Abstract
Aim
To assess the immunomodulatory effect of tonsil-derived mesenchymal stem cells (MSCs) on T-lymphocyte proliferation and cytokine production.
Methods
Tonsils were obtained from children aged 3 to 12 years (n = 15) who underwent tonsillectomy for obstructive sleep apnea from April 2012-October 2014 at the Merkur University Hospital, Zagreb. Tonsil-derived MSCs were co-cultured with peripheral blood mononuclear cells (PBMCs) and phytohemagglutinin as a mitogen. PBMCs were induced to differentiate into T helper 1 or T helper 2 cells in the presence or absence of tonsil-derived MSCs, after which the production of interferon-gamma in T helper 1 and interleukin-4 in T helper 2 cells was assessed.
Results
Tonsil-derived MSC suppressed phytohemagglutinin-induced proliferation of PBMCs. Compared with controls, tonsil-derived MSC co-culture significantly decreased interferon-gamma production (P < 0.001) and increased interleukin-4 production (P < 0.001).
Conclusion
Tonsil-derived MSCs exert immunomodulatory effects on T lymphocyte proliferation and T helper 1- and T helper 2-specific cytokine production.
Multipotent mesenchymal stem cells (MSCs) differentiate into various tissues of mesodermal origin, including bone, cartilage, and adipose and muscle tissues (1). Although MSCs do not induce immune response in an immunocompetent alloreactive host, in immunocompromised individuals they suppress T-, B-, natural killer, and dendritic cell proliferation and function (2-4). Several studies demonstrated the immunosuppressive properties of MSCs, highlighting the inhibition of T cell proliferation as their most important effect (2,5). MSCs also suppress other T cell functions, such as activation, cytokine secretion, and cytotoxicity. They inhibit the expression of T cell surface activation markers, antigen-specific T cell maturation, formation of cytotoxic T-lymphocytes, and production of proinflammatory interferon-gamma (IFN-γ) in T helper 1 (Th1) cells and immunosuppressive interleukin-4 (IL-4) in T helper 2 (Th2) cells (6,7).
MSCs for research and therapeutic use are mostly isolated from bone marrow; however, they can also be successfully isolated from many other adult tissues, including palatine tonsils (8-10). Palatine tonsils are an important alternative source of mesenchymal progenitor cells because of simple surgical access, lack of ethical dilemmas on tonsillectomy, and the fact that tonsils are generally removed in children and young adults, which provides young cells with high proliferation potential. In addition, since tonsillectomy is a common surgical procedure in children and adults, palatine tonsils are one of the most abundant surgical residual tissues (11). Previous studies have demonstrated human palatine tonsils to be a good source of MSCs (8). Furthermore, protocols for MSC isolation and their cell culture conditions have been established, the cell surface markers have been characterized, and MSC differentiation into mesodermal tissues has been demonstrated (8).
Considering that tonsil-derived MSCs have comparable phenotypic properties and differentiation characteristics to MSCs isolated from other tissues, we hypothesized that they may also exhibit immunomodulatory properties. Therefore, we assessed the immunomodulatory effect of tonsil-derived MSCs on T-lymphocyte proliferation and specific T-lymphocyte cytokine production in vitro.
Material and methods
T-MSC isolation
Tonsils were obtained from children aged 3 to 12 years (n = 15) who underwent tonsillectomy for obstructive sleep apnea in the period April 2012-October 2014 at the Merkur University Hospital but had no other metabolic, cardiac, or respiratory diseases. The study was approved by the institutional review board of the Merkur University Hospital (KBM 03/11/1259, date of approval: March 17, 2011), and parents signed the informed consent for study participation and data publication.
