulative sun damage (CSD) associated with extensive UV exposure has historically been implicated as one of the major risk factors [2, 14]. MM is particularly common in individuals who are susceptible to sun-related skin damage (red hair, blue eyes, poor tanning tendency, freckling; skin types I and II); this is also borne out in data that shows increased incidence of MM in patients who live closer to the equator.
The majority of MM on cutaneous surfaces arise de novo within the epidermis. Initially, they grow within the layers of epithelium, exhibiting a radial/horizontal growth phase. At this early stage, these clinically flat lesions should be evaluated for the ABCDE of black–brown/pigmented lesions. Lesions that are deemed suspicious exhibit: (i) asymmetry, (ii) irregular borders, (iii) variegation in color, (iv) diameter > 0.6 cm and (v) signs of growth/ulceration/erythema or evolution (Fig. 5a, b). Clinical evidence of nodule formation implies that the tumor is progressing. With accumulating defects, MM develop the potential to infiltrate and invade the underlying dermis in what is termed vertical growth/invasion; this corresponds clinically to a nodular lesion. The clinical-pathological features of surface appearance and growth patterns have historically been used to classify MM into four categories:
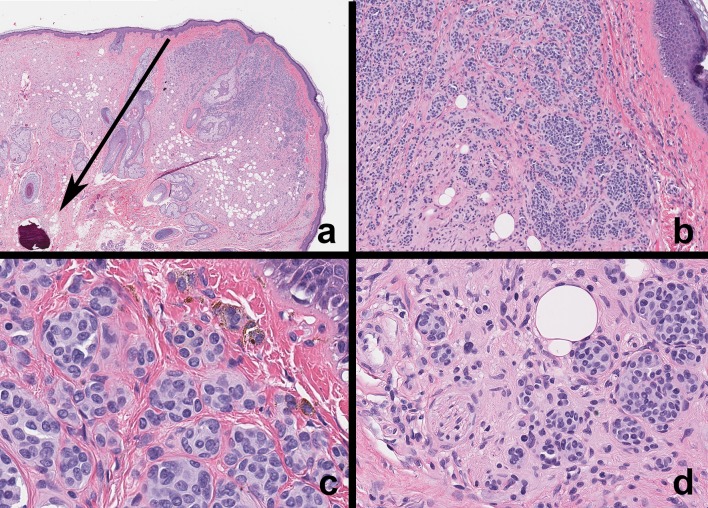
Fig. 5.
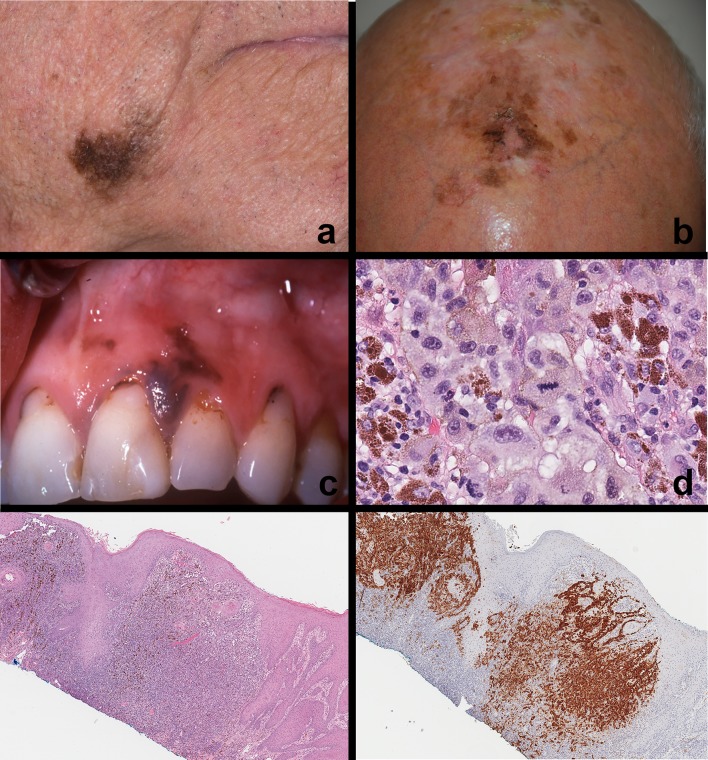
Malignant melanoma—cutaneous and mucosal. a, b Melanoma of the right lower face and scalp. Note the asymmetry, irregular borders, variegation in color and size > 1.0 cm. c Primary oral mucosal melanoma. Irregularly shaped, asymmetrical and variegated black–brown lesion on the left maxillary gingiva. Note the multiple pigmented areas. This patient complained of mobile teeth in the upper left quadrant and tumor invasion into the surrounding periodontium and alveolar bone. There was ill-defined lytic change around the canine and lateral incisor. d Mucosal melanoma (high magnification) Tumor cells are large, polygonal and exhibit marked cytoplasmic and nuclear pleomorphism with prominent nucleoli, melanin pigmentation and high mitotic index. e, f Primary mucosal melanoma (low magnification; immunohistochemistry) Melanoma arising from oral mucosa infiltrating the lamina propria and submucosa. Immunohistochemistry (HMB-45) highlights tumor cells originating from overlying epithelium. Similar expression was noted with S-100 and Melan A
- Lentigo maligna melanoma (MM arising within lentigo maligna) (Fig. 5a, b)
- Typically seen as macular areas of variegated pigmentation in chronically sun exposed areas. 10–15 year old precursor lesion prior to invasion.
