Abstract
The increasing incidence of pediatric inflammatory bowel disease, coupled with the efficiency of whole-exome sequencing, has led to the identification of tetratricopeptide repeat domain 7A (TTC7A) as a steward of intestinal health. TTC7A deficiency is an autosomal-recessively inherited disease. In the 5 years since the original description, more than 50 patients with more than 20 distinct disease-causing TTC7A mutations have been identified. Patients show heterogenous intestinal and immunologic disease manifestations, including but not limited to multiple intestinal atresias, very early onset inflammatory bowel disease, loss of intestinal architecture, apoptotic enterocolitis, combined immunodeficiency, and various extraintestinal features related to the skin and/or hair. The focus of this review is to highlight trends in patient phenotypes and to consolidate functional data related to the role of TTC7A in maintaining intestinal homeostasis. TTC7A deficiency is fatal in approximately two thirds of patients, and, as more patients continue to be discovered, elucidating the comprehensive role of TTC7A could show druggable targets that may benefit the growing cohort of individuals suffering from inflammatory bowel disease.
Keywords: Inflammatory Bowel Disease, Monogenic, Very Early Onset IBD, Genetics, Whole-Exome Sequencing, Multiple Intestinal Atresia, PI4K, Primary Immunodeficiency
Abbreviations used in this paper: CID, combined immunodeficiency; EFR3, Protein EFR3 (EFR3); HSCT, hematopoietic stem cell transplantation; MIA, multiple intestinal atresia; P4KIIIalpha, phosphatidylinositol 4-kinase III alpha; PI4P, phosphatidyl inositol 4 phosphate; PIP, phosphatidyl inositol–phosphate lipid; ROCK, RhoA kinase; TPR, tetratricopeptide repeat; TTC7A, tetratricopeptide repeat domain 7A; TTC7B, tetratricopeptide repeat domain 7B
Graphical abstract
Summary.
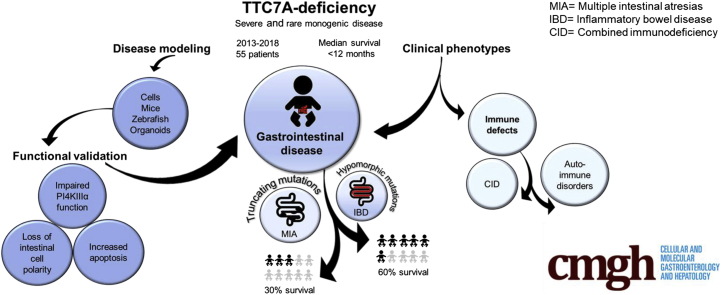
This review discusses the progress and challenges in tetratricopeptide repeat domain 7A deficiency, a monogenic cause of severe intestinal disease and immunodeficiency. Patient reports and functional studies to date are amalgamated into graphic summaries, highlighting patient mutations and the known role of tetratricopeptide repeat domain 7A in the cell.
Rare autosomal-recessive variants in tetratricopeptide repeat domain 7A (TTC7A) have been discovered in patients presenting with severe intestinal and immune diseases.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Mounting evidence has indicated that TTC7A mutations can result in various phenotypes that may or may not be associated with combined immunodeficiency (CID), including multiple intestinal atresias (MIA) and very early onset inflammatory bowel disease (VEOIBD), a form of IBD in children younger than 6 years of age.3, 5, 13 Since 2013, there have been more than 50 TTC7A cases reported in the literature, and, unfortunately, more than half of these children died of the disease, with a median survival age of younger than 12 months.10 Hematopoietic stem cell transplantation (HSCT) has been reported to recover immunologic defects; however, the intestinal disease was refractory to HSCT.10, 11 Multiple mutations in the TTC7A gene, combined with heterogenous disease presentation, has made it difficult to correlate phenotype and genotype.11 TTC7A has 9 tetratricopeptide repeat (TPR) domains, a structural motif that is involved in multiprotein scaffolding.17 Previous studies have confirmed that TTC7A acts as a scaffolding protein, where it binds and recruits phosphatidyl inositol 4 phosphatidylinositol 4-kinase III alpha to the plasma membrane, facilitating the synthesis of PI4-phosphate (PI4P).3, 18, 19 There is evidence to suggest that patients with immunodeficiency and homozygosity for mutations affecting the scaffolding TPR domains have a worse prognosis.11 Furthermore, TTC7A mutations that are predicted to result in significant protein truncation and loss of function tend to result in more severe intestinal phenotypes (ie, MIA).1, 2, 3, 5, 7, 9, 13, 14 Alternatively, nontruncating mutations, termed hypomorphic variants, tend to present as VEOIBD.1, 2, 3, 6, 8, 11, 13 Establishing an appropriate animal model to study TTC7A deficiency has been challenging, however, patient-derived intestinal organoids show defects in intestinal epithelial cell polarity.5, 6, 20 Ultimately, the comprehensive structure, interactions, targets, and pathobiology of TTC7A remain to be elucidated, however, the focus of this review is to highlight the work that is being performed to understand TTC7A deficiency, from patient genetics to functional in vitro studies. Presently, as more patients are being discovered, there is a great need to unearth more about TTC7A so that target-specific therapies may be developed.
TTC7A Biology
A Conserved TPR-Domain–Containing Protein
TTC7A is a conserved gene and has homologs in the chimpanzee, mouse, chicken, zebrafish, and frog.21 RNA expression of TTC7A is comparable in many tissues, making it unclear why patients with TTC7A mutations have phenotypes specifically related to gut and immune dysfunction.22 Although there are 2 splice isoforms, the most common TTC7A splice variant has 20 exons and is 858 amino acids long.22 TTC7A has a paralog, tetratricopeptide repeat domain 7B (TTC7B) with 49.47% sequence identity, and both (independently) are involved in the conserved PI4KIIIα complex.18, 19, 23, 24 The early onset of TTC7A deficiency symptoms may hint at differences in the expression of TTC7A and TTC7B during embryonic development. During development, TTC7B is expressed in the cerebral cortex and cerebellum, whereas TTC7A is expressed in a wide range of tissues/organs, including several tissues of the brain, bone marrow, testis, pancreas, ovaries, liver, and blood.25, 26 In adults, TTC7B is expressed ubiquitously in the body, with higher expression in the brain, fat, and small bowel. TTC7B may have some redundancy with TTC7A because the small bowel is less affected in TTC7A patients.3, 6, 24
TTC7 proteins contain TPR domains, which are helix-turn-helix structurally conserved motifs, thought to have roles in protein scaffolding.21 The highly conserved secondary and tertiary structures of TPR domains have large surface areas that accommodate multiple-protein interactions.17, 27 Although the complete structure of TTC7A has yet to be elucidated, Baskin et al19 resolved the crystal structure of TTC7B and confirmed the presence of large concave and convex surfaces formed by multiple TPR domains. TTC7B has a very large concave interface (approximately 600 nm, where anything upward of 200 nm is thought to be large) that can facilitate binding with PI4KIIIα, FAM126A, and EFR3, members of the PI4KIIIα complex.19, 24 TTC7A mutations affecting TPR domains tend to result in worse patient phenotypes and poor outcomes, implying that TPR-domain structure is critical to TTC7A’s scaffolding function.11 Interestingly, trichohepatoenteric syndrome, a severe condition defined by intractable infant diarrhea and immune abnormalities, is caused by defects in TTC37, another TPR-domain–containing protein.13, 28 Similar to TTC7A, little is known of the protein encoded by TTC37 and how mutations in both of these TPR-domain–containing proteins contribute to immune and gut dysfunction. Resolving TTC7A’s comprehensive structure may further our understanding of how TPR domains contribute to the protein’s molecular biology in the cell.
A Scaffolding Protein for the PI4KIIIα Complex
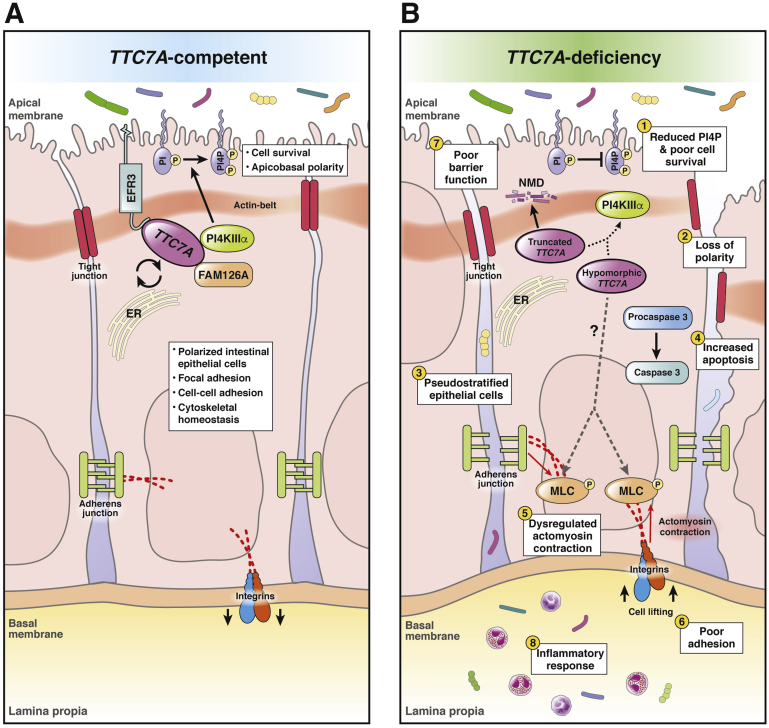
Ten years ago, Baird et al29 identified the yeast ortholog of TTC7A (Ypp1) and Efr3 as novel components of the PI4KIIIα (Stt4) complex. Avitzur et al3 subsequently showed that PI4KIIIα is a specific TTC7A binding partner in mammalian cells via co-immunoprecipitation experiments. Tandem mass spectrometry showed that spectral hit levels of PI4KIIIα are reduced significantly in TTC7A hypomorphic (having partial function) mutants, relative to hit counts with wild-type TTC7A.3 Several studies have corroborated the role of TTC7 in recruiting and targeting PI4KIIIα to the plasma membrane.18, 19, 24 The TTC7A–PI4K complex is stabilized by an adaptor protein, FAM126A, and once at the plasma membrane, tethered by EFR3B, a membrane-bound protein (Figure 1).18 TTC7A is localized diffusely in the cytosol, however, when co-expressed with EFR3, it localizes to the plasma membrane, further indicating a scaffolding role for PI4KIIIα to Protein EFR3 (EFR3).18 Formation of the PI4KIIIα complex results in the phosphorylation of PI membrane lipids to form PI4P (Figure 1), which indicates that TTC7A has an upstream role in mediating PI4P synthesis.18, 19, 24 The effect of TTC7A on PI4P levels has been shown via TTC7A knockdown experiments that show a decrease in the immunostaining for PI4P membrane lipids.3 Plasma membrane PI4P synthesis is dependent exclusively on the proper functioning of PI4KIIIα, and homeostatic levels of PI4Ps are important for plasma membrane identity, polarity, cell survival, and production of phosphatidylinositol 4,5-bisphosphate (PI[4,5]P2)/phosphatidylinositol 3,4,5-trisphosphate (PI[3,4,5]P3].30 In mice, transient loss of PI4KIIIα in fibroblasts results in cell death, and germline loss results in embryonic lethality, indicating that PI4KIIIα has a critical role in survival pathways.18 The link between PI4KIIIα, intestinal integrity, and overall viability was shown elegantly with mice harboring an inducible PI4KIIIα knockout mutation that caused fatality and gastrointestinal tract lesions, similar to TTC7A-deficiency patient defects.31 Specifically, the conditional knockout of PI4KIIIα in mice resulted in gastrointestinal distension (owing to the loss of intestinal emptying), necrosis, and extensive mucosal destruction, while other tissues (ie, brain, heart, and lungs) were unaffected, indicating an essential role for PI4KIIIα in intestinal health.31
Figure 1.
