Key Clinical Message
This case report describes management of a chronic radiation wound in a patient with multiple comorbidities using a lyopreserved placental membrane containing viable cells (vLPM). Positive outcomes suggest that vLPM provides a good conservative management option for patients with compromised wound healing due to radiation and comorbidities.
Keywords: lyopreserved, placental membrane, radiation necrosis, viable, wound
1. INTRODUCTION
Radiation therapy is commonly employed in the treatment of various cancers alone, or in a combination with surgical tumor excision, with the purpose of selectively killing cancer cells at the tumor site.1 Radiation therapy, however, is damaging to the surrounding healthy tissue and may result in tissue necrosis. Radiation can also compromise wound healing leading to a chronic ulceration.1
Standardized treatment for chronic radiation‐induced wounds is not established; however, flaps and skin grafts are two common surgical options.2 Conservative care typically includes cleaning and debridement, nutritional support, and establishment of adequate blood and oxygen supply along with vacuum‐assisted devices, hyperbaric oxygen (HBO), or advanced wound care dressings, alone or in a combination.2, 3 Unfortunately, chronic radiation wounds are often resistant to currently available treatment modalities and remain a challenge to treat.
Here we describe the use of a lyopreserved placental membrane containing viable cells (vLPM) allograft for conservative management of a radiation wound in a patient with multiple comorbidities who failed several other treatment modalities. The applications of vLPM were performed in an outpatient setting and resulted in complete closure of the refractory radiation wound.
2. CASE REPORT
A 73‐year‐old female with a history of rheumatoid arthritis (RA), systemic sclerosis, lymphedema, mild chronic venous insufficiency, and squamous cell carcinoma presented with a radiation necrosis wound on the right medial ankle as a result of cancer treatment after tumor excision. The wound duration was 1 year and had been previously treated with various collagen dressings, honey‐impregnated dressings, topical antibiotics, and multiple rounds of oral antibiotics. Due to extensive inflammation and fibrosis of the wound, this patient was not a candidate for surgical closure and was selected to receive weekly applications of vLPM for 12 weeks.
vLPM (GrafixPL PRIME®; Osiris Therapeutics, Inc, Columbia, MD) is a lyopreserved placental tissue allograft that retains the extracellular matrix, growth factors, and endogenous neonatal mesenchymal stem cells, fibroblasts and epithelial cells of the native tissue and is intended for use in the management of acute and chronic wounds. vLPM is processed aseptically following rigorous quality assurance standards and is stored and distributed for use in accordance with the regulations outlined in 21 Code of Federal Regulations (CFR) 1271 and the standard of the American Association of Tissue Banks (AATB). All donors have been extensively screened, and all tissues have been recovered, processed, stored, tested, and distributed in accordance with current US Federal Regulations, current AATB standards, and state/local regulations as required. vLPM is supplied in sheet form and packaged within a heat‐sealed pouch and is stored at room temperature.4
Figure 1A,B shows the status of the wound 10 and 5 months prior to vLPM application, respectively. During that time, the wound measured roughly 10.0 cm2 with multiple fluctuations in wound size without progress toward closure. One week prior to the first vLPM application, the wound was measured 8.95 cm2 with a depth of 0.2 cm (Figure 1C). At the initial treatment visit, the wound was measured 1.57 cm2 with a depth of 0.2 cm (Figure 1D). At each treatment visit, the wound was cleaned and debrided prior to vLPM application. The vLPM graft was covered with a non‐adherent dressing, Adaptic® (Systagenix, Gatwick, UK), followed by a hydro fiber dressing to absorb drainage. The patient also received multi‐layer compression and was instructed to leave dressings intact and keep the wound site clean and dry.
Figure 1.
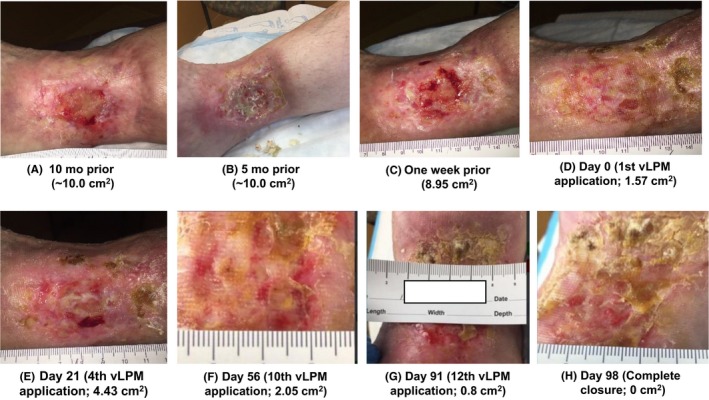
(A‐H) Progression of the wound 10 mo prior to vLPM treatment until complete closure at day 98
During the course of vLPM treatment, there were four episodes of size increase, correlating to increased areas of slough. One such example is shown in Figure 1E. The graph (Figure 2) illustrates the rate of wound closure and shows the four increases in wound size. The reduction of wound area is expressed in percent relative to the baseline wound size, which is considered 100%. The patient achieved complete wound re‐epithelialization after 12 applications of vLPM in 98 days, as shown in Figure 1H. The epithelial layer is pink and not matured at this time point; however, the wound remained closed after 3 months from initial closure. Further, the patient did not experience any adverse events (AE) related to vLPM.
