Abstract
BACKGROUND
The current case report describes successful phacoemulsification with the aid of perioperative topical ascorbic acid (AA) in two patients with corneal endothelial disorders to prevent postoperative corneal endothelial decompensation.
CASE SUMMARY
Two eyes of two patients underwent phacoemulsification with pre-existing corneal endothelial disorders including Fuchs corneal endothelial dystrophy (Patient 1) and endotheliitis (Patient 2). Topical AA was applied to both patients at least one month before and after with a frequency of four times per day. After the surgery, both eyes improved best-corrected visual acuity (BCVA) and there was limited human corneal endothelial cell loss without signs of corneal endothelial decompensation, such as deteriorated BCVA or persistent corneal edema during the follow-up of at least two years.
CONCLUSION
Perioperative administration of topical AA may be an alternative therapy to the triple procedure in patients expecting to undergo cataract surgery.
Keywords: Ascorbic acid, Fuchs corneal endothelial dystrophy, Endotheliitis, Human corneal endothelial cell, Phacoemulsification, Case report
Core tip: Perioperative topical ascorbic acid (AA) was instilled in two patients with low corneal endothelial cell density who were scheduled for cataract surgery. After the surgery, both patients showed improved visual acuity without corneal decompensation. Perioperative topical AA is promising for prevention of corneal endothelial dysfunction in high-risk patients undergoing phacoemulsification; it may be considered as an alternative therapy.
INTRODUCTION
Phacoemulsification has been used to treat cataract for decades, while human corneal endothelial cells (HCECs) loss remains a major complication[1]. Fuchs endothelial corneal dystrophy (FECD) is a dystrophic disorder of HCECs that are vulnerable to external damage[2]. Recently, corneal endotheliitis has been demonstrated to be an inflammatory process with viral origin, e.g., cytomegalovirus (CMV), that destroys HCECs and leads to severe visual impairment[3]. Because corneal decompensation with severe HCECs loss is common in patients with impaired HCECs, including corneal endothelial dystrophy, post-traumatic status and corneal endotheliitis[4,5], adequate management is advocated to prevent corneal decompensation after phacoemulsification.
Triple procedures, combining endothelial keratoplasty, phacoemulsification and intraocular lens implantation, have been successfully performed in patients with concomitant cataract and corneal decompensation[6], while limited sources of corneal donor tissues result in long waiting lists[7]. Also, several complications following triple procedure have been reported, such as secondary glaucoma, graft dislocation requiring re-surgery and graft rejection leading to graft failure[8,9]. We therefore wonder if there are potential strategies to prevent corneal endothelial loss other than surgical triple procedures.
Theoretically, ascorbic acid (AA) can be an ideal ocular nutritional supplement[10]. Two in vitro studies have shown that AA and L-AA 2-phosphate prolonged the lifespan and inhibited apoptosis of murine corneal endothelial cells[11] and HCECs[12], probably through anti-oxidant effects. On the other hand, topical AA can be used alone to manage alkali burns which decrease the incidence of corneal ulcerations and perforation in rabbits[13], or combined with topical steroids to treat alkali burns without significant side effects[14,15]. Furthermore, animal studies showed that AA applied to the irrigating solution can serve as free-radical scavengers to prevent endothelial cell damage from phacoemulsification[16,17]. Still, it remains unclear whether topical AA can prevent HCECs loss after phacoemulsification. In addition, although AA has been used for protective agent of HCECs by intraoperative or postoperative administration[17-19], the perioperative instillation of AA has not been used before.
Herein, we report the use of topical AA before and after cataract surgery to reduce HCECs loss in patients with pre-existing corneal endothelial disorders. The novel effect of perioperative topical AA, which obviated the need for corneal transplantation, is demonstrated in two patients with FECD and CMV corneal endotheliitis respectively.
CASE PRESENTATION
Chief complaint
Patient 1: A 41-year-old man presented with blurry vision in the right eye.
Patient 2: A 51-year-old man was referred to our clinic due to whitish left cornea.
History of present illness
Patient 1: The symptom persisted for months without exacerbating or relieving factors.
Patient 2: The symptom persisted for 3 wk and had gradually progressed.
