Abstract
Leptospirosis is a worldwide zoonosis with higher incidence in tropical areas and is a neglected disease in the Pacific region. French Polynesia (FP) is a French overseas territory located in the South Pacific. Data on the epidemiology in FP are scarce. In this study, we describe our understanding of leptospirosis epidemiology in FP and discuss the prospects concerning this disease and its surveillance to better address preventive actions. We report 11 years of surveillance data between 1 January 2007 and 31 December 2017. Over the study period, 1356 confirmed and probable leptospirosis cases were reported. The mean annual incidence rate was 46.0 (95% confidence interval, 43.6–48.5) cases per 100 000 inhabitants. We registered 864 (63.7%) hospitalizations; of these, at least 270 (19.9%) were in the intensive care unit, and 24 patients (1.8%) died. Even if the incidence of leptospirosis is lower in FP compared to most of other Pacific countries and territories, our data confirm that the disease is highly endemic in FP. Despite all the preventive measures taken, leptospirosis remains a major public health concern in FP, thus highlighting the need to maintain intensive leptospirosis surveillance, medical staff training and provision of information to the general population.
Keywords: Epidemiology, incidence, leptospirosis/epidemiology, polynesia/epidemiology, public health/epidemiology, zoonoses
Introduction
Leptospirosis is a worldwide bacterial zoonosis that imposes a significant health burden on economically vulnerable populations [1], [2], [3]. It is a neglected tropical disease, especially in the Pacific region [4], [5], [6].
Leptospirosis is caused by spirochetes of the genus Leptospira, bacteria belonging to the Leptospiraceae family [7], [8]. Many mammal species, wild or domestic, can carry the pathogen. Rodents are the main reservoir of Leptospira [1]. The epidemiology of leptospirosis is characterized in the Pacific islands by a potentially important role of livestock farming animals [4].
Human contamination results from contact of abraded skin or mucous membranes with urine or tissues of infected animals, directly or indirectly via contaminated water or wet soil [1], [9], [10]. The disease runs the gamut of subclinical infection, flulike symptoms, severe disease and multivisceral complications such as acute renal failure, pulmonary haemorrhage (Weil disease) and death [1], [9], [11]. Treatment is based on early antibiotic therapy. The vaccine only protects against the serovar Leptospira icterohaemorrhagiae, which is the most frequently responsible for severe human infections; it must be renewed every 2 years.
The annual worldwide incidence of leptospirosis is estimated at 1 million cases, with 5% to 15% severe disease [12] and about 60 000 deaths [13]. Leptospirosis is responsible for 2.9 million disability-adjusted life-years per annum, with a preponderant occurrence among low- and middle-income tropical countries and male subjects [2]. The burden of leptospirosis is underestimated because of the high proportion of subclinical and asymptomatic infections, lack of notification systems and poor access to accurate laboratory diagnosis in low- and middle-income countries [1], [14], [15], [16]. In 2009 the World Health Organization created the Leptospirosis Burden Epidemiology Reference Group, with the goal of conducting research and developing policies targeted towards decreasing the burden of leptospirosis [17].
Pacific islands have highly favourable environmental conditions for the transmission of leptospirosis: hot and humid tropical and subtropical climate with high rainfall, recurrent flooding, poverty, urbanization, local lifestyle (barefoot walking, swimming) and occupational activities [15]. Knowledge of the global burden of leptospirosis in the Pacific region remains incomplete, principally because of the lack of laboratory capacity to confirm cases.
French Polynesia (FP) is a French overseas territory located in the South Pacific comprising five archipelagos: Society, Marquesas, Tuamotu-Gambier and Austral Islands. The population is about 280 000 inhabitants (2017 census) spread over 72 inhibited islands, with 70% of the population living on the island of Tahiti [18]. Few data on the epidemiology of leptospirosis in FP exist [4], [19], with the first human infections reported only in 1959 [20]. The number of reported cases per year between 2006 and 2012 ranged between 79 and 127, and the annual incidence ranged 30 to 49 cases per 100 000 inhabitants [21], [22], [23].
In this study, we describe our understanding of leptospirosis epidemiology in FP and discuss the prospects concerning this disease and its surveillance to better address preventive actions.