Figure 2.
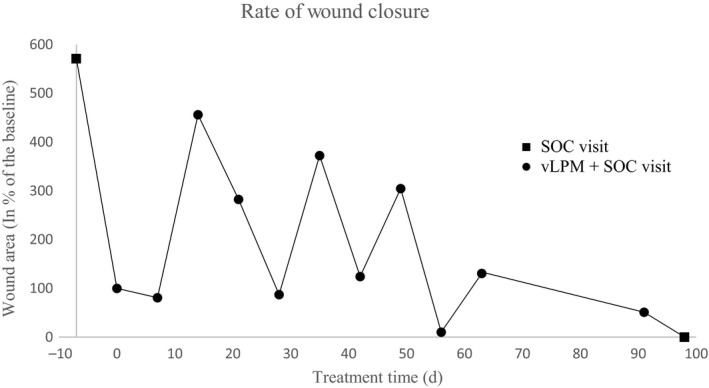
Changes in wound size during vLPM adjunct to standard of care (SOC) treatment course. Wound size at each time point is presented in % of wound area relative to the baseline. Baseline was defined as the first day of vLPM application and is marked as day 0 on the X‐axis. Visits when the patient was treated with vLPM plus SOC are represented by black circle markers on the graph. Visits when the patient was treated with SOC alone are shown with black square markers. Note: The patient received 12 applications of vLPM; however, measurements were not obtained at one visit on day 77
3. DISCUSSION
In the current study, we evaluated vLPM in the treatment of a chronic radiation necrosis wound that resulted from squamous cell carcinoma radiation therapy, post‐surgical tumor excision in a patient with multiple comorbidities. Radiation triggers cell death and impairs cell functionality including migration, proliferation, differentiation, and secretion of extracellular matrix proteins and growth factors. Radiation directly damages fibroblasts causing a decrease in collagen production and loss of collagen function.1 Further, the presence of reactive oxygen species can lead to the dysregulated production of myofibroblasts, causing an abnormal production of collagen. This is referred to as radiation‐induced fibrosis, which prevents wound healing as it inhibits inflammatory cells and impairs angiogenesis, both of which are required to remove bacteria and dead cells, and re‐establish microvasculature at the injury site.1 As all cellular activities key to the wound healing process are heavily compromised after radiation therapy, these wounds prove challenging to treat. Moreover, this patient had multiple comorbidities including RA, systemic sclerosis, lymphedema, and chronic venous insufficiency, which have all been shown to negatively impact wound healing.5, 6, 7, 8
The benefits of placental membranes, amnion, and chorion, for the management of burns and chronic wounds, have been extensively outlined in the literature.9 Fresh placental membrane is inherently anti‐inflammatory, antimicrobial, and antifibrotic.10, 11, 12 When used as a wound cover, placental membranes maintain a moist environment in the wound, reduce pain, and support angiogenesis, granulation of the wound bed, and wound epithelialization.13, 14 Development of different tissue preservation methods have led to the commercialization of placental membranes; however, the majority of these preservation methods destroy viable tissue cells. Accumulated data show that devitalization (ie killing viable tissue cells during processing) of placental membranes decreases its anti‐inflammatory, antioxidant, antimicrobial, and angiogenic potential.15, 16, 17, 18, 19 One proprietary cryopreservation method developed by Osiris Therapeutics results in high cell viability within the preserved placental membrane and is a commercially available cryopreserved placental membrane product containing viable cells.15, 16 Cryopreserved placental membrane (vCPM) retains the components of fresh placental membrane, and its clinical effectiveness has been demonstrated in various trials with hard‐to‐treat wounds using vCPM plus standard of care.20, 21, 22, 23
In 2016, Frykberg et al demonstrated positive clinical outcomes in the treatment of complex diabetic foot ulcers (DFU) with vCPM. This patient population had significant comorbidities such as heart disease, kidney disease, and previous partial foot amputations.20 Also in 2016, a retrospective review of five actively smoking diabetics with six nonhealing ulcers and peripheral arterial disease (PAD) who received serial applications of vCPM reported positive outcomes, as all patients achieved complete wound closure.21 Further, Anselmo et al22 showed positive clinical outcomes with vCPM in the treatment of three chronic wounds that had previously failed standard wound care treatments. These patients also had significant comorbidities including chronic heart failure, diabetes, peripheral vascular disease, and venous insufficiency. Lastly, Dress et al23 reported on the use of vCPM adjunct to an open fasciectomy in a patient with Dupuytren's disease, a tissue disorder resulting in progressive fibrosis and excessive collagen deposition. Though all of these types of wounds are challenging to treat, vCPM adjunct to standard of care demonstrated clinical benefits.