History of past illness
Patient 1: The patient reported no known systemic illness or previous surgery.
Patient 2: The patient revealed no known systemic illness or previous surgery.
Personal and family history
Patient 1: The patient revealed no known personal and family history.
Patient 2: The patient revealed no known personal and family history.
Physical examination
Patient 1: The best-corrected visual acuity (BCVA) in the right eye was 20/60. Slit lamp examination revealed cataract with right predominance.
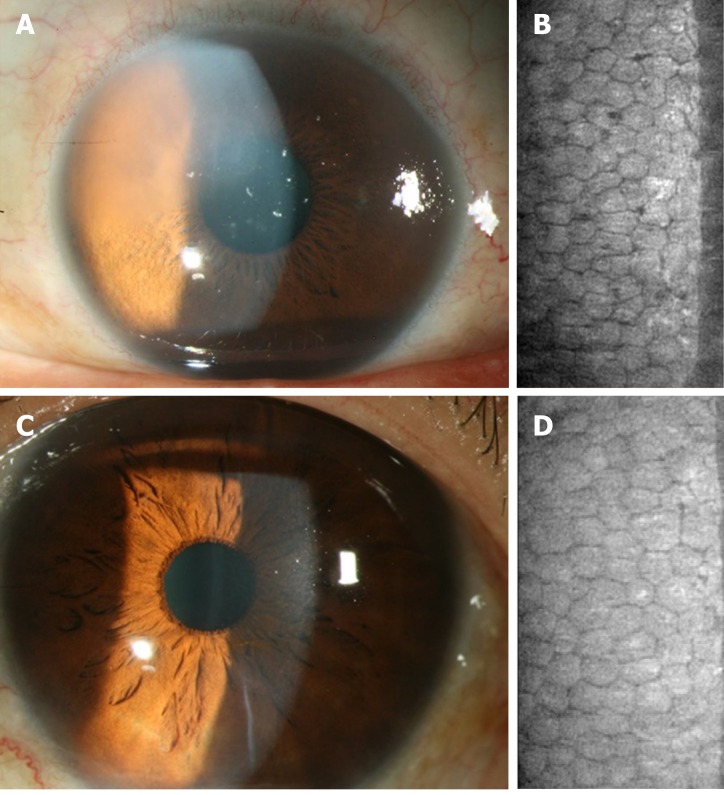
Patient 2: On examination, BCVA was 20/50 in the left eye and the silt-lamp microscope revealed bilateral cataract with mild corneal edema in the left eye (Figure 1A).
Figure 1.
The corneal endothelium condition before and after cataract surgery in the left eye of Patient 2. A: Preoperative corneal appearance via silt-lamp biomicroscope; B: Preoperative corneal endothelial cell density via specular microscope; C: Postoperative corneal appearance via silt-lamp biomicroscope; D: Postoperative corneal endothelial cell density via specular microscope.
Laboratory examinations
Patient 1: No laboratory examination was performed due to the absence of indication.
Patient 2: Intracameral tapping of left eye was arranged, and real-time quantitative polymerase chain reaction exam reported a positive result for CMV.
Imaging examinations
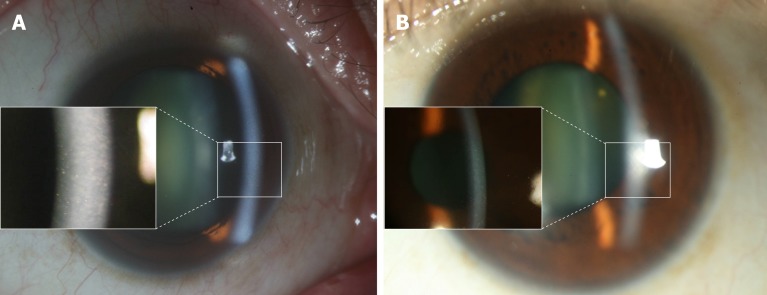
Patient 1: Specular microscopy showed bilateral guttate formations (Figure 2).
Figure 2.
The appearance of Fuchs endothelial corneal dystrophy in Patient 1. A: External eye appearance and guttate formation (left bracket) in the right eye; B: External eye appearance and guttate formation (left bracket) in the left eye.
