Abstract
The World Health Organization (WHO) defines glycogen-rich clear cell carcinoma (GRCC) of the breast as a carcinoma with glycogen accumulation in more than 90% of its tumor cells. Due to the rarity of this disease, its reported survival and clinical associations have been inconsistent due to reliance on case reports and limited case series. As a result, the prognostic implication of this cancer subtype remains unclear. Using the U.S. Surveillance, Epidemiology, and End Results (SEER) program database, we compared the incidence, demographics and prognostic factors of 155 cases of GRCC of the breast to 1,251,584 cases of other (non-GRCC) breast carcinomas. We demonstrate that GRCC is more likely to be identified as high grade, advanced stage, and more likely to have triple negative receptor status. GRCC cases display a poorer prognosis than non-GRCC carcinomas of the breast irrespective of age, AJCC staging, tumor grade, joint hormone receptor/human epidermal growth factor receptor 2 (HER2) status, and treatment. Similar to non-GRCC carcinomas, older age and higher American Joint Committee on Cancer (AJCC)/TNM staging were associated with poorer prognosis for GRCC, while treatment with surgery and radiation were associated with improved survival. Radiation, specifically in the setting of breast-conserving surgery, further improved survival compared to surgery alone. Our study highlights the poorer prognosis associated with glycogen accumulation in breast cancers and hence stresses the importance of identifying this more aggressive tumor type.
Keywords: glycogen, breast cancer, SEER program database, clear cell carcinoma, prognosis, survival
1. Introduction
Glycogen is a multibranched, carbohydrate storage molecule made up of monomers of glucose [1,2]. During periods of caloric excess, the level of plasma glucose increases and stimulates glycogen synthesis in the liver and skeletal muscle. During caloric deficit, glycogen is rapidly degraded to glucose to supply energy production via glycolysis and the tricyclic acid (TCA) cycle [2]. Coordinated regulation of glycogen synthesis and degradation is critical for cellular homeostasis [3]. Microscopic visualization of glycogen deposits in cells and tissue are important in the diagnosis of diseases with aberrant glycogen metabolism. Period acid-Schiff (PAS) positive/PAS-diastase (PAS-D) negative staining is the most commonly used method for histochemical detection of glycogen by pathologists in multiple diseases [4,5,6].
Clinicians and pathologists have identified large glycogen granules in multiple cancers, yet the significance of these deposits remains unknown. These aberrant glycogen accumulations have been identified in cancers of the breast, kidney, uterus, lung, head and neck, bladder, ovary, and recently, colorectal tumors [7,8,9,10,11,12,13,14]. Many PAS-positive tumors have earned the term “clear cell” due to the transparent and ovoid appearance left by the carbohydrate accumulations in the cytoplasm after fixation and tissue preparation processing. Several theories currently exist with regards to the mechanism underlying glycogen metabolism changes in cancer cells [15,16,17,18,19,20,21,22]. One popular explanation is that, under hypoxic conditions in the center of solid tumors, glycogen stores are increased via hypoxia-inducible factor 1α (HIF1α) mediated signaling pathways to accommodate the low oxygen levels and nutrient deprivation [13,18,23]. In recent years, cancer research has highlighted multiple aspects of metabolic re-wiring in malignant cancers with glycogen, at least in part, contributing to these changes by providing a constant supply of glucose. While multiple pharmacological modulators of glycogen metabolism are currently being tested, none have yet been clinically approved [22].
Breast cancer is a heterogeneous disease accounting for 30% of all cancers diagnosed in women [24]. Its routine classification/characterization is based on both histopathology (WHO recognizes over 20 epithelial subtypes) and immunophenotypic/molecular features such as estrogen receptor (ER), progesterone receptor (PR) and human epidermal receptor 2 (HER2) status. Intriguingly, there are several rare histological variants of breast cancer, one of which contains clear cells of epithelial origin. The optically clear cytoplasm in these cells may be due to various compounds such as carbohydrate or lipid accumulations; with glycogen as the most commonly identified [25]. Fisher et al. identified glycogen accumulation in 100% of 45 clear cell carcinoma cases [26] and Hayes et al. reported 21 of 26 clear cell carcinomas were glycogen-rich [27]. Breast carcinoma with clear cytoplasm containing glycogen (PAS+/PAS-D−) identified in more than 90% of the tumor cells is classified as glycogen-rich clear cell carcinoma of the breast (GRCC) by the WHO [28].
GRCC is considered an orphan disease as it accounts for less than 3% of all breast carcinomas cases [26,27,29,30]. There has been an ongoing debate over its prognostic implications. Fisher et al. identified 45 cases of GRCC among 1555 women with invasive breast cancer [26]. The study noted a poorer prognosis for GRCC patients, greater frequency of nodal metastasis, and higher histologic grade (III) when compared to the 1510 cases of non-clear cell carcinomas [26]. However, 24 cases reported by Ma et al. [31] showed no significant difference in overall survival between GRCC and control invasive carcinomas when matched by age, tumor size, nodal status, and immune-phenotype. Hayes et al. [27] reported 21 cases of GRCC and also found that prognosis was not different from non-GRCC when the tumor was matched by size, grade, and lymph node status. Due to the lack of sufficient cases and inconsistency in available data, classification of GRCC in terms of prognosis is currently unclear [28,32]. Given the rarity of the cases at any given institution, the Surveillance, Epidemiology, and End Results (SEER) Program database provides a unique opportunity to perform large population-based studies on this orphan disease. This study takes advantage of the SEER program database for the retrospective assessment of incidence, survival, demographics, and prognostic factors for GRCC of the breast.
2. Methods
2.1. Data Source
The Surveillance, Epidemiology and End Results (SEER) Program is the National Cancer Institute’s authoritative source for cancer incidence and survival [33]. It is considered the gold standard for cancer data collection internationally [34]. It is populated with data from national cancer registries, covering approximately 34.6% of the United States population [33]. Vital status is updated annually and the database routinely undergoes quality-control checks. The methodology was conducted as described previously [35].
2.2. Sample Selection and Coding
We queried the SEER database (November, 2017 [36] and November, 2015 submissions, including data from 1973 to 2015) [36,37] to identify all malignant cancers within the breast (International Classification of Diseases- (ICD-) O-3 code (ICD-O-3 codes C34.0–C34.3 and C34.8–C34.9), diagnosed between January 1, 1981 and December 31, 2015. Carcinomas of the breast were determined based on the adapted classification scheme for adolescents and young adults (AYA). Cases of GRCC were identified by ICD-O-3 codes 8310 (clear cell carcinoma (CC)) and 8315 (glycogen-rich carcinoma (GR)). According to the WHO Classifications of Tumors, in the case of breast carcinomas, ICD-O-3 codes 8310 (CC) and 8315 (GR) are considered equivalent and both refer to GRCC [28]. Therefore, the two histological groups, CC and GR, were combined in our analysis and henceforth referred to as GRCC. Follow-up subgroup analysis of CC and GR cases were also conducted separately.