Recently, the lyopreserved configuration of placental membrane has been developed using a novel lyophilization technique that allows storage of viable tissues at ambient temperatures. A scientific study has shown that vLPM is structurally and functionally equivalent to vCPM.24 Similar to vCPM, vLPM retains the components (extracellular matrix, growth factors, and endogenous viable cells) and properties of native placental tissue and is also intended for use in acute and chronic wounds of various etiologies and locations.4, 24 However, in contrast to vCPM, which requires ultra‐low temperatures for storage and distribution, vLPM is stored at room temperature. Based on positive clinical outcomes from various studies utilizing vCPM in the management of chronic wounds in patients with multiple comorbidities, and with the added convenience of room temperature storage, vLPM was selected in the treatment of the present radiation wound.
In this study, we show that with vLPM, the patient was able to achieve complete wound closure in 3 months when multiple other treatments had previously failed. As the surrounding skin was fibrotic and necrotic due to the radiation burn, this patient was not a candidate for traditional surgical approaches, and the clinician elected to use an advanced skin substitute. The selection of vLPM was based on the product composition and inherent properties of placental membrane previously mentioned. Chronic inflammation is a hallmark of radiation wounds.25 vLPM retains the anti‐inflammatory properties of fresh placental membrane—a feature that other advanced skin substitutes do not have.
During the course of treatment, the wound experienced four increases in wound size. According to published literature, chronic inflammation in radiation wounds often leads to subsequent waves of tissue injury leading to skin breakdown and necrosis. These waves can occur weeks, months, or years after the initial injury.25, 26, 27 Fluctuations in wound size were observed during the course of treatment prior to vLPM application. As such, it is unlikely that wound size fluctuations were a result of vLPM application.
The positive outcomes of the clinical case presented here suggest that vLPM provides a good conservative option in the treatment of chronic refractory radiation wounds in patients with impaired wound healing due to negative effects of radiation and multiple comorbidities. In the outpatient setting, vLPM provides ease of application as no thawing or reconstitution is required prior to application. Future prospective clinical trials will be needed to further establish effectiveness of vLPM.
CONFLICT OF INTEREST
MJR is a speaker and a consultant of Osiris Therapeutics. MCS and AD are employees of Osiris.
AUTHOR CONTRIBUTION
MJR: involved in conception and design of this case report, collection and assembly of case, critical revision of the article for important intellectual content and final approval of the article. AD: involved in assembly of case, drafting of the article, and critical revision of the article for important intellectual content. MCS: involved in conception and design of this case report, drafting of the article, and critical revision of the article for important intellectual content.
PATIENT CONSENT
The patient gave consent in writing for data concerning this case to be submitted for publication.
Regulski MJ, Danilkovitch A, Saunders MC. Management of a chronic radiation necrosis wound with lyopreserved placental membrane containing viable cells. Clin Case Rep. 2019;7:456–460. 10.1002/ccr3.2011
REFERENCES
- 1. Iyer S, Balasubramanian D. Management of radiation wounds. Indian J Plast Surg. 2012;45(2):325‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012;7:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. U.S. Department of Health and Human Services ; Food and Drug Administration ; Center for Drug Evaluation and Research ; Center for Biologics Evaluation and Research ; Center for Devices and Radiological Health . Guidance for industry: chronic cutaneous ulcer and burn wounds—developing products for treatment; 2006. https://www.fda.gov/downloads/drugs/guidances/ucm071324.pdf. Accessed July 18, 2018.
