Abstract
The liver has a high regenerative capacity after acute liver injury, but this is often impaired during chronic liver injury. The existence of a dedicated liver stem cell population that acts as a source of regeneration during chronic liver injury has been controversial. Recent advances in transgenic models and cellular reprogramming have provided new insights into the plasticity of the liver epithelium and directions for the development of future therapies. This article will highlight recent findings about the cellular source of regeneration during liver injury and the advances in promoting liver regeneration.
Keywords: Liver regeneration, Stem cells, Cellular plasticity
Core tip: There have been controversies regarding the existence of a true liver stem cell population. Nevertheless, more evidence is beginning to reveal that cellular plasticity in the liver plays a role in liver regeneration. This mini-review highlights recent findings about plasticity in the liver epithelium.
INTRODUCTION
The liver is the largest internal organ in humans, and has exceptional regenerating capacity. The liver epithelium mainly consists of hepatocytes and cholangiocytes, with 70%-85% of the liver consisting of hepatocytes. Hepatocytes function in drug metabolism, protein synthesis and bile secretion. The biliary tree is a three-dimensional branching structure lined by cholangiocytes to form tubular networks. These networks transport bile for storage in the gall bladder, or to the duodenum for the breakdown of fats. The liver is relatively quiescent compared to other epithelial organs such as the skin and intestines, with minimal hepatocyte proliferation during homeostasis[1,2]. The average lifespan of a hepatocyte ranges from 200-400 d[3]. It is believed that the liver has a ‘’two-tiered’’ regeneration system, where hepatocytes proliferate and regenerate the liver upon acute liver damage, and the liver progenitors or oval cells orchestrate the regeneration process during chronic liver injury when hepatocyte proliferation is impaired[4]. Recent advances in lineage-tracing and imaging techniques have provided new perspectives in addition to the original ‘’two-tiered’’ idea. This review will focus on recent advances in stem cell biology and tissue repair to highlight the plasticity of the liver epithelium during regeneration.
REGENERATIVE POTENTIAL OF CHOLANGIOCYTES
The existence and the regenerative potential of hepatic progenitor cells (HPCs) or oval cells have been debatable. HPCs are a subpopulation of cells in the liver characterised by their oval shape and high nucleus to cytoplasm ratio when hepatocyte proliferation is inhibited[5]. Expansion of HPCs was observed in rat models of chronic liver injury in which hepatocyte proliferation is inhibited by the administration of 2-acetylaminofluorene (2-AAF). This was also observed upon hepatic injury such as carbon tetrachloride (CCl4) or partial hepatectomy (PHH)[6,7]. In addition to rat studies, HPC activation was also observed in mouse studies and human chronic liver disease[8-11]. In particular, the presence and contribution of a facultative liver stem cell population in human liver regeneration are highlighted by studies showing shared mitochondrial DNA mutations between HPC and the regenerative nodules in cirrhotic patients, suggesting their common origin[12,13].
HPC activation is a part of the dynamic change of the liver in response to chronic liver injury called ductular reaction (DR). Besides HPC activation, DR also includes immune cell infiltration, remodelling of the extracellular matrix, and myofibroblast activation[10,14,15]. Label-retaining assays that determine the cycling speed of the ductular cell populations have suggested multiple potential niches for slower cycling ductular cells after injury. These include the intralobular bile ducts, areas around periductal mononuclear cells, peribiliary hepatocytes, and the Canal of Hering[16]. However, most studies that characterised DR relied on immunohistochemistry of paraffin-embedded sections, which has the limitation of being two-dimensional and lacking spatial information. This leads to the confusion that the DR observed is the activation of endogenous liver stem cells that leave their stem cell niche, migrate towards the site of injury and differentiate into hepatocytes. However, an elegant study performed by Kamimoto and colleagues used three-dimensional imaging techniques to demonstrate that instead of leaving their niche and migrating, the biliary tree undergoes complex remodelling without diverging from the main structure[17,18]. Furthermore, they also observed that cholangiocytes do not proliferate uniformly, which fits with the previous label-retaining studies suggesting heterogeneity in the proliferative capacity of cholangiocyte populations. These indicate the presence of HPC populations within the biliary epithelium with different regenerative potentials. However, the identity of HPCs within cholangiocytes remains elusive, as there are no specific markers to differentiate HPCs from cholangiocytes[19,20]. Nevertheless, subpopulations of cholangiocytes have been identified with markers such as Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5), ST14, Trop2, neural cell adhesion molecule (NCAM), MIC-1C3, CD133, etc[21-26].
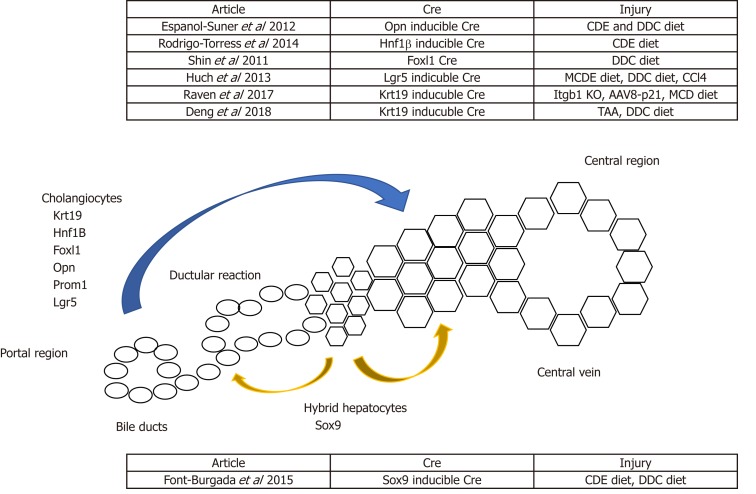
Advances in transgenic mice and gene editing technologies in the past decades have facilitated lineage-tracing studies of cholangiocytes in the background of chronic liver injury, which is mainly induced by the administration of special diets. For example, the choline-deficient, ethionine-supplemented (CDE) diet and the methionine choline-deficient (MCD) diet can induce hepatocellular injury[8,27]. Alternatively, cholestatic injury can be induced by the administration of a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet[28]. Lineage-tracing studies using the Cre-lox system and traceable proteins such as GFP, RFP and B-galactosidase facilitate the identification of the origin and regenerative potential of cholangiocytes during homeostasis, chronic liver injury and post-recovery. However, lineage-tracing studies performed by multiple groups showed mixed results about the regenerative potential of cholangiocytes[24,29-31]. Rodrigo-Torres et al[29] and Espanol-Suner et al[30] used a tamoxifen-inducible Cre system controlled under the Hepatocyte Nuclear Factor 1B (HNF1B) and Osteopontin (OPN) promoters, respectively, to label cholangiocytes during homeostasis. They both showed that cholangiocytes can differentiate into hepatocytes following chronic liver injury. However, the contribution of biliary cells towards hepatocytes are minimal in these studies (< 3%)[29,30]. On the contrary, when Cre is controlled under the transcription factor Forkhead box L1 (Foxl1) promoter, the degree of cholangiocyte-derived hepatocytes increases up to 29%, and ablation of the Foxl1 population results in impaired DR and liver regeneration[32-34]. The higher contribution from the Foxl1 population compared to the Hnf1b and the Opn population might be due to the former study’s use of a constitutive Cre system, and the latter study’s use of an inducible Cre system, in which the cell-labelling efficiency can be limited due to variable Cre penetrance. In contrast, the constitutive Cre system will always have the caveat that any cells that transiently expressed the promoter gene will be labelled, causing less accurate results. It is also worth noting that several studies do not show direct differentiation of cholangiocytes into hepatocytes following liver injury, which will be discussed in the sections below (Figure 1).