Summary of the role of TTC7A in intestinal epithelial cells and the consequences of its mutation. (A) TTC7A competent. TTC7A and FAM126A chaperone PI4KIIIα to the plasma membrane.19 EFR3 tethers the PI4KIIIα complex to the plasma membrane where PI4KIIIα catalyzes the conversion of PI to PI4P.18, 19, 24 The synthesis of PI4P is an important regulatory step in the phosphatidyl inositol–phosphate pathway, which is important for survival pathways and intestinal epithelial cell polarity.30 Phosphatidyl inositol–phosphate lipids are differentially produced and turned over on apical and basal membranes of polarized intestinal epithelial cells; thus, phosphatidyl inositol–phosphate levels aid in coordinating apicobasal polarity, which is critical for epithelium integrity.75 Cytoskeletal homeostasis is important in maintaining cell polarity and cell adhesions.5, 35 Tight junctions, adherens junctions, and integrins are regulated in part by actin dynamics and maintain cell adhesions, contributing to intestinal epithelial barrier function.36, 76 (B) TTC7A deficiency. Truncating mutations (ie, nonsense, frameshifts, large deletions) are associated with more severe phenotypes and are predicted to cause a complete loss of function in which TTC7A transcripts are thought to be degraded via nonsense-mediated decay.2, 3, 6, 7, 24 Because TTC7A is a scaffold for multiple proteins, its structure is critical to its role in binding, shuttling, and tethering the PI4KIIIα complex to the plasma membrane; thus, partial or total loss of TTC7A reduces PI4P synthesis (1) via the PI4KIIIα complex (dottedarrow).3, 19 Alterations to the structure of TTC7A (ie, truncated or hypomorphic protein) have been shown to alter the kinase activity and/or the positioning of PI4KIIIα at the membrane.24 Decreases in PI4P affects cell polarity (2), signaling, homeostasis, and survival.30 H&E staining of intestinal biopsy specimens from TTC7A-deficiency patients showed pseudostratification, which points to defects in apicobasal polarity (3). Similarly, tightly regulated apical and basal membranes, marked by the actin belt and integrins, respectively, were mislocalized in TTC7A patient-derived organoids.5, 6 Loss of apicobasal polarity jeopardizes the integrity of the epithelium, contributing to the diverse epithelial defects observed in TTC7A deficiency.5, 6 (4) An increase in intestinal epithelial cell apoptosis, activated via caspase cleavage, was a common feature in patient histology and in vitro functional studies.3, 5, 6, 8 TTC7A deficiency, through unknown mechanisms (dashed arrow), is correlated with increased phosphorylation of myosin light chain (5) and numerous other downstream targets in the RhoA pathway (omitted for simplicity).5, 6 Myosin light-chain phosphorylation initiates actomyosin contraction, which can disturb junction and integrin adhesion proteins and promote cell lifting (6).35, 36, 77, 78 Increases in intestinal epithelial cell apoptosis, actomyosin contraction, and cell lifting disrupts epithelial barrier function (7).54, 56 Furthermore, a weakened intestinal barrier results in the translocation of luminal bacteria into the lamina propria, which can trigger an inflammatory response (8).34, 76 The role of TTC7A in the cell is incompletely understood, making it difficult to link its known functions to the development of MIA, another poorly understood disease.
Cryo-electron microscopy recently showed that the PI4KIIIα complex can dimerize to form a large and stable “superassembly.”24 Two PI4KIIIα enzymes form a homodimer, in which the dimerization interface is held at 2 contact positions by TTC7, suggesting that TTC7 plays an important role in stabilizing the large PI4KIIIα–heterodimer complex.24 Co-immunoprecipitation experiments have shown that the formation of the PI4KIIIα complex is possible with hypomorphic TTC7A variants, however, minor alterations to the TTC7A–PI4KIIIα assembly nonetheless may affect kinase activity.3 Specifically, mutations in the C-terminus of TTC7A and N-terminus of PI4KIIIα result in PI4KIIIα-complex formation, whereas kinase activity is lost.24 The PI4KIIIα complex is conserved from yeast32 to human beings, where PI4KIIIα interaction with TTC7 and FAM126 restricts its conformation so that its kinase domain can interact with PIs on the plasma membrane.24 The activity and localization of the PI4KIIIα complex depends on several contact sites between the FAM126–TTC7A–PI4KIIIα–EFR3B proteins and the plasma membrane, providing grounds for how hypomorphic TTC7A mutations may have critical functional consequences for the tightly regulated PI4KIIIα complex.18, 19, 24
Although the PI4KIIIα complex is responsible for PI4P synthesis at the plasma membrane, it is important to recognize the plasticity of PI4P levels and the dynamic nature of phosphatidyl inositol–phosphate lipids (PIPs) throughout the cell.30 Despite insults to the PI4KIIIα complex, PI4Ps still can be present at the plasma membrane owing to several compensatory pathways, including but not limited to: other subcellular membranes with PI4Ps can fuse with the plasma membrane, TMEM150A (a membrane-bound protein) stabilizes PI4KIIIα at the membrane in the absence of TTC7A, TTC7B redundancy, and other PIP kinases and phosphatases potentially replenishing pools of various plasma membrane PIPs.18, 19, 24, 30 PI4P synthesis represents a critical regulatory threshold in the plasma membrane PI pathway and an important mediator of cellular homeostasis.30 PI4P and its role as a precursor in the PI pathway is partly dependent on a functional TTC7A, which is involved in tethering, shuttling, and stabilizing the conserved PI4KIIIα complex. A thorough understanding of the pathobiology related to PI4P levels in TTC7A deficiency has yet to be elucidated.
Increased Apoptosis and Loss of Polarity: Phenotypic Insights From Functional Studies
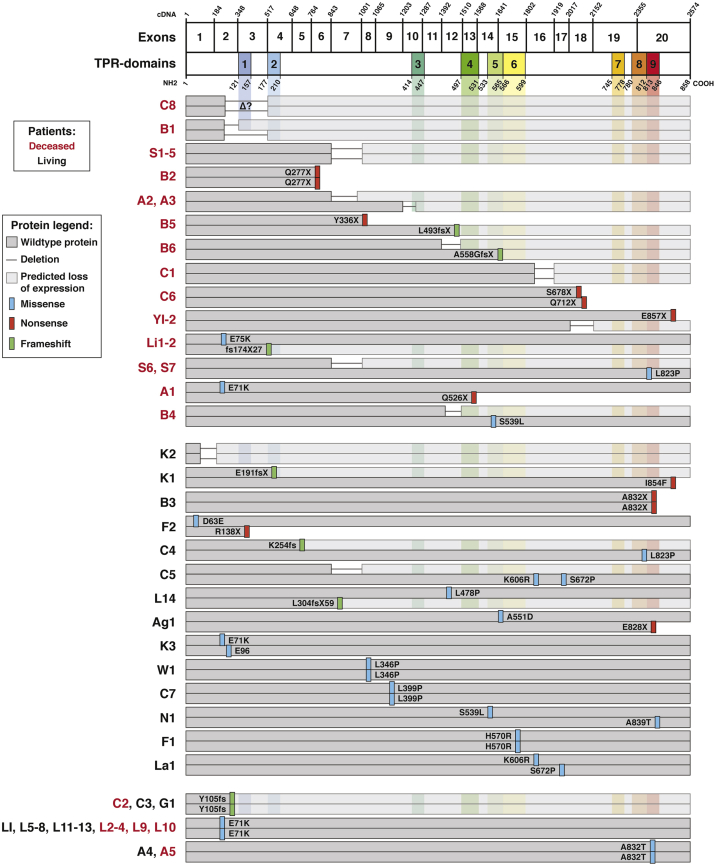
Immunohistochemical staining of healthy intestinal biopsy specimens has confirmed that TTC7A is expressed in intestinal cells and localizes intracellularly at the plasma membrane.3 Staining of patient biopsy samples with truncating TTC7A mutations show a total loss of protein, suggesting nonsense-mediated decay of TTC7A messenger RNA transcripts.1, 3 The absence of TTC7A in immunohistochemical staining also is consistent with the increased phenotypic severity in patients with MIA-CID, further associating protein truncations with complete loss of function (Figure 2).1, 2, 3, 4, 5, 7, 9, 10, 11
Figure 2.
Known TTC7A mutations grouped by patient outcomes. Patient naming codes are derived from Table 1 and are shown in red or black to indicate deceased or living patients, respectively. Within patient outcome groupings (ie, deceased, living, or mixed), patients are arranged so that deletion, frameshift, or nonsense mutations affecting both alleles are listed first, followed by patients with missense mutations on at least 1 allele. TTC7A has 20 exons that include 9 TPR domains that are represented by the complementary DNA and protein schematics, respectively (NM_020458.3 and NP_065191.2). Unaffected protein sequences are indicated in dark grey, while grey connecting lines indicate exon deletions. Light grey stretches are downstream of frameshift and deletion mutations and represent areas where there is a predicted loss of protein expression resulting from nonsense-mediated decay mechanisms.2, 3, 6, 7, 24 Specific amino acid mutations are indicated by color bars: blue, missense; red, nonsense (ie, stop codon or X); green, frameshifts. Patient M1 is not represented because of a lack of specifics regarding their deletion mutation. ER, endoplasmic reticulum; NMD, nonsense mediated decay.
