Abstract
Curcuma species have been cultivated in tropical and subtropical regions in Asia, Australia, and South America for culinary as well as medicinal applications. The biological activities of Curcuma have been attributed to the non-volatile curcuminoids as well as to volatile terpenoids. Curcuma essential oils have demonstrated a wide variety of pharmacological properties. The objective of this work was to examine the variation in the compositions of Curcuma rhizome essential oils. In this work, the volatile oils from C. longa and C. zedoaria were obtained and analyzed by gas chromatography-mass spectrometry. The chemical compositions of C. longa and C. zedoaria essential oils, including those reported in the literature, were analyzed by hierarchical cluster analysis. In addition, cluster analyses of the chemical compositions of C. aromatica and C. aeruginosa from the literature were also carried out. Curcuma longa volatiles were dominated by α-turmerone, curlone, ar-turmerone, β-sesquiphellandrene, α-zingiberene, germacrone, terpinolene, ar-curcumene, and α-phellandrene and showed four distinct chemical clusters. C. zedoaria rhizome oil contained 1,8-cineole, curzerenone/epi-curzerenone, α-copaene, camphor, β-caryophyllene, elemol, germacrone, curzerene, and β-elemene and showed two different chemical types. C. aromatica had three clearly defined clusters, and C. aeruginosa had three types.
Keywords: Curcuma aeruginosa, Curcuma longa, Curcuma zedoaria, Curcuma aromatica, rhizome essential oils
1. Introduction
The genus Curcuma L. (Zingiberaceae) consists of about 93–100 species of perennial rhizomatous herbs that originated in tropical and subtropical regions of Asia, Australia, and South America [1]. Many of these species are extensively grown on a very large scale in India, Pakistan, Indonesia, Malaysia, Bangladesh, Nepal, and Thailand [2]. Curcuma species are greatly valued for their medicinal properties. For hundreds of years, members of Curcuma have been used in traditional medicine for treating respiratory complaints, pain, digestive disorders, inflammatory conditions, wounds, hypercholesterolemia, hypertension, hematologic and circulation abnormalities, infectious diseases, and cancer prevention, among others [3,4,5]. They are also important sources of flavoring and coloring agents, cosmetics, perfumes, and ornamental plants [5,6]. Curcuma species possess a variety of pharmacological activities including anti-inflammatory, antiproliferative, anticancer, hypoglycemic, anti-hyperlipidemic, antiatherosclerotic, neuroprotective, hepatoprotective, anti-diarrheal, carminative, diuretic, antirheumatic, anticonvulsant, hypotensive, antioxidant, insecticidal, larvicidal, antimicrobial, antiviral, antivenomous, anti-thrombotic, and antityrosinase activities [7,8,9,10,11,12,13,14,15].
The rhizome, which contains a variety of terpenoids, flavonoids, and phenylpropanoids [16], is the most extensively used part of the plant [17]. Several studies indicated that the bioactive ingredients of Curcuma rhizome are the non-volatile curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) and the volatile oil (sesquiterpenoids and monoterpenoids) [14,18]. Curcumin, the most active curcuminoid in turmeric rhizome, has anticancer [19], anti-inflammatory [20], antioxidant [21], antibacterial, anti-fungal [22], analgesic, digestive, antidepressant [23], and hypoglycemic [23] properties and has shown potential against cardiovascular diseases [24] and Alzheimer’s disease [25]. Curcuma essential oil (EO) is often extracted by distillation of the fresh or dry rhizome [26], or by supercritical fluid extraction [27]. Generally, the Curcuma oils are made up of sesquiterpenoids and monoterpenoids [5]. There is a great variation in the literature on Curcuma EO due to differences in the genotype, edaphic factors, climate, time of harvest, extraction, and analysis methods [28,29,30]. Around 31 Curcuma species have been studied of which C. longa (turmeric) and C. zedoaria (zedoary) are the most extensively investigated [5]. The current study was conducted to investigate the composition and different chemotypes of the rhizome essential oils of C. longa L., C. aromatica Salisb., C. zedoaria (Christm.) Roscoe, and C. aeruginosa Roxb. from collections from different geographical origins.
2. Materials and Methods
2.1. Volatile Oils
Volatile oils from commercial suppliers were obtained from the collections of the Aromatic Plant Research Center (APRC, Lehi, UT, USA). A total of 33 Curcuma longa (turmeric) rhizome oils from the APRC collection, including 24 hydro- or steam-distilled essential oils, five supercritical CO2 extracts, and four oils of unknown origin or extraction method, were analyzed by gas chromatography–mass spectrometry (GC-MS).
2.2. Gas Chromatographic-Mass Spectral Analysis
The essential oils obtained from APRC were analyzed by gas chromatography-mass spectrometry (GC-MS) using a Shimadzu GCMS-QP2010 Ultra operated in the electron impact (EI) mode (electron energy = 70 eV), scan range = 40–400 atomic mass units, scan rate = 3.0 scans/s, and GC-MS solution software (Shimadzu Scientific Instruments, Columbia, MD, USA). The GC column was a ZB-5 fused silica capillary column with a (5% phenyl)-polymethylsiloxane stationary phase and a film thickness of 0.25 μm, a length of 30 m, and an internal diameter of 0.25 mm (Phenomenex, Torrance, CA, USA). The carrier gas was helium with a column head pressure of 552 kPa and flow rate of 1.37 mL/min. The injector temperature was 250 °C and the ion source temperature was 200 °C. The GC oven temperature was programmed for 50 °C initial temperature, then temperature was increased at a rate of 2 °C/min to 260 °C. A 7% w/v solution of the sample was prepared in dichloromethane and 0.1 μL was injected with a splitting mode (30:1). Identification of the oil components was based on their retention indices determined by reference to a homologous series of n-alkanes, and by comparison of their mass spectral fragmentation patterns with those reported in the literature [31] and our own in-house library [32].
2.3. Hierarchical Cluster Analysis
The chemical compositions of the Curcuma oils obtained from this work as well as the published literature were used in the cluster analysis. The essential oil compositions were treated as operational taxonomic units (OTUs), and the concentrations (percentages) of the major components (C. longa: α-phellandrene, p-cymene, 1,8-cineole, terpinolene, ar-curcumene, α-zingiberene, β-bisabolene, β-sesquiphellandrene, ar-turmerone (= dehydroturmerone), α-turmerone, germacrone, curlone (= β-turmerone), (6S,7R)-bisabolone, and (E)-α-atlantone; C. zedoaria: 1,8-cineole, camphor, α-copaene, β-elemene, β-caryophyllene, ar-curcumene, zingiberene, curzerene, germacrene B, β-sesquiphellandrene, curzerenone/epi-curzerenone, and germacrone; C. aromatica: α-pinene, camphene, 1,8-cineole, camphor, isoborneol, borneol, β-elemene, ar-curcumene, curzerene, β-curcumene, curzerenone, germacrone, xanthorrhizol, and curdione (= 1(10)-germacrene-5,8-dione; C. aeruginosa: camphene, β-pinene, 1,8-cineole, camphor, isoborneol, borneol, β-elemene, β-farnesene, zingiberene, curzerene, germacrene B, curzerenone, β-eudesmol, germacrone, and curcumenol) were used to determine the chemical associations between the essential oils using agglomerative hierarchical cluster (AHC) analysis using XLSTAT Premium, version 2018.5.53172 (Addinsoft, Paris, France). Dissimilarity was determined using Euclidean distance, and clustering was defined using Ward’s method.