Figure 1.
Schematic summarising potential non-hepatocyte sources of regeneration. Lineage-tracing models using Cre recombinase controlled under a cholangiocyte-specific promoter have shown cholangiocytes giving rise to hepatocytes. Alternatively, Sox9+ hybrid hepatocytes have been shown to give rise to both cholangiocytes and hepatocytes.
The existence of HPCs remains controversial, largely due to the mixed results obtained from different lineage-tracing studies[31,35,36]. Technical factors such as the selection of the promoter that controls the expression of Cre recombinase is one of the causes for this discrepancy. For example, it has been shown that some hepatocytes express Sox9 after tamoxifen injection or liver injury, which may result in the labelling of hepatocytes, potentially leading to the erroneous conclusion that overestimates the contribution from cells with biliary origin[35,37-39]. Furthermore, it is known that hepatocytes have a propensity to express certain biliary markers like osteopontin upon stress[40]. It seems that biliary markers such as Krt19 are an exception to this phenomenon, as Krt19 is not expressed by hepatocytes following injury, making it one of the most common markers used for lineage-tracing studies[39]. It is worth investigating whether other biliary markers such as EpCAM or Trop2 share similar characteristics to Krt19 during liver injury[25]. Another potential reason for the discrepancy might be the variable in injury models applied. For example, different studies have used different percentages of ethionine in water (0.1%-0.15%)[33,35]. The level of ethionine controls the severity of injury by inhibiting hepatocyte proliferation, which is crucial for the activation and differentiation of cholangiocytes into hepatocytes. The level of injury can also be affected by age, gender, the intake of ethionine-supplemented water and special diets. As the intake of diets is highly variable across individual mice, this might lead to the discrepancy observed due to the high regenerative capacity of hepatocytes. A recent study performed by Deng et al[41] showed that Krt19-positive cells can differentiate into hepatocytes following long-term liver injury induced by the DDC diet. This shows the importance of selecting an appropriate injury model to investigate the true regenerative potential of cholangiocytes.
In comparison to dietary models, lineage-tracing studies involving liver injury induced by transgenic approaches appears to result in a more robust contribution of hepatocytes from non-hepatocyte sources[42,43]. Transgenic approaches to induce hepatocyte senescence through the deletion of Mdm2, a negative regulator of p53, has been developed to simulate human chronic liver disease. In this system, where the inducible Ah-Cre is controlled by the Cyp1a promoter, hepatocytes can be targeted by the administration of B-Naphthoflavone to trigger a senescence phenotype, hence causing a robust DR and the regeneration of the liver parenchyma contributed by a non-hepatocyte source[42,44]. Besides inducing cellular senescence, hepatocyte proliferation can also be disrupted by the deletion of the ItgB1 gene[45]. Hepatocytes that have defective ItgB1 have impaired proliferation due to disrupted HGF signalling[45]. Overexpression of p21, a cyclin-dependent kinase inhibitor, has also been used to impair hepatocyte proliferation through the delivery of AAV8 vector packaged with the p21 codon[43]. When combining the inhibition of hepatocyte proliferation (Itgb1 knockout or AAV8-p21 delivery) with dietary-induced chronic liver injury, biliary cells can commit to both biliary and hepatocyte fates[43]. Although the contribution of cholangiocytes into hepatocytes does not lead to full repopulation, it is still a significant contribution (25%) compared with previous models. Furthermore, suppression of the Wnt/β-catenin pathway in hepatocytes promotes the differentiation of cholangiocytes into hepatocytes[46]. However, it remains unclear whether full repopulation of the liver from cholangiocytes can be achieved. The loss of ItgB1, the Wnt pathway or p21 overexpression in all hepatocytes do not completely resemble the mechanisms of human liver disease. Nevertheless, the impairment of hepatocyte regenerative capacity promotes the differentiation of cholangiocytes as a proof-of-principle to demonstrate the regenerative capacity of cholangiocytes. Despite this, the complex regulatory process that triggers cholangiocytes to differentiate into hepatocytes remains to be identified. It was recently reported that Histone deacetylase 1 (HDAC1) regulates the commitment of cholangiocytes to biliary epithelial and hepatocyte fates through controlling Sox9 expression in zebrafish and mouse. This reveals extra molecular pathways that work in conjunction with the previously identified Wnt and Notch pathways in regulating liver regeneration[14,47]. Nevertheless, it remains to be tested whether promoting endogenous regeneration through the activation and differentiation of biliary cells to repopulate the liver parenchyma is plausible.