Patient 2: The specular microscope revealed ECD of 1273/mm2 with disciform lesions in the left eye.
FINAL DIAGNOSIS
Patient 1: The final diagnosis of the case was bilateral cataract with right predominant and bilateral FECD.
Patient 2: The final diagnosis of the case was bilateral cataracts and CMV endotheliitis in the left eye.
TREATMENT
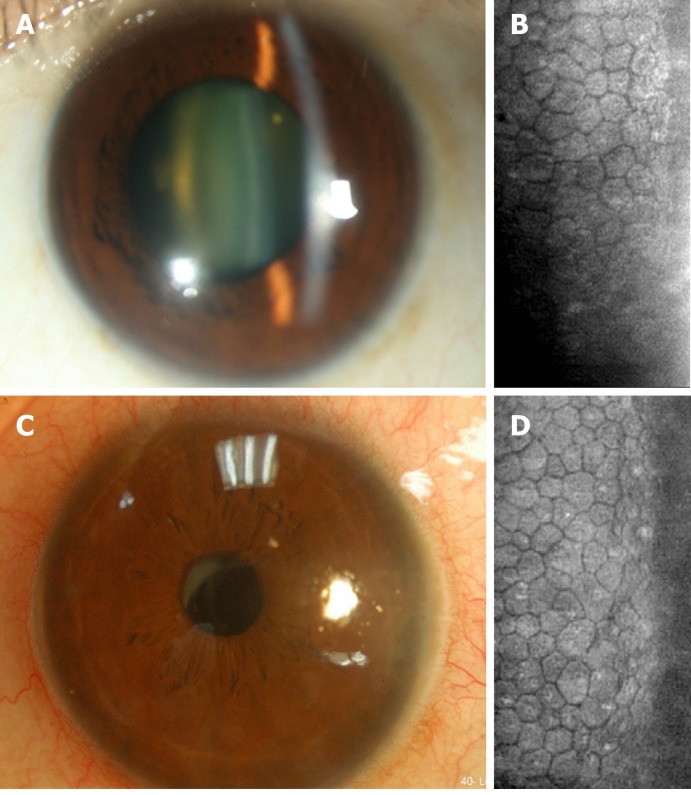
Patient 1: Phacoemulsification was performed, and postoperative BCVA in the right eye was 20/200; nevertheless, our patient continued to complain of blurry vision and tingling of the right eye. Specular microscopy revealed pseudophakic bullous keratopathy and Descemet's stripping. Automated endothelial keratoplasty was performed as salvage surgery. After keratoplasty, a clear cornea graft with enhanced endothelial cell density (ECD) of 2075/mm2 and improved BCVA of 20/25 in the right eye were observed. Two years later, a left eye cataract was found with BCVA of 20/200 (Figure 3A) and another phacoemulsification was planned. The preoperative ECD was 1365/mm2 in the left eye (Figure 3B). To prevent a similar scenario of corneal decompensation, we prescribed AA (50 mg/mL, Vitacicol, Taiwan Biotech CO., LTD., Taoyuan, Taiwan) eye drops four times daily one month before and after surgery. In addition, excessive intracameral medications, including lidocaine and carbachol, were avoided intraoperatively.
Figure 3.
The corneal endothelium condition before and after cataract surgery in the left eye of Patient 1. A: Preoperative corneal appearance via silt-lamp biomicroscope; B: Preoperative corneal endothelial cell density via specular microscope; C: Postoperative corneal appearance via silt-lamp biomicroscope; D: Postoperative corneal endothelial cell density via specular microscope.
Patient 2: Acyclovir and famciclovir were sequentially prescribed for the suspicion of herpetic simplex virus endotheliitis; however, upper corneal edema and a few keratic precipitates emerged. Topical valganciclovir and systemic ganciclovir were prescribed for the CMV endotheliitis, and the CMV endotheliitis subsided. Meanwhile, AA (50 mg/mL) was used for the scheduled cataract surgery with a frequency of four times daily one month perioperatively. The ECD was 1048/mm2 before surgery (Figure 1B).