3. Results and Discussion
Essential oils from the Curcuma species were obtained from a collection of oils from commercial sources deposited with the Aromatic Plant Research Center (APRC). Curcuma species are known for producing an array of volatile sesquiterpenes, monoterpenes, and other aromatic compounds [5,15]. Hundreds of compounds have been identified from the turmeric oils, however, the major components were α-turmerone (12.6–44.5%), curlone (9.1–37.8%), ar-turmerone (12.2–36.6%), β-sesquiphellandrene (5.0–14.6%), α-zingiberene (5.0–12.8%), germacrone (10.3–11.1%), terpinolene (10.0–10.2%), ar-curcumene (5.5–9.8%), and α-phellandrene (5.0–6.7%) (Table 1). Interestingly, Brazilian turmeric EO samples showed (Z)-γ-atlantone, ar-turmerone, and (E)-γ-atlantone as the main constituents [33], while a sample from north central Nigeria had β-bisabolene, (E)-β-ocimene, β-myrcene, 1,8-cineole, α-thujene, α-phellandrene, limonene, zingiberene, and β-sesquiphellandrene [34]. Turmeric oils of Sri Lanka and São Tomé e Principe origins had α-phellandrene, α-turmerone, 1,8-cineole, p-cymene, ar-turmerone, β-turmerone, and terpinolene as the major components [10,35].
Table 1.
Chemical compositions (major components) of Curcuma longa rhizome volatile oils.
| Sample | α-Phellandrene | p-Cymene | 1,8-Cineole | Terpinolene | ar-Curcumene | α-Zingiberene | β-Bisabolene | β-Sesquiphellandrene | ar-Turmerone | α-Turmerone | Germacrone | Curlone (= β-Turmerone) | (6S,7R)-Bisabolone | (E)-α-Atlantone |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fRh-SD-India (APRC) | 2.41 | 2.44 | 3.22 | 0.53 | 0.89 | 0.34 | 0.29 | 0.98 | 36.02 | 16.95 | 0 | 19.07 | 0.81 | 0.38 |
| Resin-SD-India (APRC) | 0.03 | 0.07 | 0.10 | 0 | 9.75 | 12.75 | 2.54 | 14.59 | 15.02 | 17.67 | 0 | 9.11 | 0.17 | 0.71 |
| Rh-SD-Nepal (APRC) | 1.08 | 0.41 | 1.17 | 0.17 | 3.26 | 5.97 | 1.01 | 7.72 | 21.20 | 28.80 | 0 | 18.20 | 1.18 | 2.68 |
| Rh-SD-Nepal (APRC) | 0.43 | 0.32 | 1.55 | 10.22 | 2.15 | 5.02 | 0.87 | 5.48 | 20.51 | 26.73 | 0 | 12.99 | 0.87 | 1.71 |
| Rh-SD-Indonesia (APRC) | 2.94 | 0.67 | 0.45 | 0.57 | 3.14 | 2.77 | 0.75 | 3.38 | 14.54 | 15.41 | 0 | 10.03 | 0 | 0.90 |
| Rh-CO2-India (APRC) | 0.41 | 0.45 | 0.35 | 0 | 3.87 | 3.35 | 0.82 | 4.69 | 33.82 | 20.62 | 0 | 14.42 | 1.29 | 3.26 |
| Rh-CO2-India (APRC) | 0.28 | 0.44 | 1.05 | 0 | 5.66 | 4.20 | 1.35 | 8.08 | 32.02 | 13.22 | 0 | 14.70 | 1.46 | 2.59 |
| Rh-CO2-India (APRC) | 0.61 | 0.45 | 1.89 | 0 | 4.41 | 6.29 | 1.49 | 9.15 | 25.23 | 19.96 | 0 | 14.63 | 1.16 | 2.02 |
| Rh-SD-Indonesia (APRC) | 3.14 | 0.66 | 0.77 | 0.50 | 1.30 | 1.80 | 0.30 | 1.95 | 21.51 | 27.90 | 0 | 17.65 | 1.17 | 1.90 |
| Rh-SD-Indonesia (APRC) | 3.10 | 1.19 | 1.15 | 0.76 | 11.07 | 3.23 | 2.16 | 8.44 | 17.45 | 13.17 | 0 | 8.81 | 0.62 | 0.72 |
| Rh-HD-Jamaica (APRC) | 2.20 | 0.70 | 1.77 | 0.66 | 1.28 | 1.76 | 0.35 | 1.76 | 22.19 | 34.24 | 0 | 16.40 | 0.74 | 0.30 |
| Rh-Unknown (APRC) | 0.45 | 0.26 | 1.14 | 0.30 | 2.46 | 4.94 | 0.79 | 4.71 | 25.32 | 25.30 | 0 | 17.49 | 0.88 | 1.04 |
| Rh-SD-India (APRC) | 0.31 | 0.47 | 1.44 | 0 | 5.50 | 4.71 | 1.46 | 7.77 | 32.12 | 15.06 | 0 | 13.18 | 1.27 | 2.11 |
| Rh-Unknown (APRC) | 1.25 | 1.40 | 4.04 | 0 | 6.59 | 6.12 | 1.57 | 9.51 | 29.00 | 13.38 | 0 | 11.97 | 1.07 | 1.50 |
| Rh-SD-Indonesia (APRC) | 1.58 | 0.50 | 0.91 | 0.32 | 2.05 | 2.41 | 0.43 | 2.39 | 21.95 | 31.05 | 0 | 18.86 | 0.71 | 1.09 |
| Rh-SD-Nepal (APRC) | 3.79 | 2.52 | 2.24 | 1.52 | 4.09 | 6.08 | 1.15 | 3.20 | 21.84 | 20.21 | 0 | 9.72 | 0.38 | 1.63 |
| Rh-SD-Nepal (APRC) | 0.05 | 0 | 0.23 | 1.58 | 0.99 | 8.81 | 0.94 | 5.65 | 12.52 | 44.51 | 0 | 14.44 | 1.06 | 0.26 |
| dRh-SD-India (APRC) | 3.36 | 2.31 | 0.79 | 0.33 | 2.62 | 1.42 | 0.44 | 2.03 | 36.64 | 23.73 | 0 | 15.74 | 0.66 | 0.19 |
| fRh-SD-India (APRC) | 1.13 | 0.44 | 0.35 | 0.21 | 2.65 | 3.64 | 0.74 | 2.94 | 28.77 | 26.50 | 0 | 13.68 | 0.61 | 2.31 |
| Rh-SD-Nepal (APRC) | 0 | 0.10 | 0.26 | 1.11 | 1.12 | 0.48 | 0.18 | 1.13 | 36.37 | 12.57 | 10.29 | 12.22 | 1.10 | 0.58 |
| Rh-HD-Nepal (APRC) | 0.03 | 0.09 | 0.38 | 1.54 | 0.87 | 0.54 | 0 | 1.06 | 35.07 | 20.50 | 11.11 | 14.18 | 0.95 | 0.26 |
| Rh-Unknown (APRC) | 0.05 | 0.03 | 0.20 | 1.54 | 0.95 | 8.58 | 0.83 | 5.51 | 12.19 | 43.30 | 0 | 14.11 | 1.03 | 0.21 |
| Rh-Unknown (APRC) | 6.73 | 0.79 | 1.49 | 0.41 | 1.70 | 3.30 | 0.43 | 2.72 | 18.20 | 37.75 | 0 | 37.75 | 0.40 | 0.64 |
| Rh-SD-Nepal (APRC) | 0.16 | 0.09 | 0.30 | 4.38 | 1.69 | 4.28 | 0.68 | 4.73 | 23.18 | 28.93 | 0 | 14.76 | 1.13 | 2.38 |
| Rh-CO2-India (APRC) | 0.36 | 0.52 | 1.26 | 0 | 5.65 | 4.13 | 1.25 | 8.00 | 35.08 | 13.67 | 0 | 15.06 | 1.31 | 2.49 |
| Rh-CO2-India (APRC) | 0 | 0 | 0.3 | 0 | 3.13 | 2.78 | 0.76 | 4.43 | 34.20 | 21.49 | 0 | 16.34 | 0 | 3.77 |
| Rh-SD-Nepal (APRC) | 0.16 | 0.16 | 0.18 | 6.04 | 0 | 2.56 | 0.27 | 2.15 | 27.36 | 32.11 | 0.92 | 16.72 | 1.27 | 0.63 |
| dRh-SD-India (APRC) | 1.13 | 0.98 | 1.20 | 8.91 | 6.14 | 5.98 | 1.50 | 3.17 | 32.16 | 9.39 | 0 | 3.96 | 0 | 0 |
| Rh-HD-Nepal (APRC) | 0 | 0.07 | 0.39 | 1.54 | 0.90 | 0.66 | 0.14 | 1.07 | 34.42 | 20.25 | 11.10 | 13.90 | 0.98 | 0.29 |
| Rh-HD-Nepal (APRC) | 0.01 | 0.01 | 0.13 | 0.76 | 1.71 | 4.53 | 0.70 | 4.04 | 23.68 | 35.42 | 0 | 14.43 | 1.04 | 0.28 |
| Rh-HD-Nepal (APRC) | 0.41 | 0.30 | 1.51 | 10.01 | 2.10 | 4.90 | 0.82 | 5.35 | 20.12 | 26.20 | 0 | 12.72 | 0.83 | 1.67 |
| Rh-HD-Nepal (APRC) | 0.03 | 0.08 | 0.49 | 2.87 | 1.29 | 1.87 | 0.37 | 2.67 | 31.45 | 26.92 | 0 | 15.65 | 1.14 | 0.36 |
Rh = rhizome; dRh = dried rhizome; fRh = fresh rhizome; HD = hydrodistillation; SD = steam distillation; CO2 = supercritical CO2 extracts; APRC = from the collection of the Aromatic Plant Research Center.