Methods
We studied 11 years of surveillance data between 1 January 2007 and 31 December 2017. In FP, all probable and confirmed leptospirosis cases are collected by the local health authority (Bureau de veille sanitaire), based on notification forms filled in by general practitioners and laboratory results provided by laboratories of the Institut Louis Malardé and of the Centre Hospitalier de la Polynésie française.
A confirmed case was defined as leptospirosis symptoms with either isolation of bacteria by culture or of its DNA by PCR or seroconversion of specific-class IgM. A probable case was defined as leptospirosis symptoms and IgM detection ELISA on a single serum sample collected more than 1 week after onset of symptoms. An excluded case was defined as leptospirosis symptoms with PCR-negative findings on sera collected within the first week after onset of symptoms, or ELISA IgM negative on sera collected more than 1 week after onset of symptoms.
Demographic data were provided by the FP statistical institute (http://www.ispf.pf/). Population estimation for the duration of the study was calculated using an exponential increase during two consecutive censuses. All confirmed and probable cases were included in the statistical analysis. Qualitative variables were described using frequency and percentages. Quantitative variables were described using mean ± standard deviation (minimum–maximum). Annual incidence rates (AIRs)—that is, number of cases per 100 000 person-years (PY)—were presented with 95% confidence intervals (CI), based on the Poisson model. We used a Z test for comparing AIRs between sexes and among archipelagos. Relative risk (RR) with corresponding 95% CI were computed. A p level of <0.05 was considered statistically significant. All statistical analyses were performed by Stata 13.1 software for Windows (StataCorp, College Station, TX, USA). Mapping was done by QGis 2.18.1 software and Google Earth Pro.
Results
Between 1 January 2007 and 31 December 2017, a total of 1356 leptospirosis cases were reported, of which 851 (62.8%) were confirmed and 505 (37.2%) probable. Over the study period, the AIR was 46.0 (95% CI, 43.6–48.5) cases per 100 000 PY (Fig. 1(a)). The median number of cases per decade was 109, with a peak in 2017 with 199 cases, corresponding to an AIR of 72.1 (95% CI, 62.5–82.9) cases per 100 000 PY.
Fig. 1.
(a) Leptospirosis cases and annual incidence rates in French Polynesia, 2007–2017. (b) Cumulative monthly cases of 11 years of leptospirosis in French Polynesia, 2007–2017.
A total of 1032 cases (76.1%) were reported in men and 324 (23.9%) in women (sex ratio, 3.2), with an overall average age of men of 33.7 ± 16.2 (range, 2–80) years and of women 35.2 ± 17.8 (range, 1–88) years. Comparison of AIRs showed a significant excess risk of contamination in men (68.5 (95% CI, 64.4–72.8) cases per 100 000 PY) vs. women (22.4 (95% CI, 20.1–25) cases per 100 000 PY) (RR = 3.1; 95% CI, 2.7–3.5; p <0.001). Analysis by age group found that the largest number of cases occurred in subjects aged 20 to 29 years, with 332 cases (24.5%) representing a peak AIR of 66.8 (95% CI, 59.8–74.4) cases per 100 000 PY. During the whole period, cases were reported throughout the year, but mostly between January and May, with a noticeable decrease from August to December (Fig. 1(b)).
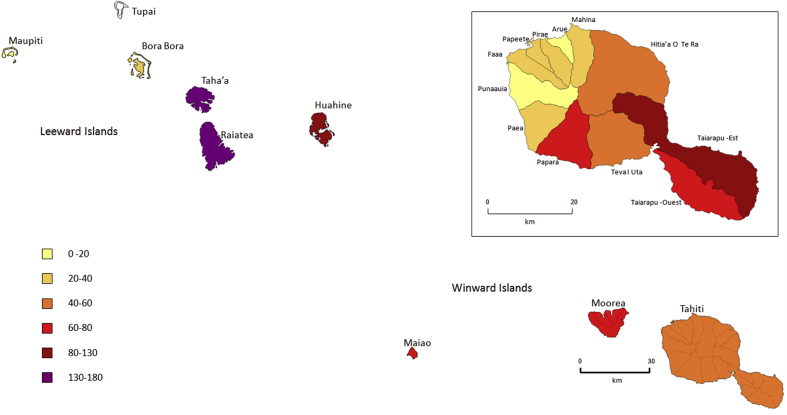
Island of residence was available for 1282 patients (94.5%). The Society archipelago accounted for 95.5% of cases, with an annual average of 114.7 ± 35.1 (range, 74–192) cases. The AIRs per archipelago were 48.8 (95% CI, 46.1–51.6) cases per 100 000 PY for the Society, 53 (95% CI, 39.8–69.2) for the Marquesas, 1.3 (95% CI, 0.0–7.4) for the Austral and 2.2 (95% CI, 0.6–5.5) for the Tuamotu Gambier islands. Map, number of cases, AIRs and RR by island in the Society archipelago are presented in Table 1 and Fig. 2.