- Superficial spreading melanoma
- Common variant seen on the leg/back. Asymmetrical, > 1.0 cm irregularly bordered, variegated colored macule which can present with nodular invasion
- Acral lentiginous melanoma
- Predominant subtype seen in individuals with darker pigmentation (Asians, Africans origin). Palmar-plantar and heavily keratinized surfaces. Mucosal surfaces (palate)
- Nodular melanoma
- Has no radial growth phase and present initially as dark colored nodules, or may present in lesions with known radial growth phase. Often ulcerated and may be non-pigmented (amelanotic melanoma).
The majority of MM have a radial growth phase before vertical growth. Radial growth is greatest in lentigo maligna, of shorter duration in superficial spreading MM, and absent in nodular melanoma. Although historically effective in diagnosis and management, the validity of this purely clinico-morphological classification is being questioned in light of emerging molecular characterization and gene profiling data [2, 14]. For instance, when considering the causal link between UV radiation and MM, one must take into consideration the classic UV-signature mutations: C>T or CC>TT transitions. Advances in the understanding of MM biology over the past couple of decades have shown that the mutations seen in the majority of MM are in common melanoma-oncogenes such as BRAF and NRAS, which are also seen in common AMN (Tables 1, 2). Notably, the common primary oncogenic mutation BRAF codon 600 T>A transition does not match documented UV-signature mutations. On the surface, this may appear to contradict the concept of UV-B radiation as a risk factor for melanomagenesis. This demonstrates that the initiating UV associated gain of function oncogenic mutations are not sufficient by themselves. These oncogenic events only mark the transition to the next progression stage with additional defects—these secondary defects are associated with loss of function alterations of tumor suppressor genes such as CDKN2A (p16, p14), TP53 (p53), PTEN [1, 2, 19] and mutations in TERT (telomerase), and a range of other secondary and tertiary defects. Future classifications of MM will likely use initiating oncogenic events to separate classes, and secondary oncogenic events may help further subclassify down the clade (Table 2). Therefore, as with AMN, the classification of MM is becoming cladistic, drawing on knowledge of initiating and secondary genetic events. Table 2 summarizes the clinical, histological and genetic profile of selected subtypes of MM seen in the head and neck region comparing CSD melanomas, Non-CSD melanomas and mucosal melanomas.
Table 2.