The following variables were collected and coded: AYA site recode, primary site, ICD-O-3 histology, age at diagnosis, sex, race, grade, ER status, PR status, HER2 status, immunohistochemistry (IHC) based intrinsic subtype of breast cancer, American Joint Commission on Cancer (AJCC) 6th Edition Staging, AJCC 6th Edition tumor size (T), lymph nodes affected (N), and metastasis (M) Staging, bone metastasis at diagnosis, brain metastasis at diagnosis, liver metastasis at diagnosis, lung metastasis at diagnosis, surgery at primary site, and radiation. Details of coding for hormone receptor and HER2 status are available on the SEER website (https://seer.cancer.gov/seerstat/databases/ssf/her2-derived.html) and in the AJCC Collaborative Stage Data Collection System Coding Manual [38]. Cases diagnosed at autopsy or that could have 0 days of follow-up were excluded.
2.3. Statistical Analysis
All statistical analyses was carried out using the IBM SPSS Statistics software package (version 25, International Business Machines Corporation, Armonk, NY, USA). Differences in demographic and clinical characteristics between GRCC and non-GRCC cases were determined using the Pearson’s chi-square test and column proportions were compared using Bonferroni correction to adjust for multiple comparisons. Median survival times were determined using the Kaplan-Meier method and the significance of the difference was determined using the log-rank test. Multivariable analyses of overall survival were conducted using the Cox Proportional Hazards Ratios (HR) model. Corresponding hazard ratios and 95% confidence intervals (CI) were estimated from the model. Two-tailed p-values <0.05 were considered statistically significant.
3. Results
3.1. Demographics and Clinical Characteristics
Our SEER Program query identified 1,256,901 cases of malignant breast carcinoma. From these cases of interest, cases diagnosed at autopsy or that could have 0 days of follow-up (n = 5162, 0.4%) were excluded. The final number of cases included in our analysis was 1,251,739. Out of these, 110 cases (0.008%) were identified as CC and 45 of the cases (0.003%) were classified as GR. According to the WHO, the two terms are synonymous when referring to breast carcinomas [28] and since each patient could only be classified by one ICD code, both CC and GR cases were combined and henceforth referred to as GRCC. We first described our findings in the GRCC combined population. We then conducted subgroup analyses on GR and CC carcinomas, separately comparing these cases to non-GRCC cases and to each other; those results will be described subsequently.
Among all carcinomas of the breast, the median follow-up time was 60 months (range: 0 to 395 months), with 414,019 recorded deaths. In the GRCC population, the median follow-up time was 54 months (range: 2 to 96 months), with 63 deaths. The demographical and clinical characteristics of the patient population are summarized in Table 1. The median age at diagnosis of GRCC of the breast was 62 years old compared to 60 years old in non-GRCC carcinomas of the breast (p = 0.46). The majority of patients with GRCC carcinoma were female (98.1%) and ethnically white (80.6%), and 92.9% of GRCC patients received surgery and 45.7% received radiation therapy. We found that grade (p < 0.001), ER status (p <0.001), PR status (p < 0.001), IHC based intrinsic subtypes (p < 0.001), AJCC 6th staging (p = 0.03), T status (p = 0.01) and brain metastasis (p = 0.03) significantly differed between GRCC and non-GRCC carcinomas of the breast; although only a single case of GRCC with brain metastasis was available in our analysis. GRCC were statistically more likely to be Grade III (GRCC: 41.3% vs. non-GRCC carcinomas: 29.2%) and grade IV (GRCC: 3.2% vs. non-GRCC carcinomas: 1.4%), ER negative (GRCC: 40.3% vs. non-GRCC carcinomas: 17.9%), PR negative (GRCC: 58.3% vs. non-GRCC carcinomas: 27.4%), triple negative (GRCC: 44.8% vs. non-GRCC carcinomas: 10.4%), T2 status (GRCC: 36.8% vs. non-GRCC carcinomas: 23.6%), and positive for brain metastasis at diagnosis (GRCC: 3.4% vs. non-GRCC carcinomas: 0.4%).
Table 1.
Demographical and clinical characteristics.
| GRCC | Non-GRCC | |||||
|---|---|---|---|---|---|---|
| Count | % | Count | % | p-Value | ||
| Age | 0–60 years | 78 | 50.3% | 592,544 | 47.3% | 0.46 |
| >60 years | 77 | 49.7% | 659,040 | 52.7% | ||
| Sex | Female | 152 | 98.1% | 1,242,647 | 99.3% | 0.07 |
| Male | 3 | 1.9% | 8937 | 0.7% | ||
| Race | American Indian/Alaska Native | 1 | 0.6% | 6099 | 0.5% | 0.98 |
| Asian or Pacific Islander | 12 | 7.7% | 85,407 | 6.8% | ||
| Black | 16 | 10.3% | 123,081 | 9.8% | ||
| Unknown | 1 | 0.6% | 5967 | 0.5% | ||
| White | 125 | 80.6% | 1,031,030 | 82.4% | ||
| Grade | Well differentiated; Grade I | 5 | 3.2% | 216,525 | 17.3% | <0.001 |