- 4. GrafixPL PRIME® Package Insert. Columbia, MD: Osiris Therapeutics, Inc.; 2017. [Google Scholar]
- 5. Vickers A. Delayed wound healing in patients with rheumatoid arthritis. Nurs Times. 2014;100(14):61‐63. [PubMed] [Google Scholar]
- 6. Hafner J, Schneider E, Burg G, Cassina PC. Management of leg ulcers in patients with rheumatoid arthritis or systemic sclerosis: the importance of concomitant arterial and venous disease. J Vasc Surg. 2000;32(2):322‐329. [DOI] [PubMed] [Google Scholar]
- 7. Mallon EC, Ryan TJ. Lymphedema and wound healing. Clin Dermatol. 1994;12(1):89‐93. [DOI] [PubMed] [Google Scholar]
- 8. Valencia I, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44(3):401‐424. [DOI] [PubMed] [Google Scholar]
- 9. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88‐99. [DOI] [PubMed] [Google Scholar]
- 10. Zare‐Bidaki M, Sadrinia S, Erfani S, Afkar E, Ghanbarzade N. Antimicrobial properties of amniotic and chorionic membranes: a comparative study of two human fetal sacs. J Reprod Infertil. 2017;18:218‐224. [PMC free article] [PubMed] [Google Scholar]
- 11. Shimmura S, Shimazaki J, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001;20:408‐413. [DOI] [PubMed] [Google Scholar]
- 12. Sant'anna LB, Hage R, Cardoso M, et al. Antifibrotic effects of human amniotic membrane transplantation in established biliary fibrosis induced in rats. Cell Transplant. 2016;25:2245‐2257. [DOI] [PubMed] [Google Scholar]
- 13. Castellanos G, Bernab‐Garcia A, Moraleda JM, Nicolas FJ. Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta. 2017;59:146‐153. [DOI] [PubMed] [Google Scholar]
- 14. Elheneidy H, Omran E, Halwagy A, Al‐Inany H, Al‐Ansary M, Gad A. Amniotic membrane can be a valid source for wound healing. Int J Womens Health. 2016;8:225‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duan‐Arnold Y, Gyurdieva A, Johnson A, Uveges TE, Jacobstein DA, Danilkovitch A. Retention of endogenous viable cells enhances the anti‐inflammatory activity of cryopreserved amnion. Adv Wound Care (New Rochelle). 2015;4:523‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson A, Gyurdieva A, Dhall S, Danilkovitch A, Duan‐Arnold Y. Understanding the impact of preservation methods on the integrity and functionality of placental allografts. Ann Plast Surg. 2017;79(2):203‐213. [DOI] [PubMed] [Google Scholar]
- 17. Duan‐Arnold Y, Gyurdieva A, Johnson A, Jacobstein DA, Danilkovitch A. Soluble factors released by endogenous viable cells enhance the antioxidant and chemoattractive activities of cryopreserved amniotic membrane. Adv Wound Care (New Rochelle). 2015;4:329‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duan‐Arnold Y, Uveges TE, Gyurdieva A, Johnson A, Danilkovitch A. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention of all tissue components in their native state. Adv Wound Care (New Rochelle). 2015;4:513‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mao Y, Hoffman T, Singh‐Varma A, et al. Antimicrobial peptides secreted from human cryopreserved viable amniotic membrane contribute to its antibacterial activity. Sci Rep. 2017;7:13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frykberg RG, Gibbons GW, Walters JL, Wukich DK, Milstein FC. A prospective, multicentre, open‐label, single‐arm clinical trial for treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: positive clinical outcomes of viable cryopreserved human placental membrane. Int Wound J. 2017;14(3):569‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smedley J, Michael GM, Tamire YG. Wound closure in smoking peripheral arterial disease patients with treatment‐refractory ulcerations. Int J Low Extrem Wounds. 2016;15:360‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anselmo DS, McGuire JB, Love E, Vlahovic T. Application of viable cryopreserved human placental membrane grafts in the treatment of wounds of diverse etiologies: a case series. Wounds. 2018;30(3):57‐61. [PubMed] [Google Scholar]
- 23. Dress CM, Tassis EK. A case of Dupuytren’s disease managed with viable cryopreserved placental membrane adjunct to open palmar fasciectomy. J Surg Case Rep. 2018;2018(3):rjy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhall S, Sathyamoorthy M, Kuang JQ, et al. Properties of viable lyopreserved amnion are equivalent to viable cryopreserved amnion with the convenience of ambient storage. PLoS ONE. 2018;13(10):e0204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Linard C, Brachet M, L’homme B, et al. Long‐term effectiveness of local BM‐MSCs for skeletal muscle regeneration: a proof of concept obtained on a pig model of severe radiation burn. Stem Cell Res Ther. 2018;9(1):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bey E, Prat M, Duhamel P, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow‐derived stem cell administrations. Wound Repair Regen. 2010;18(1):50‐58. [DOI] [PubMed] [Google Scholar]
- 27. Rodgers K, Jadhav SS. The application of mesenchymal stem cells to treat thermal and radiation burns. Adv Drug Deliv Rev. 2018;123:75‐81. [DOI] [PubMed] [Google Scholar]