Table 1.
Leptospirosis cases by Society Islands archipelago, 2007–2017
| Island | N (%) | AIRa (95% CI) | RR (95% CI) | pb |
|---|---|---|---|---|
| Tahiti | 731 (57.9) | 40.0 (37.1–43.0) | 1.00 (reference group) | |
| Moorea-Maiao | 121 (9.6) | 63.8 (53.0–76.3) | 1.6 (1.3–1.9) | <0.001 |
| Raiatea | 191 (15.1) | 141.9 (132.3–163.5) | 3.6 (3.0–4.2) | <0.001 |
| Tahaa | 94 (7.5) | 163.7 (132.3–200.4) | 4.1 (3.3–5.1) | <0.001 |
| Huahine | 87 (6.9) | 125.5 (100.5–154.8) | 3.1 (2.5–3.9) | <0.001 |
| Bora-Bora | 37 (2.9) | 35.1 (24.7–48.3) | 0.9 (0.6–1.2) | 0.437 |
| Maupiti | 1 (0.1) | 7.6 (0.2–42.4) | 0.2 (0.03–1.4) | 0.098 |
CI, confidence interval; AIR, annual incidence rate; RR, relative risk.
AIR expressed as number of cases per 100 000 persons per year.
Comparison of RR with reference group.
Fig. 2.
Map of Society archipelago with a focus on island of Tahiti, annual incidence rate of leptospirosis by island and municipality, 2007–2017.
Over the study period, we recorded 864 hospitalizations (63.7%); among them, at least 270 (19.9%) were in the intensive care unit, and 24 patients (1.8%) died.
Information on risk factors for leptospirosis was obtained from 920 cases (67.8%) as being occupational risk, 885 (65.3%) leisure activity, 835 (61.6%) contact with animals and 324 (23.9%) high-risk facilities (Table 2).
Table 2.
Potential risk factors of leptospirosis, French Polynesia, 2007–2017
| Category | Variable | No. of cases | Percentage over total no. of cases provided for this category | Missing values for this category, n (%) |
|---|---|---|---|---|
| Occupational risk | Farmer | 383 | 41.6% | 436 (32.2) |
| Sanitation and waste treatment | 60 | 6.5% | ||
| Animal husbandry | 134 | 14.6% | ||
| Cattle breeding | 23 | 2.5% | ||
| Horse breeding | 17 | 1.8% | ||
| Pig farming | 43 | 4.7% | ||
| Leisure activity/fresh water contact | Fishing | 131 | 14.8% | 471 (34.7) |
| Hunting | 40 | 4.5% | ||
| Gardening | 319 | 36.0% | ||
| Hiking | 101 | 11.4% | ||
| Walking barefoot | 497 | 56.2% | ||
| Freshwater swimming | 277 | 31.3% | ||
| River mouth surfing | 67 | 7.6% | ||
| Contact with animals | Cats | 292 | 35.0% | 521 (38.4) |
| Dogs | 466 | 55.8% | ||
| Rats | 545 | 65.3% | ||
| High-risk facilities | Dump in vicinity | 126 | 38.9% | 1032 (76.1) |
| Water abstraction | 48 | 14.8% |
Discussion
The worldwide AIR of leptospirosis is about 15 cases per 100 000 PY; the highest incidence is reported in the Oceania region, with 150 cases per 100 000 PY and a mortality rate of 9.6 per 100 000 PY. In this region, AIR of up to 844 cases per 100 000 PY over an 11-year period from 2004 to 2014 has been reported in Futuna [24], with a peak of 1945 cases per 100 000 PY in 2008 [4]. The AIR of leptospirosis in FP between 2007 and 2017 was 46.0 (95% CI, 43.6–48.5) cases per 100 000 PY, in the lower range of AIR reported in the region (range, 40–270) [13]. This incidence is low compared to other Pacific islands, probably because, unlike FP, most of them are low- and middle-income countries. However, the AIR of leptospirosis in FP is most probably underestimated, especially in the most remote islands (Marquesas, Tuamotu Gambier and Austral). The main factors responsible for underestimation of leptospirosis in FP are misdiagnosis with other diseases, especially dengue, a high number of patients with few symptoms and geographic constraint (isolation, multiplicity of islands, lack of medical personnel to carry out sampling, difficulties in accessing healthcare facilities, lack of transport or communication within and between islands) [25]. Similar clinical symptoms between leptospirosis and arboviral infection is a main issue during arboviral outbreaks, leading to failure to detect leptospirosis cases and delayed treatment, which may potentially be responsible for fatal cases [26].