TTC7A knockdown in intestinal Henle-407 cells with short hairpin RNA disrupts their characteristic cobblestone morphology and impairs adhesion to collagen/fibronectin.3 These features are in line with endoscopic observations from patients showing loss of epithelial integrity and mucosal sloughing.3, 5, 6, 11 TTC7A deficiency also results in increased caspase 3 cleavage, which is a cysteine–aspartic acid protease that is important in mediating apoptosis once cleaved.3, 5 The findings show that TTC7A deficiency results in increased susceptibility to apoptosis, a phenotype consistent with patient histology showing higher intestinal epithelial cell apoptosis.3, 5, 6, 8
The polarized monolayer of intestinal epithelial cells is an elegant example of form and function because it creates a barrier that protects against inappropriate immune responses and facilitates nutrient absorption. In TTC7A deficiency, stenosis can arise from alterations to the integrity of the intestinal epithelium.33, 34 Intestinal epithelial cell polarity, for instance, normally restricts epithelial cell growth to a monolayer, however, when highly organized epithelial cells lose an aspect of their polarity, the epithelium may become stratified or pseudostratified, characteristics associated with intestinal pathologies.33, 34 Bigorgne et al5 showed that TTC7A dysfunction results in aberrant intestinal epithelial cell polarity. Ileum-derived intestinal organoids (or enteroids) were cultured from a patient with homozygous p. A832fsX1 mutations that disrupt the final exon, however, it is unknown whether TTC7A is hypomorphic, or absent, because no expression levels were provided.5 The organoid donor was the only surviving member of the study cohort and presented with intestinal atresias from birth, lymphocytopenia, hypogammaglobulinemia, and progressive skin conditions from 4 years of age.5 The organoids were stained for intestinal epithelial cell polarity markers, filamentous actin and α6 integrin, which designate apical and basal membranes, respectively.5 The TTC7A patient-derived organoids showed several abnormalities: filamentous actin staining on the basal membrane, an ill-defined luminal space, a disorganized pattern of α6 integrin, significantly reduced proliferation, dense aggregates with no obvious luminal space, and reduced budding.5 Taken together, TTC7A-deficient organoids showed disrupted apicobasal polarity and poor epithelial cell integrity.
Remarkably, exposure of the TTC7A patient organoids to a RhoA kinase (ROCK) inhibitor (10 μmol/L Y-27632 for 5 days) corrected the polarity defect and resulted in increased proliferation.5 Apicobasal polarity is partly regulated by the RhoA pathway via cytoskeletal proteins, where downstream effectors (such as myosin light chain and ezrin–radixin–moesin) are important in filamentous actin dynamics, stress fiber contractility, and adhesion (ie, tight junctions, integrins).5, 35 Higher protein expression of the downstream targets of ROCK were uncovered in these organoids, and subsequently reduced with ROCK-inhibitor treatment.5 Thus, the data infer that TTC7A, through an unknown mechanism, acts to regulate cytoskeletal dynamics via myosin light-chain activation (Figure 1). Alternatively, ROCK inhibition may be acting on a TTC7A-independent pathway. It is intriguing to suggest that ROCK activity may mediate intestinal epithelial cell polarity, although the exact mechanism by which TTC7A is involved in the RhoA/ROCK pathway is unknown.35, 36
In contrast to the intestinal epithelial cell phenotypes associated with TTC7A dysfunction, Lemoine et al6 found that lymphocytes lacking TTC7A have increased proliferation, adhesion, and migration, all of which were reduced with ROCK-inhibitor treatment. The data suggest that TTC7A also regulates the actin cytoskeleton in lymphocytes. Increases in the activation of effectors downstream of ROCK in lymphocytes and intestinal epithelial cells seem to disrupt normal cell homeostasis and epithelial-immune dynamics contributing to inflammation.6, 37 Elucidating the cell-specific nuances of TTC7A deficiency may provide insight into how therapies could have differential effects on epithelial and immune cells.
Underlying Genetics of TTC7A Deficiency
The role of genetics in the development of IBD has been well established via genome-wide association studies, which have identified more than 200 IBD susceptibility loci contributing to polygenic or typical IBD.38 Genome-wide association studies, a statistically based genetic analysis, is blind to rare variants, especially those that cause severe disease and increased mortality, which further dilutes these rare alleles from the population’s gene pool.39, 40 In contrast, whole-exome sequencing often is used for diagnosis when single genetic mutations are suspected to be causative. For example, the majority of known rare diseases are attributed to defects in a single gene, as opposed to being driven by multiple variants or an unknown etiology.41 Similarly, more than 50 genes have been uncovered that ascribe monogenic IBD-like phenotypes.42 Of those genes, single variants in TTC7A are shown to cause intestinal and immune disease with high penetrance.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 16 Recently, Lien et al11 performed TTC7A genotype/phenotype comparisons via Kaplan–Meier analysis. They showed that patients with autoimmune defects and biallelic missense mutations (not affecting TPR domains) had better survival outcomes.11 Ultimately, it remains difficult to correlate genotype with phenotype because there are more than 20 TTC7A mutations linked with heterogenous disease features (Table 1), which, in turn, are associated with varying morbidities and mortalities.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 16
Table 1.
Mutations, Clinical Phenotypes, and Outcomes of Reported TTC7A-Deficiency Patients
| Patient | Cited mutationsa | TPR Δ | Ethnicity | MIA | IBD | CID | Alive/deceased, ageb; cause |
|---|---|---|---|---|---|---|---|
| S1-51 | Hom c.1000+ 3del AAGT, Ex7 del | + | French Canadian | + | Deceased, <7 days; MIA | ||
| S61 | Het c.1000+ 3del AAGT, Ex7 del; c. 133074 A>G Ex20; p.L823P | + | French Canadian | + | Deceased, 47 days; MIA | ||
| S71 | Het c.1000+ 3del AAGT, Ex7 del; c. 133074 A>G Ex20; p.L823P | + | French Canadian | + | + | + | Deceased, 1 y; MIA |
| C12 | Hom c.1919+1G>A Ex16 del | + | Arabic | + | + | Deceased, 3 mo; Candida sepsis | |
| C22 | Hom c. 313 del TATC, Ex2/Ex3 del p.Y105fs | + | Serbian | + | + | Deceased, 1 mo; MIA | |
| C32 | Hom c. 313 del TATC, Ex2/Ex3 del p.Y105fs | + | Bosnian | + | + | Alive, 2.8 y | |
| C42 | Het c.762 del G Ex5;p.K254fs c.2468T>C Ex20; p.L823P | + | Unknown | + | + | Alive, 2 y | |
| C52 | Het c.1817A>G Ex16, p.K606Rc c.2014 T>C Ex17, p.S672Pc c.1000+ 3del AAGT, Ex7 del |
French Canadian, mixed European | + | + | Alive, 22 mo | ||
| C62 | Het c.2033C>A Ex18; p.S678X; c.2134C>T Ex18; p.Q712X | + | Italian | + | + | + | Deceased, 10 mo; pneumonitis |
| C72 | Hom c.1196T>C Ex9; p.L399P | Italian | + | + | Alive, 9 mo | ||
| C82 | Unknown mutations Ex2 Ex3 del |
+ | Italian | + | + | Deceased, 8 mo; sepsis | |
| A13 | Het c.211G>A Ex2; p.E71K; c.1578C>T Ex14; p.Q526X | + | Caucasian/Sudanese | + | + | + | Deceased, 11 mo; parainfluenza pneumonia, ARDS |
| A23 | Het c.844-1G>T, Ex 7 del; c.1204-2A>G, Ex 10 del | + | Caucasian | + | + | + | Deceased, 3 mo; pulmonary embolism |
| A33 | Het c.844-1G>T, Ex 7 del; c.1204-2A>G, Ex 10 del | + | Caucasian | + | + | Deceased, 19 mo; cardiac arrest | |
| A43 | Hom c.2494 G>A Ex20; p.A832T | + | Caucasian | + | Alive, 14 mo | ||
| A53 | Hom c.2494 G>A Ex20; p.A832T | + | Caucasian | + | Deceased, 11 mo; Candida sepsis | ||
| Ag14 | Het c.1652 C>A Ex15; p.A551D c.2482C>T Ex20; p.E828X | + | Irish/Ashkenazi Jewish | + | + | Alive, 7 mo | |
| B15 | Het c.185-348 del Ex2; p.D62-S116 del c.185-517 del Ex2/3; p.D62-173G del | Mixed European | + | + | Deceased, 2.5 y; sepsis | ||
| B25 | Hom c. 829 C>T; p.Q277X Ex 6 del | Saudi Arabian | + | + | Deceased, 9 mo; pulmonary hemorrhage | ||
| B35 | Hom c.2496 del CG Ex20, p.A832X | + | Sri Lankan | + | + | Alive, 73 mo | |
| B45 | Het c.1288-1392 del Ex 12 del; c.1616C>T Ex 14; p.S539L | Norwegian | + | + | Deceased, 8 mo; sepsis | ||
| B55 | Het c.1008C>G Ex8; p.Y336X c.1479 delG Ex 12; p.L493 fsX13 | Mixed European | + | + | Deceased, 3.9 y; virus pneumonia | ||
| B65 | Het c.1510+105 T>A Ex12 del; c.1673 insG Ex15; p.A558GfsX7 | Mixed European | + | + | Deceased, 7 mo; cytomegalovirus pneumonitis | ||
| L16 | Hom c.211G>A Ex2, p.E71K | French | + | + | Alive, 5 mo | ||
| L26 | Hom c.211G>A Ex2, p.E71K | Deceased, 6 mo; HSCT | |||||
| L36 | Hom c.211G>A Ex2, p.E71K | Deceased, 9 mo; HSCT | |||||
| L46 | Hom c.211G>A Ex2, p.E71K | Deceased, 8 mo; enteropathy | |||||
| L56 | Hom c.211G>A Ex2, p.E71K | Alive, 10 mo | |||||
| L66 | Hom c.211G>A Ex2, p.E71K | Alive, 10 mo | |||||
| L76 | Hom c.211G>A Ex2, p.E71K | Alive, 2 y | |||||
| L86 | Hom c.211G>A Ex2, p.E71K | Alive, 4 y | |||||
| L96 | Hom c.211G>A Ex2, p.E71K | Deceased, 4 y; sepsis | |||||
| L106 | Hom c.211G>A Ex2, p.E71K | Deceased, 14 y; gastric carcinoma | |||||
| L116 | Hom c.211G>A Ex2, p.E71K | Alive, 14 y | |||||
| L126 | Hom c.211G>A Ex2, p.E71K | Alive, 28 y | |||||
| L136 | Hom c.211G>A Ex2, p.E71K | Alive, 50 y | |||||
| L146 | Het c.911T Ex7; p.L304 fsX59; c.1433T>C Ex12, p. L478P | French | + | Alive, 18 mo | |||
| G17 | Hom c. 313 del TATC, Ex2/Ex3 del p.Y105fs | + | Bosnian | + | + | Alive, 3 y | |
| W18 | Hom c.1037 T>C Ex8; p.L346P | Turkish | + | + | Deceased, 15 mo, sepsis | ||
| Y19 | Het c.2018-2 A>G; Ex18 del c.2569 G>T Ex20; p.E857X | + | Malaysian | + | Deceased, 27 mo, sepsis | ||
| Y29 | Het c.2018-2 A>G; Ex18 del c.2569G>T Ex20; p.E857X | + | Malaysian | + | Deceased, 3 days, sepsis | ||
| K110 | Het p.E191FsX; Ex4 p.I854F Ex20 | + | Mediterranean | + | Alive, 77 mo | ||
| K210 | Hom p.G45_A55 del Ex1 | Middle Eastern | + | + | Alive, 86 mo | ||
| K310 | Het p.E71K Ex2 p.E96 Ex2 | British | + | + | Alive, 20 mo | ||
| Li111 | Het c.223 G>A p.E75K Ex2 c. 520-521 del CT fs174X27 Ex4 | + | Taiwanese | + | + | Deceased, 8 mo, sepsis | |
| Li211 | Het c.223 G>A p. E75K Ex 2 c. 520-521 del CT fs174X27 Ex4 | + | Taiwanese | + | + | Deceased, 4 mo, liver failure | |
| La112 | Het c.1817A>G; p.K606R Ex16 c.2014 T>C; p.S672P Ex17 | European | CVID | Alive, 15 y | |||
| N113 | Het c.1616 C>T p. S539L Ex14 c. 2515 G>A p.A839T Ex20 | + + |
Portuguese | + | + | + | Alive, 3 y |
| M114 | Hom c. 53344_53347 del | Unknown Consanguineous |
+ | + | Deceased, 8 mo; graft-versus-host disease, liver failure | ||
| F115 | Hom c.1709A>G; p.H570R Ex15 | + | North African | + | + | + | Alive, 43 mo |
| F215 | Het c. 189C>G; p.D63E Ex2 c. 412 C>T; p.R138X Ex3 | + | Unknown | + | + | Alive, 18 mo |
NOTE. Superscript numbers in Patient column refer to reference citations.