| Moderately differentiated; Grade II | 53 | 34.2% | 446,827 | 35.7% | ||
| Poorly differentiated; Grade III | 64 * | 41.3% | 365,903 | 29.2% | ||
| Anaplastic; Grade IV | 5 * | 3.2% | 16,937 | 1.4% | ||
| Unknown | 28 | 18.1% | 205,392 | 16.4% | ||
| ER Status a | Negative | 58 * | 40.3% | 203,173 | 17.9% | <0.001 |
| Positive | 67 | 46.5% | 807,640 * | 71.1% | ||
| Borderline | 0 | 0.0% | 2837 | 0.2% | ||
| Unknown | 19 | 13.2% | 121,600 | 10.7% | ||
| PR Status a | Negative | 84 * | 58.3% | 310,973 | 27.4% | <0.001 |
| Positive | 40 | 27.8% | 683,610 * | 60.2% | ||
| Borderline | 0 | 0.0% | 5794 | 0.5% | ||
| Unknown | 20 | 13.9% | 134,873 | 11.9% | ||
| HER2 Status c | Negative | 25 | 86.2% | 291,238 | 78.5% | 0.56 |
| Positive | 2 | 6.9% | 52,021 | 14.0% | ||
| Borderline | 0 | 0.0% | 8250 | 2.2% | ||
| Unknown | 2 | 6.9% | 19,359 | 5.2% | ||
| IHC based intrinsic Subtypes c | HER2−/HR+ | 12 | 41.4% | 251,946 * | 67.9% | <0.001 |
| HER2+/HR− | 1 | 3.4% | 15,597 | 4.2% | ||
| HER2+/HR+ | 1 | 3.4% | 36,269 | 9.8% | ||
| Triple Negative | 13 * | 44.8% | 38,750 | 10.4% | ||
| Unknown | 2 | 6.9% | 28,306 | 7.6% | ||
| AJCC 6th Stage | I | 54 | 34.8% | 525,652 | 42.0% | 0.03 |
| II | 59 * | 38.1% | 365,536 | 29.2% | ||
| III | 17 | 11.0% | 139,296 | 11.1% | ||
| IV | 11 | 7.1% | 54,270 | 4.3% | ||
| Unknown | 14 | 9.0% | 166,830 | 13.3% | ||
| T | T0 | 0 | 0.0% | 1110 | 0.1% | 0.01 |
| T1 | 63 | 40.6% | 647,840 * | 51.8% | ||
| T2 | 57 * | 36.8% | 295,198 | 23.6% | ||
| T3 | 7 | 4.5% | 49,233 | 3.9% | ||
| T4 | 3 | 1.9% | 33,931 | 2.7% | ||
| Tis | 0 | 0.0% | 1791 | 0.1% | ||
| Unknown | 25 | 16.1% | 222,481 | 17.8% | ||
| N | N0 | 91 | 58.7% | 718,198 | 57.4% | 0.79 |
| N1 | 32 | 20.6% | 234,449 | 18.7% | ||
| N2 | 9 | 5.8% | 68,563 | 5.5% | ||
| N3 | 6 | 3.9% | 48,862 | 3.9% | ||
| Unknown | 17 | 11.0% | 181,512 | 14.5% | ||
| M | M0 | 132 | 85.2% | 1,083,182 | 86.5% | 0.21 |
| M1 | 11 | 7.1% | 54,270 | 4.3% | ||
| Unknown | 12 | 7.7% | 114,132 | 9.1% | ||
| Bone Metastasis c | No Metastasis | 27 | 93.1% | 351,795 | 94.9% | 0.72 |
| Metastasis | 1 | 3.4% | 13,264 | 3.6% | ||
| Unknown | 1 | 3.4% | 5809 | 1.6% | ||
| Brain Metastasis c | No Metastasis | 27 | 93.1% | 363,212 | 97.9% | 0.03 |
| Metastasis | 1 * | 3.4% | 1501 | 0.4% | ||
| Unknown | 1 | 3.4% | 6155 | 1.7% | ||
| Liver Metastasis c | No Metastasis | 27 | 93.1% | 359,794 | 97.0% | 0.46 |
| Metastasis | 1 | 3.4% | 5079 | 1.4% | ||
| Unknown | 1 | 3.4% | 5995 | 1.6% | ||
| Lung Metastasis c | No Metastasis | 27 | 93.1% | 358,514 | 96.7% | 0.56 |
| Metastasis | 1 | 3.4% | 6,198 | 1.7% | ||
| Unknown | 1 | 3.4% | 6,156 | 1.7% | ||
| Surgery b | No Surgery | 7 | 5.5% | 70,372 | 7.3% | 0.38 |
| Surgery | 118 | 92.9% | 889,255 | 92.0% | ||
| Unknown | 2 | 1.6% | 6822 | 0.7% | ||
| Extent of Surgery b | No Surgery | 8 | 6.3% | 75,877 | 7.9% | 0.51 |
| Sub-Mastectomy # | 70 | 55.1% | 485,610 | 50.2% | ||
| Mastectomy | 48 | 37.8% | 401,380 | 41.5% | ||
| Surgery, Unknown | 0 | 0.0% | 1358 | 0.1% | ||
| Unknown | 1 | 0.8% | 2224 | 0.2% | ||
| Radiation b | No Radiation | 64 | 50.4% | 426,196 | 44.1% | 0.21 |
| Radiation | 58 | 45.7% | 513,445 | 53.1% | ||
| Unknown | 5 | 3.9% | 26,808 | 2.8% | ||
Bolded are statistically significant p-values when comparing between clear cell/glycogen rich to other carcinomas of the breast. * Statistically significant differences between column proportions. # Defined as any surgery less aggressive than total mastectomy. a,b,c Variable available for cases diagnosed after 1990, 1998 and 2010 respectively. GRCC—glycogen-rich clear cell; ER—estrogen receptor; PR—progesterone receptor; IHC—immunohistochemistry; HER2—human epidermal growth factor receptor 2; HER2+—human epidermal growth factor receptor 2 positive; HER2−—human epidermal growth factor receptor 2 negative; HR+—hormone receptor positive; HR−—hormone receptor negative; AJCC—American Joint Committee on Cancer; T, N, M refers to tumor size, nodal status, and metastasis classifications of malignant tumor staging system, respectively.
3.2. Survival
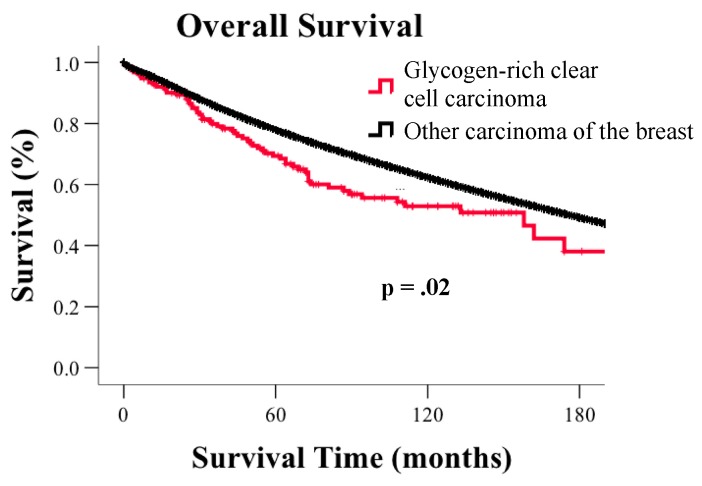
The median survival time for GRCC patients was 158 months vs. 176 months for non-GRCC breast carcinomas (p = 0.02, Figure 1). The corresponding 5-, 10- and 15-year survival rates for GRCC were 70%, 53%, and 44%, respectively, whereas the 5-, 10-, 15-year survival rate for non-GRCC carcinomas was 79%, 64%, and 51%. After adjusting for age, disease stage, tumor grade, ER status, PR status, HER2 status, surgery status, and radiation treatment, survival for GRCC remained significantly poorer compared to non-GRCC carcinomas (HR: 1.33; 95% CI: 1.04–1.67; p = 0.025).
Figure 1.
Kaplan-Meier curves for overall survival based on histological subtype.