Classification and summary of malignant melanoma—head and neck cutaneous and mucosal
| Melanoma type | CSD Melanoma | Non-CSD melanoma | Mucosal melanoma—oral |
|---|---|---|---|
| Age distribution | 7th decade and older | 3rd to 6th decade | 5th decade and older |
| Anatomic site | Chronically sun exposed sites of the head, neck, arms and legs | Glabrous sites, conjunctiva, intermittently sun-exposed skin | Oral mucosa, Nasal cavity, Sinuses Oral—palate (40%) gingiva (30%) |
| Initial Clinical presentation | Common in patients with non-melanoma skin cancers (SCC, BCC). Irregularly pigmented, asymmetrical patch or nodule | More common in patients with AMN. Irregularly pigmented asymmetrical patch or nodule | Pigmented brown-black macule, rapid nodular growth, ulceration. Oral neoplasms infiltrate surrounding periodontal structures and alveolar bone—mobile teeth |
| Histological features | Lentiginous growth seen in early lesions followed by vertical growth. Pronounced solar elastosis of stromal tissues | Prominent pagetoid scatter, enlarged, pleomorphic round melanocytes with pigmentation | Limited lentiginous growth in early lesions. Prominent vertical infiltration of lamina propria. Tumor nests in stroma, periodontium and bone |
| Incidence per million | 55 | 135 | 2.2 |
| Role of UV | YES + + + | YES + | NO |
| Primary oncogenic alteration | NRAS (15%), KIT (10%) | BRAF (70%), NRAS (15%) | c-KIT (15%), NRAS |
| Secondary genetic alteration | TERT (telomerase) and p53 mutations | TERT mutations, CDKN2A (p16, p14) deletion, PTEN mutations | TERT (telomerase) amplification |
| Benign precursor | Questionable | Acquired Melanocytic Nevus | Questionable to none |
| Chromosome abberation | Losses of 9p, 6q, 8p Gains of 6p, 11q13, 8q, 1q, 20q, 17q |
Losses of 9p, 10, 6q, 8p Gains of 6p, 7, 8q, 1q, 20q, 17q |
Not characterized |
CSD cumulative sun damage associated
Mucosal Melanomas
Mucosal melanomas (MuM) of the head and neck account for < 0.2–8% of all MM arising in all sites—the variation in incidence is based on differences in racial predisposition with a higher rate of MuMs seen in individuals of Asian, Native American, African or Central/South American origin [1, 14–16, 18]. Mucosal sites in the head and neck include the sinonasal cavity, oral mucosa, pharynx, larynx and esophagus in decreasing order of frequency. In contrast to cutaneous melanoma, MuM involving the head and neck region often present with an earlier nodular/vertical growth phase with invasion of the underlying submucosal tissues [1, 16]. Radial growth is fairly short lived. The presenting symptoms of MuM differs in relation to the site of origin. In the sinonasal tract, the early signs of epistaxis, sinonasal obstruction, fullness, facial pain may be overlooked and confused with inflammatory conditions [1, 16]. Endoscopy may reveal findings that lead to biopsies and eventual diagnosis. On endoscopic examination, sinonasal MuM may present as polypoid, fleshy, non-pigmented lesions and confused with a range of other sinonasal neoplasms. The most common sites involved include the inferior turbinate/lateral nasal wall and nasal septum (43% and 24% respectively) [16]. MuM of the sinonasal tract do not exhibit some of the salient features of other pigmented melanomas. Conversely, lesions arising within the oral cavity lend themselves to complete examination owing to the accessibility of the oral mucosal membranes.
Melanomas of the oral cavity are exceedingly rare and represent either primary oral mucosal melanomas that arise from within melanocytes that reside within oral mucosal epithelium, or represent an oral manifestation of metastatic melanoma that arose within cutaneous surfaces [1, 15, 18, 20, 21]. Primary oral MuM present as asymptomatic, irregularly shaped black–brown or tan macular lesions (Fig. 5c), or may appear blue or purple owing to deep seated pigment. Amelanotic lesions may appear dark red or pink. The surface may be ulcerated, erythematous and nodular. The most common locations within the oral cavity are the palate and gingiva (Table 2). Given their early vertical growth tendency, oral MuM frequently invade the underlying periodontium, alveolar bone and associated with local lytic bone loss. Patients may present with tooth mobility and associated ill-defined radiographic findings. Given their advanced stage at discovery, oral MuM do not fit the same classification schemes set out for cutaneous MM [1]. Oral MuM are classified as:
Superficial spreading/In-situ MM
Nodular/invasive MM
Combined (superficial + nodular) MM
The discovery of clinically suspicious black–brown oral lesions must trigger a biopsy and specific diagnoses made on histological examination.
