Abstract
This study was conducted to investigate the serum levels of membrane-bound mucin 2 (MUC2) in breast cancer (BC) patients and the relationship with tumour progression and known prognostic parameters. We enrolled 127 female patients with histopathologically diagnosed BC who did not receive chemotherapy (CT) or radiotherapy. Serum MUC2 levels were measured by the enzyme-linked immunosorbent assay (ELISA) method and compared with those of 40 age and sex-matched healthy controls. Median age of diagnosis was 50 (range: 26–78). Twenty-eight (22%) patients were metastatic and the most frequent site of metastasis was bone (n = 17, 61%). The median serum MUC2 level of BC patients was significantly higher than that of the controls (198 vs. 54 ng/mL, p < 0.001). There was no significant difference between patients and controls according to known disease-related clinicopathological or laboratory parameters (p > 0.05). Serum MUC2 levels were not associated with survival (p = 0.65). Although serum MUC2 levels might have a diagnostic role, their predictive and prognostic role in survival in BC patients was not detected. Serum levels of MUC2 should be investigated for diagnostic or screening purposes on a larger scale.
Keywords: breast cancer, MUC2, serum, diagnostic
1. Introduction
For females in both developed and developing countries, breast cancer (BC) has the second highest mortality rate after lung cancer. More than 1.3 million individuals are diagnosed with BC each year, and the mortality rate is 60% in developing countries [1,2]. Some studies have reported that in American women, the probability of developing BC is 12.3% [3]. Presently, various factors such as genetic predisposition, hormones, lifestyle, and age play an etiological role in this disease [4]. Approximately 7% of patients with BC are diagnosed before age 40 years and the risk for BC increases with age. The most critical factors affecting the survival of patients with BC are the early diagnosis, tumour stage, and age [3,5].
Mucins are high molecular weight glycoproteins, and are sub-classified into two structurally different groups: the secreted and the membrane-bound (transmembrane) mucins [6]. Membrane-bound mucins act as a cell surface receptor for various signal transduction pathways. Secreted mucins form a protective layer and form a barrier against pathogens. Mucins are found in the mammary glands, liver, and in pancreas and kidney secretions, primarily in the respiratory and digestive system [7]. Many studies have shown the altered expression of mucins in different pathological conditions, including cancer. The hypersecretion of mucins in cancers is due to their distinctive pattern of glycosylation, that serves as a binding platform for various growth factors and cytokines and thereby promotes the proliferation and metastasis of malignant cells through several signalling cascades. Each mucin has a specific role in tumour formation; some of the membrane-bound mucin (MUC) genes show hyperactivation and others show inactivation in pathological conditions. Membrane-bound mucin-2 (MUC2) is a secretory mucin, which is mainly in the gel format and is usually excreted in the digestive and respiratory tract [8,9]. MUC2 overexpression is seen in mucinous carcinomas in organs such as the pancreas, breast, and prostate [10,11,12]. Dhanisha et al. suggested that an increased level of mucin in the serum can be used as a potential marker for disease diagnosis and prognosis [6].
In some preclinical studies, MUC2 expression was evaluated in breast cancer tissue [12,13,14,15,16,17,18]. However, to the best of our knowledge, the clinical significance of serum MUC2 levels in breast cancer has not been previously examined. Therefore, this study was conducted to investigate the serum levels of MUC2 in BC patients and the relationship with tumour progression and known prognostic parameters.
2. Materials and Methods
2.1. Patients
In total, 127 female breast cancer patients admitted to Istanbul University, Institute of Oncology were enrolled in the study. Serum samples were obtained on the first admission before any type of treatment (chemotherapy, radiotherapy, hormonotherapy, or targeted therapy) was given to the patients. Written informed consent was obtained from all patients and healthy controls in order to obtain blood and to conduct MUC2 tests for their blood. The pretreatment evaluation included detailed clinical history and physical examination with a series of biochemistry tests, including serum lactate dehydrogenase (LDH) and Cancer Antigen 15-3 (CA15-3) levels. Diagnosis of breast cancer was histologically proven either with tru-cut biopsy of the primary lesion or metastatic site and staged according to the seventh edition of American Joint Committee on Cancer (AJCC). All the patients were treatment-naive for at least 3 months before accrual.