Over the period 2007–2012, we noticed a low variation in AIR. A peak of incidence was recorded in 2010. At the end of January 2010, cyclonic depressions (Hurricane Oli) caused heavy rainfall and mudslides in many FP islands, especially Tahiti [22]. These exceptional climatic conditions led to an increase in the number of cases in the first quarter of 2010. The population was quickly made aware by the local health authority of the probable increase in the risk of leptospirosis. Health professionals were encouraged to collect blood samples of patients with acute fever and diffuse pain syndromes. From 2013, the AIR increased as a result of an increase in screening linked to an arbovirus’s epidemic context. Dengue and Zika epidemics occurred simultaneously between September 2013 and March 2014, followed by a major chikungunya epidemic between October 2014 and March 2015 [27], [28]. These three arboviruses are the main differential diagnoses of leptospirosis in endemic areas [11], [26]. Concomitantly, the development of affordable molecular diagnosis in FP increased the number of tests and then the number of diagnosed cases. The significant increase of AIR in 2017 is mainly related to the heavy rains and floods at the beginning of the year [29].
Leptospirosis is known to be seasonal and is strongly associated with rainfall in tropical settings [9]. The number of cases increases during the hot and rainy season and decreases during the cold and dry season [30], [31]. Heavy rains and floods are associated with a sixfold increased risk of developing leptospirosis and are a direct cause of epidemics, as for the year 2017 in our cohort [30]. In FP, rainfall usually increases from December with a peak in January, then decreases until April. In our cohort, 60% of cases occurred during the first 5 months of the year; cases increased from January with a peak in March and remained high until June. Thus, the recrudescence of reported cases of leptospirosis seems to be delayed by 2 months compared to peak rainfall. This phenomenon had already been observed in Futuna and Reunion Island [24], [32]. In two studies in Mayotte, cases followed the evolution of rainfall with a 3-month delay. The importance of seasonal peaks has been found to be more related to the number of consecutive months with heavy rains than to the total rainfall [33], [34]. The discrepancy observed between the incidence of leptospirosis and peak rainfall is explained by the soaking of the soil after the peak rainfall—conditions that favour the survival of leptospires in the environment [32], [34].
We have found a geographical disparity in the incidence of leptospirosis in FP. AIR were higher in high islands: Leeward Islands, Windward Islands and Marquesas. In contrast, the lower islands (atolls) of Tuamotu-Gambier were almost free of cases because of their geomorphology: coral atolls without relief and very little freshwater standing on the surface, no river or sludge because of the porous soils and low rainfall. In the high islands, the development of agricultural and industrial operations and the higher population density create waste and activities that may attract rodents and thus increase the risk of contamination [30], [31]. In the Society archipelago, AIRs are higher in some Windward Islands and in the southeast part of Tahiti, which are agricultural regions and where the main pig, cattle, horse and poultry farms are located.
Most cases occurred in male subjects, in accordance with what is described elsewhere. It probably only reflects higher exposure of the male population through agricultural, farming and outdoor leisure activities. The highest AIR was in the 20- to 29-year-old age group. Like the sex ratio, a high number of cases in the younger age classes possibly reflects the frequent exposure of active young men involved in agriculture and farming activities. However, the hypothesis that older classes are relatively protected by the immunity resulting from previous exposures also deserves consideration.