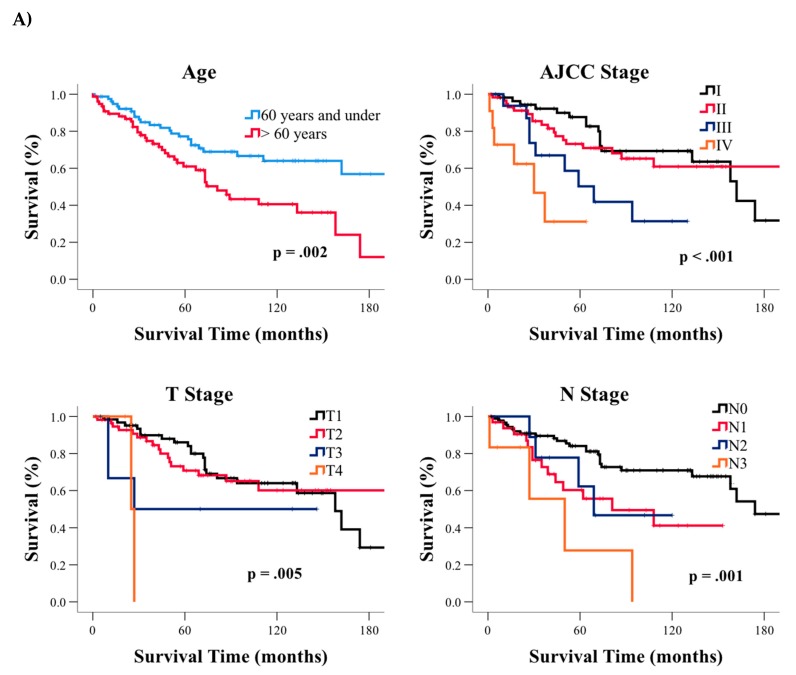
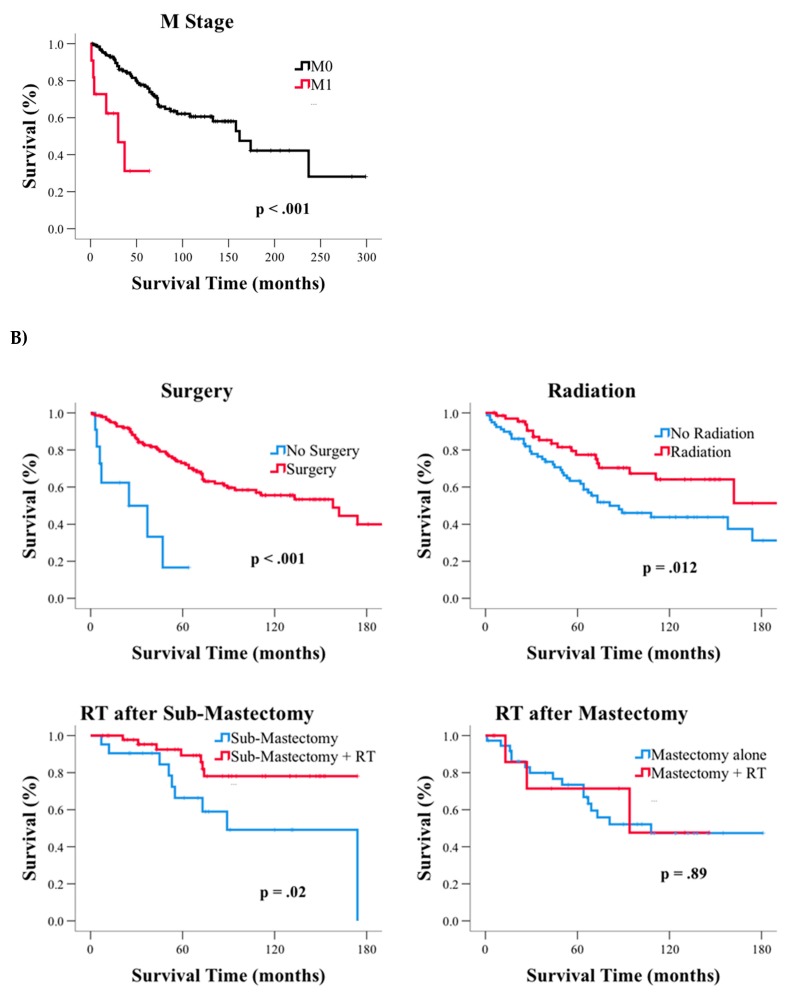
Among GRCC patients, older age (p = 0.002), higher AJCC stage (p < 0.001), T status (p < 0.001), N status (p = 0.001), and M status (p < 0.001) were also associated with significantly poorer survival (Table 2, Figure 2A), whereas surgery (p < 0.001) and radiation treatments (p = 0.02) significantly improved survival (Figure 2B left two panels). We further assessed the combination of surgery and radiation treatment on patient survival (Figure 2B, right two panels). We identified a significant survival improvement in patients who underwent sub-mastectomy—defined as any surgery less than a total mastectomy, plus radiation treatment compared to individuals who only underwent sub-mastectomy (p = 0.02). However, when total mastectomy was combined with radiation treatment, no improvement in survival was observed (p = 0.89). We found that age (HR: 1.05; 95% CI: 1.02–1.07; p < 0.001); AJCC stage III (HR: 4.67; 95% CI: 1.84–11.87; p = 0.001); AJCC stage IV (HR: 15.84; 95% CI: 4.11–60.97; p < 0.001); and radiation (HR: 0.47; 95% CI: 0.25–0.90; p = 0.02) remained significant prognostic factors for survival in our subsequent multivariable analysis after accounting for age, AJCC stage, surgery, or radiation (Table 3).
Table 2.
Survival durations for glycogen-rich clear cell (GRCC) carcinoma patients.