ARDS, acute respiratory distress syndrome; c, complementary DNA location; CVID, common variable immune deficiency; Het, compound heterozygous mutation; Hom, homozygous mutation; p. protein or amino acid location.
National Center for Biotechnology Information reference sequences for TTC7A were absent or varied between publications. Among the 15 reports citing TTC7A-deficiency patients, 63, 5, 6, 8, 9, 15 referenced mutations with NM_020458.3 (transcript variant 2, coding sequence from 369 to 2945 nucleotides) and NP_065191.2 (isoform 2, 858 amino acids). Lien et al11 and Neves et al13 reported mutations based on NM_001288951.1 (transcript variant 1, coding sequence from 369 to 3017 nucleotides) and NP_001275880.1 (isoform 1, 882 amino acids). Samuels et al1 based DNA annotations on NC_000002.11 because one of the mutations affected an intronic region, however, the protein annotation was based on NP_065191.2. To our knowledge, the remaining 62, 4, 7, 10, 12, 14 publications did not report the National Center for Biotechnology Information coding sequence and protein reference codes.
Surviving patient age is as reported in the original publication.
Maternal.
Lessons from Hypomorphic and Loss-of-Function TTC7A Variants
Missense mutations, resulting in the substitution of one amino acid for another, are thought to result in hypomorphic TTC7A variants, oftentimes presenting with clinical features reminiscent of monogenic IBD, or, more specifically, VEOIBD given the age of patients.1, 3, 5, 6, 8, 11, 13, 43 The MIA-CID phenotype is associated with greater morbidity and mortality and tends to be associated with complete loss of function because of truncating TTC7A mutations (ie, nonsense, frameshifts, large deletions).1, 2, 3, 4, 5, 7, 9, 10, 11 For example, a patient compound heterozygous for missense (p. E71K) and truncation (p. Q526X) mutations showed no clinical or pathologic evidence of atresias.3 Patients with mutations affecting splice acceptor sites, resulting in the skipping of exons and premature stop codons, have large peptide stretches missing from TTC7A and present with atresias, severe inflammation, and increased intestinal cell apoptosis.1
Analysis of patient outcomes (Figure 2) loosely correlate poor survival to truncating mutations, which are predicted to result in complete loss of TTC7A function because of transcript degradation via nonsense-mediated decay.2, 3, 6, 7 At odds with the prediction of nonsense-mediated decay, Yang et al9 reported that a 45–amino acid in-frame deletion and a nonsense mutation in the final exon of TTC7A had similar reverse-transcription polymerase chain reaction amplifications to control DNA, suggesting that these transcripts were not subject to nonsense-mediated decay mechanisms. However, no protein expression data were provided for these patients, who ultimately died of their disease.9 Furthermore, the French Canadian patients reported by Samuels et al1 also had a large deletion causing the loss of exon 7 and similarly were found to have “sufficient” RNA levels, although no protein expression was reported. Interestingly, fibroblasts and B cells derived from TTC7A-deficiency patients harboring the same deletion presented in the Samuels et al1 report showed that TTC7A protein expression is undetectable, suggesting nonsense-mediated decay of messenger RNA transcripts.24 As illustrated in Figure 2, all deceased TTC7A patients had at least 1 allele affected by deletion, nonsense, or frameshift mutations. Approximately half of this group had truncating or deleterious mutations on both alleles, providing a rationale for the poor survival outcomes of these patients and suggesting a total loss of TTC7A function.
Despite the previous examples, we are not yet able to draw any hard and fast rules regarding correlations between patient genotypes and phenotypes. TTC7A deficiency seems to result in a range of complex phenotypes not easily attributed to physical alternations on the TTC7A gene. For example, Woutsas et al8 reported a patient with MIA-CID who was homozygous for a substitution mutation at a conserved site (p. L346P). HEK293T cells expressing the p. L346P mutation confirmed that the variant had expression levels that were stable and similar to wild-type TTC7A levels, making this a hypomorphic variant, yet the patient presented with the MIA phenotype.8 These findings highlight the discrepancies between genotype and phenotype in TTC7A patients and indicate that the hypomorphic/truncated dichotomous framework may be incomplete.
To understand the effects of specific TTC7A mutations that contribute to complete loss of protein function, more protein analysis via immunostaining or immunoblotting is required. For example, protein analysis was limited among the 15 publications discussed here: 3 provided TTC7A immunohistochemical staining of the thymus,2 small bowel,12 and cecum,3 and 2 provided immunoblot protein analysis from TTC7A mutant overexpression cell lines.3, 8 To fully appreciate the pathogenicity of specific TTC7A mutations, site-specific and knock-in mutagenesis studies in disease models will aid in understanding the relationship between genetics and disease phenotypes.
Clinical Presentation of TTC7A Deficiency
Advancements in diagnostic genetic screening has uncovered mutations in the TTC7A gene that manifest as severe intestinal disease (the focus of this review) and primary immunodeficiency in pediatric patients.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 16 Young children (age, <2 y) experiencing aggressive and refractory intestinal disease and with a family history of immunologic and/or intestinal disease are candidates for genetic testing and diagnosis.44 Identification of an underlying monogenic cause of disease is typically a collaborative effort involving the patient, their family, clinical teams (ie, endoscopy, biopsy), and laboratory testing (ie, histology, blood processing, candidate gene screening, and identification).42 Although many TTC7A-deficiency reports have identified mutations that are suspected to drive the disease, few studies have performed functional analysis to confirm the phenotype and pathogenicity of specific variants. Within 5 years, there have been 55 TTC7A cases described in 15 reports (Table 1), and, unfortunately, many of these patients died of their disease with a median survival age younger than 12 months.10
MIAs With or Without CID
In 2013, a French Canadian cohort of patients with hereditary MIA, a severe and fatal form of congenital bowel obstruction, were found to have TTC7A deficiency.1 MIA presents as discontinuity of the intestinal lumen caused by obstructions found anywhere along the pylorus to the rectum, and despite surgical resections the obstructions tend to be recurrent.45 Intestinal atresias are estimated to occur in 1 in 5000 newborns, in whom jejunoileal atresias are most prevalent.46 Idiopathic intestinal obstruction and stenosis have been reported in IBD, severe CID, and graft-versus-host disease, conditions involving aberrant inflammatory responses. In particular, obstructions are relatively infrequent in ulcerative colitis, yet are observed in the small bowel (25% of cases) and colon (10% of cases) in patients with Crohn’s disease.46, 47 TTC7A-deficiency patients have been reported to have widespread atresias affecting the pylorus, small bowel, appendix, ileocecal valve, colon, and anal regions.1, 2, 3, 5, 7, 9, 13, 14 In 2 cases in which substantial resections were performed early, a detailed analysis of the resected tissues showed that the lumen was strictured or completely lost, intestinal layers showed fibrosis, calcified fibrotic areas, reduced lymphoid follicle size, and a thickened bowel wall suggestive of chronic intestinal inflammation.15 Furthermore, tissue analysis of resected duodenal atretic areas showed ulcerations in the mucosa, loss of epithelial polarity, mucosal sloughing, apoptotic bodies, and loss of villi architecture, which were commonly reported features in patient histology.15 Along with MIA, patients showed other intestinal defects, including meconium peritonitis, omphalocele, and bowel distension.1, 7, 15
Less than 20% of TTC7A patients presented with MIA alone.11 In the general population, the development of MIA-CID is considered sporadic, where 10%–15% of MIA patients concomitantly present with CID.2, 11, 14 The incidence of MIA accompanying CID is higher, approximately 50%, in TTC7A patients.11, 14 Although no TTC7A-deficient patients were reported to have isolated CID, the immune defect accompanied intestinal disease in more than 75% of cases, pointing toward a crucial role for TTC7A in maintaining intestinal and immune homeostasis.5, 11, 16 Intestinal disease manifestations can present as early as in utero, whereas CID may be difficult to recognize at very early ages. The high incidence of CID in reported cases suggests that rigorous testing must be performed to exclude CID in TTC7A-deficiency confirmed patients. Lymphocytopenia in combination with disruption of the epithelial barrier results in increased risk for pathogenic proliferation and infection, and, consequently, many TTC7A-related fatalities have resulted from sepsis.3, 4, 5, 6, 11, 14 MIA-CID patients were reported to have infections caused by various forms of enteric microbes, including but not limited to: Escherichia coli, Enterococcus fecalis, Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans, and Klebsiella pneumoniae.2, 3, 4, 5, 6, 7, 9, 11, 14, 15, 48 Several groups attributed barrier dysfunction as a possible cause for recurrent infections.2, 7, 11, 15
Patients with CID were found to have a hypoplastic thymus, which is thought to disrupt T-cell maturation and result in lymphocytopenia.3, 5, 6 To that end, Chen et al2 observed that patients with milder T-cell lymphocytopenia had increased thymic function and integrity; furthermore, they hypothesized that CD8+ T-cell lymphocytopenia points to a defect in thymopoiesis, a chief function of the thymus in which thymocytes differentiate to mature T cells. Low blood immunoglobulin levels (ie, IgA, IgM, IgG) also were suggestive of reduced B cells or B-cell functioning.3, 6, 8, 11, 13, 14 In light of the presence of naive B cells, Woutsas et al8 suggested that patients with hypomorphic mutations may have compromised B-cell development owing to increases in the relative levels of transitional B cells. Regulating PI4P levels via scaffolding of the PI4KIIIα complex likely is compromised in patients with hypomorphic forms of TTC7A, which is thought to down-regulate activities in the PIP pathway, and in turn may affect B-cell–receptor assembly and organelle synthesis in activated B cells.8, 49 Ultimately, more research is needed to understand how TTC7A supports lymphocytic immunity and whether CID stems from errors in intrinsic lymphocytic functioning or extrinsic factors influencing lymphocytic progenitors, proliferation, and/or maturation.