Tonsils were de-epithelialized and digested in the Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen, Carlsbad, CA, USA) containing 210 U/mL collagenase type I (Invitrogen) and 90 kU/mL DNase I (Sigma-Aldrich, St. Louis, MO, USA) for 30 minutes at 37°C. The cells were washed twice in 20% normal human serum (NHS)-RPMI and once with 10% NHS-RPMI after filtration through a wire mesh. The supernatant was discarded after centrifugation (centrifuged for 10 minutes at 4000 RPM), and the cell pellet was resuspended in 50 mL phosphate buffered saline (PBS); after dilution, 10 mL suspension was distributed in five polypropylene tubes. Mononuclear cells were isolated by Ficoll-Paque (Amersham, GE Healthcare, Little Chalfont, Buckinghamshire, UK) density gradient centrifugation of processed cell pellet, which was plated for 24 to 48 hours in T-150 tissue culture flasks (Corning Incorporated, Corning, NY, USA). Non-adherent cells were subsequently removed by flushing with serum-free MSC expansion medium (R&D Systems, Minneapolis, MN, USA).
T-MSC expansion
Serial passages of the cell culture were performed when tonsil-derived MSCs attained 80% confluence (as determined by phase contrast microscopy). Cells were washed twice with PBS, detached using 0.25% trypsin-EDTA solution (Gibco-BRL, Carlsbad, CA, USA), washed twice with expansion medium using centrifugation (1200 RPM for 5 minutes), and replated at 1:3 dilution under the same culture conditions. Cells obtained at passages 2 to 5 were used.
Cell surface epitope profiling – flow cytometry analysis
Tonsil-derived MSCs (>1 × 105 cells) were harvested and mixed with PBS containing 1% fetal bovine serum (FBS) (P+F mixture) with dilutions (1:100) of subsequent conjugated mouse IgG1κ anti-human monoclonal antibodies: CD14-phycoerythrin (PE), CD31-PE, CD45- fluorescein isothiocyanate (FITC), CD73-PE, CD90-FITC, CD105-PE (BD Biosciences, San Jose, CA, USA), HLA-A, B, C-PE (MHC I), HLA-DR, DP, DQ-FITC (MHC II) (R&D Systems) over 1 hour (at 4°C). Cell suspensions were washed twice with P+F and resuspended in P+F for analysis on a flow cytometer (FACSCalibur, BD Biosciences) using the CellQuest ProTM software (BD Biosciences). Positive staining was defined as fluorescence emission higher than the levels obtained in more than 99% of the cells in a population stained with isotype controls.
In vitro differentiation
Tonsil-derived MSCs were induced for adipogenic, osteogenic, and chondrogenic differentiation. For adipogenic differentiation, cells were incubated for 3 weeks in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 1 μM dexamethasone, 1 μg/mL insulin, and 0.5 mM 3-isobutyl-1-methylxanthine (all Sigma-Aldrich). For osteogenic differentiation, cells were incubated for 3 weeks in DMEM supplemented with 10% FBS, 10 nM dexamethasone, 50 μg/mL ascorbic acid-2-phosphate, 10 mM β-glycerophosphate, and 10 nM 1,25 dihydroxyvitamin D3 (Biomol International L.P., Plymouth Meeting, PA, USA). For chondrogenic differentiation, pelleted cultures were incubated for 3 weeks in high-glucose DMEM supplemented with 100 nM dexamethasone, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, 50 μg/mL ascorbic acid-2-phosphate, 10 ng/mL recombinant human transforming growth factor-β3 (R & D Systems), and 50 mg/mL insulin-transferrin-selenium-premix stock (BD Biosciences).
Total RNA isolation and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA samples were obtained from tonsil-derived MSCs after 3 weeks of culture using Trizol reagent (Invitrogen Corporation) and reverse-transcribed using random hexamers. Ten nanograms of complementary DNA (cDNA) and SYBR Green mix (Bio-Rad Laboratories, Irvine, CA, USA) was used for qRT-PCR with gene-specific primers (forward/reverse) designed using GenBank cDNA sequences (Table 1). Specific transcript levels were normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and presented as fold increase over GAPDH levels using the 2(ΔCt) method, where ΔCt = Ct of target gene - Ct of GAPDH.
Table 1.