The rhizome of Curcuma aromatica (commonly known as wild turmeric) is a traditional medicine used to alleviate pain, eliminate blood stasis, and slow ageing [36]. The Japanese C. aromatica oil was reported to have curdione (32.2–44.0%), 1,8-cineole (7.5–25.3%), and germacrone (4.6–9.6%) [37], while a sample from Thailand contained camphor (26.9%), ar-curcumene (23.2%), and xanthorrhizol (18.7%) as the main components [38]. Indian samples of C. aromatica had camphor (18.2–48.3%), β-curcumene (28.4–31.4%), ar-curcumene (22.1–24.1%), xanthorrhizol (4.8–16.2%), 1,8-cineole (5.5–15.9%), isoborneol (8.2–12.2%), curzerenone (5.5–11.0%), germacrone (4.9–10.6%), camphene (7.4–10.2%), curdione (4.8–8.0%), borneol (4.9–8.2%), β-elemene (7.5%), curzerene (4.6–6.0%), α-pinene (5.7–5.9%), and terpinolene (5.2%) [15,37,39,40,41,42] (Table 2).
Table 2.
Chemical composition of Curcuma aromatica rhizome essential oils.
| Compound | Car India [15] | Car India [42] | Car India [42] | Car Thailand [38] | Car Japan [37] | Car Japan [37] | Car India [37] | Car India [39] | Car India [39] | Car India [40] | Car India [41] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Pinene | 1.5 | 5.9 | 5.7 | 0.5 | 0.4 | 0.9 | 0.2 | 0.4 | 0.3 | 0.3 | 0.8 |
| Camphene | 10.2 | 0.9 | 1.1 | 2.0 | 0 | 0 | 0.3 | 0.9 | 0.8 | 0.7 | 7.4 |
| Myrcene | 1.2 | 0 | 0 | 0.4 | 0.1 | 0.3 | 0.1 | 0.2 | 0.2 | 0.2 | 1.0 |
| 1,8-Cineole | 10.1 | 13.7 | 15.9 | 0.3 | 7.5 | 25.3 | 1.0 | 0.1 | 0.1 | 5.5 | 9.3 |
| Terpinolene | 0 | 5.2 | 3.9 | 0 | 0 | 0 | 0 | tr | tr | 0 | 0.1 |
| Linalool | 2.1 | 0 | 0 | 0.6 | 2.2 | 2.8 | 0.1 | 0 | 0 | 0.2 | 1.2 |
| Camphor | 18.8 | 48.3 | 45.7 | 26.9 | 0 | 0 | 3.9 | 3.9 | 3.3 | 32.3 | 25.6 |
| Isoborneol | 1.8 | 12.2 | 10.1 | 2.3 | 0 | 0 | 0.3 | 0 | 0 | 3.4 | 8.2 |
| Borneol | 8.2 | 5.0 | 4.9 | 1.7 | 0 | 0 | 0.3 | 1.8 | 1.1 | 1.1 | 2.5 |
| α-Terpineol | 0 | 0 | 0 | 0 | 0.4 | 1.3 | 1.4 | 0 | tr | 0.6 | 1.0 |
| β-Elemene | 7.5 | 0 | 0 | 0.1 | 4.0 | 2.5 | 1.0 | 0.2 | 0.2 | 1.4 | 1.4 |
| β-Caryophyllene | 2.0 | 0 | 0 | 0 | 1.9 | 1.7 | 0.3 | 0 | 0 | 0.3 | 0.3 |
| α-Humulene | 0 | 0 | 0 | 1.9 | 2.1 | 1.0 | 0 | 0 | 0 | tr | tr |
| β-Farnesene | 0 | 0 | 0 | 0 | 0 | 0 | 2.6 | 0 | 0 | tr | 0 |
| ar-Curcumene | 0 | 0 | 0 | 23.2 | 0 | 0 | 22.1 | 23.6 | 24.1 | 3.1 | 0 |
| Germacrene D | 1.8 | 0 | 0 | 0 | 1.1 | 0.7 | 0.2 | tr | 0.3 | 0 | 0.9 |
| Curzerene | 0 | 0.3 | 0.4 | 1.4 | 0 | 0 | 3.2 | 4.6 | 6.0 | 0.2 | 2.7 |
| β-Curcumene | 0 | 0 | 0 | 3.9 | 0 | 0 | 29.9 | 28.4 | 31.4 | 0 | 0 |
| Germacrene B | 2.8 | 0.2 | 0.4 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0.4 |
| Caryophyllene oxide | 0 | 0 | 0 | 0 | 1.4 | 2.0 | 0 | 0 | 0 | tr | tr |
| Curzerenone | 0 | 0 | 0 | 3.8 | 0 | 0 | 3.6 | 7.3 | 5.5 | 11.0 | 10.9 |
| Germacrone | 0 | 0.3 | 0.3 | 0.3 | 9.6 | 4.6 | 4.9 | 3.6 | 6.1 | 0.5 | 10.6 |
| Xanthorrhizol | 4.8 | 0 | 0 | 18.7 | 0 | 0 | 16.2 | 8.0 | 5.3 | 0 | 0 |
| Curdione | 8 | 4.8 | 6.8 | 0 | 44.0 | 32.2 | 0 | 0 | 0 | 0 | 0 |
tr = “trace” (<0.05%)
Zedoary (Curcuma zedoaria) rhizome is also called “white turmeric” because of its similarity to ginger from the outside and to turmeric from the inside. Zedoary EO is generally made of sesquiterpenoids (80–85%) and monoterpenoids (15–20%). The major components of C. zedoaria rhizome oil are 1,8-cineole (7.0–38.4%), curzerenone/epi-curzerenone (20.9–29.4%), α-copaene (17.4%), camphor (8.6–8.8%), β-caryophyllene (8.8%), elemol (6.8%), germacrone (6.7%), curzerene (5.9%), and β-elemene (5.5%) (Table 3). The main components of C. zedoaria rhizome oil reported in the literature were curzerenone/epi-curzerenone (19.0–31.6%), curzerene (8.0%), ar-curcumene (12.1%), zingiberene (12.0%), germacrone (10.8%), camphor (10.3%), β-sesquiphellandrene (9.8%), and germacrene B (6.0%) [15,43].
Table 3.