The main risk factor for leptospirosis in FP is professional or private agricultural activity; prevention measures should therefore target this population. Secondary risk factors are mostly recreational, with freshwater contact during leisure time (barefoot walking in mud, river bathing, hiking, gardening or fishing). Other factors such as contact with rats, pigs and dogs (there are large populations of stray dogs), living in poor sanitary conditions or living in family farms in semiurban areas near watercourses can contribute to the transmission of the disease [4], [19].
Conclusion
Leptospirosis is a major public health concern in FP, where it will become soon a reportable disease. All laboratories and medical facilities, including intensive care units, are available to diagnose cases and care for patients. Prevention includes guidelines for healthcare workers, prevention measures for the general population broadcast by once every 2 months bulletin and a reminder of the risk sent to the media after each heavy rainfall or flooding. In addition, following the World Health Organization One Health approach, collaboration between animal and human health practitioners has been initiated to fight against zoonoses such as leptospirosis. Enhancement of rodent control by improving waste management is needed. Despite all the preventive measures taken, leptospirosis remains highly endemic in FP, thus highlighting the need to maintain leptospirosis surveillance, medical staff training and provision of information to the general population.
Conflict of Interest
None declared.
References
- 1.Haake D.A., Levett P.N. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65. doi: 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torgerson P.R., Hagan J.E., Costa F., Calcagno J., Kane M., Martinez-Silveira M.S. Global burden of leptospirosis: estimated in terms of disability adjusted life years. PLoS Negl Trop Dis. 2015;9:e0004122. doi: 10.1371/journal.pntd.0004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Leptospirosis Environmental Action Network (GLEAN) Mission. https://sites.google.com/site/gleanlepto/website-builder [DOI] [PMC free article] [PubMed]
- 4.Guernier V., Goarant C., Benschop J., Lau C.L. A systematic review of human and animal leptospirosis in the Pacific Islands reveals pathogen and reservoir diversity. PLoS Negl Trop Dis. 2018;12:e0006503. doi: 10.1371/journal.pntd.0006503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kline K., McCarthy J.S., Pearson M., Loukas A., Hotez P.J. Neglected tropical diseases of Oceania: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis. 2013;7:e1755. doi: 10.1371/journal.pntd.0001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Victoriano A.F.B., Smythe L.D., Gloriani-Barzaga N., Cavinta L.L., Kasai T., Limpakarnjanarat K. Leptospirosis in the Asia Pacific region. BMC Infect Dis. 2009;9:147. doi: 10.1186/1471-2334-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler B., de la Peña Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010;140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Cerqueira G.M., Picardeau M. A century of Leptospira strain typing. Infect Genet Evol. 2009;9:760–768. doi: 10.1016/j.meegid.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Levett P.N. Leptospirosis. Clin Microbiol Rev. 2001;14:296. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . World Health Organization; Geneva: 2003. Human leptospirosis: guidance for diagnosis, surveillance and control. [Google Scholar]
- 11.Bharti A.R., Nally J.E., Ricaldi J.N., Matthias M.A., Diaz M.M., Lovett M.A. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . 2018. Managing epidemics.http://www.who.int/emergencies/diseases/managing-epidemics/en/ [Google Scholar]
- 13.Costa F., Hagan J.E., Calcagno J., Kane M., Torgerson P., Martinez-Silveira M.S. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9:e0003898. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed A., Grobusch M.P., Klatser P.R., Hartskeerl R.A. Molecular approaches in the detection and characterization of Leptospira. J Bacteriol Parasitol. 2012;3:133. [Google Scholar]
- 15.Lau C. Combating infectious diseases in the Pacific Islands: sentinel surveillance, environmental health, and geospatial tools. Rev Environ Health. 2014;29:113–117. doi: 10.1515/reveh-2014-0028. [DOI] [PubMed] [Google Scholar]