For histological evaluation, tissue sections (2 mm) were deparaffinized and stained using haematoxylin and eosin. Grading of tumours was established according to the modified Bloom-Richardson grading system. Estrogen receptor (ER), progesterone receptor (PR), and HER2 status was evaluated in the sample sections using appropriate antibodies. The immunohistochemical staining was assessed upon visual inspection with an optical microscope and considered as positive if the percentage of cells staining positive was more than 5%. Regarding HER2 evaluation, in the case of 2(+) staining by immunohistochemistry, HER2 gene amplification was analysed by fluorescent in situ hybridization (FISH).
All patients were treated with standard treatment modalities according to NCCN guidelines. Sixty-two patients received adjuvant treatment after mastectomy or lumpectomy. Another sixty-five patients with locally-advanced (n = 37) and metastatic disease (n = 28) were treated with anthracycline and/or taxane-based chemotherapy (CT) or single-agent capecitabine, and trastuzumab (if HER2 positive) with/without radiotherapy depending on the stage of the disease. Follow-up programs included clinical, laboratory, and radiological assessments performed at 8-week intervals during CT. Response to treatment was evaluated in patients who had metastatic disease or receiving neoadjuvant CT according to clinical examination and the revised RECIST criteria version 1.1. Those with complete or partial responses were considered as CT-responsive. Tumour (T) and Nodal (N) stages were recorded for only non-metastatic patients. While breast cancer has four transcriptional subtypes in the prediction analysis of microarray 50 (PAM50) scheme—luminal A, luminal B, basal-like, and human epidermal growth factor receptor 2 (HER2)-enriched (HER2E)—these subtypes overlap with immunohistochemical (IHC) staining of three protein markers, ER, PR, and HER2, supplemented with in situ hybridization (ISH) of HER2. The HER2E subtype captures some but not all HER2+ tumours [19]. However, we categorized the patients according to molecular subtypes as; Her2-negative luminal (ER positive, HER2 negative), triple positive (ER, PR, and HER2 positive), triple negative (ER, PR, and HER2 negative), and HER2 disease (ER and PR negative, HER2 positive).
For comparison of serum levels of MUC2, age and sex-matched 40 healthy women controls were included in the analysis. Our study was found suitable and was approved on 9 March 2018 by the Ethics Committee of Istanbul Faculty of Medicine, Istanbul University with project number 2018/359.
2.2. Measurement of Serum MUC2 Levels
Blood samples were obtained from patients with breast cancer and healthy controls by venipuncture and clotted at room temperature. The sera were collected following centrifugation and frozen immediately at –80 °C until analysis. Serum MUC2 (USCN, Wuhan, China) levels were determined by the solid-phase sandwich ELISA method. The microtiter plate has been pre-coated with an antibody specific to MUC2. Standards and samples were added to the wells with a biotin-conjugated antibody specific to MUC2. Next, avidin conjugated to horseradish peroxidase (HRP) was added to each well and incubated. After tetramethylbenzidine (TMB) substrate solution was added, the wells that contain MUC2, biotin-conjugated antibody, and enzyme-conjugated avidin exhibited a change in colour. The enzyme substrate reaction was terminated by the addition of sulphuric acid solution and the colour change was measured spectrophotometrically (ChroMate® 4300; Awareness Technology, Inc., Palm City, FL, USA) at a wavelength of 450 nm. The concentration of MUC2 in the samples was then determined by comparing the optical density (OD) of the samples to the standard curve.
2.3. Statistical Analysis
Continuous variables were categorized using median values as the cut-off point. For assessment of relationships, comparisons between various clinical/laboratory parameters and serum levels of MUC2 assays were accomplished using Mann–Whitney U test since serum MUC2 had non-normal distribution in all groups. Survival was calculated from the date of the first admission to hospital to the death resulting from any cause or to the last contact with the patient or any family member. Kaplan–Meier method was used for estimation of survival of patient and differences in survivals were assessed by the log-rank statistic. A p-value less than 0.05 was accepted as statistically significant. Statistical analysis was carried out using IBM SPSS Statistics for Windows, Version 21.0 (Armonk, NY: IBM Corp. Released 2012, USA).