Chemical composition of Curcuma zedoaria rhizome essential oils.
| Compound | Cz Nepal-1 (APRC) | Cz Nepal-2 (APRC) | Cz India (APRC) | Cz India [15] | Cz India [43] |
|---|---|---|---|---|---|
| 1,8-Cineole | 8.77 | 38.39 | 7.00 | 0 | 1.9 |
| Camphor | 8.79 | 0 | 8.26 | 3.3 | 10.3 |
| Borneol/Isoborneol | 1.81 | 0.07 | 3.17 | 0.2 | 2.7 |
| α-Terpineol | 1.49 | 1.17 | 0.47 | 1.7 | 0.3 |
| α-Terpinyl acetate | 2.29 | 0 | 0 | 0 | 0 |
| α-Copaene | 17.35 | 0.42 | 0 | 0 | 0 |
| β-Elemene | 2.89 | 0.21 | 5.54 | 0.3 | tr |
| β-Caryophyllene | 8.28 | 1.37 | 1.46 | 0 | 0.4 |
| γ-Elemene | 0.29 | 0 | 0.84 | 2.5 | 0.1 |
| ar-Curcumene | 0 | 0.51 | 0 | 12.1 | 0 |
| Zingiberene | 0 | 0 | 0 | 12.0 | 0 |
| Curzerene | 2.36 | 0 | 5.93 | 8.0 | 0 |
| α-Farnesene | 0 | 0 | 0 | 2.3 | 0 |
| γ-Cadinene | 0 | 2.20 | 0 | 0 | 0 |
| δ-Cadinene | 3.83 | 3.85 | 0.25 | 0 | 0 |
| Germacrene B | 0.38 | 0 | 1.08 | 6.0 | 0.6 |
| β-Sesquiphellandrene | 0 | 0 | 0 | 9.8 | 0 |
| Elemol | 0 | 6.84 | 0 | 0 | 0 |
| Curzerenone/epi-Curzerenone | 20.89 | 0 | 29.41 | 19.0 | 31.6 |
| Germacrone | 2.59 | 0 | 6.65 | 0 | 10.8 |
| Curlone (= β-Turmerone) | 0 | 0 | 0 | 4.0 | 0 |
| Curdione | 0.10 | 0 | 1.23 | 0 | 1.3 |
| Curcumenol | 0 | 0 | 1.57 | 0 | 2.2 |
Curcuma aeruginosa (also known as “black curcuma”) is characterized by its distinctive ginger-like scent [44]. The volatile oil of C. aeruginosa is known to contain relatively equal amounts of monoterpenes and sesquiterpenes. Two black turmeric samples from Malaysia had curzerenone (24.6–30.4%), 1,8-cineole (11.2–25.2%), camphor (6.8–10.5%), and curcumenol (5.6%) [45,46], while from India the oil was dominated by curcumenol (38.7%) and β-pinene (27.5%) [15] (Table 4). A C. aeruginosa oil sample from Thailand was dominated by curzerenone (41.6%) followed by 1,8-cineole (9.6%) and β-pinene (7.7%) [38], whereas another sample had camphor (29.4%), germacrone (21.2%), isoborneol (7.3%), germacrene B (5.2%), and curzerene (4.8%) [4].
Table 4.
Chemical composition of Curcuma aeruginosa rhizome essential oils reported in the literature.
| Compound | Cae Thailand [4] | Cae India [15] | Cae Thailand [38] | Cae Malaysia [46] | Cae Malaysia [45] |
|---|---|---|---|---|---|
| Camphene | 1.2 | 0.18 | 0.3 | 1.6 | 0.2 |
| β-Pinene | 0.4 | 27.5 | 7.7 | 1.6 | 0.4 |
| 1,8-Cineole | 2.7 | 0.42 | 9.6 | 25.2 | 11.2 |
| Camphor | 29.4 | 0 | 0 | 6.8 | 10.5 |
| Isoborneol | 7.3 | 0 | 0.6 | 1.5 | 3.2 |
| Borneol | 2.9 | 0 | 0.5 | 0.5 | 1.3 |
| β-Elemene | 1.4 | 0 | 0.2 | 1.7 | 2.2 |
| β-Farnesene | 0 | 1.5 | 0 | 0.5 | 1.0 |
| Zingiberene | 0 | 1.2 | 0 | 0.1 | 0 |
| Curzerene | 4.8 | 0 | 1.1 | 0 | 0 |
| Germacrene B | 5.2 | 0 | 0.5 | 0 | 0 |
| Curzerenone | 0 | 0 | 41.6 | 30.4 | 24.6 |
| β-Eudesmol | 0 | 3.6 | 0 | 0 | 0 |
| Germacrone | 21.2 | 0 | 1.0 | 2.8 | 2.7 |
| Curcumenol | 0 | 38.7 | 0 | 0 | 5.6 |
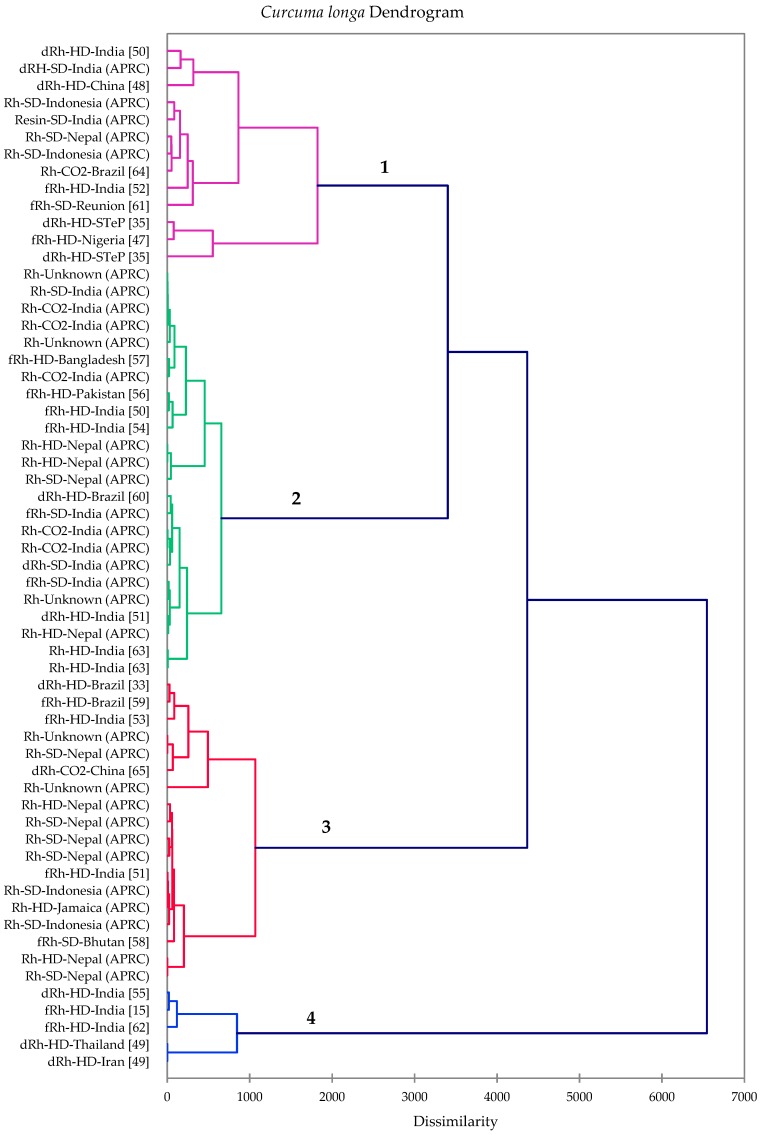
A hierarchical cluster analysis was carried out based on the C. longa essential oil compositions. For comparison, we included C. longa rhizome oils that were reported in the literature in this analysis, including 23 steam- or hydrodistilled essential oils and two supercritical CO2 extracts (Table 5). Although C. longa rhizome oils were all rich in ar-turmerone, α-turmerone, and β-turmerone, the cluster analysis revealed four clearly defined clusters based on the relative concentrations of these major components (Figure 1). The cluster centroids of the major components of C. longa rhizome oils are summarized in Table 6, illustrating the chemical differences in the four clusters. Cluster 2 was the largest, representing 21 samples dominated by the turmerones (particularly ar-turmerone). Cluster 1 represents samples with relatively large concentrations of components other than turmerones; therefore, lower concentrations of turmerones. The third cluster was also a large cluster, representing 15 samples dominated by the turmerones (predominantly α-turmerone). The fourth cluster had very large concentrations of ar-turmerone.