Primers for reverse transcription-polymerase chain reaction of differentiation-specific genes
| Gene* | Primer sequences (5′–3′) | Position (bp) | Predicted size (bp) |
|---|---|---|---|
| Housekeeping gene | |||
|
GAPDH |
Sense:
GGGCTGCTTTTAACTCTGGT
Antisense:
GCAGGTTTTTCTAGACGG |
134–835 |
702 |
| Adipose tissue-specific genes | |||
|
LPL |
Sense:
GAGATTTCTCTGTATGGCACC
Antisense:
CTGCAAATGAGACACTTTCTC |
1261–1536 |
276 |
|
PPARγ |
Sense:
GCTGTTATGGGTGAAACTCTG
Antisense:
ATAAGGTGGAGATGCAGGCTC |
153–503 |
351 |
| Bone-specific genes | |||
|
ALP |
Sense:
TGGAGCTTCAGAAGCTCAACACCA
Antisense:
ATCTCGTTGTCTGAGTACCAGTCC |
122–575 |
454 |
|
OC |
Sense:
ATGAGAGCCCTCACACTCCTC
Antisense:
GCCGTAGAAGCGCCGATAGGC |
19–312 |
294 |
| Cartilage-specific genes | |||
|
AGN |
Sense:
TGAGGAGGGCTGGAACAAGTACC
Antisense:
GGAGGTGGTAATTGCAGGGAACA |
6591–6910 |
350 |
| COL2A1 | Sense: CAGGTCAAGATGGTC Antisense: TTCAGCACCTGTCTCACCA | 1341–1717 | 377 |
*GAPDH – glyceraldehyde-3-phosphate dehydrogenase; LPL – lipoprotein lipase; PPARγ – peroxisome proliferator-activated receptor-γ; ALP – alkaline phosphatase; OC – osteocalcin; AGN – aggrecan; COL2A1 – collagen type II α1.
Primary mixed lymphocyte reaction
Peripheral blood was obtained from healthy human donors (n = 15). Peripheral blood mononuclear cells (PBMCs) were segregated by using Ficoll-Hypaque density gradient centrifugation and subsequently mixed with RPMI-1640 medium containing 10% FBS, 100 μg/mL streptomycin, 100 U/mL penicillin, 2 mM glutamine, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 20 mM HEPES, and 50 μM 2-mercaptoethanol. 1 × 105 PBMCs/100 μL (in triplicates) were seeded in 96-well round-bottom plates (BD Biosciences). T cell activator phytohemagglutinin was used as a positive control mitogen (5 μg/mL). 5 × 104 of tonsil-derived MSCs were added to obtain a final volume of suspension of 300 μL per well. Plates were incubated for 72 hours, after which 1 μCi/[3H]-thymidine (GE Healthcare) was added to each well. After overnight incubation, incorporation of radioactivity was measured by liquid scintillation counting. All tests were done in triplicates and repeated at least twice.
MSCs-induced T lymphocyte co-culture
Tonsil-derived MSCs and PBMCs were co-cultured in 12-well plates (tonsil-derived MSCs to PBMCs ratio, 1:10) under Th1- or Th2-inducing conditions. Control plates contained tonsil-derived MSCs or PBMCs only. Th1-inducing medium contained anti-CD3 [5 μg/mL], anti-CD28 [1μg/mL], recombinant human IL-2 [rhIL-2, 4 ng/mL], rhIL-12 [1 μg/mL], and anti-IL-4 [1μg/mL]. Th2-inducing medium contained anti-CD3 [5 μg/mL], anti-CD28 [1 μg/mL], rhIL-2 [4 ng/mL], rhIL-4 [1 μg/mL], and anti-IFN-γ [1 μg/mL] (Sigma-Aldrich). After two days of incubation, the non-adherent cells were collected from co-cultures and mixed with phytohemagglutinin (5 μg/mL) for further 24 hours. The levels of IFN-γ in Th1 and IL-4 in Th2 co-cultures were analyzed using commercial enzyme-linked immunosobent assay kits (R&D Systems) as per manufacturer’s instructions and compared with controls.
Statistical analysis
All data are presented as mean ± standard deviation unless otherwise indicated. The sample size was based on literature search and is similar to that in other studies (3,7). The study power was calculated from sample size, effect size (Cohen's d), and significance level. The lowest power was obtained for the assessment of IFN-γ secretion. For IFN-γ levels, the Cohen's d was 2.9 (signifying the large effect), so the significance level was set at 0.01 to correct for multiple comparisons (since there were three groups). The calculated power based on these data was at least 85%, while the power for other comparisons was even higher.