The clinical-radiographic and histological features of metastatic melanoma to the oral cavity region is similar to that of any metastatic disease to the jaws and mouth. Patients typically present with a rapidly progressing expansile mass of the jaws (mandible > maxilla) potentially with mobile teeth, paresthesia, pain, weight loss, and known history of metastatic melanoma. Intraorally, the mass may appear nodular black–brown in color with an ulcerated, erythematous surface. Radiographically, metastatic melanoma to the jaws presents with an ill-defined lytic radiolucency with root resorption, wipeout of alveolar bone structures and effacement of borders. The microscopic and genetic features of metastatic melanoma will be similar to that of the original tumor (Fig. 6a, b).
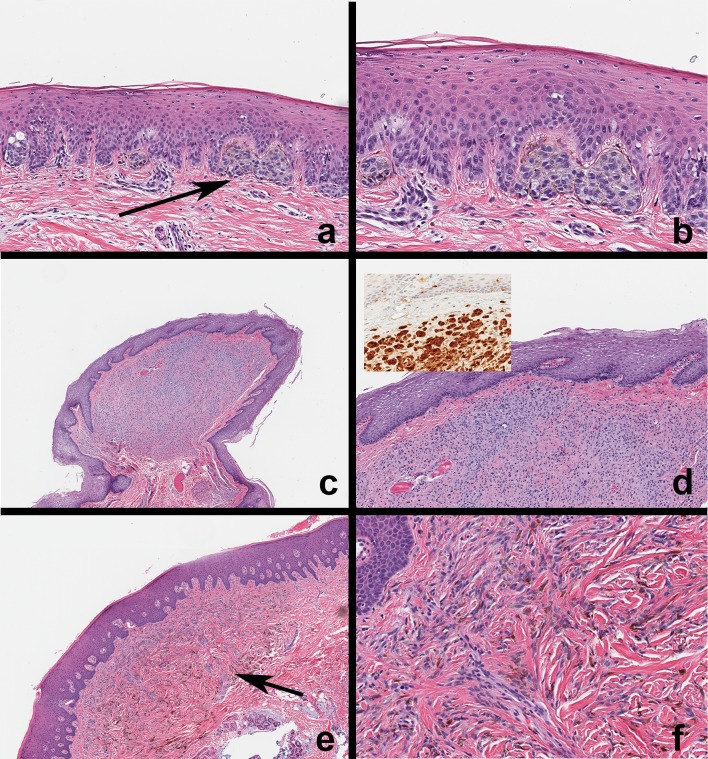
Fig. 6.
Melanoma, Melanotic neuroectodermal tumor and other cutaneous pigmented neoplasms. a, b Metastatic melanoma—examples of melanoma metastatic to the jaw bone. Tumor cells resemble original tumor, infiltrate alveolar bone and exhibit melanoma immunohistochemical markers (HMB-45, Melan-A, S-100; insets), c Melanotic neuroectodermal tumor of infancy (MNTI) Rapidly growing, pigmented, ulcerated mass of the anterior mandible in an infant, d MNTI microscopic features. Biphasic tumor population—clusters of large polygonal epithelioid melanosome containing cells (yellow arrow), loosely arranged clusters of small, round cells with blue-black nuclei (black arrow), e Seborrheic keratosis—pigmented. Solitary, discrete, pigmented exophytic waxy cutaneous nodule on the left upper face, f Basal cell carcinoma—pigmented. Ulcerated, umbilicated nodule on the right tip of the nose with black–brown pigmentation
Microscopic Features of Melanoma
Central to one’s understanding of MM microscopy is the concept of radial growth (horizontal) and invasive growth (vertical) phases. Radial growth is characterized by invasion the epithelium laterally and superiorly (pagetoid) by pleomorphic, cytologically abnormal neoplastic melanocytes. Tumor cells typically exhibit marked nuclear pleomorphism, clear cytoplasm, prominent nucleoli and a tendency to infiltrate the layers of the epithelium in a scattershot manner (contrast with cohesive junctional activity seen in nevi) (Fig. 5d). Mitotic figures are not uncommon. Vertical growth phase of melanoma is characterized by infiltrative sheets and single-celled, non-cohesive tumor cells that are polygonal/spindle shaped or with differences in morphology. Nuclear pleomorphism is prominent. Typically, there is no evidence of melanocyte regression. Tumor related stromal desmoplasia and chronic lymphocyte-dominated inflammatory infiltrates are often evident (these features are absent in melanocytic nevi). Tumor cells can exhibit a range of features and patterns—they may appear epithelioid or spindled on H&E stained sections. Immunohistochemical markers S-100, HMB-45 and Melan-A are often used in combination to confirm diagnoses of MM (Figs. 5e, f, 6a, b insets). Cutaneous MM are extensively documented to evaluate for potential prognostic factors. Clinical prognostic factors include age, sex, site of primary tumor, age of patient and high-risk sites back, upper arm, neck and scalp (BANS). When reporting melanomas, pathologists also record and comment on the following variables: (i) tumor thickness in mm (Breslow method) (ii) tumor invasion level (Clark method) (iii) growth phase (radial or vertical) (iv) mitotic index (v) ulceration (vi) lymphovascular invasion (vii) perineural invasion (viii) microsatellosis (ix) tumor infiltrating lymphocytes [1, 14, 16]. Of these, the Breslow method, presence of ulceration and tumor infiltrating lymphocytes are important independent prognostic factors. Tumor associated inflammation is characterized as brisk, nonbrisk or absent. The presence of tumor associated lymphocytes is a positive prognostic factor whereas the absence of tumor associated lymphocytes correlated with metastasis to lymph nodes and poor prognosis.