Table 5.
Chemical composition of Curcuma longa rhizome volatile oils from the published literature.
| Sample | α-Phellandrene | p-Cymene | 1,8-Cineole | Terpinolene | ar-Curcumene | α-Zingiberene | β-Bisabolene | β-Sesquiphellandrene | ar-Turmerone | α-Turmerone | Germacrone | Curlone (= β-Turmerone) | (6S,7R)-Bisabolone | (E)-α-Atlantone |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fRh-HD-Nigeria [47] | 15.5 | 2.1 | 10.3 | 3.2 | 0.7 | 2.0 | 0 | 1.8 | 10.0 | 35.9 | 0 | 12.9 | 0 | 0 |
| dRh-HD-China [48] | 0 | 0.5 | 0.5 | 0.3 | 6.1 | 20.1 | 5.1 | 15.5 | 27.5 | 0.1 | 0.3 | 1.7 | 0 | 0 |
| fRh-HD-India [15] | 3.1 | 0.3 | 0.7 | 0.1 | 3.5 | 4.0 | 0 | 0.8 | 49.8 | 9.1 | 0 | 7.9 | 0 | 0 |
| dRh-HD-Iran [49] | 2.2 | 0.4 | 0.4 | 1.5 | 0.8 | 1.5 | 0.4 | 1.3 | 68.9 | 20.9 | 0 | 0 | 0 | 0 |
| fRh-HD-India [50] | 0.1 | 0.3 | 0.4 | 2.7 | 1.6 | 2.5 | 0.8 | 2.9 | 24.4 | 20.5 | 1.0 | 11.1 | 1.7 | 0.9 |
| dRh-HD-India [50] | 0 | 0.1 | 0.1 | tr | 6.6 | 0.8 | 4.1 | 4.2 | 21.4 | 0.6 | 2.6 | 4.3 | 0.8 | 2.6 |
| fRh-HD-India [51] | 2.0 | 0.6 | 0.8 | 0.2 | 1.9 | 2.6 | 0.4 | 2.4 | 21.0 | 33.5 | 0 | 18.9 | 0 | 0 |
| dRh-HD-India [51] | tr | 0 | tr | 0 | 1.2 | 2.2 | 1.5 | 2.8 | 30.3 | 26.5 | 0 | 19.1 | 0 | 0 |
| fRh-HD-India [52] | 8.0 | 4.3 | 11.2 | 0.7 | 4.4 | 5.6 | 2.8 | 7.1 | 7.3 | 11.1 | 0.1 | 5.0 | 0.1 | 0.2 |
| fRh-HD-India [53] | 9.4 | 1.2 | 1.9 | 1.2 | 0.5 | 2.3 | 0 | 1.8 | 5.4 | 44.1 | 0.4 | 18.5 | 0 | 1.1 |
| fRh-HD-India [54] | 0.1 | 0.1 | 2.6 | 0.1 | 0.2 | 1.3 | 0.2 | 0 | 31.7 | 12.9 | 0.9 | 12.0 | 0.2 | 1.5 |
| dRh-HD-India [55] | 2.2 | 1.0 | 0 | 0 | 4.8 | 0 | 0 | 0 | 53.1 | 6.2 | 0 | 6.4 | 0 | 0 |
| dRh-HD-Thailand [49] | 2.2 | 0.4 | 0.4 | 1.5 | 0.8 | 1.5 | 0.4 | 1.3 | 68.9 | 20.9 | 0 | 0 | 0 | 0 |
| fRh-HD-Pakistan [56] | 0.4 | 0 | 1.6 | 0 | 0 | 0 | 0 | 0 | 25.3 | 18.4 | 0 | 12.5 | 0 | 0 |
| fRh-HD-Bangladesh [57] | 0.5 | 0.2 | 0 | 0 | 3.3 | 4.4 | 0.2 | 5.6 | 27.8 | 17.2 | 0 | 13.8 | 0 | 0 |
| fRh-SD-Bhutan [58] | 1.7 | 0.5 | 7.6 | 0.7 | 1.4 | 4.2 | 0.7 | 3.6 | 16.7 | 30.1 | 0 | 14.7 | 1.0 | 1.2 |
| fRh-HD-Brazil [59] | 6.5 | 0.9 | 3.2 | 1.4 | 1.0 | 1.9 | 0.3 | 1.4 | 12.9 | 42.6 | 0.5 | 16.0 | 0.3 | 0.5 |
| dRh-HD-Brazil [60] | 1.7 | 0.8 | 0.7 | 0 | 2.6 | 1.0 | 0 | 2.4 | 33.2 | 23.5 | 0 | 22.7 | 3.1 | 1.4 |
| dRh-HD-S. Tomé e Principe [35] | 15.5 | 2.5 | 10.2 | 3.1 | 0.8 | 1.1 | 0 | 1.0 | 12.8 | 23.9 | 0 | 11.5 | 0 | 0.6 |
| dRh-HD-S. Tomé e Principe [35] | 30.4 | 5.5 | 23.0 | 4.5 | 1.1 | 2.4 | 0 | 2.0 | 4.0 | 12.2 | 0 | 4.3 | 0 | 0 |
| dRh-HD-Brazil [33] | 2.7 | 0 | 1.4 | 0 | 1.0 | 2.4 | tr | 1.9 | 18.0 | 44.0 | 0 | 18.3 | 0.6 | 0.6 |
| fRh-SD-Reunion [61] | 1 | 0.6 | 2 | 15.8 | 4.5 | 11.8 | 1.9 | 8.8 | 7.7 | 21.4 | 0 | 7.1 | 0 | 0 |
| fRh-HD-India [62] | 5.3 | 0 | 2.6 | 0 | 3.5 | 0 | 0.6 | 1.7 | 49.1 | 0 | 0 | 16.8 | 0 | 0 |
| dRh-HD-India [63] | 1.8 | 1.3 | 1.3 | 0 | 1.4 | 1.7 | 0 | 1.7 | 34.0 | 34.0 | 0 | 15.0 | 0 | 0 |
| dRh-HD-India [63] | 1.4 | 0.9 | 1.3 | 0 | 1.5 | 1.9 | 0 | 1.9 | 35.0 | 35.0 | 0 | 12.0 | 0 | 0 |
| Rh-CO2-Brazil [64] | 4.1 | 1.5 | 4.0 | 1.3 | 3.6 | 6.4 | 1.7 | 7.7 | 15.5 | 20.3 | 0 | 15.6 | 0.3 | 0.6 |
| dRh-CO2-China [65] | 0 | 0 | 0 | 2.2 | 1.9 | 16.9 | 1.5 | 10.0 | 11.0 | 40.8 | 0 | 14.1 | 0 | 0 |
Rh = rhizome; dRh = dried rhizome; fRh = fresh rhizome; HD = hydrodistillation; SD = steam distillation; CO2 = supercritical CO2 extracts.
Figure 1.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of 60 Curcuma longa volatile oil samples.
Table 6.