Besides the advances achieved in lineage-tracing studies, the development of the organoid culture system pioneered by the Clevers group revolutionised the field of regenerative medicine. First established in 2009 by Sato et al[48], organoid culture was used to culture intestinal stem cells that express the Wnt target gene leucine-rich-repeat-containing G protein-coupled receptor 5 (Lgr5). This technology was then applied by the same group to the liver to form liver organoids, and is now widely used by researchers for in vitro modelling, drug screening and gene sequencing[24,49,50]. Endogenous lineage-tracing of Lgr5-expressing cells using the Lgr5-IRES-creERT2 reporter mice showed that Lgr5-expressing cells can contribute to both cholangiocytes and hepatocytes after liver damage[24]. Interestingly, Lgr5 expression is not detected in healthy liver, but only detected in cholangiocytes after injury. This indicates that Lgr5 is transiently expressed in a subpopulation of cholangiocytes that activate Wnt signalling and repopulate the liver when required. However, the origin and identity of this trans-amplifying population in the liver during quiescence remains to be investigated. Identifying the origin of the Lgr5 population will reveal whether either there are predetermined residential liver stem cells, or cholangiocytes obtain regenerative capacity stochastically and regenerate the liver parenchyma during injury. Although the controversy regarding the existence of a dedicated liver stem cell population remains, subpopulations of cholangiocytes have been identified. Furthermore, it appears that the cells of the liver epithelium (i.e. cholangiocytes and hepatocytes) can obtain cellular plasticity and differentiate into the other compartment when one compartment is significantly damaged.
EXOGENOUS REPOPULATING CAPACITY OF CHOLANGIOCYTES
In contrast to lineage-tracing studies, there are more convincing results in transplanting isolated or in vitro-cultured cholangiocytes[42,51-53]. Transplantation assays have been widely used by the liver research community to prove the repopulating capacity of different cell populations. As the most effective treatment for liver disease is whole organ transplantation, it is common to seek alternatives using cells isolated from the liver to overcome the challenges faced in allogenic transplantations. Most transplantation assays are designed in a way that selective advantages are provided to the donor population, and this is often achieved by causing defects to the host hepatocytes. The most widely used models are the fumarylacetoacetate hydrolasemice (Fah)-deficient and the urokinase-type plasminogen activator-overexpressing (uPA) mice[54,55].
The Fah-deficient mice lack the functional fumarylacetoacetate hydrolase (Fah), a metabolic enzyme required for the last step of the tyrosine catabolism pathway. Fah mice develop liver diseases and die within 12 hours after birth from hypoglycaemia[54]. This defect can be corrected by blocking the accumulation of hepatotoxins through the administration of 2-(2-nitro-4-trifluoro-methylbenzyol)-1,3-cyclohexanedione (NTBC) to partially recover liver function[56]. The administration of NTBC prolongs the lifespan of Fah mice and enables this strain to be suitable for liver repopulation[57]. On the other hand, the uPA transgenic mice have the mouse urokinase-type plasminogen activator (uPA) gene under the control of the mouse albumin enhancer/promotor. Hepatocyte-specific expression of uPA causes liver toxicity and chronic liver injury, hence creating a selective advantage for transplanted hepatocytes[55,58]. However, the limitations of this model are the narrow time transplantation window after birth and the poor breeding efficiency of this strain. In addition, only homozygous animals are good recipients for liver cell transplantation[59]. Immunocompromised variants of these strains have also been developed, and transplantation of human or rodent hepatocytes has shown promising repopulation capacity after xenotransplantation[60-62].
With hepatocyte transplantation as the gold-standard for the liver-repopulating transplantation assay, the liver-repopulating capacity of isolated HPCs were investigated using similar assays. HPCs can be enriched by density gradient centrifugation from the liver non-parenchymal fraction after protease digestion[21,25,53]. Fluorescence-activated cell sorting (FACS) is often used to isolate a pure HPC population. However, HPCs do not have a specific marker and often share surface markers (e.g., c-kit, Thy-1) with other cell types such as haematopoietic cells[6,63,64]. This raised doubts about whether HPCs originate from the bone marrow, but transplantation assays and lineage-tracing studies have proven that HPCs originate from cholangiocytes and not the bone marrow[42,53]. Multiple surface markers have been used to isolate cholangiocyte subpopulations with liver-repopulating capacity, which include EpCAM, Trop2, CD24, CD133, LGR5, c-kit, CD44, Thy-1, etc[22,25,42,51,53,57]. Due to the relatively low numbers of isolated cells, most studies culture the isolated cells in vitro before transplantation. Upon transplantation, cells can differentiate into hepatocytes and self-renew[21,22,24,25,42,51,53,63]. However, the magnitude of repopulation is still relatively low compared to primary hepatocyte transplantation, which remains a challenge for using HPCs in cell therapy[65]. Refinements to increase the degree of repopulation, either by targeting the engraftment efficiency or the differentiation capacity, are required. Nevertheless, an advantage of using biliary-derived cells for transplantation is that cholangiocytes are more resistant to protease digestion than hepatocytes and can be cultured in vitro, which is difficult to achieve for primary hepatocytes. In addition to regenerating damaged hepatocytes, human liver organoids derived from cholangiocytes have also been used to regenerate extrabiliary bile ducts in vivo after being seeded with biodegradable scaffolds[66]. The use of cultured cholangiocytes to regenerate damaged liver epithelium seems promising. However, the heterogeneity of cells within the culture, and whether prolonged culture alters the characteristics and the long-term stability of the cells, need to be further investigated.
THE PLASTICITY OF HEPATOCYTES
The liver is deemed a highly regenerative organ mainly due to the remarkable regenerative capacity of hepatocytes. The proliferative capacity of hepatocytes is well characterised such that when acute liver injury occurs, hepatocytes initiate a series of pathways to restore the lost mass[67]. These studies are mostly performed using the PHH model, where a substantial portion of the liver is resected. It was commonly presumed that proliferation is the main mechanism used by hepatocytes to compensate for the loss of liver mass. However, a study by Miyaoka and colleagues show that hepatocyte hypertrophy (i.e. increase in hepatocyte size) contributes concurrently with proliferation to compensate for the loss of liver mass[68,69]. However, this regeneration process does not promote regrowth of the original resected lobes, but instead, the replacement of the original mass occurs at the remnant lobes. It has been showed that YAP/Hippo signalling plays a major role in controlling the restoration of the liver mass to its original size[70]. However, it needs to be noted that in the PHH model, the remnant lobes are not affected by any injury, which is uncommon in most clinical cases of acute liver failure. Patients have substantial inflammatory effects or senescence in the liver, such as paracetamol overdose.