In contrast to cutaneous melanoma, standardized correlation of the above variables has not been thoroughly studied for oral MuM owing to challenges posed by: (i) small biopsy size, (ii) unrepresentative sampling (confines of the mouth), (iii) late stage of presentation and rapid vertical growth [1, 14–18]. The most important features to document for management and prognostic purposes when reporting on an oral melanoma are as follows: (i) site of involvement, (ii) mucosal ulceration, (iii) extension along salivary gland ducts, (iv) vascular or neural invasion, (v) involvement of underlying skeletal structures and (vi) whether the oral melanoma is primary or metastatic in origin (this is the most critical variable).
Pathogenesis of Mucosal Melanoma
Unlike cutaneous melanoma, MuM of the oral cavity, sinonasal membranes and other mucosal sites are unrelated to UV radiation. Moreover, several recent studies have identified alterations in the receptor tyrosine kinase KIT in a host of mucosal melanomas (and in acral and selected CSD melanomas) [1–3, 14–16, 19] (Table 2). Activating point mutations found in c-KIT (as seen in GISTs) and copy number increases of the c-KIT gene were found in patterns mutually exclusive from the BRAF and NRAS mutations associated with CSD and non-CSD cutaneous MM. While initial case reports showed promising results with KIT inhibitors such as imatinib, recent clinical trials of selected patients have failed to show consistent efficacy with c-KIT inhibitors [14, 16].
Mucosal Melanomas: Principles of Management and Prognosis
Surgery is considered the mainstay of treatment for MuM of the head and neck region despite the lack of randomized trials supporting this approach. Diagnostic imaging, staging and radical tumor excision with disease free margins is the first goal of surgery in patients with local disease. The importance of clear margins is increasingly being balanced with adjunctive considerations. Despite tumor-free margins, a significant proportion of patients suffer from recurrent or persistent disease. The progress made in characterizing melanoma pathogenesis has yielded several promising options in managing patients. A host of options have been developed for patients with melanoma with targeted therapies and immunotherapies. Targeted therapies include BRAF inhibitors (dabrafenib, vemurafenib), MEK inhibitors (trametinib) and KIT inhibitors. Patients with type-1 evidence BRAFv600 mutations receive BRAF inhibitors or MEK inhibitors, especially those patients who have unresectable or metastatic disease. KIT inhibitors imatinib, dasatinib and nilotinib are recommended as individualized options and are considered for selected mucosal melanomas. Immunotherapy options include ipilimumab (monoclonal antibody targeting cytotoxic T-lymphocyte associated molecule-4—CTLA4), PD-1 ligand pathway inhibitors, nivolumab and pembrolizumab. The latter three are recommended as standard options for patients with unresectable or metastatic melanoma. Despite the recognition of different biology between cutaneous and mucosal MM, several patients are still managed the same as cutaneous melanoma absent clinical trials for exclusively mucosal melanomas. Additional radiotherapy and supplemental surgical techniques are employed on an individualized and stage-based basis [1, 3, 14, 16, 22]. A detailed discussion of therapeutic options is beyond the scope of this article.