OUTCOME AND FOLLOW-UP
Patient 1: Only mild stromal edema was observed postoperatively without bullae, while improved visual acuity was reported by the patient. The BCVA in the left eye was 20/30 with clear cornea and ECD of 1239/mm2 two years postoperatively (Figure 3C and D).
Patient 2: After the surgery, the visual acuity had improved without signs of corneal decompensation. The postoperative ECD was 1017/mm2 in the left eye with BCVA of 20/20 at the latest visit (Figure 1C and D).
DISCUSSION
In the corneal endothelium, oxidative stress may increase lipid peroxidation, leading to cellular impairment and apoptosis of HCECs[20]. FECD is featured by elevated cell apoptosis resulting from higher oxidative stress and oxygen-induced damage on DNA[20,21]. Although oxidative stress has not been demonstrated in CMV corneal endotheliitis, apoptosis has been detected in CMV retinitis in cell line models[22]. In addition, oxygen free radicals generated by high-intensity ultrasound oscillations in water during phacoemulsification have been shown to damage the corneal endothelium[23]. Since HCECs of the two patients enrolled were impaired with ECD below 1500 cell/mm2, the double damage of phacoemulsification and concurrent FECD or CMV endotheliitis increased the risks of developing corneal decompensation or even pseudophakic bullous keratopathy. To prevent the oxidative damage, we arranged anti-oxidant therapy with topical AA and reached acceptable visual outcome with fair corneal condition. To our knowledge, this is the first experience to show the effectiveness of topical AA in the prevention of phacoemulsification-related corneal endothelial damage.
The AA has been widely applied in immune and inflammatory disorders due to its antioxidant properties recently[24]. In the field of ophthalmology, previous studies illustrated that AA may be associated with the development of cataract, age-related macular degeneration and diabetic macular edema and owns the potential as a therapeutic agent[10,25-27]. About the cornea, postoperatively topical instillation and oral administration of AA preserve the integrity of corneal stroma in refractive procedures[18,19], while intracameral irrigation of AA during phacoemulsification may decrease corneal endothelial injury in animal studies[17,28]. Still, the effectiveness of perioperative AA instillation on the protection of HCECs demonstrated in the current study has rarely been reported elsewhere. The AA we administrated in the current study was 50 mg/mL, which was much higher than the concentration of AA in the general culture medium. However, the corneal endothelium is immersed in an environment where aqueous humor is recycled every one to two hours and the bioavailability of topical medications is only approximately one to five percent[29]. As a result, we adopted a much higher concentration of AA than that of the ordinary culture medium and followed a humanly possible administration protocol of four times a day in the hope to reach similar concentration of AA in the culture medium. The favorable preservation of HCECs and absence of complications in our two patients verifies the current concentration of AA as acceptable whether in terms of efficacy or safety.
The first patient was diagnosed with FECD. In a previous study, 35 of 89 eyes needed to undergo endothelial keratoplasty, but no improvement in BCVA was found in those eyes that underwent endothelial keratoplasty[6]. By comparison, BCVA in the left eye of the first patient recovered prominently. Our findings suggest that perioperative anti-oxidant supplements can improve and preserve HCECs and visual outcomes compatible with the degree of the traditional approach. Furthermore, graft dislocation and pupillary block glaucoma occasionally occur in patients undergoing triple surgery despite excellent visual outcome[30], while current management avoids these complications.
For patients with corneal endotheliitis who require cataract surgery, previous studies focused only on postoperative infection[31], or did not provide adequate details regarding postoperative status[5]. In the current study, the second patient was diagnosed with CMV endotheliitis and low ECD, while the final BCVA was 20/20, showing much improvement with similar ECD values observed postoperatively. A possible explanation is that phacoemulsification-related oxidative stress can be alleviated by AA and that the anti-inflammatory effect of AA may also reduce the inflammation from both phacoemulsification and CMV endotheliitis[32].