Normality of data distribution was tested by the Kolmogorov-Smirnov test. The differences between the groups in data from flow cytometry analysis of surface epitope profiles and real-time PCR analysis of differentiation-specific genes were assessed with the t test. The differences in cytokine secretion were assessed with one-way ANOVA with Bonferroni post hoc correction. The significance level was set at P < 0.05. Statistical analysis was performed with the SPSS software, v. 23 (IBM Corp., Armonk, NY, USA).
Results
Cell isolation and proliferation
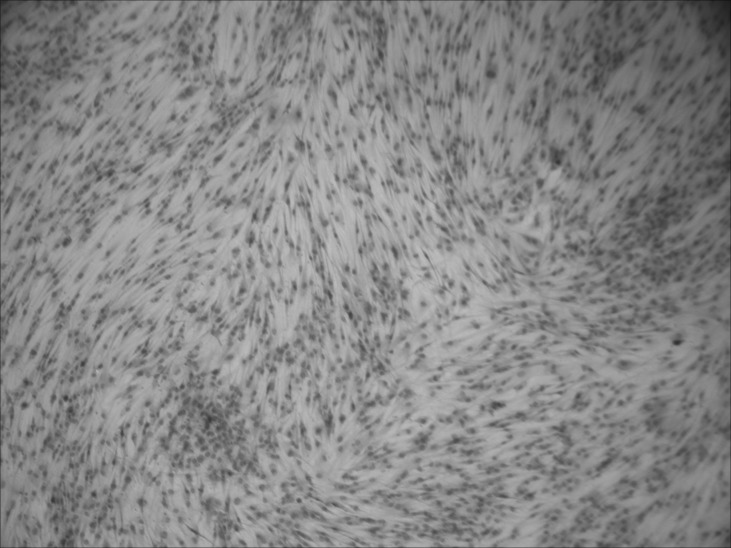
The total cell yield from each tonsil (n = 15) was in the range of 1-5 × 109. The majority of these cells were non-adherent; most likely of hematopoietic origin. Approximately 0.1%-1% of the isolated cells remained adherent to flasks after repetitive irrigations with PBS and expansion-medium changes. Tonsil-derived MSCs were homogenous fibroblast-like with extended cytoplasmic processes (Figure 1).
Figure 1.
Microscopic view of unstained fibroblast-like tonsil-derived mesenchymal stem cells with flat polygonal morphology at passage 1. Magnification: 40 × .
Flow cytometry analysis of surface epitope profiles
Flow cytometric analysis showed non-hematopoietic and non-endothelial origin of tonsil-derived MSCs as they did not express CD45 and CD31. However, they were positive for characteristic MSC surface epitope markers, such as CD105, CD73, and CD90 (Figure 2). They also expressed MHC class I but not MHC class II molecules.
Figure 2.
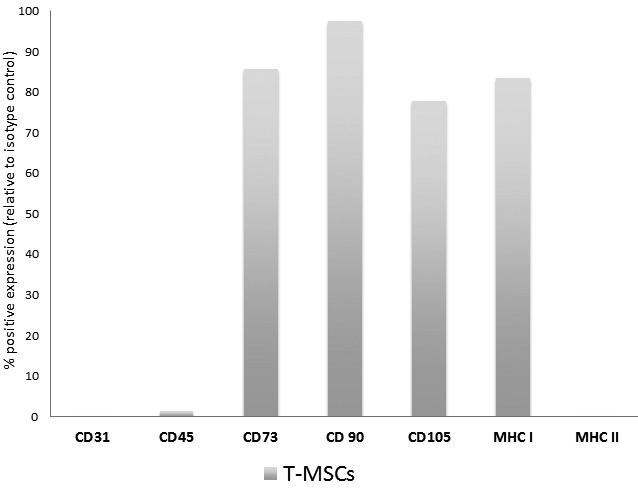
Flow cytometry analysis of tonsil-derived mesenchymal stem cells (T-MSC). Cluster of differentiation (CD)14, CD34, CD45, CD73, CD90, CD105, major histocompatibility complex (MHC) I, and MHC II were analyzed by fluorescent conjugated antibodies. The expression of each epitope is presented as the percentage of positive expression relative to the isotype control.