The use of the Adeno-associated Virus (AAV) vector, a small (approximately 5 kb long), non-enveloped virus for transgene delivery specifically into hepatocytes, allows researchers to lineage-trace hepatocytes during homeostasis and injury[71]. Gao et al[72] isolated AAV serotype 8 (AAV8), which has a 10-100-fold higher liver tropism compared to other AAV serotypes. Malato et al[71] used the hepatotropism characteristic of the AAV8 to introduce Cre recombinase controlled under the hepatocyte-specific transthyretin (Ttr) promoter to target hepatocytes, which has a higher specificity than the widely used Alb-Cre. They lineage-traced the labelled hepatocytes under the context of homeostasis, acute liver injury, and biliary injury. During homeostasis and acute liver injury, they did not find any evidence that hepatocytes are derived from the biliary compartment. However, they observed a small amount of biliary-derived, periportal hepatocytes following bile duct ligation and DDC injury, suggesting a population within the cholangiocyte population that can differentiate into hepatocytes following injury. However, this conclusion is based on the authors’ observation of unlabelled hepatocytes located at the periportal region, instead of positively lineage-traced biliary cells. The same group went further to investigate whether cholangiocytes can differentiate into hepatocytes following chronic liver injury using the CDE diet regime, which predominantly damages hepatocytes[36]. Surprisingly, in contrast to the DDC model, the authors observed minimal (less than 1%) production of hepatocytes by cholangiocytes after chronic liver injury. This was further confirmed with a positive lineage-tracing model using CK19-CreER;R26R-RFP mice. Furthermore, the authors also showed that mesenchymal cells cannot differentiate into hepatocytes after chronic liver injury. Yanger et al[31] further investigated the source of newly formed hepatocytes following chronic liver injury using CK19-CreER; R26R-YFP mice by applying different types of injury, including DDC, CDE and CCl4 administration, as well as an alpha-naphthyl-isothiocyanate (ANIT) diet. Similar to what Schaub et al[36] observed, they did not detect any production of hepatocytes from YFP-labelled biliary cells after injury, regardless of the timing of cell labelling before or during injury. These suggest that quiescent cholangiocytes or activated atypical duct cells cannot differentiate into hepatocytes following injury. They further characterised the source of regenerating hepatocytes following liver injury using an AAV8 vector packaged with a Cre recombinase controlled under a hepatocyte-specific Thyroid Binding Globulin promoter (AAV8-TBG-Cre). Hepatocytes from R26R-YFP mice are labelled with YFP after AAV8-TBG-Cre administration. They observed no reduction in the amount of YFP-labelled hepatocytes following treatment with the panel of injury models mentioned above, including the widely used CDE and DDC. This suggested that the newly formed hepatocytes derived from self-duplication of pre-existing hepatocytes rather than from other cell populations[31].
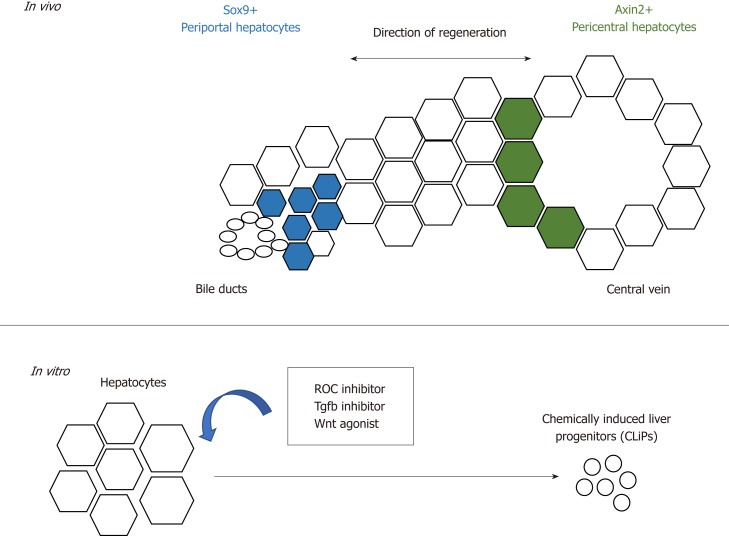
These observations require further follow-up investigation as to whether there is a population of hepatocytes that possess a higher regenerative capacity[31,35,36,71]. Wang et al[73] reported that a population of pericentral hepatocytes that express the Wnt responsive gene Axin2 migrate towards the periportal region during homeostasis. This Axin2+ population also expresses the hepatoblast marker Tbx3, and differentiates into Tbx3-negative hepatocytes. Furthermore, the Axin2+ population proliferates faster, is mostly diploid and its Axin2 expression is dependent on the Wnt signals provided by the endothelium at the central vein. This reawakened the streaming liver hypothesis proposed by Zajicek et al[74] 30 years ago, which claims that hepatocytes from the portal region gradually advance towards the central region during homeostasis. In this study, however, the stream starts from the opposite direction instead (Figure 2). However, recent studies showed that despite the presence of Wnt activity in the pericentral hepatocytes, this population does not possess proliferative advantages over other hepatocyte populations during homeostasis and acute injury[75]. Instead, proliferation rates across the three acinar zones are similar, and regeneration is performed by hepatocytes with high levels of telomerase expression[76].
Figure 2.
Schematic summarising the regenerative potential of hepatocytes. In vivo, periportal and pericentral hepatocytes regenerate the liver with different regenerative mechanisms. In vitro, hepatocytes can be converted into liver progenitors with extrinsic factors.
Another report by Font-Burgada et al[77] suggests that a population of hepatocytes named hybrid hepatocytes (HybHP) resides close to the biliary epithelium and expresses Sox9 during homeostasis. In both chronic liver injury models of CCl4 administration as well as the MUP-uPA transgenic model, HybHP repopulate the damaged population and manage to differentiate into zone 3 hepatocytes with the expression of glutamine synthetase. Interestingly, under the CDE diet regime, they found that most HybHP were killed, but no contribution of hepatocytes from the biliary cells was observed albeit the existence of extensive DR. Furthermore, they found HybHP can transdifferentiate into biliary-like cells that express biliary markers like Sox9, Opn, and Ck19 when induced with cholestatic injury from a DDC diet. Transcriptional analysis revealed the hybrid character of HybHP, as this population expresses a cluster but not all of genes that are usually expressed by cholangiocytes. However, it remains to be investigated whether this population can fully differentiate into mature cholangiocytes, and this might explain the differences in the observations made by Malato et al[71] and Yanger et al about whether hepatocytes can differentiate into cholangiocytes after injury. Upon transplantation into the immunodeficient Fah-/-Rag2-/-Il2rg-/- transgenic model, HybHP exhibit higher repopulation capacity than conventional hepatocytes or HPC. Despite their regenerative capacity, neither HybHP nor HPC preferentially give rise to hepatocellular carcinoma (HCC), suggesting tumorigenesis is a random event that occurs equally in all cell types rather than preferentially in certain cell populations. In addition, the HybHP phenotype was also observed by Tarlow et al[78] after chronic liver injury, in which hepatocytes can obtain a biliary phenotype with the expression of the cholangiocyte marker MIC-1C3 (hepPD). Although they share similar surface marker expression, the transcriptional profile of hepatocytes and biliary-derived MIC-1C3-expressing cells are different, and these two populations behave differently in vitro, with the former maintaining a more hepatic-like profile. These Sox9-expressing hepPD cells can also differentiate back into hepatocytes after injury[78]. Both the Tarlow and Font-Burgada studies demonstrated the plasticity of hepatocytes after injury, and the heterogeneity of hepatocytes within the liver does not only restrict to their zonation and drug metabolic functions, but also occurs in the context of their regenerative potential. Interestingly, the conversion of hepatocytes into biliary-like cells was observed in the DDC model but not in the Thioacetamide (TAA) model[18]. This might due to the pathophysiology caused by different injury models, in which the DDC is a model for cholestatic liver injury whilst the TAA model is a hepatotoxic injury model. This highlights the plasticity in the liver when cholestatic injury occurs, and that hepatocytes can transdifferentiate into biliary-like cells due to damage in the biliary compartment. On the other hand, when hepatocyte proliferation is impaired, cholangiocytes can differentiate into hepatocytes.