| Median (Months) | 95% CI | p-Value | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| All GRCC cases | 158 | 96.5 | 219.5 | NA | |
| Age | 60 years and under | 158 | 96.6 | 219.4 | 0.002 |
| >60 year | 73 | 18.6 | 127.4 | ||
| Sex | Female | - | - | - | 0.56 |
| Male | 81 | 62.5 | 99.5 | ||
| Race | American Indian/Alaska Native | 27 | - | - | 0.16 |
| Asian or Pacific Islander | - | - | - | ||
| Black | - | - | - | ||
| White | 111 | 45.6 | 176.4 | ||
| Grade | Well to Moderately Differentiated | 162 | 105.1 | 218.9 | 0.19 |
| Poorly Differentiated to Anaplastic | 111 | - | - | ||
| ER Status | Negative | 158 | 69.3 | 246.7 | 0.82 |
| Positive | 174 | 61.2 | 286.8 | ||
| PR Status | Negative | 158 | 86.3 | 229.7 | 0.15 |
| Positive | - | - | - | ||
| AJCC Stage | I | 162 | 125.7 | 198.3 | <0.001 |
| II | - | - | - | ||
| III | 69 | 38.4 | 99.6 | ||
| IV | 30 | 7.9 | 52.1 | ||
| T | T1 | 158 | 125.4 | 190.6 | 0.005 |
| T2 | - | - | - | ||
| T3 | 27 | - | - | ||
| T4 | 25 | - | - | ||
| N | N0 | 174 | - | - | 0.001 |
| N1 | 81 | 15.3 | 146.7 | ||
| N2 | 69 | - | - | ||
| N3 | 50 | 12.3 | 87.7 | ||
| M | M0 | 162 | 121.1 | 202.9 | <0.001 |
| M1 | 30 | 7.9 | 52.1 | ||
| Surgery | No Surgery | 25 | 0.0 | 58.1 | <0.001 |
| Surgery | 158 | 100.4 | 215.6 | ||
| Extent of Surgery | No Surgery | 25 | 0.0 | 58.1 | 0.02 |
| Sub-Mastectomy # | 174 | 116.0 | 232.0 | ||
| Mastectomy | 108 | - | - | ||
| Radiation | No Radiation | 81 | 40.4 | 121.6 | 0.012 |
| Radiation | - | - | - | ||
HER2 Status was only available for cases diagnosed after 2010 and was therefore excluded from the analysis. Bolded are statistically significant p-values. “-” Median survival time not reached or could not be calculated. # Defined as any surgery less aggressive than total mastectomy. CI—confidence interval; ER—estrogen receptor; PR—progesterone receptor; AJCC—American Joint Committee on Cancer; NA—not applicable; T, N, M refers to tumor size, nodal status, and metastasis classifications of malignant tumor staging system, respectively.
Figure 2.
Kaplan-Meier curves for glycogen-rich clear cell (GRCC) based on (A) clinical factors and (B) treatment. AJCC—American Joint Committee on Cancer; T, N, M refers to tumor size, nodal status, and metastasis classifications of malignant tumor staging system, respectively; RT—radiation therapy.
Table 3.
Multivariable analysis of overall survival for glycogen-rich clear cell (GRCC.) patients.
| Variable | Reference | p-Value | HR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | <0.001 | 1.05 | 1.02 | 1.07 | |
| AJCC Stage | I | <0.001 | |||
| II | 0.64 | 1.18 | 0.60 | 2.34 | |
| III | 0.001 | 4.67 | 1.84 | 11.87 | |
| IV | <0.001 | 15.84 | 4.11 | 60.97 | |
| Surgery | No Surgery | 0.39 | 2.05 | 0.40 | 10.45 |
| Radiation | No Radiation | 0.02 | 0.47 | 0.25 | 0.90 |
Bolded are statistically significant p-values. CI—confidence interval; HR—hazards ratio; AJCC—American Joint Committee on Cancer.
3.3. Subgroup Analysis of GR and CC Carcinoma of the Breast
To confirm our finding in the combined GRCC cases, we also conducted subgroup analyses on the GR and CC cases, comparing them to non-GRCC separately. The 45 cases of GR carcinoma had a median age at diagnosis of 61 years. The demographical and clinical characteristics of this patient subpopulation showed similar trends to those previously described in GRCC carcinomas of the breast (Supplemental Table S1). We found that grade (p = 0.04), ER status (p = 0.02), PR status (p = 0.003), receptor subtype (p = 0.002), T status (p = 0.02) and brain metastasis (p < 0.001) remained significantly differed between GR and non-GRCC carcinomas of the breast. AJCC 6th staging had a borderline significance (p = 0.06). The median survival time for GR was 133 months, with 5-, 10- and 15-year survival rates of 75%, 56%, and 45%, respectively. However, these survival durations no longer differed significantly from non-GRCC patients (p = 0.50, Supplemental Figure S1a). When comparing survival based on various demographic/clinical factors for the GR subgroup, only N staging reached statistical significance (p < 0.02, Supplemental Table S2). AJCC stage (p = 0.12), T status (p = 0.06), surgery (p = 0.06), and radiation (p = 0.10), factors which were identified as prognostic factors in our analysis of GRCC carcinomas, showed a consistent trend towards an effect on survival in the GR subgroup (Supplemental Table S2). Radiation after sub-mastectomy, however, remained significant in improving survival in GR patients (p = 0.04, Supplemental Figure S1b).
The 110 CC cases also displayed similar trends in demographical and clinical characteristics as the GRCC combined group (Supplemental Table S3). The only exceptions were that CC patients were more likely to be male than non-GRCC carcinoma patients of the breast (2.7% vs. 0.7%, p =0.012) and that they were no longer significantly different from non-GRCC patients with regards to brain metastasis at diagnosis (p = 0.31). Among CC cases, 5-, 10, and 15- year survival rates were 75%, 56%, and 45%, respectively. Median survival duration was 158 months, which was significantly worse than non-GRCC carcinomas of the breast (176 months in other carcinomas, p = 0.02, Supplemental Figure S2). CC patients displayed poorer survival compared to non-GRCC patients after accounting for age, stage, tumor grade, ER/PR/HER2 status, surgery and radiation (HR: 1.33; 95% CI: 1.00–1.76; p = 0.047). Prognostic factors for CC cases included age (p = 0.006), AJCC stage (p < 0.001), T status (p = 0.04), N status (p = 0.02), M status (p < 0.001), surgery (p < 0.001) and radiation (p = 0.05) (Supplemental Table S4). Age (p < 0.001) and stage (p < 0.001) remained independent prognostic factors of poorer survival in multivariable analysis (Supplemental Table S5).
When comparing CC directly to the GR subgroup, there were no significant differences in demographical and clinical characteristics (Supplemental Table S6) or survival durations (p = 0.59, Supplemental Figure S3).
4. Discussion
Using the SEER Program database, our study identified that GRCC is an aggressive histology with a poorer prognosis than non-GRCC carcinomas of the breast. Most importantly, the poorer prognosis was irrespective of the AJCC stage, tumor grade, patient age, treatment, and molecular subtypes defined by ER/PR/HER2 status. Our findings of poorer prognosis in GRCC carcinomas of the breast mirrors the poorer outcomes of clear cell carcinomas of other origins, such as the kidney [39], uterus [40], and ovaries [41]. We further identified that prognostic factors for non-GRCC breast carcinomas, such as age and AJCC staging, including TNM staging, were also relevant for GRCC tumors. Moreover, treatment with radiation and surgery showed improvements in survival for GRCC cases. Specifically, radiation was associated with improved survival when less aggressive surgical procedures (i.e., breast-conserving surgeries) were performed. This finding is consistent with the standard of care for other breast carcinomas [42] and suggests that GRCC may be treated similarly. While similar trends were observed in the GR subgroup, statistical significance was not reached, likely due to the smaller number of cases as compared to the GRCC analysis. In the CC cases, the significance of two demographic/clinical factors differed from our GRCC analysis possibly due to variations in the selected cases.