Multilineage differentiation potential
Tonsil-derived MSCs in passages 2-5 were treated with adipogenic, osteogenic, and chondrogenic supplements. After 3 weeks of culture, qRT-PCR analysis of the differentiated cells revealed a significant increase in lipoprotein lipase and peroxisome proliferator-activated receptor-γ expression in adipogenic cultures; osteocalcin and alkaline phosphatase expression in osteogenic cultures; and aggrecan and collagen type II α1 expression in chondrogenic cultures (Figure 3).
Figure 3.
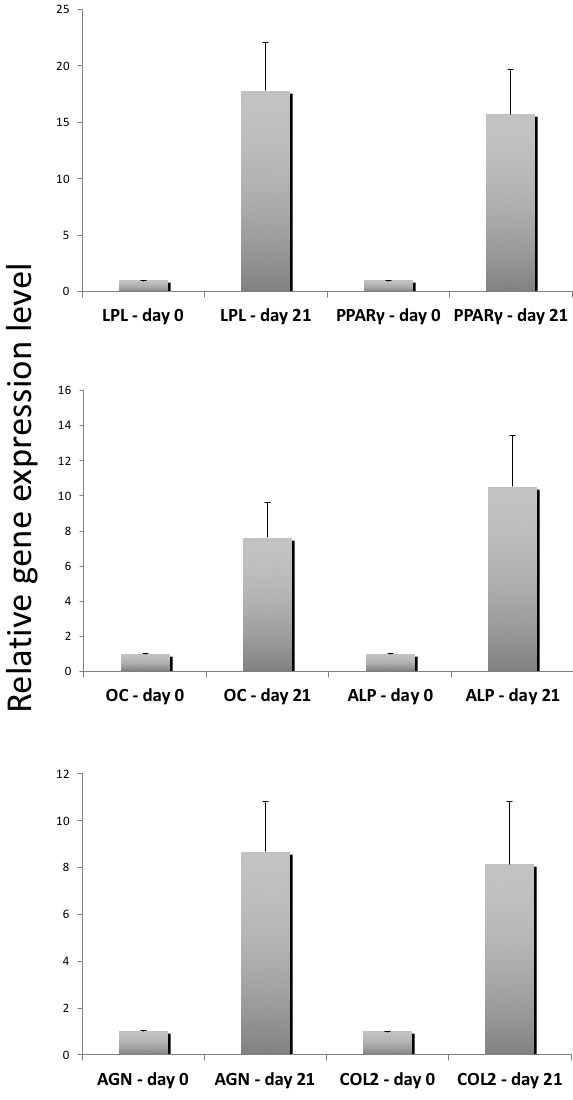
Gene expression analysis of differentiated tonsil-derived mesenchymal stem cells. Lipoprotein lipase (LPL) and proliferation-activated receptor-gamma (PPARγ) are genes involved in adipogenesis, osteocalcin (OC) and alkaline phosphatase (ALP) are genes involved in osteogenesis, and aggrecan (AGN) and collagen type II α1 (COL2) are genes involved in chondrogenesis. Gene expression analysis was performed at the beginning of culture (day 0) and at day 21. Expression levels were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the results are reported as marker gene vs GAPDH ratios using the formula 2ΔCT ( × 100). Values are mean ± standard deviation (n = 2). Error bars represent standard deviation.
Inhibition of T cell proliferation
To assess the immunomodulatory effect of tonsil-derived MSCs on T cell responses, we first used in vitro assays of proliferative T cell activity with phytohemagglutinin as a mitogen. Tonsil-derived MSC addition to cultured and phytohemagglutinin-stimulated PBMCs robustly inhibited PBMC proliferation (Figure 4A). We observed a dose-dependent effect, except at 1:5 tonsil-derived MSC-to-PBMC ratio, when the effect of tonsil-derived MSCs was largely absent (Figure 4B). Paired t test showed that the immunosuppressive effect of tonsil-derived MSCs on phytohemagglutinin-stimulated proliferation of T cells was significant (P < 0.001). There was no significant self-induced effect (P = 0.150)
Figure 4.