The plasticity of hepatocytes after chronic liver injury is also recapitulated in vitro by the conversion of mature hepatocytes in mice and rats into progenitor-like cells using a cocktail of small molecules that consists of ROCK-inhibitor(Y-27632), TGF-β inhibitor (A-83-01) and Wnt agonist (CHIR99021)[79] (Figure 2). The chemically-induced liver progenitors (CLiPs) can be cultured long-term, as well as differentiate into hepatocytes and biliary cells in vitro and in vivo after transplantation. The development of CLiPs has overcome the limitation that primary hepatocytes could not maintained be in prolonged culture in vitro, and the low cell number of the regenerative progenitor cells in vivo. This opens a new possibility of using CLiPs for cell therapy. Whether all hepatocytes or only a subpopulation can be reprogrammed into CLiPs, and the applicability of using human hepatocytes for CLiPs generation, remains to be investigated in order to translate this into future therapy.
CELLULAR REPROGRAMMING FOR LIVER REGENERATION
The 2006 discovery of induced pluripotent stem cells (iPSCs) by the expression of Yamanaka factors (Oct3/4, Sox2, Klf4, c-Myc) in fibroblasts sparked broad interest in cellular reprogramming[80]. Hepatocyte-like cells have been generated from embryonic stem cells (ESCs) and iPSCs using multistep protocols in vitro[81-83]. When co-cultured with other cell types such as mesenchymal and endothelial cells, iPSC-derived hepatocytes can form three-dimensional ‘’liver buds’’. The liver buds can form vascular networks with the host vasculature after cranial transplantation[84]. These cells are potentially promising for drug screening, disease modelling, and cell replacement therapy. However, ethical issues, efficacy and long-term stability of these cells in vivo remains a challenge to translate these into therapy[85,86]. Besides using ESC/iPSC-derived hepatocytes, researchers also considered the possibility of bypassing the reprogramming into pluripotency by shortening the multistep reprogramming protocol, or even directly converting adult somatic cells into functional hepatocytes[87,88]. The overexpression of two transcription factors including Hnf4a with the combination of either Foxa1, Foxa2, or Foxa3 can induce reprogramming in vitro of cultured mouse embryonic and adult fibroblasts into hepatocyte-like (iHep) cells. These cells maintained hepatic function in vitro and contribute to the regeneration of the liver parenchyma after transplantation[88]. Direct reprogramming of hepatic myofibroblasts into hepatocytes in vivo with adenovirus has also shown decreased fibrosis in mice[89]. Advances in cellular reprogramming have opened a new area of regenerative medicine. However, the efficiency, epigenetic memory and stability of transdifferentiated hepatocytes remains a challenge for the therapeutic use of cells generated by genetic reprogramming.
CONCLUSION AND FUTURE DIRECTIONS
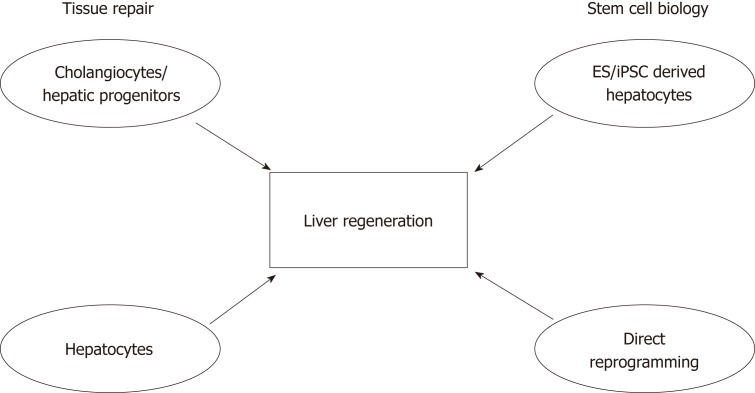
A lot has been learned about liver plasticity in recent years, especially regarding hepatocytes. It appears that cellular plasticity in the liver is bidirectional and injury-dependent, where hepatocytes are able to transdifferentiate into biliary-like cells under certain conditions. However, when the proliferative capacity of hepatocytes is impaired, cholangiocytes become the facultative liver stem cells that differentiate into hepatocytes. It remains to be investigated whether compartmentalised stem cell populations exist within the liver as in other epithelial organs, or if the acquisition of stem cell properties by cholangiocytes is a stochastic event[90,91]. Nevertheless, it seems that the plasticity of hepatocytes and cholangiocytes reflects what has been observed in other organs such as the skin and intestines, where residential stem cells acquire plasticity and differentiate after injury[90,92]. Future studies involving next generation sequencing at the single-cell level will provide us with new perspectives on cell identity and the involvement of epigenetic alterations during regeneration. Advancements in our understanding of tissue repair, cellular reprogramming and bioengineering will be beneficial for developing models for drug screening, and may even translate into cell therapy. Modulation of the regenerative niche will hopefully enhance the endogenous regenerative capacity of the liver (Figure 3).
Figure 3.