It is unclear whether the disease pathogenesis results from abnormalities in embryonic development, the immune system, or the intestinal epithelium. To shed light on what could be driving the disease, 1 study showed that despite lacking lymphocytic immunity, mice with the flaky skin mutation (Ttc7 fsn/fsn) acquire epithelial defects, whereas transplantation of Ttc7fsn/fsn hematopoietic cells alone did not confer disease phenotypes.50 Moreover, Ttc7fsn/fsn lymphocytes intensified epithelial hyperplasia and inflammation.50 Taken together, these results imply that immune defects caused by TTC7A deficiency worsen epithelial disease, but may not be the primary cause.
VEOIBD With or Without CID
Approximately half of the reported TTC7A cases involve symptoms that align with clinical features associated with monogenic IBD or VEOIBD.1, 2, 3, 6, 8, 11, 13 The designation of VEOIBD is given to children younger than age 6 years presenting with combinations of autoimmunity; primary immunodeficiency; lymphoid mucosal infiltration; ileal/general colonic inflammation; histologic features in line with Crohn's disease, ulcerative colitis, or inflammatory bowel disease unclassified; growth delay; penetrating lesions; stricturing; perianal disease; and/or diarrhea.42, 51 Rare variants in TTC7A have been uncovered in the most severe forms of VEOIBD.3, 13, 51 Fewer than 10% of TTC7A-deficiency patients have VEOIBD alone and the majority have chronic intestinal inflammation accompanying CID and/or MIA.11 The severity and extent of gastrointestinal tract inflammation varies between the adult and pediatric IBD subset, in which approximately 43% of children have extensive ileocolonic inflammation compared with 3% in the adult group.40 Furthermore, the younger the children at the age of diagnosis, the greater the proportion (one third) with inflammatory bowel disease unclassified phenotypes, highlighting the diverse phenotypic range of VEOIBD disease features.42 TTC7A-deficiency patients with VEOIBD showed chronic intestinal inflammation, secretory diarrhea from birth, hematochezia, loss of intestinal architecture affecting villi/microvilli, pseudostratified epithelia, intestinal epithelial cell sloughing, friable mucosa, ulcerations, apoptotic enterocolitis, lymphocytopenia, and hypogammaglobulinemia.3, 13, 51 In an attempt to treat the intestinal disease, many TTC7A patients receive total parenteral nutrition;3, 5, 6, 11, 14 however, avoiding enteral feeding alone does not treat the severe intestinal disease.
The histologic and endoscopic analyses of some patients were reported to be reminiscent of intestinal graft-versus-host disease, a destructive pathologic condition associated with apoptotic crypts and resulting from allogenic HSCT.3, 8 In particular, disease features tied to a loss of epithelial barrier function, such as diarrhea, intestinal sloughing, and loss of intestinal architecture, were reported frequently and are present in both IBD and graft-versus-host disease.52, 53 Consequently, it is difficult to differentiate between the source of barrier dysfunction in IBD and graft-versus-host disease. Although IBD and graft-versus-host disease share similarities, several differences exist; for example, bloody and watery diarrhea tend to be associated with IBD and graft-versus-host disease, respectively, and this may serve to explain why many TTC7A-deficiency patients have been classified as having symptoms more in line with monogenic IBD or VEOIBD.52 More patient cases and disease modeling will help to better define the intestinal pathogenicity driving TTC7A deficiency, which may be useful in the identification of effective therapies.
Increased Intestinal Epithelial Cell Apoptosis
Histologic analysis of patient biopsy samples, via H&E staining, shows that increases in intestinal epithelial cell apoptosis is a pathophysiologic feature shared among TTC7A-deficient patients.3, 5, 6, 8 Concomitant with MIA, CID, and IBD, intestinal epithelial cell apoptosis in crypts (ie, gland destruction) and villi are thought to contribute significantly to the enteropathy.3, 8, 14 Apoptotic enterocolitis, used to describe several TTC7A patients, is a feature pathologically similar to necrotizing enterocolitis as well as graft-versus-host disease, both of which are life-threatening conditions resulting in epithelium deterioration.53, 54 It is tempting to surmise that the increase in apoptosis, in contrast to necrosis, points to TTC7A having a role in promoting cell viability or antagonizing apoptotic activation pathways. Tightly regulated apoptosis is fundamental to epithelial cell renewal and epithelium homeostasis, however, increased intestinal epithelial cell apoptosis can disrupt epithelial barriers, allowing translocation of bacteria and antigens into the lamina propria, leading to inflammation and tissue destruction.55, 56
Extraintestinal Manifestations
Extending the phenotypic repertoire associated with TTC7A deficiency, several extraintestinal manifestations related to integumentary hyperplasia (ie, affecting the dermis) and poor hepatic function have been reported.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 16 Although many systems seem to be affected in TTC7A deficiency, immune and intestinal tract defects are the most formidable, hinting that compensatory mechanisms may exist in other specialized cells, reducing the apparent severity of extraintestinal phenotypes. However, data functionally validating extraintestinal disease features are lacking and it remains to be confirmed whether TTC7A deficiency is the single underlying cause for the diverse range of symptoms reported in this cohort. Neves et al13 reported a TTC7A patient presenting with VEOIBD, MIA-CID, and trichohepatoenteric syndrome, a rare genetic condition typically caused by mutations in TTC37. Trichohepatoenteric syndrome is associated with multiple abnormalities of the hair and liver, as well as chronic diarrhea, thyroiditis, hypogammaglobulinemia, ichthyosis (scaly skin), café au lait spots, facial dysmorphia, and enamel erosion of teeth.13 The complexity associated with TTC7A pathophysiology is exemplified further by a large consanguineous family of 13 affected individuals, possessing the same genotype, and presenting with enterocolitis-lymphopenia-alopecia syndrome.6 Although the severity of intestinal phenotypes varied within this family, morbidity typically decreased with age. Patients older than 4 years of age no longer required total parenteral nutrition, however, they became affected by dermatologic features such as epidermal hyperplasia and ichthyosis.6 Extraintestinal disease phenotypes seem to be more abundant in older surviving patients, suggestive of an inverse relationship between intestinal disease severity and extraintestinal manifestations.
Therapies and Outcomes
There is no standard of care for TTC7A deficiency and traditional IBD therapies do little to treat the intestinal disease (ie, MIA and VEOIBD).10, 11 To date, there is no known treatment for MIA and surgical resections do not prevent the formation of new atresias.57 Chronic diarrhea and intestinal epithelial cell apoptosis typically are refractory to immunosuppressives, steroids, and biologics.3, 58 Recently, Fayard et al15 reported 2 unrelated patients with mild MIA, severe and progressive IBD, and severe CID who were treated successfully with early total enterectomy, parenteral nutrition, and prophylaxis immunoglobulin (Privigen, Ottawa, Ontario) and antibiotic (Bactrim, Philadelphia, PA) treatments. Patients with MIA-IBD-CID typically have poor outcomes (mean survival, 24 mo), yet at publication, these patients were ages 48 and 18 months and showing positive clinical responses. Alternatively, a small-bowel–liver transplant restored intestinal function, normal feeding, and improved CD3+ and CD19+ lymphocytes in a 16-month-old patient with MIA-CID; unfortunately, no genetic background was provided for this patient.59 According to reported cases, no TTC7A-deficiency patient has received an intestinal transplant, however, apart from few donors, this intervention is associated with high mortality rates.60
HSCT Does Not Treat the Intestinal Disease in TTC7A Deficiency
Identifying the cause of immune dysfunction can inform clinical action. For example, HSCT can be completely restorative for VEOIBD patients with interleukin 10 defects, whereas the benefit of the same intervention seems to be unclear for TTC7A patients.61 Beyond the benefit of immune reconstitution, it was shown that HSCT potentially could benefit recipient intestinal epithelial cells through donor/recipient cell fusion.62 In a TTC7A patient follow-up evaluation after HSCT, it was reported that 4 patients, at various ages (2 patients younger than 1 year and 2 patients younger than 2 years), likely had immune reconstitution after transplant.10 However, 3 of these patients required subsequent resection surgeries for recurrent stricturing, 2 required immunosuppression therapy, all patients required intravenous nutrition, and all patients had unresolved intestinal inflammation. Six more TTC7A patients with HSCT were reported, but aberrant intestinal phenotypes persisted beyond the transplant and 5 of 6 patients died.2, 4, 5 HSCT is thought to correct immune defects and may increase the survival of patients with immunodeficiency, however, it does not seem to improve phenotypes related to intestinal epithelial defects.10, 11 To further complicate the risk-benefit analysis of HSCT, the deaths of 2 TTC7A-deficiency patients were attributed to HSCT, while graft-versus-host disease and sepsis are potential complications that can exacerbate existing intestinal phenotypes.6, 11 Aside from the possibility of small-bowel transplantation, no other therapy exists to target and resolve TTC7A-related epithelial defects.