Comparison of the current study to two previous studies concerning the application of phacoemulsification in low ECD patients is illustrated in Table 1. The two previous studies applied a soft-shell technique to reduce possible mechanical impact[4,5]. We did not perform the soft-shell technique but rather used an ophthalmic Viscoat viscoelastic device and irrigation with balanced salt solution. Furthermore, we did not alter the perioperative medications except AA among all eyes undergoing cataract surgery mentioned in this report. Collectively, the use of perioperative topical AA alone ended up with similar HCECs loss compared to previous experiences[4,5]. Moreover, the major etiology of low ECD in a previous study was primary angle closure glaucoma with acute attack or laser iridotomy[4], possibly inducing less damage to HCECs than HCECs-origin lesions such as FECD.
Table 1.
Comparison of different modifications of cataract surgery in patients with low endothelial count
| Hayashi 2011 | Yamazoe 2011 | Lee 2019 | |
| Technique to preserve EC | Soft shell technique | Soft shell technique | Perioperative AA |
| Case number | 50 | 61 | 2 |
| Mean age (yr) | 68.9 | 72.3 | 45.5 |
| Gender (M to F, %) | 24 to 76 | 23 to 77 | 100 to 0 |
| Leading etiology (%) | PACG (38) | FCED (33) | FCED and CMV endotheliitis (50) |
| Mean preOP ECD | 805.0 | 692.9 | 1206.5 |
| Energy (mJ) | 27.3 ± 10.1 | NA | 30.4 |
| Time (Sec) | 57.5 ± 28.3 | NA | 72.8 |
| Mean EC loss (%) | 5.1 | 11.5 | 6.1 |
EC: Endothelial cells; AA: Ascorbic acid; M: Male; F: Female; PACG: Primary angle closure glaucoma; FCED: Fuchs corneal endothelial dystrophy; CMV: Cytomegalovirus; preOP: Preoperative; ECD: Endothelial cell density.
CONCLUSION
Perioperative topical AA showed both safety and promise for prevention of HCECs dysfunction in high-risk patients undergoing phacoemulsification and may be considered an alternative therapy. Further large prospective studies are required to determine the optimal dose.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Peer-review started: November 19, 2018
First decision: December 29, 2018
Article in press: January 26, 2019
P- Reviewer: Teragawa H; Kim CH S- Editor: Dou Y L- Editor: A E- Editor: Bian YN
Contributor Information
Chia-Yi Lee, Department of Ophthalmology, Show Chwan Memorial Hospital, Changhua 50093, Taiwan; Department of Optometry, College of Medicine and Life Science, Chung Hwa University of Medical Technology, Tainan 71241, Taiwan.
Hung-Ta Chen, Department of Internal Medicine, Taipei City Hospital- Heping Branch, Taipei 10341, Taiwan.
Yi-Jen Hsueh, Department of Ophthalmology, Chang Gung Memorial Hospital, Linkou 20012, Taiwan; Center for Tissue Engineering, Chang Gung Memorial Hospital, Linkou 20012, Taiwan.
Hung-Chi Chen, Department of Ophthalmology, Chang Gung Memorial Hospital, Linkou 20012, Taiwan; Center for Tissue Engineering, Chang Gung Memorial Hospital, Linkou 20012, Taiwan; Department of Medicine, College of Medicine Chang Gung University, Taoyuan 33305, Taiwan. mr3756@cgmh.org.tw.
Chieh-Cheng Huang, Center for Tissue Engineering, Chang Gung Memorial Hospital, Linkou 20012, Taiwan; Institute of Biomedical Engineering, National Tsing Hua University, Hsinchu 30010, Taiwan.
Yaa-Jyuhn James Meir, Department of Biomedical Sciences, College of Medicine Chang Gung University, Taoyuan 33305, Taiwan.
Chao-Min Cheng, Center for Tissue Engineering, Chang Gung Memorial Hospital, Linkou 20012, Taiwan; Institute of Biomedical Engineering, National Tsing Hua University, Hsinchu 30010, Taiwan.
Wei-Chi Wu, Department of Ophthalmology, Chang Gung Memorial Hospital, Linkou 20012, Taiwan; Department of Medicine, College of Medicine Chang Gung University, Taoyuan 33305, Taiwan.