Schematic showing the importance of understanding tissue repair and stem cell biology, and their contributions to developing future therapies to promote liver regeneration. Studies focusing on understanding the mechanisms of tissue repair have suggested that cell populations with high cellular plasticity include both cells of the biliary tree and hepatocytes. The fields of stem cell biology and regenerative medicine have used signals and key factors required during embryonic development to produce hepatocyte-like cells derived from either adult or embryonic sources. In conjunction, these will advance the liver regeneration field.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: All authors declare no conflicts-of-interest related to this article.
Peer-review started: October 26, 2018
First decision: November 29, 2018
Article in press: January 26, 2019
P- Reviewer: Liu AD, Ohkoshi S S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
Contributor Information
Atsunori Tsuchiya, Division of Gastroenterology and Hepatology, Graduate school of medical and dental sciences, Niigata University, Chuo-ku, Niigata 951-8510, Japan.
Wei-Yu Lu, Centre for Liver and Gastrointestinal Research, Institute of Immunology and Immunotherapy, the University of Birmingham, Birmingham B15 2TT, United Kingdom. w.lu.3@bham.ac.uk.
References
- 1.Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561–574. doi: 10.1016/j.stem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald RA. "Lifespan" of liver cells. Autoradio-graphic study using tritiated thymidine in normal, cirrhotic, and partially hepatectomized rats. Arch Intern Med. 1961;107:335–343. doi: 10.1001/archinte.1961.03620030023003. [DOI] [PubMed] [Google Scholar]
- 4.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692–694. doi: 10.1016/j.jhep.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–148. [PubMed] [Google Scholar]
- 6.Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- 7.Petersen BE, Zajac VF, Michalopoulos GK. Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology. 1998;27:1030–1038. doi: 10.1002/hep.510270419. [DOI] [PubMed] [Google Scholar]
- 8.Akhurst B, Croager EJ, Farley-Roche CA, Ong JK, Dumble ML, Knight B, Yeoh GC. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology. 2001;34:519–522. doi: 10.1053/jhep.2001.26751. [DOI] [PubMed] [Google Scholar]
- 9.Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol. 1998;29:455–463. doi: 10.1016/s0168-8278(98)80065-2. [DOI] [PubMed] [Google Scholar]
- 10.Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15:259–269. [PubMed] [Google Scholar]
- 11.Theise ND, Kuwahara R. The tissue biology of ductular reactions in human chronic liver disease. Gastroenterology. 2007;133:350–352. doi: 10.1053/j.gastro.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 12.Lin WR, Lim SN, McDonald SA, Graham T, Wright VL, Peplow CL, Humphries A, Kocher HM, Wright NA, Dhillon AP, Alison MR. The histogenesis of regenerative nodules in human liver cirrhosis. Hepatology. 2010;51:1017–1026. doi: 10.1002/hep.23483. [DOI] [PubMed] [Google Scholar]
- 13.Fellous TG, Islam S, Tadrous PJ, Elia G, Kocher HM, Bhattacharya S, Mears L, Turnbull DM, Taylor RW, Greaves LC, Chinnery PF, Taylor G, McDonald SA, Wright NA, Alison MR. Locating the stem cell niche and tracing hepatocyte lineages in human liver. Hepatology. 2009;49:1655–1663. doi: 10.1002/hep.22791. [DOI] [PubMed] [Google Scholar]
- 14.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bird TG, Lu WY, Boulter L, Gordon-Keylock S, Ridgway RA, Williams MJ, Taube J, Thomas JA, Wojtacha D, Gambardella A, Sansom OJ, Iredale JP, Forbes SJ. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci USA. 2013;110:6542–6547. doi: 10.1073/pnas.1302168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47:1994–2002. doi: 10.1002/hep.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneko K, Kamimoto K, Miyajima A, Itoh T. Adaptive remodeling of the biliary architecture underlies liver homeostasis. Hepatology. 2015;61:2056–2066. doi: 10.1002/hep.27685. [DOI] [PubMed] [Google Scholar]
- 18.Kamimoto K, Kaneko K, Kok CY, Okada H, Miyajima A, Itoh T. Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. Elife. 2016:5. doi: 10.7554/eLife.15034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki A, Sekiya S, Onishi M, Oshima N, Kiyonari H, Nakauchi H, Taniguchi H. Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology. 2008;48:1964–1978. doi: 10.1002/hep.22558. [DOI] [PubMed] [Google Scholar]
- 20.Paku S, Schnur J, Nagy P, Thorgeirsson SS. Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol. 2001;158:1313–1323. doi: 10.1016/S0002-9440(10)64082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, Smirnova O, Duncan AW, Finegold MJ, Sander M, Kaestner KH, Grompe M. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. 2011;25:1193–1203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rountree CB, Barsky L, Ge S, Zhu J, Senadheera S, Crooks GM. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25:2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Dorrell C, Canaday PS, Pelz C, Haft A, Finegold M, Grompe M. Adult Mouse Liver Contains Two Distinct Populations of Cholangiocytes. Stem Cell Reports. 2017;9:478–489. doi: 10.1016/j.stemcr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, Nakamura K, Miyajima A. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–1960. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya A, Lu WY, Weinhold B, Boulter L, Stutchfield BM, Williams MJ, Guest RV, Minnis-Lyons SE, MacKinnon AC, Schwarzer D, Ichida T, Nomoto M, Aoyagi Y, Gerardy-Schahn R, Forbes SJ. Polysialic acid/neural cell adhesion molecule modulates the formation of ductular reactions in liver injury. Hepatology. 2014;60:1727–1740. doi: 10.1002/hep.27099. [DOI] [PubMed] [Google Scholar]
- 27.Itagaki H, Shimizu K, Morikawa S, Ogawa K, Ezaki T. Morphological and functional characterization of non-alcoholic fatty liver disease induced by a methionine-choline-deficient diet in C57BL/6 mice. Int J Clin Exp Pathol. 2013;6:2683–2696. [PMC free article] [PubMed] [Google Scholar]