Patient Outcomes
TTC7A patients are a phenotypically diverse group, and although some symptoms are less severe than others, young patients tend to have refractory intestinal disease features with high mortality rates.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 13, 14, 16 Lien et al11 analyzed 49 previously reported TTC7A patients to identify genotype and phenotype associations. In the cohort, 41% of TTC7A patients survived and approximately half of this group had IBD. Prominent phenotypes among the 49 patients included the following: MIA-CID (33%), IBD-CID (29%), MIA (16%), MIA-CID-IBD (16%), and IBD (6%). Sepsis, bowel obstruction, viral pneumonia, and HSCT were among the top causes of mortality in these patients. In the (aforementioned) consanguineous family of 13, 5 patients died of the following causes: HSCT (2 patients, <1 y), enteropathy (1 patient, <1 y), sepsis (1 patient, 4 y), and gastric adenocarcinoma (1 patient, 14 y).6 From this large family, the range of outcomes affecting the intestinal and immunologic milieu can be appreciated, showing that TTC7A disease severity can depend on, but is not limited to, genetics.
Disease Modeling to Study TTC7A Deficiency
Finding a suitable animal model to study TTC7A deficiency has not been straightforward. For example, a spontaneous mutation in the Ttc7 gene resulted in a prominent flaky skin (ie, fsn) phenotype in mice, which resembled human psoriasis.20 The Ttc7fsn mice also presented with multisystem defects, including anemia, testicular degeneration, CD4/CD8 imbalance, and apoptotic cecal intestinal epithelial cells.20 Sequencing of Ttc7 showed an insertion of an additional exon into a TPR domain, resulting in decreased transcripts and reduced function of Ttc7 in fsn mice.20 Furthermore, Takabayashi et al63 compared Ttc7fsn mutants and found that the mutants with large deletions spanning exons 1–14, specifically affecting exons 9 and 10, had worse disease phenotypes and mortality. Although psoriasis is a condition driven by autoimmunity, the lack of single-nucleotide polymorphisms in TTC7A associated with psoriasis in human beings, and the lack of intestinal atresias in mice, suggests varying functional specificities between the human and mouse orthologs.64, 65 Although the dermatologic abnormalities in Ttc7fsn mice are reminiscent of the ichthyosis and psoriasis in some TTC7A-deficiency patients,6, 66 the mice do not seem to recapitulate the intestinal disease in patients. Establishing mutant mice with a wide variety of knock-in Ttc7 mutations, developed via clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (CAS9) editing for example, will allow for a thorough evaluation of mice as a disease model to study the intestinal defects caused by TTC7A deficiency.
In the past 20 years, zebrafish (Danio rerio) have been established as relevant models to study human disease.67 Zebrafish serve as an economic model to bridge the gap between cellular and classic mammalian models. The intestinal tracts of zebrafish and human beings have homologous structures, functions, tissues, and cell types, however, the transparency of zebrafish during the larval stage makes them an advantageous model for intestinal research.68 Zebrafish have been established as an effective model for studying intestinal development and IBD.69, 70 Furthermore, the epithelial changes seen in zebrafish after chemically induced inflammation are reminiscent of the changes observed in mouse models.69, 70 Likewise, the use of anti-inflammatories, steroids, and antibiotics also were successful in abrogating inflammatory features.69, 70 Similar to mice that incur gastrointestinal defects via Pi4kIIIα induced knockout, zebrafish lacking de novo synthesis of phosphatidyl inositol (ie, cdipthi559 mutants), a precursor for PI4P, had aberrant intestinal histopathologic features, including villous atrophy, loss of intestinal architecture, intestinal epithelial cell apoptosis, and a narrow intestinal lumen.71 Ttc7a mutant zebrafish were described in a thesis entitled Congenital Diseases of the Intestine by Halim in 2016, in which the author created mutant zebrafish with indels in exon 3 of ttc7a (no genotyping provided).72 Motility assays using fluorescent microspheres showed that 46% of homozygous mutants, compared with 37% wild-type/heterozygous fish, failed to excrete the fluorescent microspheres, showing increased transit times (and decreased gut motility) in homozygous ttc7a fish.72 The ttc7a mutant zebrafish further implicate TTC7A in driving intestinal defects, and provide a precedence for using zebrafish as a vertebrate model to study TTC7A deficiency.72
As an ex vivo model, enteroids serve to bridge the gap between cell-based and in vivo models. The seminal work of the Clevers group established protocols for culturing primary tissue-derived intestinal organoids from mouse intestinal tissue samples.73 Because organoids can be cultured directly from patient stem cells, they provide a tremendous platform for the development of personalized medicine.74 Limited data have been published on TTC7A patient-derived organoids, partly because of culturing difficulties. To date, organoids derived from patients with hypomorphic TTC7A variants (in contrast to truncated variants) seem to be the most viable in ex vivo culture.5, 6 TTC7A organoids have been used in 2 studies from Bigorgne et al5 and Lemoine et al,6 and have confirmed that the epithelial defect exists in isolation from the microbiome and aberrant immune responses. Morphology and proliferation of intestinal organoids have been assessed via immunofluorescence staining with various markers. Polarity markers have included filamentous actin, α6 integrin, zonula occulens tight junction protein, and E-cadherin, all showing mislocalization in TTC7A organoids.5, 6 Rescue of the polarity defect in TTC7A patient-derived organoids with a small molecule ROCK inhibitor provides proof-of-principle that epithelial phenotypes specific to TTC7A can be abrogated therapeutically.5, 6
Conclusions and Future Directions
Rare autosomal-recessive variants in TTC7A have been uncovered in the most severe and fatal forms of pediatric intestinal disease. Advancements in genetic diagnosis have facilitated the identification of more than 50 patients with TTC7A-derived monogenic disease, presenting with diverse phenotypes, including but not limited to, intestinal and immune aberrations. Although the specific targets and interactions of TTC7A remain unknown, existing biochemical and organoid research provides evidence for TTC7A in maintaining intestinal epithelial cell integrity. Zebrafish represent an emerging animal model with the potential to recapitulate intestinal disease features caused by TTC7A deficiency. Currently, no treatment exists that can maintain clinical remission in TTC7A patients. Given that rare monogenic diseases represent good candidates for the development of personalized medicine, zebrafish and patient-derived organoids are possible platforms for drug discovery.
Many important questions exist for future researchers and clinicians to address: how do specific mutations alter TTC7A’s secondary structure and scaffolding ability? What animal models can appropriately recapitulate the spectrum of intestinal disease features? Given that patients had various combinations of MIA and/or IBD, with or without CID, what is the threshold for the development of atresias, inflammation, and immunodeficiency? How does TTC7A deficiency result in T-cell and B-cell lymphocytopenia, is it a lymphocyte production, destruction, and/or maturation defect? What is the scope of TTC7A-deficiency etiology and how do other factors (ie, genetic milieu, environment, lifestyle, microbiome, immune system) influence patient outcomes? What phenotypes and/or key cellular players might be targets in TTC7A-deficiency drug discovery? Given the rarity of diseases such as TTC7A deficiency, we are provided with clear challenges in that we know relatively little regarding the breadth and depth of TTC7A function and that patient cases to date are too few to make significant genotype/phenotype correlations. Nevertheless, as the global prevalence of IBD is increasing, decoding the pathways and targets of TTC7A may provide more insight into the subtle inner workings of intestinal health and its interplay with the immune system. Understanding TTC7A’s pathobiology may lead to the development of effective therapies capable of improving intestinal health and that have the potential to benefit patients in the broader IBD pool.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Funded by the Canadian Institute for Health Research and the Leona M. and Harry B. Helmsley Charitable Trust (A.M.M.).