Concentration (%) of centroids used in the cluster analysis of Curcuma longa rhizome oils.
| Compound | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 |
|---|---|---|---|---|
| α-Phellandrene | 6.58 | 0.71 | 2.13 | 2.99 |
| 1,8-Cineole | 5.11 | 1.11 | 1.39 | 0.82 |
| ar-Curcumene | 4.77 | 2.72 | 1.49 | 2.68 |
| α-Zingiberene | 6.23 | 2.72 | 4.68 | 1.4 |
| β-Sesquiphellandrene | 6.22 | 3.89 | 3.92 | 1.02 |
| ar-Turmerone | 15.94 | 31.68 | 18.31 | 57.96 |
| α-Turmerone | 15.49 | 20.56 | 35.11 | 11.41 |
| Curlone | 8.01 | 14.75 | 17.20 | 6.22 |
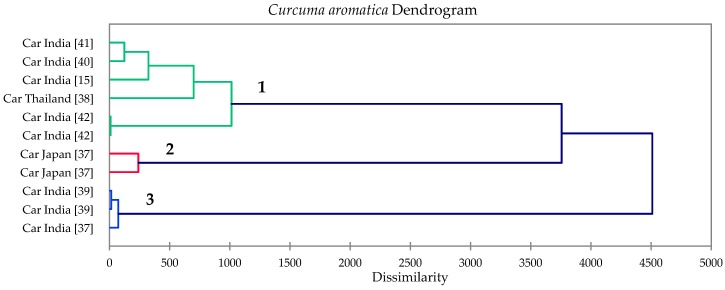
Hierarchical cluster analysis of C. aromatica essential oils clearly identified three clusters based on dissimilarity (Figure 2). Cluster 1 had a relatively high camphor concentration, represented by the C. aromatica EO sample from Thailand [38]; cluster 2 was dominated by curdione followed by 1,8-cineole, represented by two samples from Japan [37]; and cluster 3 represents samples with large concentrations of ar-curcumene and β-curcumene [15,37,39,40,41,42]. Table 7 summarizes the cluster centroids of the major components of C. aromatica rhizome oils.
Figure 2.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of eight Curcuma aromatica essential oil samples.
Table 7.
Concentration (%) of centroids used in the cluster analysis of Curcuma aromatica rhizome oils.
| Compound | Cluster 1 | Cluster 2 | Cluster 3 |
|---|---|---|---|
| Camphor | 28.28 | 0 | 3.69 |
| ar-Curcumene | 8.76 | 0 | 23.25 |
| Curdione | 0 | 38.08 | 0 |
| β-Curcumene | 1.30 | 0 | 29.93 |
| 1,8-Cineole | 5.02 | 16.41 | 0.38 |
| Xanthorrhizol | 6.23 | 0 | 9.83 |
| Curzerenone | 8.57 | 0 | 5.43 |
| Germacrone | 3.80 | 7.09 | 4.85 |
For C. zedoaria essential oils, the cluster analysis showed two clusters based on dissimilarity (Figure 3): (1) a cluster dominated by curzerenone/epi-curzerenone followed by camphor, germacrone, 1,8-cineole, and α-copaene; and (2) a cluster represented by a single sample with very large concentrations of 1,8-cineole. The cluster centroids of the main constituents of C. zedoaria rhizome oils are summarized in Table 8.
Figure 3.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of five Curcuma zedoaria essential oil samples.
Table 8.
Concentration (%) of centroids used in the cluster analysis of Curcuma aromatica rhizome oils.
| Cluster 1 | Cluster 2 | |
|---|---|---|
| Curzerenone/epi-Curzerenone | 27.3 | 0 |
| 1,8-Cineole | 4.42 | 38.39 |
| Camphor | 7.66 | 0 |
| Germacrone | 5.01 | 0 |
| α-Copaene | 4.43 | 0.42 |
| Curzerene | 4.07 | 0 |
| ar-Curcumene | 3.03 | 0.51 |
| Zingiberene | 3.00 | 0 |
| β-Sesquiphellandrene | 2.45 | 0 |
Curcuma aeruginosa essential oils showed three classes in hierarchical cluster analysis based on dissimilarity (Figure 4): (1) a camphor/germacrone rich cluster with large concentrations of isoborneol, curzerene, and germacrone B; (2) a curcumenol/β-pinene rich cluster; and (3) a curzerenone/1,8-cineole cluster. Table 9 summarizes the concentrations of cluster centroids of the major components of C. aeruginosa rhizome oils. Although there are only five essential oil samples of C. zedoaria and C. aeruginosa, which is too few to give a comprehensive chemotaxonomic representation of these species, this analysis does provide initial insights into the potential chemotypes.
Figure 4.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of five Curcuma aeruginosa essential oil compositions.
Table 9.
Concentration (%) of centroids used in the cluster analysis of Curcuma aeruginosa rhizome oils.
| Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|
| Curzerenone | 0 | 0 | 32.21 |
| 1,8-Cineole | 2.68 | 0.42 | 15.35 |
| Camphor | 29.39 | 0 | 5.77 |
| Curcumenol | 0 | 38.70 | 1.87 |
| β-Pinene | 0.35 | 27.50 | 3.24 |
| Germacrone | 21.21 | 0 | 2.16 |
| Isoborneol | 7.27 | 0 | 1.76 |
| Curzerene | 4.84 | 0 | 0.36 |
| Germacrene B | 5.20 | 0 | 0.17 |
4. Conclusions
The rhizome essential oils of Curcuma longa, C. aromatica, C. zedoaria, and C. aeruginosa from the APRC collection, compared to the published literature, were analyzed by GC-MS. α-Turmerone, curlone, ar-turmerone, β-sesquiphellandrene, α-zingiberene, germacrone, terpinolene, ar-curcumene, and α-phellandrene were the major components of C. longa. C. zedoaria rhizome oil contained 1,8-cineole, curzerenone/epi-curzerenone, α-copaene, camphor, β-caryophyllene, elemol, germacrone, curzerene, and β-elemene. The cluster analysis revealed four clearly defined clusters for C. longa, three clusters for C. aromatica and C. aeruginosa, and two types for C. zedoaria.
In the case of C. longa, there are no apparent correlations based on extraction method (steam distillation, hydrodistillation, or supercritical CO2 extraction) or country or region of origin. Furthermore, the differences between the clusters are not that great, and therefore, the clusters do not likely represent distinct chemotypes but rather just reflect the chemical variation within each species. The data do provide a baseline for comparison of C. longa rhizome oils, however. These are important points when considering sources of either essential oils or rhizomes. There are still too few data to draw conclusions about the possible chemotypes of C. aromatica, C. aeruginosa, or C. zedoaria; more data are required.
Acknowledgments
This work was carried out as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/). We are grateful to Loren Bangerter for help with distillation of C. longa samples and to Prasun Satyal for supplying the Curcuma samples from Nepal.