- 16.Musso D., La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. 2013;46:245–252. doi: 10.1016/j.jmii.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Leptospirosis Burden Epidemiology Reference Group (LERG) 2010. http://www.who.int/zoonoses/diseases/lerg/en/ Available at:
- 18.Décret du n° 2017-1681 du 13 décembre 2017 authentifiant les résultats du recensement de la population 2017 de Polynésie française. 2017. [Google Scholar]
- 19.Guernier V., Richard V., Nhan T., Rouault E., Tessier A., Musso D. Leptospira diversity in animals and humans in Tahiti, French Polynesia. PLoS Negl Trop Dis. 2017;11:e0005676. doi: 10.1371/journal.pntd.0005676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heuls J. Leptospirosis caused by Leptospira icterohemorrhagiae in Tahiti. Bull Soc Pathol Exot Filiales. 1959;52:22–26. [PubMed] [Google Scholar]
- 21.Berry A., Mallet H.P., Pescheux J. Situation épidémiologique de la leptospirose en Pf (2006–2012) Bull Inform Sanit Epidemiol. 2013:4–6. [Google Scholar]
- 22.Daudens E., Frogier E., Mallet H.P. Recrudescence of leptospirosis in French Polynesia in early 2010. Inform’Action 2010:3–6. http://phs.spc.int/ENGLISH/Publications/InformACTION/IA32/Leptospirosis_2010_French_Polynesia.pdf Available at:
- 23.Hirschauer C. Épidémiologie de la leptospirose en Polynésie française de 2006 à 2008. Bull Epidemiol Hebd. 2009;48–50:4. [Google Scholar]
- 24.Massenet D., Yvon J.-F., Couteaux C., Goarant C. An unprecedented high incidence of leptospirosis in Futuna, South Pacific, 2004–2014, evidenced by retrospective analysis of surveillance data. PLoS One. 2015;10:e0142063. doi: 10.1371/journal.pone.0142063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coudert C., Beau F., Berlioz-Arthaud A., Melix G., Devaud F., Boyeau E. Human leptospirosis in French Polynesia. Epidemiological, clinical and bacteriological features. Med Trop Rev Corps Sante Colon. 2007;67:137–144. [PubMed] [Google Scholar]
- 26.Nhan T.X., Bonnieux E., Rovery C., De Pina J.J., Musso D. Fatal leptospirosis and chikungunya co-infection: do not forget leptospirosis during chikungunya outbreaks. IDCases. 2016;5:12–14. doi: 10.1016/j.idcr.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aubry M., Teissier A., Huart M., Merceron S., Vanhomwegen J., Roche C. Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg Infect Dis. 2017;23:669–672. doi: 10.3201/eid2304.161549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao-Lormeau V.M., Roche C., Teissier A., Robin E., Berry A.L., Mallet H.P. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis. 2014;20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bureau de Veille Sanitaire . 2017. La leptospirose en Polynésie française Rapport annuel.https://www.service-public.pf/dsp/wp-content/uploads/sites/12/2018/03/Rapport-leptospirose-2017_DirectionSantePolynésieFrançaise.pdf Available at: [Google Scholar]
- 30.Mwachui M.A., Crump L., Hartskeerl R., Zinsstag J., Hattendorf J. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9:e0003843. doi: 10.1371/journal.pntd.0003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau C.L., Watson C.H., Lowry J.H., David M.C., Craig S.B., Wynwood S.J. Human leptospirosis infection in Fiji: an eco-epidemiological approach to identifying risk factors and environmental drivers for transmission. PLoS Negl Trop Dis. 2016;10:e0004405. doi: 10.1371/journal.pntd.0004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desvars A., Jégo S., Chiroleu F., Bourhy P., Cardinale E., Michault A. Seasonality of human leptospirosis in Reunion Island (Indian Ocean) and its association with meteorological data. PLoS One. 2011;6:e20377. doi: 10.1371/journal.pone.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lernout T., Bourhy P., Achirafi A., Giry C., Picardeau M. Epidemiology of human leptospirosis in Mayotte: an emerging public health problem on the island? Arch Inst Pasteur Madag. 2013;70(1):1–6. [Google Scholar]
- 34.Pagès F., Collet L., Margueron T., Achirafi A., Bourhy P., Picardeau M. Leptospirose à Mayotte: apports de la surveillance épidémiologique, 2008–2015. Bull Epidemiol Hebd. 2017;8-9:147–156. http://opac.invs.sante.fr/doc_num.php?explnum_id=10725 Available at: [Google Scholar]