Several biomolecular theories have been investigated as the reasoning for poorer prognosis of GRCC tumors. Aberrant glycogen can act as an additional fuel source in nutrient-deprived tumor microenvironments. Tumor cells have been shown to have the ability to mobilize glycogen to fuel glycolysis and cellular proliferation via a p38α mitogen-activated protein kinase (p38α-MAPK) signaling pathway [43]. Hypoxia-induced glycogen phosphorolysis (the breakdown of glycogen into glucose) has also been found to enhance tumorigenesis by suppressing reactive oxygen species levels and p53-dependent senescence [13]. Furthermore, glycogen accumulation in tumor-associated stroma was identified recently to play key roles in tumor microenvironment by supplying carbohydrate for tumor proliferation [43]. Although further investigations are needed, elevated levels of glycogen content in breast cancer may participate in similar signaling pathways to potentiate tumor growth.
Triple-negative breast tumors account for 15–20% of all breast cancers and their highly aggressive nature is well documented [44,45]. These tumors have poorer prognosis due to both the lack of receptors available for targeted therapies and they also have higher metabolic demands for cell division. Basu et al. demonstrated significantly higher fluorine-18 fluorodeoxyglucose (FDG) uptake in triple negative tumors compared with uptake in ER+/PR+/HER2− tumors using FDG-positron emission tomography [46]. Studies have also demonstrated a greater glycolytic phenotype and altered mitochondrial respiration in triple negative patient tumors and cell lines compared with other tumor subtypes [47,48,49]. These data are suggestive of a more metabolically active phenotype, likely to compensate for increased cellular proliferation rates. High glycogen content could be utilized to satisfy these demands when the solid tumor grows beyond the limit of nutrient diffusion. Although our multivariable analysis showed that the poorer prognosis of GRCC was irrespective of IHC intrinsic receptor subtype, the increased metabolic needs of triple negative tumors suggest that it may still be a factor in influencing the poorer prognosis of GRCC. However, only 29 cases were available for analysis as HER2 receptor status was only available after 2010 in the SEER database. Therefore the relationship between glycogen and triple negative tumors requires further examination.
The most widely accepted technique for glycogen histochemistry is PAS staining [50]. However, PAS not only detects glycogen; it also stains other polysaccharides such as glycoprotein, proteoglycans, and mucins [51,52,53]. Another disadvantage of PAS is the lack of sensitivity; only large polysaccharide inclusions can be identified by PAS. Given that our database analysis identified only a small number of GR (45 cases) compared to CC (110 cases), we speculate that glycogen accumulations may be under-identified due to the lack of PAS sensitivity. Routine glycogen staining may also be underutilized as clinicians do not feel the need to additionally assess the specific intracytoplasmic moiety as no specific treatment guidelines currently exist for GRCC. However, the poorer prognostic outcome for GRCC tumors illustrated in our study highlights the utility of accurate subtyping of invasive carcinomas in order to improve our understanding of the prognostic variables. Detailed histological characterization will also provide clinicians with an opportunity for detailed assessment of treatment outcomes depending on the subtype of the disease. Given the importance of glycogen in patient survival and cancer prognosis, more specific and accurate methods for glycogen detection may be warranted.
While less common than glycogen accumulations, various histological subtypes of CC carcinoma without glycogen can exist. Apocrine, lipid-rich, pleomorphic lobular (histiocytoid) or secretory carcinomas all can have a histologically “clear cell” appearance [27,32]. Consistent with previous studies, the lack of differentiation between the CC and GR cases in our subgroup analysis provides further evidence that the majority of CC cases are expected to be GR. As we were unable to confirm the presence of glycogen accumulation in the CC carcinomas of our retrospective database analysis, larger studies of CC carcinomas with validated GR cases are needed to confirm the clinical and prognostic factors identified in our study. Due to the rarity of GRCC cases, only a single positive case was available for our analysis of bone, brain, liver and lung metastasis at the time of diagnosis. Although GRCC reached a statistically higher chance of brain metastasis when compared to non-GRCC, future studies with a larger number of positive cases are needed to confirm these results. Furthermore, chemotherapy information was not available in the SEER program database. A follow-up study that includes chemotherapy treatment would further our understanding of the disease and establish better treatment options.
5. Conclusions
While it has previously been difficult to accurately assess clinical characteristics and prognosis of GRCC due to the rarity of their presentation, using the SEER program database we were able to examine 155 of these cases. Our results demonstrated that GRCC is an aggressive histology with a poorer prognosis irrespective of AJCC stage, tumor grade, patient age, treatment, and ER/PR/HER2 status. To further clarify the characteristics and prognosis of GRCC of the breast, a large, systematic study of histologically confirmed glycogen-rich cases with long-term follow up will be necessary. As it has not been possible to study these rare tumors in randomized clinical trials to determine optimal surgical, radiation or chemotherapeutic strategies, more sensitive histological characterization methods may be warranted in order to further identify prognostic variables and to promote the development of targeted therapies for these patients.
Acknowledgments
The authors would like to thank members of the Gentry Lab for their assistance and support.
Supplementary Materials
The following are available online at http://www.mdpi.com/2077-0383/8/2/246/s1, Supplemental Table S1. Demographical and clinical characteristics of GR subgroup. Supplemental Table S2. Survival duration in the GR subgroup. Supplemental Table S3. Demographical and clinical characteristics of CC subgroup. Supplemental Table S4. Survival duration in the CC subgroup. Supplemental Table S5. Multivariable analysis of overall survival for CC carcinomas. Supplemental Table S6. Comparison of demographical and clinical characteristics between GR and CC carcinomas. Supplemental Figure S1. Kaplan-Meier survival curves for (A) overall survival and (B) with or without radiation therapy after sub-mastectomy in the GR subgroup. Supplemental Figure S2. Kaplan-Meier survival curves for CC subgroup. Supplemental Figure S3. Kaplan-Meier survival curves comparing GR and CC carcinomas.
Author Contributions
Z.Z. and C.J.K. drafted and edited the manuscript. C.J.K. conducted data analysis. S.K.C., C.H., H.H. and H.G. provided clinical and population insights and edited the manuscript. M.S.G. and R.C.S. conceptualized and edited the manuscript. All authors approved the final manuscript.