Author Contributions
Conceptualization: N.S.D.; software: P.S.; validation: W.N.S.; formal analysis: W.N.S.; investigation: N.S.D., P.S., and W.N.S.; writing—original draft preparation: N.S.D.; writing—review and editing: N.S.D., P.S. and W.N.S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ravindran P.N., Babu K.N., Shiva K.N. Botany and crop improvement of tumeric. In: Ravindran P.N., Babu K.N., Sivaraman K., editors. Turmeric: The Genus Curcuma. CRC Press; Boca Raton, FL, USA: 2007. pp. 15–70. [Google Scholar]
- 2.Chuakul W., Boonpleng A. Ethnomedical uses of Thai Zingiberaceous plant (1) Thai J. Phytopharm. 2003;10:33–39. [Google Scholar]
- 3.Tushar, Basaka S., Sarma G.C., Rangan L. Ethnomedical uses of Zingiberaceous plants of northeast India. J. Ethnopharmacol. 2010;132:286–296. doi: 10.1016/j.jep.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Akarchariya N., Sirilun S., Julsrigival J., Chansakaowa S. Chemical profiling and antimicrobial activity of essential oil from Curcuma aeruginosa Roxb., Curcuma glans K. Larsen & J. Mood and Curcuma cf. xanthorrhiza Roxb. collected in Thailand. Asian Pac. J. Trop. Biomed. 2017;7:881–885. [Google Scholar]
- 5.Dosoky N.S., Setzer W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients. 2018;10:1196. doi: 10.3390/nu10091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leong-Skornikova J., Newman M. Gingers of Cambodia, Laos & Vietnam. Oxford Graphic Printers Pte Ltd.; Singapore: 2015. [Google Scholar]
- 7.Sikha A., Harini A., Prakash H. Pharmacological activities of wild turmeric (Curcuma aromatica Salisb): A review. J. Pharmacogn. Phytochem. 2015;3:1–4. [Google Scholar]
- 8.Afzal A., Oriqat G., Khan M.A., Jose J., Afzal M. Chemistry and biochemistry of terpenoids from Curcuma and related species. J. Biol. Act. Prod. Nat. 2013;3:1–55. [Google Scholar]
- 9.Krup V., Prakash H.L., Harini A. Pharmacological activities of turmeric (Curcuma longa Linn): A review. J. Tradit. Med. Clin. Naturop. 2013;2:1206–2167. doi: 10.4172/2167-1206.1000133. [DOI] [Google Scholar]
- 10.Herath H.M.I.C., Wiyasiriwardene T.D.C.M.K., Premakumara G.A.S. Comparative GC-MS analysis of all Curcuma species grown in Sri Lanka by multivariate test. Ruhunu J. Sci. 2017;8:1–9. doi: 10.4038/rjs.v8i2.29. [DOI] [Google Scholar]
- 11.Chen I.N., Chang C.C., Ng C.C., Wang C.Y., Shyu Y.T., Chang T.L. Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plants Food Hum. Nutr. 2008;63:15–20. doi: 10.1007/s11130-007-0063-7. [DOI] [PubMed] [Google Scholar]
- 12.Reanmongkol W., Subhadhirasakul S., Khaisombat N., Fuengnawakit P., Jantasila S., Khamjun A. Investigation the antinociceptive, antipyretic and anti-inflammatory activities of Curcuma aeruginosa Roxb. extracts in experimental animals. Songklanakarin J. Sci. Technol. 2006;28:999–1008. [Google Scholar]
- 13.Wilson B., Abraham G., Manju V.S., Mathew M., Vimala B., Sundaresan S., Nambisan B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J. Ethnopharmacol. 2005;99:147–151. doi: 10.1016/j.jep.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Mau J., Lai E.Y.C., Wang N.P., Chen C.C., Chang C.H., Chyau C.C. Composition and antioxidant activity of the essential oil from Curcuma zedoaria. Food Chem. 2003;82:583–591. doi: 10.1016/S0308-8146(03)00014-1. [DOI] [Google Scholar]
- 15.Angel G.R., Menon N., Vimala B., Nambisan B. Essential oil composition of eight starchy Curcuma species. Ind. Crops Prod. 2014;60:233–238. doi: 10.1016/j.indcrop.2014.06.028. [DOI] [Google Scholar]
- 16.Simoh S., Zainal A. Chemical profiling of Curcuma aeruginosa Roxb. rhizome using different techniques of solvent extraction. Asian Pac. J. Trop. Biomed. 2015;5:412–417. doi: 10.1016/S2221-1691(15)30378-6. [DOI] [Google Scholar]
- 17.Lakshmi S., Padmaja G., Remani P. Antitumour effects of isocurcumenol isolated from Curcuma zedoaria rhizomes on human and murine cancer cells. Int. J. Med. Chem. 2011;2011:253962. doi: 10.1155/2011/253962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayaprakasha G.K., Jagan L., Rao M., Sakariah K.K. Chemistry and biological activity of Curcuma longa. Trend Food Sci. Technol. 2005;16:533–548. doi: 10.1016/j.tifs.2005.08.006. [DOI] [Google Scholar]
- 19.Kunnumakkara A.B., Anand P., Aggarwal B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Satoskar R.R., Shah S.J., Shenoy S.G. Evaluation of antiinflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharma. Ther. Toxicol. 1986;24:651–654. [PubMed] [Google Scholar]
- 21.Masuda T., Kidaka K., Shinihara A., Mackawa T., Takeda Y., Yamaguchi H. Chemical studies on antioxidant mechanism of curcuminoid: Analysis of radical reaction products from curcumin. J. Agric. Food Chem. 1999;47:71–77. doi: 10.1021/jf9805348. [DOI] [PubMed] [Google Scholar]
- 22.Negi P.S., Jayaprakasha G.K., Jagan Mohan Rao L., Sakariah K.K. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J. Agric. Food Chem. 1999;47:4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 23.Jacob J.N. Comparative studies in relation to the structure and biochemical properties of the active compounds in the volatile and nonvolatile fractions of turmeric (C. longa) and ginger (Z. officinale) In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry. Volume 48. Elsevier; Amsterdam, The Netherlands: 2016. pp. 101–135. [Google Scholar]
- 24.Tilak J.C., Banerjee H.M., Mohan T.P.A. Antioxidant availability of turmeric in relation to its medicinal and culinary uses. Phytother. Res. 2004;18:798–804. doi: 10.1002/ptr.1553. [DOI] [PubMed] [Google Scholar]
- 25.Park S.Y., Kim D. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002;65:1227–1231. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- 26.Weiss E.A. Spice Crops. CAB International Publishing; Oxon, UK: 2002. [Google Scholar]
- 27.Gopalan B., Goto M., Kodama A., Hirose T. Supercritical carbon dioxide extraction of turmeric (Curcuma longa) J. Agric. Food Chem. 2000;48:2189–2192. doi: 10.1021/jf9908594. [DOI] [PubMed] [Google Scholar]
- 28.Sanghamitra N., Sujata M., Nagar K. Differential effect of soil and environment on metabolic expression of turmeric (Curcuma longa cv. Roma) Indian J. Exp. Biol. 2015;53:406–411. [PubMed] [Google Scholar]
- 29.Srinivasan V., Thankamani C.K., Dinesh R., Kandiannan K., Zachariah T.J., Leela N.K., Hamza S., Shajina O., Ansha O. Nutrient management systems in turmeric: Effects on soil quality, rhizome yield and quality. Ind. Crops Prod. 2016;85:241–250. doi: 10.1016/j.indcrop.2016.03.027. [DOI] [Google Scholar]
- 30.Dosoky N.S., Setzer W.N. Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci. 2018;19:1966. doi: 10.3390/ijms19071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 32.Satyal P. Ph.D. Thesis. University of Alabama in Huntsville; Huntsville, AL, USA: 2015. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. [Google Scholar]
- 33.Braga M.E.M., Leal P.F., Carvalho J.E., Meireles M.A.A. Comparison of yield, composition and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J. Agric. Food Chem. 2003;51:6604–6611. doi: 10.1021/jf0345550. [DOI] [PubMed] [Google Scholar]
- 34.Usman L.A., Hamid A.A., George O.C., Ameen O.M., Muhammad N.O., Zubair M.F., Lawal A. Chemical composition of rhizome essential oil of Curcuma longa L. growing in north central Nigeria. World J. Chem. 2009;4:178–181. [Google Scholar]
- 35.Martins A.P., Salgueiro L., Gonçalves M.J., da Cunha A.P., Vila R., Canigueral S., Mazzoni V., Tomi F., Casanova J. Essential oil composition and antimicrobial activity of three Zingiberaceae from S. Tomé e Principe. Planta Med. 2001;67:580–584. doi: 10.1055/s-2001-16494. [DOI] [PubMed] [Google Scholar]