3. Results
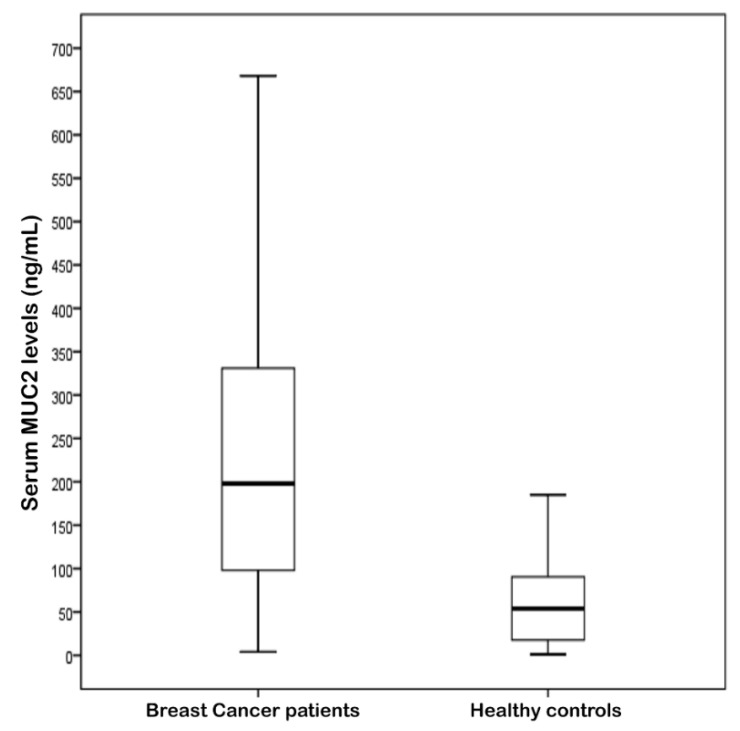
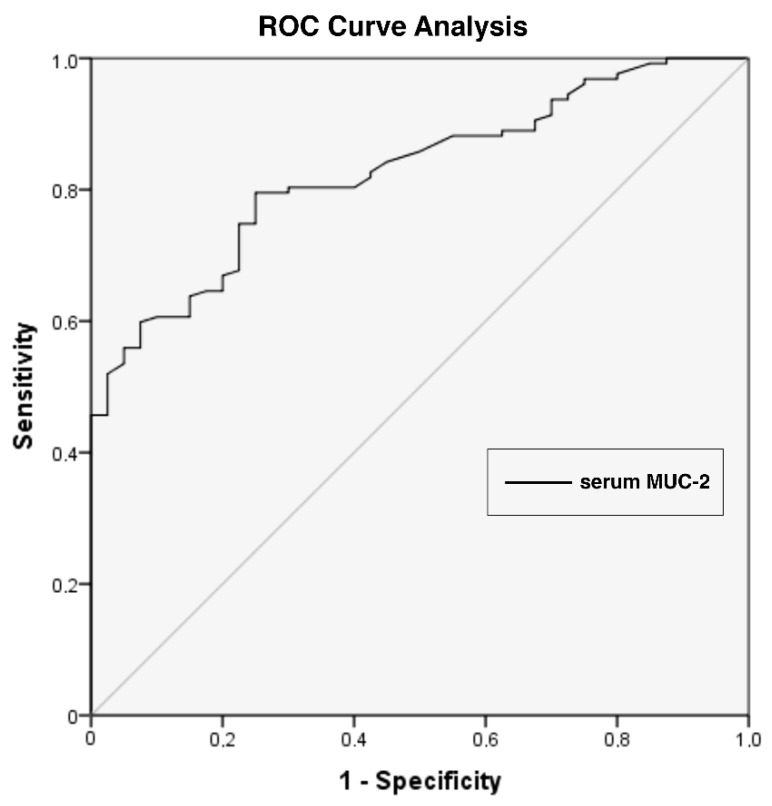
Between July 2006 and June 2016, 127 female breast cancer patients were enrolled in the study. Median age of diagnosis was 50 (range: 26-–8) (Table 1). Twenty-eight (22%) patients were metastatic and the most frequent site of metastasis was bone (n = 17, 61%). The majority of the patients with non-metastatic disease had node-positive (n = 64, 65%) disease and most of them (n = 34, 34%) were of the N1 stage. The serum MUC2 levels showed a strong difference between the patients and the control group (Table 2, Figure 1). Serum MUC2 levels were significantly higher than the controls (median level of 198 vs. 54 ng/mL, p < 0.001). As a diagnostic test, the sensitivity and specificity of serum MUC2 levels was 74.8% and 75%, respectively, if the cut-off value was taken as 97.5 ng/mL (p < 0.001) (Figure 2). Positive predictive value was 90.5% and negative predictive value was 48.4% according to the receiver operating characteristic (ROC) analysis.
Table 1.