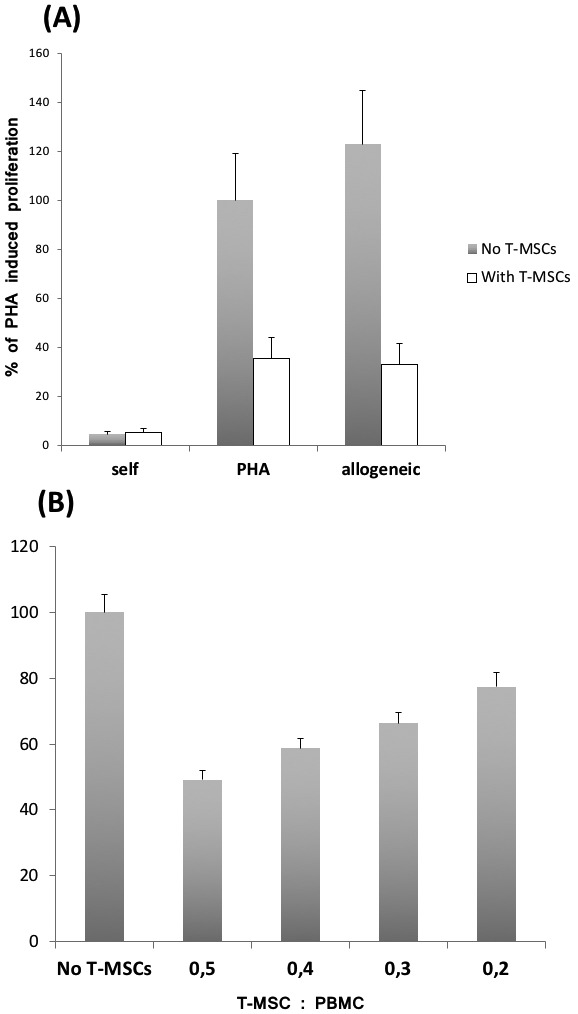
Tonsil-derived mesenchymal stem cells (T-MSCs) inhibited phytohemagglutinin (PHA)-stimulated T cell proliferation in a dose-dependent manner. Peripheral blood mononuclear cells (105 cells) with 5 μg/mL PHA were incubated for 72 hours in the absence or presence of T-MSCs (5 × 104 or varying ratios). (A) Cell proliferation based on [3H]-thymidine uptake. T-MSCs inhibited the T cell proliferation. The value of 100% was set at the proliferative response (counts per minute per culture) of PHA-stimulated T cell proliferation. All values are mean ± standard deviation of triplicates. (B) T-MSCs demonstrated a dose-dependent suppression of PHA-stimulated T cell proliferation. Results (n = 3) are expressed as percentage relative to T cell proliferation without addition of T-MSCs (the value of 100%). Error bars represent standard deviation. Gray bars – without T-MSCs; white bars – with T-MSCs.
MSC-T cell interaction
We specifically focused on the production of Th1- and Th2-specific cytokines in T cells generated under polarizing in vitro conditions. PBMCs undergoing Th1 differentiation without addition of tonsil-derived MSCs produced moderate levels of IFN-γ. After the addition of tonsil-derived MSCs, these levels significantly decreased (P < 0.001) (Figure 5A). In Th2 cells, addition of tonsil-derived MSCs significantly increased IL-4 production (P < 0.001) when compared with baseline IL-4 levels.
Figure 5.