References
- 1.Rosado-Adames N, Afshari NA. The changing fate of the corneal endothelium in cataract surgery. Curr Opin Ophthalmol. 2012;23:3–6. doi: 10.1097/ICU.0b013e32834e4b5f. [DOI] [PubMed] [Google Scholar]
- 2.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs' endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38:149–168. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 3.Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Corneal endotheliitis associated with evidence of cytomegalovirus infection. Ophthalmology. 2007;114:798–803. doi: 10.1016/j.ophtha.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi K, Yoshida M, Manabe S, Hirata A. Cataract surgery in eyes with low corneal endothelial cell density. J Cataract Refract Surg. 2011;37:1419–1425. doi: 10.1016/j.jcrs.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Yamazoe K, Yamaguchi T, Hotta K, Satake Y, Konomi K, Den S, Shimazaki J. Outcomes of cataract surgery in eyes with a low corneal endothelial cell density. J Cataract Refract Surg. 2011;37:2130–2136. doi: 10.1016/j.jcrs.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 6.van Cleynenbreugel H, Remeijer L, Hillenaar T. Cataract surgery in patients with Fuchs' endothelial corneal dystrophy: when to consider a triple procedure. Ophthalmology. 2014;121:445–453. doi: 10.1016/j.ophtha.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld E, Varssano D. The corneal transplant score: a simple corneal graft candidate calculator. Graefes Arch Clin Exp Ophthalmol. 2013;251:1771–1775. doi: 10.1007/s00417-013-2358-8. [DOI] [PubMed] [Google Scholar]
- 8.Sridhar MS, Murthy S, Bansal AK, Rao GN. Corneal triple procedure: indications, complications, and outcomes: a developing country scenario. Cornea. 2000;19:333–335. doi: 10.1097/00003226-200005000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Covert DJ, Koenig SB. New triple procedure: Descemet's stripping and automated endothelial keratoplasty combined with phacoemulsification and intraocular lens implantation. Ophthalmology. 2007;114:1272–1277. doi: 10.1016/j.ophtha.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett H, Eperjesi F. An ideal ocular nutritional supplement? Ophthalmic Physiol Opt. 2004;24:339–349. doi: 10.1111/j.1475-1313.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 11.Serbecic N, Beutelspacher SC. Vitamins inhibit oxidant-induced apoptosis of corneal endothelial cells. Jpn J Ophthalmol. 2005;49:355–362. doi: 10.1007/s10384-005-0209-9. [DOI] [PubMed] [Google Scholar]
- 12.Shima N, Kimoto M, Yamaguchi M, Yamagami S. Increased proliferation and replicative lifespan of isolated human corneal endothelial cells with L-ascorbic acid 2-phosphate. Invest Ophthalmol Vis Sci. 2011;52:8711–8717. doi: 10.1167/iovs.11-7592. [DOI] [PubMed] [Google Scholar]
- 13.Pfister RR, Paterson CA. Ascorbic acid in the treatment of alkali burns of the eye. Ophthalmology. 1980;87:1050–1057. doi: 10.1016/s0161-6420(80)35126-9. [DOI] [PubMed] [Google Scholar]
- 14.Brodovsky SC, McCarty CA, Snibson G, Loughnan M, Sullivan L, Daniell M, Taylor HR. Management of alkali burns : an 11-year retrospective review. Ophthalmology. 2000;107:1829–1835. doi: 10.1016/s0161-6420(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 15.Davis AR, Ali QK, Aclimandos WA, Hunter PA. Topical steroid use in the treatment of ocular alkali burns. Br J Ophthalmol. 1997;81:732–734. doi: 10.1136/bjo.81.9.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemet AY, Assia EI, Meyerstein D, Meyerstein N, Gedanken A, Topaz M. Protective effect of free-radical scavengers on corneal endothelial damage in phacoemulsification. J Cataract Refract Surg. 2007;33:310–315. doi: 10.1016/j.jcrs.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Rubowitz A, Assia EI, Rosner M, Topaz M. Antioxidant protection against corneal damage by free radicals during phacoemulsification. Invest Ophthalmol Vis Sci. 2003;44:1866–1870. doi: 10.1167/iovs.02-0892. [DOI] [PubMed] [Google Scholar]