Funding
This study was supported by the University of Kentucky Center for Cancer and Metabolism, National Institute of General Medical Sciences COBRE program (grant ID: P20 GM121327). Additionally, R.S. is aided by grant #16-182-28 from the American Cancer Society and funding from the University of Kentucky Markey Cancer Center. C.H. is supported by the University of Kentucky Markey Cancer Center (P30CA177558). Z.Z. is supported by the NIH National Center for Advancing Translational Sciences (grant number: UL1TR001998).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem. J. 2008;414:1–18. doi: 10.1042/BJ20080595. [DOI] [PubMed] [Google Scholar]
- 2.Adeva-Andany M.M., Gonzalez-Lucan M., Donapetry-Garcia C., Fernandez-Fernandez C., Ameneiros-Rodriguez E. Glycogen metabolism in humans. BBA Clin. 2016;5:85–100. doi: 10.1016/j.bbacli.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roach P.J., Depaoli-Roach A.A., Hurley T.D., Tagliabracci V.S. Glycogen and its metabolism: Some new developments and old themes. Biochem. J. 2012;441:763–787. doi: 10.1042/BJ20111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss R.D. A microchemical reaction resulting in the staining of polysaccharide structures in fixed tissue preparations. Arch. Biochem. 1948;16:131–141. [PubMed] [Google Scholar]
- 5.Crignis G.S., Abreu L., Bucard A.M., Barcaui C.B. Polarized dermoscopy of mammary Paget disease. An. Bras. Dermatol. 2013;88:290–292. doi: 10.1590/S0365-05962013000200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warnock M.L., Stoloff A., Thor A. Differentiation of adenocarcinoma of the lung from mesothelioma. Periodic acid-Schiff, monoclonal antibodies B72.3, and Leu M1. Am. J. Pathol. 1988;133:30–38. [PMC free article] [PubMed] [Google Scholar]
- 7.Rousset M., Zweibaum A., Fogh J. Presence of glycogen and growth-related variations in 58 cultured human tumor cell lines of various tissue origins. Cancer Res. 1981;41:1165–1170. [PubMed] [Google Scholar]
- 8.Rousset M., Chevalier G., Rousset J.-P., Dussaulx E., Zweibaum A. Presence and cell growth-related variations of glycogen in human colorectal adenocarcinoma cell lines in culture. Cancer Res. 1979;39:531–534. [PubMed] [Google Scholar]
- 9.Rousset M., Chevalier G., Rousset J.-P., Robine-Leon S., Dussaulx E., Zweibaum A. Kinetics of glycogen levels in asynchronous and synchronous cultures of a human colon carcinoma cell line, HT 29. Front. Gastrointest. Res. 1979;4:73–79. doi: 10.1159/000402287. [DOI] [PubMed] [Google Scholar]
- 10.Rousset M., Robine-Leon S., Dussaulx E., Chevalier G., Zweibaum A. Glycogen storage in foetal and malignant epithelial cells of the human colon. Front. Gastrointest. Res. 1979;4:80–85. doi: 10.1159/000402288. [DOI] [PubMed] [Google Scholar]
- 11.Staedel C., Beck J.-P. Resurgence of glycogen synthesis and storage capacity in cultured hepatoma cells. Cell Differ. 1978;7:61–71. doi: 10.1016/0045-6039(78)90007-6. [DOI] [PubMed] [Google Scholar]
- 12.Sato A., Kawasaki T., Kashiwaba M., Ishida K., Nagashima Y., Moritani S., Ichihara S., Sugai T. Glycogen-rich clear cell carcinoma of the breast showing carcinomatous lymphangiosis and extremely aggressive clinical behavior. Pathol. Int. 2015;65:674–676. doi: 10.1111/pin.12321. [DOI] [PubMed] [Google Scholar]
- 13.Favaro E., Bensaad K., Chong M.G., Tennant D.A., Ferguson D.J., Snell C., Steers G., Turley H., Li J.-L., Günther U.L., et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab. 2012;16:751–764. doi: 10.1016/j.cmet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Altemus M.A., Yates J.A., Wu Z., Bao L., Merajver S.D. Glycogen accumulation in aggressive breast cancers under hypoxia [abstract]; Proceedings of the American Association for Cancer Research Annual Meeting; Chicago, IL, USA. 14–18 April 2018. [Google Scholar]
- 15.Hochachka P. Defense strategies against hypoxia and hypothermia. Science. 1986;231:234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- 16.Crass M.F., Pieper G. Lipid and glycogen metabolism in the hypoxic heart: Effects of epinephrine. Am. J. Physiol. 1975;229:885–889. doi: 10.1152/ajplegacy.1975.229.4.885. [DOI] [PubMed] [Google Scholar]
- 17.Iida Y., Aoki K., Asakura T., Ueda K., Yanaihara N., Takakura S., Yamada K., Okamoto A., Tanaka T., Ohkawa K. Hypoxia promotes glycogen synthesis and accumulation in human ovarian clear cell carcinoma. Int. J. Oncol. 2012;40:2122–2130. doi: 10.3892/ijo.2012.1406. [DOI] [PubMed] [Google Scholar]
- 18.Pescador N., Villar D., Cifuentes D., Garcia-Rocha M., Ortiz-Barahona A., Vazquez S., Ordonez A., Cuevas Y., Saez-Morales D., Garcia-Bermejo M.L., et al. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS ONE. 2010;5:e9644. doi: 10.1371/journal.pone.0009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen G.M., Zhang F.L., Liu X.L., Zhang J.W. Hypoxia-inducible factor 1-mediated regulation of PPP1R3C promotes glycogen accumulation in human MCF-7 cells under hypoxia. FEBS Lett. 2010;584:4366–4372. doi: 10.1016/j.febslet.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 20.Chen J., Lee H.-J., Wu X., Huo L., Kim S.-J., Xu L., Wang Y., He J., Bollu L.R., Gao G., et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 2015;75:554–565. doi: 10.1158/0008-5472.CAN-14-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J., Gao L., Zhang H., Wang D., Wang M., Zhu J., Pang C., Wang C. Succinate dehydrogenase 5 (SDH5) regulates glycogen synthase kinase 3β-β-Catenin-mediated lung cancer metastasis. J. Biol. Chem. 2013;288:29965–29973. doi: 10.1074/jbc.M113.450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zois C.E., Harris A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016;94:137–154. doi: 10.1007/s00109-015-1377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelletier J., Bellot G., Gounon P., Lacas-Gervais S., Pouyssegur J., Mazure N.M. Glycogen synthesis is induced in hypoxia by the hypoxia-inducible factor and promotes cancer cell survival. Front. Oncol. 2012;2:18. doi: 10.3389/fonc.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 25.Shirley S.E., Escoffery C.T., Titus I.P., Williams E.E., West A.B. Clear cell carcinoma of the breast with immunohistochemical evidence of divergent differentiation. Ann. Diagn. Pathol. 2002;6:250–256. doi: 10.1053/adpa.2002.35399. [DOI] [PubMed] [Google Scholar]
- 26.Fisher E.R., Tavares J., Bulatao I.S., Sass R., Fisher B. Glycogen-rich, clear cell breast cancer: With comments concerning other clear cell variants. Hum. Pathol. 1985;16:1085–1090. doi: 10.1016/S0046-8177(85)80175-1. [DOI] [PubMed] [Google Scholar]
- 27.Hayes M.M., Seidman J.D., Ashton M.A. Glycogen-rich clear cell carcinoma of the breast. A clinicopathologic study of 21 cases. Am. J. Surg. Pathol. 1995;19:904–911. doi: 10.1097/00000478-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Lakhani S.R., Ellis I.O., Schnitt S.J., Tan P.H., van de Vijver M.J. World Health Organization Classification of Tumors of the Breast. 4th ed. IARC Press; Lyon, France: 2012. pp. 74–75. [Google Scholar]
- 29.Kuroda H., Sakamoto G., Ohnisi K., Itoyama S. Clinical and pathological features of glycogen-rich clear cell carcinoma of the breast. Breast Cancer. 2005;12:189–195. doi: 10.2325/jbcs.12.189. [DOI] [PubMed] [Google Scholar]
- 30.Toikkanen S., Joensuu H. Glycogen-rich clear-cell carcinoma of the breast: A clinicopathologic and flow cytometric study. Hum. Pathol. 1991;22:81–83. doi: 10.1016/0046-8177(91)90066-X. [DOI] [PubMed] [Google Scholar]
- 31.Ma X., Han Y., Fan Y., Cao X., Wang X. Clinicopathologic characteristics and prognosis of glycogen-rich clear cell carcinoma of the breast. Breast J. 2014;20:166–173. doi: 10.1111/tbj.12231. [DOI] [PubMed] [Google Scholar]
- 32.Yerushalmi R., Hayes M.M., Gelmon K.A. Breast carcinoma—Rare types: Review of the literature. Ann. Oncol. 2009;20:1763–1770. doi: 10.1093/annonc/mdp245. [DOI] [PubMed] [Google Scholar]
- 33.Overview of the SEER Program. [(accessed on 30 July 2018)]; Available online: https://seer.cancer.gov/about/overview.html.