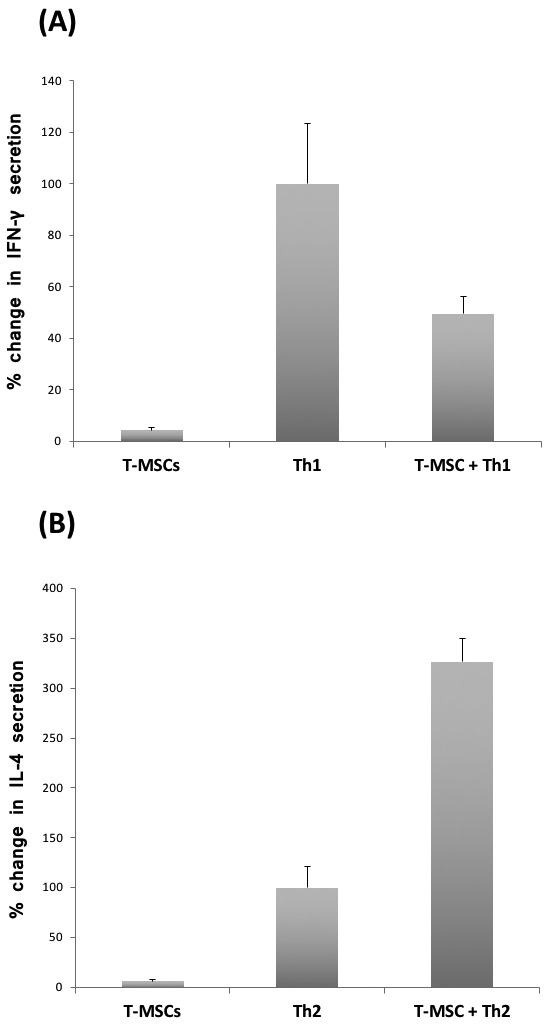
Tonsil-derived mesenchymal stem cells (T-MSCs) were co-cultured with peripheral blood mononuclear cells under T helper 1 (Th1) or T helper 2 (Th2)-inducing conditions. (A) In the presence of T-MSCs, Th1 cells showed >50% decrease in interferon-gamma (IFN-γ) production and (B) Th2 showed >300% increase in interleukin-4 (IL-4) production compared with controls without T-MSCs. Values indicate the change in cytokine secretion (mean ± standard deviation) in the absence or presence of T-MSCs in three independent experiments. Error bars represent standard deviation.
Discussion
Our study identified a potent immunomodulatory effect of human tonsil-derived MSCs on proliferative and cytokine production. In the presence of tonsil-derived MSCs, T cells exhibited significantly decreased proliferation, decreased IFN-γ production, and increased IL-4 production, which differs from the typical inflammatory profile.
Our findings extend the results of earlier studies that showed that other MSCs (bone marrow, Wharton's jelly, and fat tissue) exerted immunomodulatory effects on CD4+ and CD8+ T cells (12) and that tonsil-derived MSCs in mouse models modulated human immune responses (13).
Compared with controls, we observed >50% decrease in IFN-γ secretion in Th1 cells and >300% increase in IL-4 production in Th2 cells when they were co-cultured with tonsil-derived MSCs. This is similar to other studies that showed that MSCs can alter immune cell response by inhibiting proinflammatory TNF-α and IFN-γ and increasing anti-inflammatory IL-4 and IL-10 secretion (6).
Both autologous and allogeneic MSCs suppress CD4+ Th cells and CD8+ cytotoxic T-lymphocytes (14,15). This suppression of T cells may be direct, or indirect through modulatory action on antigen-presenting cells (16). Another mechanism is the arrest in the G0/G1 phase of the T cell cycle and reduction in IFN-γ, IL-2, and TNF-α production (6). MSCs can also suppress many other T cell mediators (14,15,17).
Several clinical studies investigated the hypoimmunogenic and immunosuppressive nature of allogeneic MSCs. Transplantation of allogeneic MSCs showed favorable results in patients with osteogenesis imperfecta (18), metachromatic leukodystrophy (19), and severe idiopathic aplastic anemia (20). They were also used for graft vs host disease prevention in the transplantation of hematopoietic stem cells (21). Therefore, identification of MSCs’ immunomodulatory properties may facilitate their application in hematology and organ transplantation protocols.
We demonstrated that tonsil-derived MSCs suppressed the growth of T-lymphocytes and secretion of two T-lymphocyte-specific cytokines. However, MSCs have also been shown to impact other T helper subsets involved in diseases characterized by immune dysregulation (such as IL-17-producing T helper 17 or FoxP3-expressing regulatory T cells) (22). Additionally, MSCs suppress DC maturation, and inhibit B cell proliferation, differentiation, and chemotaxis. Therefore, additional studies are required to completely understand the hierarchy of tonsil-derived MSC-associated immunomodulators and determine whether tonsil-derived MSCs can trigger the harmful T-cell effector behavior (23).