- 18.Stojanovic A, Ringvold A, Nitter T. Ascorbate prophylaxis for corneal haze after photorefractive keratectomy. J Refract Surg. 2003;19:338–343. doi: 10.3928/1081-597X-20030501-11. [DOI] [PubMed] [Google Scholar]
- 19.Kasetsuwan N, Wu FM, Hsieh F, Sanchez D, McDonnell PJ. Effect of topical ascorbic acid on free radical tissue damage and inflammatory cell influx in the cornea after excimer laser corneal surgery. Arch Ophthalmol. 1999;117:649–652. doi: 10.1001/archopht.117.5.649. [DOI] [PubMed] [Google Scholar]
- 20.Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am J Pathol. 2010;177:2278–2289. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czarny P, Kasprzak E, Wielgorski M, Udziela M, Markiewicz B, Blasiak J, Szaflik J, Szaflik JP. DNA damage and repair in Fuchs endothelial corneal dystrophy. Mol Biol Rep. 2013;40:2977–2983. doi: 10.1007/s11033-012-2369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cinatl J, Jr, Blaheta R, Bittoova M, Scholz M, Margraf S, Vogel JU, Cinatl J, Doerr HW. Decreased neutrophil adhesion to human cytomegalovirus-infected retinal pigment epithelial cells is mediated by virus-induced up-regulation of Fas ligand independent of neutrophil apoptosis. J Immunol. 2000;165:4405–4413. doi: 10.4049/jimmunol.165.8.4405. [DOI] [PubMed] [Google Scholar]
- 23.Augustin AJ, Dick HB. Oxidative tissue damage after phacoemulsification: influence of ophthalmic viscosurgical devices. J Cataract Refract Surg. 2004;30:424–427. doi: 10.1016/S0886-3350(03)00577-7. [DOI] [PubMed] [Google Scholar]
- 24.Sorice A, Guerriero E, Capone F, Colonna G, Castello G, Costantini S. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev Med Chem. 2014;14:444–452. doi: 10.2174/1389557514666140428112602. [DOI] [PubMed] [Google Scholar]
- 25.Schmidl D, Garhöfer G, Schmetterer L. Nutritional supplements in age-related macular degeneration. Acta Ophthalmol. 2015;93:105–121. doi: 10.1111/aos.12650. [DOI] [PubMed] [Google Scholar]
- 26.May JM. Ascorbic acid repletion: A possible therapy for diabetic macular edema? Free Radic Biol Med. 2016;94:47–54. doi: 10.1016/j.freeradbiomed.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalal D, Koorosh F, Fereidoun H. Comparative study of plasma ascorbic acid levels in senile cataract patients and in normal individuals. Curr Eye Res. 2009;34:118–122. doi: 10.1080/02713680802632599. [DOI] [PubMed] [Google Scholar]
- 28.M Padua IR, P Valdetaro G, B Lima T, K Kobashigawa K, E S Silva P, Aldrovani M, M Padua PP, Laus JL. Effects of intracameral ascorbic acid on the corneal endothelium of dogs undergoing phacoemulsification. Vet Ophthalmol. 2018;21:151–159. doi: 10.1111/vop.12490. [DOI] [PubMed] [Google Scholar]
- 29.Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57:2010–2032. doi: 10.1016/j.addr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Terry MA, Shamie N, Chen ES, Phillips PM, Shah AK, Hoar KL, Friend DJ. Endothelial keratoplasty for Fuchs' dystrophy with cataract: complications and clinical results with the new triple procedure. Ophthalmology. 2009;116:631–639. doi: 10.1016/j.ophtha.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Zarei-Ghanavati S, Alizadeh R, Yoo SH. Herpes Simplex Virus Endotheliitis following Descemet's Membrane Endothelial Keratoplasty. J Ophthalmic Vis Res. 2015;10:184–186. doi: 10.4103/2008-322X.163764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellulu MS, Rahmat A, Patimah I, Khaza'ai H, Abed Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: a randomized controlled trial. Drug Des Devel Ther. 2015;9:3405–3412. doi: 10.2147/DDDT.S83144. [DOI] [PMC free article] [PubMed] [Google Scholar]