- 28.Fickert P, Stöger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, Tsybrovskyy O, Jaeschke H, Zatloukal K, Denk H, Trauner M. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigo-Torres D, Affò S, Coll M, Morales-Ibanez O, Millán C, Blaya D, Alvarez-Guaita A, Rentero C, Lozano JJ, Maestro MA, Solar M, Arroyo V, Caballería J, van Grunsven LA, Enrich C, Ginès P, Bataller R, Sancho-Bru P. The biliary epithelium gives rise to liver progenitor cells. Hepatology. 2014;60:1367–1377. doi: 10.1002/hep.27078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Español-Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, Lemaigre F, Leclercq IA. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575.e7. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, Pikarsky E, Stanger BZ. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sackett SD, Gao Y, Shin S, Esterson YB, Tsingalia A, Hurtt RS, Brondell K, Kaestner KH, Greenbaum LE. Foxl1 promotes liver repair following cholestatic injury in mice. Lab Invest. 2009;89:1387–1396. doi: 10.1038/labinvest.2009.103. [DOI] [PubMed] [Google Scholar]
- 33.Shin S, Upadhyay N, Greenbaum LE, Kaestner KH. Ablation of Foxl1-Cre-labeled hepatic progenitor cells and their descendants impairs recovery of mice from liver injury. Gastroenterology. 2015;148:192–202.e3. doi: 10.1053/j.gastro.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, Smirnova O, Dorrell C, Erker L, Chu AS, Wells RG, Grompe M, Greenbaum LE, Kaestner KH. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev. 2011;25:1185–1192. doi: 10.1101/gad.2027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarlow BD, Finegold MJ, Grompe M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology. 2014;60:278–289. doi: 10.1002/hep.27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaub JR, Malato Y, Gormond C, Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–939. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpentier R, Suñer RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438, 1438.e1-1438.e4. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 39.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, Wells RG, Greenbaum LE, Stanger BZ. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coombes J, Syn WK. Utility of osteopontin in lineage tracing experiments. Gastroenterology. 2013;145:254–255. doi: 10.1053/j.gastro.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 41.Deng X, Zhang X, Li W, Feng RX, Li L, Yi GR, Zhang XN, Yin C, Yu HY, Zhang JP, Lu B, Hui L, Xie WF. Chronic Liver Injury Induces Conversion of Biliary Epithelial Cells into Hepatocytes. Cell Stem Cell. 2018;23:114–122.e3. doi: 10.1016/j.stem.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, Wojtacha D, Man TY, Mackinnon A, Ridgway RA, Kendall T, Williams MJ, Jamieson T, Raven A, Hay DC, Iredale JP, Clarke AR, Sansom OJ, Forbes SJ. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raven A, Lu WY, Man TY, Ferreira-Gonzalez S, O'Duibhir E, Dwyer BJ, Thomson JP, Meehan RR, Bogorad R, Koteliansky V, Kotelevtsev Y, Ffrench-Constant C, Boulter L, Forbes SJ. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, Winton DJ. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Speicher T, Siegenthaler B, Bogorad RL, Ruppert R, Petzold T, Padrissa-Altes S, Bachofner M, Anderson DG, Koteliansky V, Fässler R, Werner S. Knockdown and knockout of β1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat Commun. 2014;5:3862. doi: 10.1038/ncomms4862. [DOI] [PubMed] [Google Scholar]
- 46.Russell JO, Lu WY, Okabe H, Abrams M, Oertel M, Poddar M, Singh S, Forbes SJ, Monga SP. Hepatocyte-Specific β-Catenin Deletion During Severe Liver Injury Provokes Cholangiocytes to Differentiate Into Hepatocytes. Hepatology. 2018 doi: 10.1002/hep.30270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ko S, Russell JO, Tian J, Gao C, Kobayashi M, Feng R, Yuan X, Shao C, Ding H, Poddar M, Singh S, Locker J, Weng HL, Monga SP, Shin D. Hdac1 Regulates Differentiation of Bipotent Liver Progenitor Cells During Regeneration via Sox9b and Cdk8. Gastroenterology. 2019;156:187–202.e14. doi: 10.1053/j.gastro.2018.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 49.Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R, Sidorova O, Gaspersz MP, Georgakopoulos N, Koo BK, Dietmann S, Davies SE, Praseedom RK, Lieshout R, IJzermans JNM, Wigmore SJ, Saeb-Parsy K, Garnett MJ, van der Laan LJ, Huch M. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–1435. doi: 10.1038/nm.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J, van de Wetering M, Sasaki N, Boers SJ, Kemperman H, de Jonge J, Ijzermans JN, Nieuwenhuis EE, Hoekstra R, Strom S, Vries RR, van der Laan LJ, Cuppen E, Clevers H. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu Q, Hernandez JC, Dean AM, Rao PH, Darlington GJ. CD24-positive cells from normal adult mouse liver are hepatocyte progenitor cells. Stem Cells Dev. 2011;20:2177–2188. doi: 10.1089/scd.2010.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanimizu N, Ichinohe N, Yamamoto M, Akiyama H, Nishikawa Y, Mitaka T. Progressive induction of hepatocyte progenitor cells in chronically injured liver. Sci Rep. 2017;7:39990. doi: 10.1038/srep39990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grompe M, al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 1993;7:2298–2307. doi: 10.1101/gad.7.12a.2298. [DOI] [PubMed] [Google Scholar]
- 55.Heckel JL, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Neonatal bleeding in transgenic mice expressing urokinase-type plasminogen activator. Cell. 1990;62:447–456. doi: 10.1016/0092-8674(90)90010-c. [DOI] [PubMed] [Google Scholar]
- 56.Grompe M, Lindstedt S, al-Dhalimy M, Kennaway NG, Papaconstantinou J, Torres-Ramos CA, Ou CN, Finegold M. Pharmacological correction of neonatal lethal hepatic dysfunction in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1995;10:453–460. doi: 10.1038/ng0895-453. [DOI] [PubMed] [Google Scholar]
- 57.Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 58.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- 59.Tateno C, Yoshizane Y, Saito N, Kataoka M, Utoh R, Yamasaki C, Tachibana A, Soeno Y, Asahina K, Hino H, Asahara T, Yokoi T, Furukawa T, Yoshizato K. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol. 2004;165:901–912. doi: 10.1016/S0002-9440(10)63352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dandri M, Burda MR, Török E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, Greten H, Petersen J. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- 62.Meuleman P, Vanlandschoot P, Leroux-Roels G. A simple and rapid method to determine the zygosity of uPA-transgenic SCID mice. Biochem Biophys Res Commun. 2003;308:375–378. doi: 10.1016/s0006-291x(03)01388-3. [DOI] [PubMed] [Google Scholar]