- 34.Davis F.G., McCarthy B.J., Berger M.S. Centralized databases available for describing primary brain tumor incidence, survival, and treatment: Central Brain Tumor Registry of the United States; Surveillance, Epidemiology, and End Results; and National Cancer Data Base. Neuro Oncol. 1999;1:205–211. doi: 10.1093/neuonc/1.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinslow C.J., Bruce S.S., Rae A.I., Sheth S.A., McKhann G.M., Sisti M.B., Bruce J.N., Sonabend A.M., Wang T.J.C. Solitary-fibrous tumor/hemangiopericytoma of the central nervous system: A population-based study. J. Neurooncol. 2018;138:173–182. doi: 10.1007/s11060-018-2787-7. [DOI] [PubMed] [Google Scholar]
- 36.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database Incidence—SEER 9 Regs Research Data, Nov 2017 Sub (1973–2015)—Linked To County Attributes—Total U.S., 1969–2016 Counties. released April 2018, based on the November 2017 submission. [(accessed on 1 December 2018)]; Available online: https://seer.cancer.gov/data/citation.html.
- 37.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database Incidence—SEER 9 Regs Research Data, Nov 2015 Sub (1973–2013)—Linked To County Attributes—Total U.S., 1969–2014 Counties. released April 2016, based on the November 2015 submission. [(accessed on 1 December 2018)]; Available online: https://seer.cancer.gov/data/citation.html.
- 38.Collaborative Stage Work Group of the American Joint Committee on Cancer . Collaborative Stage Data Collection System Coding Manual and Instructions. American Joint Committee on Cancer; Chicago, IL, USA: 2011. Part I Section 2: Site-Specific Notes. [Google Scholar]
- 39.Cheville J.C., Lohse C.M., Zincke H., Weaver A.L., Blute M.L. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am. J. Surg. Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Gadducci A., Cosio S., Spirito N., Cionini L. Clear cell carcinoma of the endometrium: A biological and clinical enigma. Anticancer Res. 2010;30:1327–1334. [PubMed] [Google Scholar]
- 41.Sugiyama T., Kamura T., Kigawa J., Terakawa N., Kikuchi Y., Kita T., Suzuki M., Sato I., Taguchi K. Clinical characteristics of clear cell carcinoma of the ovary: A distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. doi: 10.1002/1097-0142(20000601)88:11<2584::AID-CNCR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Darby S., McGale P., Correa C., Taylor C., Arriagada R., Clarke M., Cutter D., Davies C., Ewertz M., Godwin J., et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis M., Kenny H.A., Ashcroft B., Mukherjee A., Johnson A., Zhang Y., Helou Y., Batlle R., Liu X., Gutierrez N., et al. Fibroblasts mobilize tumor cell glycogen to promote proliferation and metastasis. Cell Metab. 2019;29:141–155. doi: 10.1016/j.cmet.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jitariu A.A., Cimpean A.M., Ribatti D., Raica M. Triple negative breast cancer: The kiss of death. Oncotarget. 2017;8:46652–46662. doi: 10.18632/oncotarget.16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elsawaf Z., Sinn H.P. Triple-negative breast cancer: Clinical and histological correlations. Breast Care. 2011;6:273–278. doi: 10.1159/000331643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basu S., Chen W., Tchou J., Mavi A., Cermik T., Czerniecki B., Schnall M., Alavi A. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: A potentially useful method for disease characterization. Cancer. 2008;112:995–1000. doi: 10.1002/cncr.23226. [DOI] [PubMed] [Google Scholar]
- 47.Pelicano H., Zhang W., Liu J., Hammoudi N., Dai J., Xu R.H., Pusztai L., Huang P. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: Role of mTOR pathway and therapeutic potential. Breast Cancer Res. 2014;16:434. doi: 10.1186/s13058-014-0434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen L., O’Shea J.M., Kaadige M.R., Cunha S., Wilde B.R., Cohen A.L., Welm A.L., Ayer D.E. Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP. Proc. Natl. Acad. Sci. USA. 2015;112:5425–5430. doi: 10.1073/pnas.1501555112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun R.C., Fadia M., Dahlstrom J.E., Parish C.R., Board P.G., Blackburn A.C. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res. Treat. 2010;120:253–260. doi: 10.1007/s10549-009-0435-9. [DOI] [PubMed] [Google Scholar]
- 50.Mc M.J. Histological and histochemical uses of periodic acid. Stain Technol. 1948;23:99–108. doi: 10.3109/10520294809106232. [DOI] [PubMed] [Google Scholar]
- 51.Mc M.J. Histological demonstration of mucin after periodic acid. Nature. 1946;158:202. doi: 10.1038/158202a0. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto K., Gross B.G., Lever W.F. Angiokeratoma Corporis Diffusum (Fabry). Histochemical and Electron Microscopic Studies of the Skin. J. Investig. Dermatol. 1965;44:119–128. doi: 10.1038/jid.1965.22. [DOI] [PubMed] [Google Scholar]
- 53.Dahr W., Uhlenbruck G., Janssen E., Schmalisch R. Heterogeneity of human cell membrane sialoglycoproteins. Blut. 1976;32:171–184. doi: 10.1007/BF00995910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.