Characteristics of the patients and disease.
| Variables | n |
|---|---|
| Number of patients | 127 |
| Age, years Median (range): 50 (26–78) <50/≥50 |
62/65 |
| Grade 1/2/3 |
5/50/44 |
| Estrogen Receptor Negative/Positive |
31/96 |
| Progesteron Receptor Negative/Positive |
48/79 |
| HER-2 Negative/Positive |
97/30 |
| classification Luminal/Triple-positive/Her-2 disease/Triple-negative |
77/19/11/20 |
| T stage * 1/2/3/4 |
34/58/4/3 |
| N stage * 0/1/2/3 |
35/34/21/9 |
| M stage 0/1 |
99/28 |
| Serum LDH level (ULN: 450 U/L) Normal/Elevated |
83/44 |
| Serum CA15-3 level (ULN: 35 U/mL) Normal/Elevated |
97/30 |
| Response to CT Yes/No |
58/7 |
| Last status Alive/Dead |
105/22 |
* in 99 non-metastatic patients. LDH: lactate dehydrogenase, ULN: upper limit of normal, CT: chemotherapy.
Table 2.
The values of membrane-bound mucin 2 (MUC2) in patients with breast cancer and healthy controls.
| Data | Value |
|---|---|
| Assay | MUC2 level (ng/mL) |
| Patients Median (range) |
198 (4–468) |
| Controls Median (range) |
54 (1–210) |
| p | <0.001 ** |
** p < 0.05.
Figure 1.
The values of serum MUC2 levels in patients with breast cancer and healthy controls (p < 0.001).
Figure 2.
The receiver operating characteristic (ROC) analysis for serum MUC-2 levels (p < 0.001). AUC (CI) = 0.82 (0.76–0.89), cut-off value = 97.5 ng/mL, PPV= 90.5%, NPV= 48.4%. AUC: area under the curve; CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value.
Table 3 shows the correlation between the serum levels of MUC2 and clinicopathological factors. There were no significant correlations between serum MUC2 levels and any type of clinic-pathological and laboratory parameters (p > 0.05).
Table 3.
Results (median and range) of comparisons between the factors and various clinical/laboratory parameters.
| Variables | MUC2 (ng/mL) | p |
|---|---|---|
| Age, years | ||
| Younger (<50 years) | 185.5 (8–640) | 0.90 |
| Older (≥50 years) | 200.0 (4-668) | |
| Grade | ||
| I–II (Good–medium) | 215 (10–668) | 0.42 |
| III (Poor) | 183.5 (4–560) | |
| Estrogen Receptor | ||
| Negative | 135 (4–560) | 0.21 |
| Positive | 202.5 (8–668) | |
| Progesteron Receptor | ||
| Negative | 184 (4–668) | 0.43 |
| Positive | 205 (10–560) | |
| HER-2 | ||
| Negative | 205 (8–668) | 0.51 |
| Positive | 160 (4–560) | |
| Classification HER-2 -negative luminal Others |
205 (8–668) 160 (4–560) |
0.46 |
| Classification Triple-positive Others |
183 (50–560) 201.5 (4–668) |
0.73 |
| Classification | ||
| HER-2 disease Others |
96 (4–475) 202.5 (8–668) |
0.13 |
| Classification Triple-negative Others |
196 (38–560) 198 (4–668) |
0.77 |
| T stage | ||
| 1 (small) | 234 (10–496) | 0.41 |
| 2-4 (large) | 185 (4–668) | |
| N status | ||
| Negative | 234 (10–490) | 0.66 |
| Positive | 190.5 (4–668) | |
| M stage | ||
| M0 | 198 (4–668) | 0.65 |
| M1 | 195 (20–640) | |
| Serum LDH level (ULN: 450 U/L) | ||
| Normal | 200 (10–668) | 0.47 |
| Elevated | 175 (4–640) | |
| Serum CA15-3 level (ULN: 35 U/mL) | ||
| Normal | 198 (4–668) | 0.84 |
| Elevated | 200.5 (14–560) | |
| Response to CT | ||
| Yes No |
184.5 (8–668) 270 (50–400) |
0.53 |
LDH: lactate dehydrogenase, ULN: upper limit of normal; CT: chemotherapy.