References
- 1.Samuels M.E., Majewski J., Alirezaie N., Fernandez I., Casals F., Patey N., Decaluwe H., Gosselin I., Haddad E., Hodgkinson A., Idaghdour Y., Marchand V., Michaud J.L., Rodrigue M.A., Desjardins S., Dubois S., Le Deist F., Awadalla P., Raymond V., Maranda B. Exome sequencing identifies mutations in the gene TTC7A in French-Canadian cases with hereditary multiple intestinal atresia. J Med Genet. 2013;50:324–329. doi: 10.1136/jmedgenet-2012-101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen R., Giliani S., Lanzi G., Mias G.I., Lonardi S., Dobbs K., Manis J., Im H., Gallagher J.E., Phanstiel D.H., Euskirchen G., Lacroute P., Bettinger K., Moratto D., Weinacht K., Montin D., Gallo E., Mangili G., Porta F., Notarangelo L.D., Pedretti S., Al-Herz W., Alfahdli W., Comeau A.M., Traister R.S., Pai S.Y., Carella G., Facchetti F., Nadeau K.C., Snyder M. Whole exome sequencing identifies TTC7A mutations for combined immunodeficiency with intestinal atresias. J Allergy Clin Immunol. 2013;132:656–664. doi: 10.1016/j.jaci.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avitzur Y., Guo C., Mastropaolo L.A., Bahrami E., Chen H., Zhao Z., Elkadri A., Dhillon S., Murchie R., Fattouh R., Huynh H., Walker J.L., Wales P.W., Cutz E., Kakuta Y., Dudley J., Kammermeier J., Powrie F., Shah N., Walz C., Nathrath M., Kotlarz D., Puchaka J., Krieger J.R., Racek T., Kirchner T., Walters T.D., Brumell J.H., Griffiths A.M., Rezaei N., Rashtian P., Najafi M., Monajemzadeh M., Pelsue S., McGovern D.P., Uhlig H.H., Schadt E., Klein C., Snapper S.B., Muise A.M. Mutations in tetratricopeptide repeat domain 7A result in a severe form of very early onset inflammatory bowel disease. Gastroenterology. 2014;146:1028–1039. doi: 10.1053/j.gastro.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal N.S., Northrop L., Anyane-Yeboa K., Aggarwal V.S., Nagy P.L., Demirdag Y.Y. Tetratricopeptide repeat domain 7A (TTC7A) mutation in a newborn with multiple intestinal atresia and combined immunodeficiency. J Clin Immunol. 2014;34:607–610. doi: 10.1007/s10875-014-0067-7. [DOI] [PubMed] [Google Scholar]
- 5.Bigorgne A.E., Farin H.F., Lemoine R., Mahlaoui N., Lambert N., Gil M., Schulz A., Philippet P., Schlesser P., Abrahamsen T.G., Oymar K., Davies E.G., Ellingsen C.L., Leteurtre E., Moreau-Massart B., Berrebi D., Bole-Feysot C., Nischke P., Brousse N., Fischer A., Clevers H., de Saint Basile G. TTC7A mutations disrupt intestinal epithelial apicobasal polarity. J Clin Invest. 2014;124:328–337. doi: 10.1172/JCI71471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemoine R., Pachlopnik-Schmid J., Farin H.F., Bigorgne A., Debre M., Sepulveda F., Heritier S., Lemale J., Talbotec C., Rieux-Laucat F., Ruemmele F., Morali A., Cathebras P., Nitschke P., Bole-Feysot C., Blanche S., Brousse N., Picard C., Clevers H., Fischer A., de Saint Basile G. Immune deficiency-related enteropathy-lymphocytopenia-alopecia syndrome results from tetratricopeptide repeat domain 7A deficiency. J Allergy Clin Immunol. 2014;134:1354–1364. doi: 10.1016/j.jaci.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Guanà R., Garofano S., Teruzzi E., Vinardi S., Carbonaro G., Cerrina A., Morra I., Montin D., Mussa A., Schleef J. The complex surgical management of the first case of severe combined immunodeficiency and multiple intestinal atresias surviving after the fourth year of life. Pediatr Gastroenterol Hepatol Nutr. 2014;17:257–262. doi: 10.5223/pghn.2014.17.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woutsas S., Aytekin C., Salzer E., Conde C.D., Apaydin S., Pichler H., Memaran-Dadgar N., Hosnut F.O., Förster-Waldl E., Matthes S., Huber W.D., Lion T., Holter W., Bilic I., Boztug K. TTC7A causes combined immunodeficiency with mild structural intestinal defects. Blood. 2015;125:1674–1676. doi: 10.1182/blood-2014-08-595397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W., Lee P.P., Thong M.K., Ramanujam T.M., Shanmugam A., Koh M.T., Chan K.W., Ying D., Wang Y., Shen J.J., Yang J., Lau Y.L. Compound heterozygous mutations in TTC7A cause familial multiple intestinal atresias and severe combined immunodeficiency. Clin Genet. 2015;88:542–549. doi: 10.1111/cge.12553. [DOI] [PubMed] [Google Scholar]
- 10.Kammermeier J., Lucchini G., Pai S.Y., Worth A., Rampling D., Amrolia P., Silva J., Chiesa R., Rao K., Noble-Jamieson G., Gasparetto M., Ellershaw D., Uhlig H., Sebire N., Elawad M., Notarangelo L., Shah N., Veys P. Stem cell transplantation for tetratricopeptide repeat domain 7A deficiency: long-term follow-up. Blood. 2016;128:1306–1308. doi: 10.1182/blood-2016-01-696385. [DOI] [PubMed] [Google Scholar]
- 11.Lien R., Lin Y.F., Lai M.W., Weng H.Y., Wu R.C., Jaing T.H., Huang J.L., Tsai S.F., Lee W.I. Novel mutations of the tetratricopeptide repeat domain 7A gene and phenotype/genotype comparison. Front Immunol. 2017;8:1066. doi: 10.3389/fimmu.2017.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawless D., Mistry A., Wood P.M., Stahlschmidt J., Arumugakani G., Hull M., Parry D., Anwar R., Carter C., Savic S. Bialellic mutations in tetratricopeptide repeat domain 7A (TTC7A) cause common variable immunodeficiency-like phenotype with enteropathy. J Clin Immunol. 2017;37:617–622. doi: 10.1007/s10875-017-0427-1. [DOI] [PubMed] [Google Scholar]
- 13.Neves J.F., Afonso I., Borrego L., Martins C., Cordeiro A.I., Neves C., Lacoste C., Badens C., Fabre A. Missense mutation of TTC7A mimicking tricho-hepato-enteric (SD/THE) syndrome in a patient with very-early onset inflammatory bowel disease. Eur J Med Genet. 2018;61:185–188. doi: 10.1016/j.ejmg.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Mandiá N., Perez-Muñuzuri A., Lopez-Suarez O. Congenital intestinal atresias with multiple episodes of sepsis. Medicine (Baltimore) 2018;97:e10939. doi: 10.1097/MD.0000000000010939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fayard J., Collardeau S., Bertrand Y., Cordier M.P., Malcus C., Dubois R., Mure P.Y., de Saint Basile G., Louazon T., Rohmer B., Lachaux A., Duclaux R., Peretti N. TTC7A mutation must be considered in patients with repeated intestinal atresia associated with early inflammatory bowel disease: two new case reports and a literature review. Arch Pediatr. 2018;25:334–339. doi: 10.1016/j.arcped.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Fullerton B.S., Velazco C.S., Hong C.R., Carey A.N., Jaksic T. High rates of positive severe combined immunodeficiency screening among newborns with severe intestinal failure. JPEN J Parenter Enteral Nutr. 2018;42:239–246. doi: 10.1002/jpen.1013. [DOI] [PubMed] [Google Scholar]
- 17.Blatch G.L., Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Chung J., Nakatsu F., Baskin J.M., De Camilli P. Plasticity of PI4KIIIalpha interactions at the plasma membrane. EMBO Rep. 2015;16:312–320. doi: 10.15252/embr.201439151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baskin J.M., Wu X., Christiano R., Oh M.S., Schauder C.M., Gazzerro E., Messa M., Baldassari S., Assereto S., Biancheri R., Zara F., Minetti C., Raimondi A., Simons M., Walther T.C., Reinisch K.M., De Camilli P. The leukodystrophy protein FAM126A (hyccin) regulates PtdIns(4)P synthesis at the plasma membrane. Nat Cell Biol. 2016;18:132–138. doi: 10.1038/ncb3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helms C., Pelsue S., Cao L., Lamb E., Loffredo B., Taillon-Miller P., Herrin B., Burzenski L.M., Gott B., Lyons B.L., Keppler D., Shultz L.D., Bowcock A.M. The tetratricopeptide repeat domain 7 gene is mutated in flaky skin mice: a model for psoriasis, autoimmunity, and anemia. Exp Biol Medicine (Maywood) 2005;230:659–667. doi: 10.1177/153537020523000908. [DOI] [PubMed] [Google Scholar]
- 21.TTC7A tetratricopeptide repeat domain 7A [Homo sapiens (human)] - gene - NCBI. https://www.ncbi.nlm.nih.gov/pubmed Available from: Accessed June 18, 2018.
- 22.Tissue expression of TTC7A - summary - The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000068724-TTC7A/tissue Available from: Accessed June 18, 2018.
- 23.Gene: TTC7A (ENSG00000068724) - paralogues - Homo sapiens - Ensembl genome browser 92. http://useast.ensembl.org/Homo_sapiens/Gene/Compara_Paralog?db=core Available from: Accessed June 18, 2018.
- 24.Lees J.A., Zhang Y., Oh M.S., Schauder C.M., Yu X., Baskin J.M., Dobbs K., Notarangelo L.D., De Camilli P., Walz T., Reinisch K.M. Architecture of the human PI4KIIIalpha lipid kinase complex. Proc Natl Acad Sci U S A. 2017;114:13720–13725. doi: 10.1073/pnas.1718471115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Database GeneCards Human Gene TTC7B gene - GeneCards | TTC7B protein | TTC7B antibody. https://genecards.weizmann.ac.il/v3/cgi-bin/carddisp.pl?gene=TTC7B&search=0f410229cce13b4d7879d05c344f93fd Available from: Accessed October 21, 2018.
- 26.Database GeneCards Human Gene TTC7A gene - GeneCards | TTC7A protein | TTC7A antibody. https://genecards.weizmann.ac.il/v3/cgi-bin/carddisp.pl?gene=TTC7A&lm_expand=all&search=ttc7a#lifemap_expression Available from: Accessed October 21, 2018.