- 63.Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest. 1994;70:511–516. [PubMed] [Google Scholar]
- 64.Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632–640. doi: 10.1053/jhep.2003.50104. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Shao Y, Li L, Tian F, Cen J, Chen X, Hu D, Zhou Y, Xie W, Zheng Y, Ji Y, Liu M, Li D, Hui L. Efficient liver repopulation of transplanted hepatocyte prevents cirrhosis in a rat model of hereditary tyrosinemia type I. Sci Rep. 2016;6:31460. doi: 10.1038/srep31460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sampaziotis F, Justin AW, Tysoe OC, Sawiak S, Godfrey EM, Upponi SS, Gieseck RL, 3rd, de Brito MC, Berntsen NL, Gómez-Vázquez MJ, Ortmann D, Yiangou L, Ross A, Bargehr J, Bertero A, Zonneveld MCF, Pedersen MT, Pawlowski M, Valestrand L, Madrigal P, Georgakopoulos N, Pirmadjid N, Skeldon GM, Casey J, Shu W, Materek PM, Snijders KE, Brown SE, Rimland CA, Simonic I, Davies SE, Jensen KB, Zilbauer M, Gelson WTH, Alexander GJ, Sinha S, Hannan NRF, Wynn TA, Karlsen TH, Melum E, Markaki AE, Saeb-Parsy K, Vallier L. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017;23:954–963. doi: 10.1038/nm.4360. [DOI] [PubMed] [Google Scholar]
- 67.Alison MR, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. J Pathol. 2009;217:282–298. doi: 10.1002/path.2453. [DOI] [PubMed] [Google Scholar]
- 68.Miyaoka Y, Miyajima A. To divide or not to divide: revisiting liver regeneration. Cell Div. 2013;8:8. doi: 10.1186/1747-1028-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22:1166–1175. doi: 10.1016/j.cub.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 71.Malato Y, Naqvi S, Schürmann N, Ng R, Wang B, Zape J, Kay MA, Grimm D, Willenbring H. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest. 2011;121:4850–4860. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao GP, Lu F, Sanmiguel JC, Tran PT, Abbas Z, Lynd KS, Marsh J, Spinner NB, Wilson JM. Rep/Cap gene amplification and high-yield production of AAV in an A549 cell line expressing Rep/Cap. Mol Ther. 2002;5:644–649. doi: 10.1006/mthe.2001.0591. [DOI] [PubMed] [Google Scholar]
- 73.Wang B, Zhao L, Fish M, Logan CY, Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zajicek G, Oren R, Weinreb M., Jr The streaming liver. Liver. 1985;5:293–300. doi: 10.1111/j.1600-0676.1985.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 75.Planas-Paz L, Orsini V, Boulter L, Calabrese D, Pikiolek M, Nigsch F, Xie Y, Roma G, Donovan A, Marti P, Beckmann N, Dill MT, Carbone W, Bergling S, Isken A, Mueller M, Kinzel B, Yang Y, Mao X, Nicholson TB, Zamponi R, Capodieci P, Valdez R, Rivera D, Loew A, Ukomadu C, Terracciano LM, Bouwmeester T, Cong F, Heim MH, Forbes SJ, Ruffner H, Tchorz JS. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol. 2016;18:467–479. doi: 10.1038/ncb3337. [DOI] [PubMed] [Google Scholar]
- 76.Lin S, Nascimento EM, Gajera CR, Chen L, Neuhöfer P, Garbuzov A, Wang S, Artandi SE. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature. 2018;556:244–248. doi: 10.1038/s41586-018-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Font-Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, Taniguchi K, Nakagawa H, Valasek MA, Ye L, Kopp JL, Sander M, Carter H, Deisseroth K, Verma IM, Karin M. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Katsuda T, Kawamata M, Hagiwara K, Takahashi RU, Yamamoto Y, Camargo FD, Ochiya T. Conversion of Terminally Committed Hepatocytes to Culturable Bipotent Progenitor Cells with Regenerative Capacity. Cell Stem Cell. 2017;20:41–55. doi: 10.1016/j.stem.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 80.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 81.Sampaziotis F, de Brito MC, Madrigal P, Bertero A, Saeb-Parsy K, Soares FAC, Schrumpf E, Melum E, Karlsen TH, Bradley JA, Gelson WT, Davies S, Baker A, Kaser A, Alexander GJ, Hannan NRF, Vallier L. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat Biotechnol. 2015;33:845–852. doi: 10.1038/nbt.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hay DC, Zhao D, Fletcher J, Hewitt ZA, McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA, Wolf R, Cui W. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- 83.Cameron K, Tan R, Schmidt-Heck W, Campos G, Lyall MJ, Wang Y, Lucendo-Villarin B, Szkolnicka D, Bates N, Kimber SJ, Hengstler JG, Godoy P, Forbes SJ, Hay DC. Recombinant Laminins Drive the Differentiation and Self-Organization of hESC-Derived Hepatocytes. Stem Cell Reports. 2015;5:1250–1262. doi: 10.1016/j.stemcr.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 85.Woo DH, Kim SK, Lim HJ, Heo J, Park HS, Kang GY, Kim SE, You HJ, Hoeppner DJ, Kim Y, Kwon H, Choi TH, Lee JH, Hong SH, Song KW, Ahn EK, Chenoweth JG, Tesar PJ, McKay RD, Kim JH. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology. 2012;142:602–611. doi: 10.1053/j.gastro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 86.Baxter M, Withey S, Harrison S, Segeritz CP, Zhang F, Atkinson-Dell R, Rowe C, Gerrard DT, Sison-Young R, Jenkins R, Henry J, Berry AA, Mohamet L, Best M, Fenwick SW, Malik H, Kitteringham NR, Goldring CE, Piper Hanley K, Vallier L, Hanley NA. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 2015;62:581–589. doi: 10.1016/j.jhep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu S, Rezvani M, Harbell J, Mattis AN, Wolfe AR, Benet LZ, Willenbring H, Ding S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508:93–97. doi: 10.1038/nature13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 89.Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, Reetz J, Brandes S, Dai Z, Pützer BM, Araúzo-Bravo MJ, Steinemann D, Luedde T, Schwabe RF, Manns MP, Schöler HR, Schambach A, Cantz T, Ott M, Sharma AD. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell Stem Cell. 2016;18:797–808. doi: 10.1016/j.stem.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Donati G, Rognoni E, Hiratsuka T, Liakath-Ali K, Hoste E, Kar G, Kayikci M, Russell R, Kretzschmar K, Mulder KW, Teichmann SA, Watt FM. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat Cell Biol. 2017;19:603–613. doi: 10.1038/ncb3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ritsma L, Ellenbroek SIJ, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]