Melanotic Neuroectodermal Tumor of Infancy
Melanotic Neuroectodermal Tumor of Infancy (MNTI) is a rare benign neoplasm that can present as a brown-black pigmented oral mucosal mass [23–25]. This unusual tumor is seen almost exclusively in infants with a few reports in older children and adults. The tumor favors the head and neck region, but has been reported at other sites including the brain, mediastinum, epididymis, and femur. The posited theory for the origin of MNTI is that it is a congenital benign dysembryogenic neoplasm that arises from neural crest cells [24]. This is supported by embryologic, ultrastructural, immunohistochemical and genetic studies. Given the pluripotent nature of neural crest cells, the tumor cells display mesodermal and ectodermal features at different stages of their ontogeny, resulting in the biphasic cellular phenotype associated with these tumors. The neuroectodermal origin of this tumor is supported by reports of catecholamine and vaniyllmandelic acid secretion.
Clinically, these tumors typically present in infants or young children as a painless, smooth-surfaced black–brown or blue colored oral soft tissue mass that is firm on palpation, and that often involves the underlying bone (Fig. 6c). MNTIs grow rapidly, destroy local tissues, and given their predilection for the maxilla (common location) and mandible these tumors infiltrate and resorb the underlying alveolar bone, teeth, and surrounding structures. Radiographically, MNTI’s present as expansile areas of radiolucency with poorly demarcated margins and foci of radiopaque osteogenic reaction, a pattern that can be mistaken for osteosarcoma. Teeth are often displaced and resorbed.
Microscopically, MNTIs are biphasic in that there are 2 cell types that are appreciated. The first population is composed of clusters of large, polygonal epithelioid cells with clear cytoplasm containing melanosomes (Fig. 6d). These cells are positive for cytokeratin and HMB-45 on immunohistochemistry. The second cell population is composed of loose clusters of small, round cells with black-blue nuclei and limited cytoplasm (Fig. 6d). These cells resemble neuroblasts and express neuron specific enolase (NSE), and selected neuroendocrine markers like GFAP and synaptophysin on immunohistochemistry. The clinical presentation of MNTIs can be rather alarming and include differential diagnoses such as rhabdomyosarcoma, neuroblastoma, Ewing sarcoma and lymphoma. However, clinical context, imaging and tissue findings help establish definitive diagnosis. The standard of care for MNTI is surgical excision with negative margins. Although the morbidity associated with this tumor can be substantial, there are only isolated reports of malignant transformation and mortality associated with MNTI.
Ectodermal Neoplasms with Pigmentation
There is a range of benign and malignant ectodermal tumors that can present secondary black–brown melanin associated pigmentation. Several of these tumors can present on the skin of the head, face and neck. Although none of these neoplasms presents within the oral mucosa or other head and neck mucosal sites, we will briefly discuss these entities given how commonly one encounters them during a routine head and neck examination.
Seborrheic Keratosis: Pigmented
Seborrheic keratosis is a benign cutaneous neoplasm composed of a clonal proliferation of basaloid epidermal cells with variable degree of squamous differentiation, keratin production and horn cyst formation. Seborrheic keratoses can be solitary or numerous and appear as sharply delineated, round or oval, flesh colored or occasionally brown-black warty nodules or plaques with a greasy surface texture (Fig. 6e). They are particularly common on the skin of the face, chest and back. Deeply black–brown pigmented or secondarily traumatized seborrheic keratoses may be mistaken for melanoma. They are lesions that are typically seen in middle-aged and adults. Thorough clinical, dermoscopic and histological examination should help distinguish seborrheic keratoses from nevi and melanoma.