Our study did not include children with histopathological diagnosis or microanatomical architecture other than hypertrophic tonsils. While the existing data show only limited impact of distinct tonsillar histopathology on morphologic changes (hypertrophic changes in palatine tonsil are driven only by the germinal center expansion) (24), further studies are needed to determine the cell-intrinsic properties of palatine tonsil-derived MSCs removed for different non-malignant reasons.
The limitations of our study include a limited insight into T-MSC mechanisms involved in the immune response suppression and selective focus on Th1 and Th2 functions, whereas other populations with defined pathogenic role in human disease (eg, Th17 and regulatory T cells) remained unexplored (22). Recent murine model demonstrated that tonsil-derived MSCs expressed both the membrane-bound and soluble forms of programmed death-ligand 1 (PD-L1), which distinguishes them from MSCs derived from other organs. It also found that T-MSC-derived PD-L1 effectively suppressed Th17 differentiation in autoimmune diseases such as psoriasis (13).
Despite these limitations, our study offers an initial assessment of immunomodulatory properties of tonsil-derived MSCs in human samples. Further research is warranted to better explain the mechanisms behind the observed immunosuppressive effect and to provide a foundation for translational consideration.
Acknowledgments
Funding None.
Ethical approval received from the institutional review board of the Merkur University Hospital (KBM 03/11/1259, date of approval: March 17, 2011).
Declaration of authorship DP and SJ conceived and designed the study; AB acquired the data; AB and SJ analyzed and interpreted the data; all authors drafted the manuscript; DP and SJ critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; all authors agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 3.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 4.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–73. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 5.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 7.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 8.Janjanin S, Djouad F, Shanti RM, Baksh D, Gollapudi K, Prgomet D, et al. Human palatine tonsil: a new potential tissue source of multipotent mesenchymal progenitor cells. Arthritis Res Ther. 2008;10:R83. doi: 10.1186/ar2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multi-potent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–75. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 11.Vital and health statistics: ambulatory and inpatient procedures in the United States, 1996. U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. 1998.
- 12.Najar M, Raicevic G, Boufker HI, Fayyad Kazan H, De Bruyn C, Meuleman N, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cell Immunol. 2010;264:171–9. doi: 10.1016/j.cellimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Park M, Kim YH, Ryu KH, Lee KH, Cho KA, et al. Tonsil-derived mesenchymal stem cells (T-MSCs) prevent Th17-mediated autoimmune response via regulation of the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway. J Tissue Eng Regen Med. 2018;12:e1022–33. doi: 10.1002/term.2423. [DOI] [PubMed] [Google Scholar]
- 14.English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–42. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 16.Cutler AJ, Limbani V, Girdlestone J, Navarrete CV. Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J Immunol. 2010;185:6617–23. doi: 10.4049/jimmunol.1002239. [DOI] [PubMed] [Google Scholar]
- 17.Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14:252–65. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 19.Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant. 2002;30:215–22. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 20.Fouillard L, Bensidhoum M, Bories D, Bonte H, Lopez M, Moseley AM, et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17:474–6. doi: 10.1038/sj.leu.2402786. [DOI] [PubMed] [Google Scholar]
- 21.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 22.Zambrano-Zaragoza JF, Romo-Martínez EJ, Durán-Avelar Mde J, García-Magallanes N, Vibanco-Pérez N. Th17 cells in autoimmune and infectious diseases. Int J Inflam. 2014;2014:651503. doi: 10.1155/2014/651503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2:34–42. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reis LG, Almeida EC, da Silva JC, Pereira Gde A, Barbosa Vde F, Etchebehere RM. Tonsillar hyperplasia and recurrent tonsillitis: clinical-histological correlation. Braz J Otorhinolaryngol. . 2013;79:603–8. doi: 10.5935/1808-8694.20130108. [DOI] [PMC free article] [PubMed] [Google Scholar]