- 36.Al-Reza S.M., Rahman A., Sattar M.A., Rahman M.O., Fida H.M. Essential oil composition and antioxidant activities of Curcuma aromatica Salisb. Food Chem. Toxicol. 2010;48:1757–1760. doi: 10.1016/j.fct.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Kojima H., Yanai T., Toyota A. Essential oil constituents from Japanese and Indian Curcuma aromatica rhizomes. Planta Med. 1998;64:380–381. doi: 10.1055/s-2006-957458. [DOI] [PubMed] [Google Scholar]
- 38.Jarikasem S., Thubthimthed S., Chawananoraseth K., Suntorntanasat T. Essential oils from three Curcuma species collected in Thailand. Acta Hortic. 2005;677:37–41. doi: 10.17660/ActaHortic.2005.675.4. [DOI] [Google Scholar]
- 39.Catalan C.A.N., Bardon A., Retamar J.A., Gros E.G., Verghese J., Joy M.T. The essential oil of Curcuma aromatica Salisb. Flavor Fragr. J. 1989;4:25–30. doi: 10.1002/ffj.2730040106. [DOI] [Google Scholar]
- 40.Bordoloi A.K., Sperkova J., Leclercq P.A. Essential oils of Curcuma aromatica Salisb. from northeast India. J. Essent. Oil Res. 1999;11:537–540. doi: 10.1080/10412905.1999.9701209. [DOI] [Google Scholar]
- 41.Choudhury S.N., Ghosh A.C., Saikia M., Choudhury M., Leclercq P.A. Volatile constituents of the aerial and underground parts of Curcuma aromatica Salisb from India. J. Essent. Oil Res. 1996;8:633–638. doi: 10.1080/10412905.1996.9701031. [DOI] [Google Scholar]
- 42.Gopichand, Singh R.D., Meena R.L., Singh M.K., Kaul V.K., Lal B., Acharya R., Prasad R. Effect of manure and plant spacing on crop growth, yield and oil-quality of Curcuma aromatica Salisb. in mid hill of western Himalaya. Ind. Crops Prod. 2006;24:105–112. doi: 10.1016/j.indcrop.2005.06.006. [DOI] [Google Scholar]
- 43.Singh P., Singh S., Kapoor I.P.S., Singh G., Isidorov V., Szczepaniak L. Chemical composition and antioxidant activities of essential oil and oleoresins from Curcuma zedoaria rhizomes, part-74. Food Biosci. 2013;3:42–48. doi: 10.1016/j.fbio.2013.06.002. [DOI] [Google Scholar]
- 44.Srivastava S., Chitranshi N., Srivastava S., Dan M., Rawat A.K.S., Pushpangadan P. Pharmacognostic evaluation of Curcuma aeruginosa Roxb. Nat. Prod. Sci. 2006;12:162–165. [Google Scholar]
- 45.Sirat M.H., Jamil S., Hussain J. Essential oil of Curcuma aeruginosa Roxb. from Malaysia. J. Essent. Oil Res. 1998;10:453–458. doi: 10.1080/10412905.1998.9700942. [DOI] [Google Scholar]
- 46.bin Jantan I., Ahmad A.S., Ali N.A.M., Ahmad A.R., Ibrahim H. Chemical composition of the rhizome oils of four Curcuma species from Malaysia. J. Essent. Oil Res. 1999;11:719–723. doi: 10.1080/10412905.1999.9712004. [DOI] [Google Scholar]
- 47.Oyemitan I.A., Elusiyan C.A., Onifade A.O., Akanmu M.A., Oyedeji A.O., McDonald A.G. Neuropharmacological profile and chemical analysis of fresh rhizome essential oil of Curcuma longa (turmeric) cultivated in southwest Nigeria. Toxicol. Rep. 2017;4:391–398. doi: 10.1016/j.toxrep.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Yang Z., Chen F., Su P., Chen D., Pan W., Fang Y., Dong C., Zheng X., Du Z. Composition and bioactivity assessment of essential oils of Curcuma longa L. collected in China. Ind. Crops Prod. 2017;109:60–73. doi: 10.1016/j.indcrop.2017.08.009. [DOI] [Google Scholar]
- 49.Asghari G., Mostajeran A., Shebli M. Curcuminoid and essential oil components of turmeric at different stages of growth cultivated in Iran. Res. Pharm. Sci. 2009;4:55–61. [Google Scholar]
- 50.Singh G., Kapoor I.P.S., Singh P., de Heluani C.S., de Lampasona M.P., Catalan C.A.N. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.) Food Chem. Toxicol. 2010;48:1026–1031. doi: 10.1016/j.fct.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Kutti Gounder D., Lingamallu J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind. Crops Prod. 2012;38:124–131. doi: 10.1016/j.indcrop.2012.01.014. [DOI] [Google Scholar]
- 52.Raina V.K., Srivastava S.K., Jain N., Ahmad A., Syamasundar K.V., Aggarwal K.K. Essential oil composition of Curcuma longa L. cv. Roma from the plains of northern India. Flavour Fragr. J. 2002;17:99–102. doi: 10.1002/ffj.1053. [DOI] [Google Scholar]
- 53.Raina V.K., Syamsundar S.K., Srivastava K.V. Rhizome and leaf oil composition of Curcuma longa from the lower Himalayan region of northern India. J. Essent. Oil Res. 2005;17:556–559. doi: 10.1080/10412905.2005.9698993. [DOI] [Google Scholar]
- 54.Awasthi P.K., Dixit S.C. Chemical composition of Curcuma longa leaves and rhizome oil from the plains of northern India. J. Young Pharm. 2009;1:312–316. [Google Scholar]
- 55.Naveen Kumar K., Venkataramana M., Allen J.A., Chandranayaka S., Murali H.S., Batra H.V. Role of Curcuma longa L. essential oil in controlling the growth and zearalenone production of Fusarium graminearum. LWT Food Sci. Technol. 2016;69:522–528. doi: 10.1016/j.lwt.2016.02.005. [DOI] [Google Scholar]
- 56.Naz S., Ilyas S., Parveen Z., Javed S. Chemical analysis of essential oils from turmeric (Curcuma longa) rhizome through GC-MS. Asian J. Chem. 2010;22:3153–3158. [Google Scholar]
- 57.Chowdhury J.U., Nandi N.C., Bhuiyan M.N.I., Mobarok M.H. Essential oil constituents of the rhizomes of two types of Curcuma longa of Bangladesh. Bangladesh J. Sci. Ind. Res. 2008;43:259–266. doi: 10.3329/bjsir.v43i2.970. [DOI] [Google Scholar]
- 58.Sharma R.K., Mishra B.P., Sharma T.C., Bordloi A.K., Pathak M.G., Leclercq P.A. Essential oil of Curcuma longa L. from Bhutan. J. Essent. Oil Res. 1997;9:589–592. doi: 10.1080/10412905.1997.9700783. [DOI] [Google Scholar]
- 59.Avanço G.B., Ferreira F.D., Bomfimb N.S., de Souza Rodrigues dosSantos P.A., Peralta R.M., Brugnari T., Mallmann C.A., de Abreu Filho B.A., Mikcha J.M.G., Machinski M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control. 2017;73:806–813. [Google Scholar]
- 60.Ferreira F.D., Kemmelmeier C., Arrotéia C.C., Da Costa C.L., Mallmann C.A., Janeiro V., Ferreira F.M.D., Mossini S.A.G., Silva E.L., Machinski M. Inhibitory effect of the essential oil of Curcuma longa L. and curcumin on aflatoxin production by Aspergillus flavus Link. Food Chem. 2013;136:789–793. doi: 10.1016/j.foodchem.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Chane-Ming J., Vera R., Chalchat J.C., Cabassu P. Chemical composition of essential oils from rhizomes, leaves and flowers of Curcuma longa L. from Reunion Island. J. Essent. Oil Res. 2002;14:249–251. doi: 10.1080/10412905.2002.9699843. [DOI] [Google Scholar]
- 62.Singh S., Sankar B., Rajesh S., Sahoo K., Subudhi E., Nayak S. Chemical composition of turmeric oil (Curcuma longa L. cv. Roma) and its antimicrobial activity against eye infecting pathogens. J. Essent. Oil Res. 2011;23:11–18. doi: 10.1080/10412905.2011.9712275. [DOI] [Google Scholar]
- 63.Chatterjee S., Variyar P.S., Gholap A.S., Bongirwar D.R. Effect of γ-irradiation on the volatile oil constituents of turmeric (Curcuma longa) Food Res. Int. 2000;33:103–106. doi: 10.1016/S0963-9969(00)00012-0. [DOI] [Google Scholar]
- 64.Chassagnez-mendez A.L., Machado N.T., Araujo M.E., Maia J.G., Meireles A.A. Supercritical CO2 extraction of curcumins and essential oil from the rhizomes of turmeric (Curcuma longa L.) Ind. Eng. Chem. Res. 2000;39:4729–4733. doi: 10.1021/ie000171c. [DOI] [Google Scholar]
- 65.Ling J., Wei B., Lv G., Ji H., Li S. Anti-hyperlipidaemic and antioxidant effects of turmeric oil in hyperlipidaemic rats. Food Chem. 2012;130:229–235. doi: 10.1016/j.foodchem.2011.07.039. [DOI] [Google Scholar]