Basal Cell Carcinoma: Pigmented
Basal cell carcinomas (BCC) are the most prevalent cutaneous malignant neoplasm with an estimated incidence of 600,000–900,000 cases in the United States. The face is the most commonly involved site. BCCs are the result of cumulative sun damage (CSD). Overexposure to UV radiation in patients with fair skin is associated with activating mutations in the Shh pathway. Gain of function mutations or activation of GLI-1 transcription factor is a consistent finding in many sporadic BCCs. Basal cell carcinomas classically appear as pearly/opalescent nodules on the skin of the face (above the ala-tragal line) with central umbilicated, rolled ulceration and surface telangiectasias. The majority of basal cell carcinomas can be diagnosed on clinical and dermoscopic examination. Of the various subtypes of BCC (nodular, diffuse, superficial etc.), the pigmented variant of basal cell carcinoma can appear as a black–brown nodule on the skin of the head and neck (Fig. 6f). The variegated pigmentation associated with an ulcerated, black–brown cutaneous nodule can cause clinical confusion with a primary melanoma. Thorough clinical, dermoscopic and histological examination should help distinguish between these processes.
Discussion and Conclusion
The discovery of brown-black colored muco-cutaneous lesions in the head and neck region can be disconcerting and almost always brings up the fearful prospect of malignant melanoma. As concerning as it may be, it is essential to note that the vast majority of neoplastic black–brown lesions discovered on the cutaneous surfaces of the head and neck, and importantly the black brown lesions within the oral cavity are benign, and unrelated to melanoma. Focusing specifically on the oral and perioral region, the vast majority of pigmented lesions in this area are oral/labial melanotic macules, amalgam tattoos, followed by oral melanocytic nevi and other pigmented lesions. Melanomas constitute less than 0.9% of all pigmented lesions in this region. Nevertheless, as clinicians, it is important to be thorough in one’s approach to diagnosis, treatment planning and management. Equally critical during one’s approach to diagnosis and patient education is a foundational understanding of neoplastic pathogenesis, and the ability to correlate this at the clinical, microscopic, genetic and molecular level.
Acknowledgements
We thank Dr. Justin Finch, Assistant Professor and Director of Clinical Photography in the Department of Dermatology at UCONN Health for kindly permitting use several excellent photographs of head and neck pigmented lesions.
Funding
This article did not receive any funding.
Conflict of interest
The author declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individuals whose photographs are used in this article.
References
- 1.Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000;36(2):152–169. doi: 10.1016/S1368-8375(99)00085-8. [DOI] [PubMed] [Google Scholar]
- 2.Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol. 2014;9:239–271. doi: 10.1146/annurev-pathol-012513-104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damsky WE, Bosenberg M. Melanocytic nevi and melanoma: unraveling a complex relationship. Oncogene. 2017;36(42):5771–5792. doi: 10.1038/onc.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers T, Marino ML, Raciti P, Jain M, Busam KJ, Marchetti MA, et al. Biologically distinct subsets of nevi. G Ital Dermatol Venereol. 2016;151(4):365–384. [PMC free article] [PubMed] [Google Scholar]
- 5.Krengel S. Nevogenesis–new thoughts regarding a classical problem. Am J Dermatopathol. 2005;27(5):456–465. doi: 10.1097/01.dad.0000175532.27368.3f. [DOI] [PubMed] [Google Scholar]
- 6.MacKie RM, English J, Aitchison TC, Fitzsimons CP, Wilson P. The number and distribution of benign pigmented moles (melanocytic naevi) in a healthy British population. Br J Dermatol. 1985;113(2):167–174. doi: 10.1111/j.1365-2133.1985.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 7.Stegmaier OC. Natural regression of the melanocytic nevus. J Invest Dermatol. 1959;32(3):413–421. doi: 10.1038/jid.1959.70. [DOI] [PubMed] [Google Scholar]
- 8.Carrera C, Marghoob AA. Discriminating Nevi from Melanomas: Clues and Pitfalls. Dermatol Clin. 2016;34(4):395–409. doi: 10.1016/j.det.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchner A, Hansen LS. Pigmented nevi of the oral mucosa: a clinicopathologic study of 36 new cases and review of 155 cases from the literature. Part II: analysis of 191 cases. Oral Surg Oral Med Oral Pathol. 1987;63(6):676–682. doi: 10.1016/0030-4220(87)90370-7. [DOI] [PubMed] [Google Scholar]
- 10.Buchner A, Hansen LS. Pigmented nevi of the oral mucosa: a clinicopathologic study of 36 new cases and review of 155 cases from the literature. Part I: a clinicopathologic study of 36 new cases. Oral Surg Oral Med Oral Pathol. 1987;63(5):566–572. doi: 10.1016/0030-4220(87)90229-5. [DOI] [PubMed] [Google Scholar]
- 11.Buchner A, Merrell PW, Carpenter WM. Relative frequency of solitary melanocytic lesions of the oral mucosa. J Oral Pathol Med. 2004;33(9):550–557. doi: 10.1111/j.1600-0714.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira L, Jham B, Assi R, Readinger A, Kessler HP. Oral melanocytic nevi: a clinicopathologic study of 100 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(3):358–367. doi: 10.1016/j.oooo.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Meleti M, Mooi WJ, Casparie MK, van der Waal I. Melanocytic nevi of the oral mucosa—no evidence of increased risk for oral malignant melanoma: an analysis of 119 cases. Oral Oncol. 2007;43(10):976–981. doi: 10.1016/j.oraloncology.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Calonje EB, Lazar T, McKee AP. McKee’s Pathology of the Skin. 4th ed. Elsevier: Saunders; 2012.