- 27.Zeytuni N., Zarivach R. Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure. 2012;20:397–405. doi: 10.1016/j.str.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Leung G., Muise A.M. monogenic intestinal epithelium defects and the development of inflammatory bowel disease. Physiology (Bethesda, MD) 2018;33:360–369. doi: 10.1152/physiol.00020.2018. [DOI] [PubMed] [Google Scholar]
- 29.Baird D., Stefan C., Audhya A., Weys S., Emr S.D. Assembly of the PtdIns 4-kinase Stt4 complex at the plasma membrane requires Ypp1 and Efr3. J Cell Biol. 2008;183:1061–1074. doi: 10.1083/jcb.200804003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan J., Brill J.A. Cinderella story: PI4P goes from precursor to key signaling molecule. Crit Rev Biochem Mol Biol. 2014;49:33–58. doi: 10.3109/10409238.2013.853024. [DOI] [PubMed] [Google Scholar]
- 31.Vaillancourt F.H., Brault M., Pilote L., Uyttersprot N., Gaillard E.T., Stoltz J.H., Knight B.L., Pantages L., McFarland M., Breitfelder S., Chiu T.T., Mahrouche L., Faucher A.M., Cartier M., Cordingley M.G., Bethell R.C., Jiang H., White P.W., Kukolj G. Evaluation of phosphatidylinositol-4-kinase IIIalpha as a hepatitis C virus drug target. J Virol. 2012;86:11595–11607. doi: 10.1128/JVI.01320-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flower W., Clark-Dixon C., Metoyer C., Hui Y., Runhua S., Zhaojie Z., Stephan N. YGR198w (YPP1) targets A30P α-synuclein to the vacuole for degradation. J Cell Biol. 2007;177:1091–1104. doi: 10.1083/jcb.200610071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allaire J.M., Crowley S.M., Law H.T., Chang S.Y., Ko H.J., Vallance B.A. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 35.Amano M., Nakayama M., Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Citalan-Madrid A.F., Vargas-Robles H., Garcia-Ponce A., Shibayama M., Betanzos A., Nava P., Salinas-Lara C., Rottner K., Mennigen R., Schnoor M. Cortactin deficiency causes increased RhoA/ROCK1-dependent actomyosin contractility, intestinal epithelial barrier dysfunction, and disproportionately severe DSS-induced colitis. Mucosal Immunol. 2017;10:1237–1247. doi: 10.1038/mi.2016.136. [DOI] [PubMed] [Google Scholar]
- 37.Wittkopf N., Neurath M.F., Becker C. Immune-epithelial crosstalk at the intestinal surface. J Gastroenterol. 2014;49:375–387. doi: 10.1007/s00535-013-0929-4. [DOI] [PubMed] [Google Scholar]
- 38.de Lange K.M., Moutsianas L., Lee J.C., Lamb C.A., Luo Y., Kennedy N.A., Jostins L., Rice D.L., Gutierrez-Achury J., Ji S.G., Heap G., Nimmo E.R., Edwards C., Henderson P., Mowat C., Sanderson J., Satsangi J., Simmons A., Wilson D.C., Tremelling M., Hart A., Mathew C.G., Newman W.G., Parkes M., Lees C.W., Uhlig H., Hawkey C., Prescott N.J., Ahmad T., Mansfield J.C., Anderson C.A., Barrett J.C. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49:256–261. doi: 10.1038/ng.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen B.S., Fredrich B., Hoeppner M.P., Ellinghaus D., Franke A. Opportunities and challenges of whole-genome and -exome sequencing. BMC Genet. 2017;18:e1471–e2156. doi: 10.1186/s12863-017-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peloquin J.M., Goel G., Villablanca E.J., Xavier R.J. Mechanisms of pediatric inflammatory bowel disease. Annu Rev Immunol. 2016;34:31–64. doi: 10.1146/annurev-immunol-032414-112151. [DOI] [PubMed] [Google Scholar]
- 41.Sun W., Zheng W., Simeonov A. Drug discovery and development for rare genetic disorders. Am J Med Genet A. 2017;173:2307–2322. doi: 10.1002/ajmg.a.38326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhlig H.H., Schwerd T., Koletzko S., Shah N., Kammermeier J., Elkadri A., Ouahed J., Wilson D.C., Travis S.P., Turner D., Klein C., Snapper S.B., Muise A.M., Colors in IBD Study Group and Neopics The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990–1007 e1003. doi: 10.1053/j.gastro.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uhlig H.H., Booth C.A. Spectrum of genetic variants contributes to immune defects and pathogenesis of inflammatory bowel diseases. Gastroenterology. 2018;154:2022–2024. doi: 10.1053/j.gastro.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Uhlig H.H., Muise A.M. Clinical genomics in inflammatory bowel disease. Trends Genet. 2017;33:629–641. doi: 10.1016/j.tig.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Lambrecht W., Kluth D. Hereditary multiple atresias of the gastrointestinal tract: report of a case and review of the literature. J Pediatr Surg. 1998;33:794–797. doi: 10.1016/s0022-3468(98)90225-1. [DOI] [PubMed] [Google Scholar]
- 46.Amin S.C., Pappas C., Iyengar H., Maheshwari A. Short bowel syndrome in the NICU. Clin Perinatol. 2013;40:53–68. doi: 10.1016/j.clp.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang C.W., Wong J.M., Tung C.C., Shih I.L., Wang H.Y., Wei S.C. Intestinal stricture in Crohn's disease. Intest Res. 2015;13:19–26. doi: 10.5217/ir.2015.13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngan B., Merico D., Marcus N., Kim V.H.D., Upton J., Bates A., Herbrick J., Nalpathamkalam T., Thiruvahindrapuram B., Cox P., Roifman C.M. Mutations in tetratricopeptide repeat domain 7A (TTC7A) are associated with combined immunodeficiency with dendriform lung ossification but no intestinal atresia. LymphoSign Journal. 2014;01:10–26. [Google Scholar]
- 49.Brewer J.W. Phospholipids: "greasing the wheels" of humoral immunity. Biochim Biophys Acta. 2013;1831:642–651. doi: 10.1016/j.bbalip.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nüesch U., Mauracher A.A., Opitz L., Volkmer B., Michalak-Mićka K., Kamarashev J., Hartwig T., Reichmann E., Becher B., Vavassori S., Pachlopnik Schmid J. Epithelial proliferation in inflammatory skin disease is regulated by tetratricopeptide repeat domain 7 (Ttc7) in fibroblasts and lymphocytes. J Allergy Clin Immunol. 2019;143:292–304. doi: 10.1016/j.jaci.2018.02.057. [DOI] [PubMed] [Google Scholar]
- 51.Moran C.J., Klein C., Muise A.M., Snapper S.B. Very early-onset inflammatory bowel disease: gaining insight through focused discovery. Inflamm Bowel Dis. 2015;21:1166–1175. doi: 10.1097/MIB.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nalle S.C., Turner J.R. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. 2015;8:720. doi: 10.1038/mi.2015.40. [DOI] [PubMed] [Google Scholar]
- 53.Akpek G., Chinratanalab W., Lee L.A., Torbenson M., Hallick J.P., Anders V., Vogelsang G.B. Gastrointestinal involvement in chronic graft-versus-host disease: a clinicopathologic study. Biol Blood Marrow Transplant. 2003;9:46–51. doi: 10.1053/bbmt.2003.49999. [DOI] [PubMed] [Google Scholar]
- 54.Jilling T., Lu J., Jackson M., Caplan M.S. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622–629. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 55.Schnoor M. Inflammatory mediators contributing to intestinal epithelial cell apoptosis and barrier disruption in IBD. J Clin Cell Immunol. 2011 doi: 10.4172/2155-9899.S3-003. S3:003. [DOI] [Google Scholar]
- 56.Schulzke J.D., Bojarski C., Zeissig S., Heller F., Gitter A.H., Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann N Y Acad Sci. 2006;1072:288–299. doi: 10.1196/annals.1326.027. [DOI] [PubMed] [Google Scholar]
- 57.Ali Y.A., Rahman S., Bhat V., Al Thani S., Ismail A., Bassiouny I. Hereditary multiple intestinal atresia (HMIA) with severe combined immunodeficiency (SCID): a case report of two siblings and review of the literature on MIA, HMIA and HMIA with immunodeficiency over the last 50 years. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.05.2010.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thiagarajah J.R., Kamin D.S., Acra S., Goldsmith J.D., Roland J.T., Lencer W.I., Muise A.M., Goldenring J.R., Avitzur Y., Martin M.G., Pedi C.C. Advances in evaluation of chronic diarrhea in infants. Gastroenterology. 2018;154:2045–2059 e2046. doi: 10.1053/j.gastro.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilroy R.K., Coccia P.F., Talmadge J.E., Hatcher L.I., Pirruccello S.J., Shaw B.W., Jr., Rubocki R.J., Sudan D.L., Langnas A.N., Horslen S.P. Donor immune reconstitution after liver-small bowel transplantation for multiple intestinal atresia with immunodeficiency. Blood. 2004;103:1171–1174. doi: 10.1182/blood-2003-04-1187. [DOI] [PubMed] [Google Scholar]
- 60.Small bowel transplant: an evidence-based analysis. Ontario health technology assessment series. 2003;3:1–72. [PMC free article] [PubMed] [Google Scholar]
- 61.Crowley E., Muise A. Inflammatory bowel disease: what very early onset disease teaches us. Gastroenterol Clin North Am. 2018;47:755–772. doi: 10.1016/j.gtc.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Silk A.D., Gast C.E., Davies P.S., Fakhari F.D., Vanderbeek G.E., Mori M., Wong M.H. Fusion between hematopoietic and epithelial cells in adult human intestine. PLoS One. 2013;8:e55572. doi: 10.1371/journal.pone.0055572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takabayashi S., Iwashita S., Hirashima T., Katoh H. The novel tetratricopeptide repeat domain 7 mutation, Ttc7fsn-Jic, with deletion of the TPR-2B repeat causes severe flaky skin phenotype. Exp Biol Medicine (Maywood) 2007;232:695–699. [PubMed] [Google Scholar]
- 64.Fiorino G., Omodei P.D. Psoriasis and inflammatory bowel disease: two sides of the same coin? J Crohns Colitis. 2015;9:697–698. doi: 10.1093/ecco-jcc/jjv110. [DOI] [PubMed] [Google Scholar]
- 65.Parisi R., Symmons D.P., Griffiths C.E., Ashcroft D.M. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 66.Leclerc-Mercier S., Lemoine R., Bigorgne A.E., Sepulveda F., Leveau C., Fischer A., Mahlaoui N., Hadj-Rabia S., de Saint Basile G. Ichthyosis as the dermatological phenotype associated with TTC7A mutations. Br J Dermatol. 2016;175:1061–1064. doi: 10.1111/bjd.14644. [DOI] [PubMed] [Google Scholar]
- 67.Ali S., Champagne D.L., Spaink H.P., Richardson M.K. Zebrafish embryos and larvae: a new generation of disease models and drug screens. Birth Defects Res C Embryo Today. 2011;93:115–133. doi: 10.1002/bdrc.20206. [DOI] [PubMed] [Google Scholar]
- 68.Ng A.N., de Jong-Curtain T.A., Mawdsley D.J., White S.J., Shin J., Appel B., Dong P.D., Stainier D.Y., Heath J.K. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol. 2005;286:114–135. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 69.Brugman S. The zebrafish as a model to study intestinal inflammation. Dev Comp Immunol. 2016;64:82–92. doi: 10.1016/j.dci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 70.Fleming A., Jankowski J., Goldsmith P. In vivo analysis of gut function and disease changes in a zebrafish larvae model of inflammatory bowel disease: a feasibility study. Inflamm Bowel Dis. 2010;16:1162–1172. doi: 10.1002/ibd.21200. [DOI] [PubMed] [Google Scholar]
- 71.Thakur P.C., Davison J.M., Stuckenholz C., Lu L., Bahary N. Dysregulated phosphatidylinositol signaling promotes endoplasmic-reticulum-stress-mediated intestinal mucosal injury and inflammation in zebrafish. Dis Model Mech. 2014;7:93–106. doi: 10.1242/dmm.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halim D. Congenital diseases of the intestine. Doctoral Thesis, Erasmus University Medical Center, Rotterdam, The Netherlands. 2016.
- 73.Peters Hans T.S., Robert G.V., Hugo J.S., Marc van de W., Nick B., Daniel E.S., Johan HvE., Arie A., Pekka K., Peter J. Single Lgr5 stem cells build crypt–villus structures. Nature. 2009;459:262. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 74.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 75.St Johnston D., Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 76.Cunningham K.E., Turner J.R. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci. 2012;1258:34–42. doi: 10.1111/j.1749-6632.2012.06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen G., Hou Z., Gulbranson D.R., Thomson J.A. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi J., Wu X., Surma M., Vemula S., Zhang L., Yang Y., Kapur R., Wei L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis. 2013;4:e483. doi: 10.1038/cddis.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]