- 15.Rawal YB, Dodson TB, Bal HS. Oral melanoma: relevance to the dental team members. J Am Dent Assoc. 2017;148(2):113–119. doi: 10.1016/j.adaj.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Ascierto PA, Accorona R, Botti G, Farina D, Fossati P, Gatta G, et al. Mucosal melanoma of the head and neck. Crit Rev Oncol Hematol. 2017;112:136–152. doi: 10.1016/j.critrevonc.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Eisen D, Voorhees JJ. Oral melanoma and other pigmented lesions of the oral cavity. J Am Acad Dermatol. 1991;24(4):527–537. doi: 10.1016/0190-9622(91)70077-F. [DOI] [PubMed] [Google Scholar]
- 18.Femiano F, Lanza A, Buonaiuto C, Gombos F, Di Spirito F, Cirillo N. Oral malignant melanoma: a review of the literature. J Oral Pathol Med. 2008;37(7):383–388. doi: 10.1111/j.1600-0714.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 19.Bandarchi B, Jabbari CA, Vedadi A, Navab R. Molecular biology of normal melanocytes and melanoma cells. J Clin Pathol. 2013;66(8):644–648. doi: 10.1136/jclinpath-2013-201471. [DOI] [PubMed] [Google Scholar]
- 20.Sortino-Rachou AM, Cancela Mde C, Voti L, Curado MP. Primary oral melanoma: population-based incidence. Oral Oncol. 2009;45(3):254–258. doi: 10.1016/j.oraloncology.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Tavares TS, Meirelles DP, de Aguiar MCF, Caldeira PC. Pigmented lesions of the oral mucosa: a cross-sectional study of 458 histopathological specimens. Oral Dis. 2018 doi: 10.1111/odi.12924. [DOI] [PubMed] [Google Scholar]
- 22.Del Vecchio M, Di Guardo L, Ascierto PA, Grimaldi AM, Sileni VC, Pigozzo J, et al. Efficacy and safety of ipilimumab 3 mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer. 2014;50(1):121–127. doi: 10.1016/j.ejca.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Azarisamani A, Petrisor D, Wright J, Ghali GE. Metastatic melanotic neuroectodermal tumor of infancy: report of a case and review of the literature. J Oral Maxillofac Surg. 2016;74(12):2431–2440. doi: 10.1016/j.joms.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Gaiger de Oliveira M, Thompson LD, Chaves AC, Rados PV, da Silva Lauxen I, Filho MS. Management of melanotic neuroectodermal tumor of infancy. Ann Diagn Pathol. 2004;8(4):207–212. doi: 10.1053/j.anndiagpath.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Rachidi S, Sood AJ, Patel KG, Nguyen SA, Hamilton H, Neville BW, et al. Melanotic neuroectodermal tumor of infancy: a systematic review. J Oral Maxillofac Surg. 2015;73(10):1946–1956. doi: 10.1016/j.joms.2015.03.061. [DOI] [PubMed] [Google Scholar]