Survival Analysis
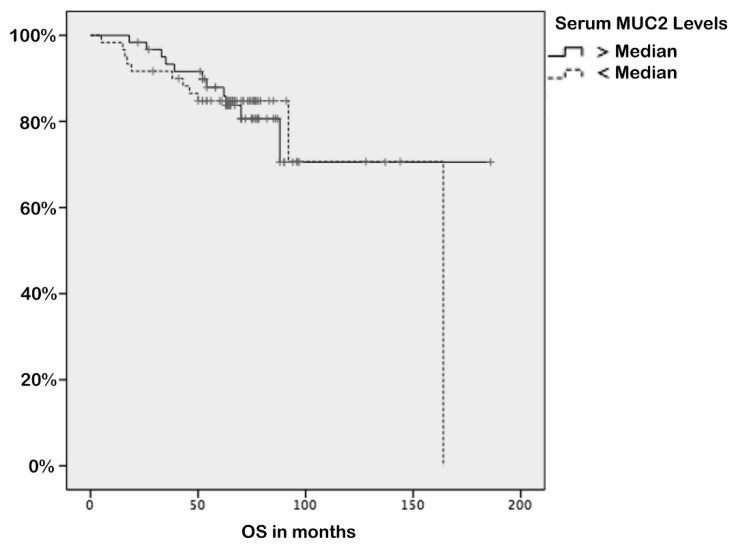
The median follow-up time was 65 months (range: 5–97 months). At the end of the observation period, 22 (17%) patients were dead. The mean survival for all patients was 85.0 ± 2.4 months (95% CI = 28.05–43.14) and 1-, 3-, and 5-year overall survival rates were 99% (95% CI = 87–97), 92% (95% CI = 97–101), and 85% (95% CI = 79–92), respectively. Negativity of ER and PR (p = 0.03 and p = 0.03, respectively), presence of triple-negative histology (p = 0.01), higher T stage (p = 0.03), elevated CA15-3 (p = 0.004), unresponsiveness to chemotherapy (p = 0.001), and presence of metastasis (p < 0.001) were associated with worse survival (Table 4). However, serum MUC2 levels were not associated with survival (p = 0.65) (Figure 3).
Table 4.
Univariate analyses of survival.
| Variables | Mean Survival (SE) Months |
3-Year Survival Rate (SE) % |
p |
|---|---|---|---|
| Age of patients | |||
| <50 years | 84.9 (3.1) | 94.7 (3.0) | 0.69 |
| ≥50 years | 84.8 (3.4) | 89.6 (4.0) | |
| Grade | |||
| I–II | 89.8 (2.4) | 97.4 (2.5) | 0.26 |
| III | 82.0 (3.1) | 91.8 (3.9) | |
| Estrogen Receptor | |||
| Negative | 77.7 (5.7) | 82.8 (7.0) | 0.03 ** |
| Positive | 87.2 (2.5) | 95.3 (2.3) | |
| Progesteron Receptor | |||
| Negative | 80.5 (4.5) | 84.8 (5.3) | 0.03 ** |
| Positive | 87.0 (2.4) | 97.1 (2.0) | |
| HER-2 | |||
| Negative | 84.0 (2.8) | 89.8 (3.2) | 0.56 |
| Positive | 87.7 (3.8) | 92.6 (5.0) | |
| Classification | |||
| Luminal | 86.9 (2.8) | 94.3 (2.8) | 0.19 |
| Others | 81.8 (4.1) | 88.9 (4.7) | |
| Classification | |||
| Triple-positive | 83.1 (4.4) | 100 | 0.49 |
| Others | 84.4 (2.6) | 90.9 (2.9) | |
| Classification | |||
| HER2 disease | 86.2 (6.3) | NR | 0.86 |
| Others | 84.7 (2.6) | 91.4 (2.8) | |
| Classification Triple-negative Others |
69.1 (7.5) 87.3 (2.3) |
72.2 (10.6) 95.9 (2.0) |
0.01 ** |
| T stage | |||
| T1 | 90.0 (0.8) | NR | 0.03 ** |
| T2–T4 | 84.7 (3.0) | 91.7 (3.6) | |
| Node status | |||
| Negative | 96.8 (3.2) | 87.3 (2.5) | 0.24 |
| Positive | 93.2 (3.3) | 85.1 (2.9) | |
| M stage | |||
| M0 | 86.9 (2.1) | 94.5 (2.4) | <0.001 ** |
| M1 | 70.6 (6.6) | 82.6 (7.9) | |
| Serum LDH level (ULN: 450 U/L) | |||
| Normal | 87.6 (2.7) | 92.2 (3.1) | 0.07 |
| Elevated | 77.1 (3.8) | 92.3 (4.3) | |
| Serum CA15-3 level (ULN: 35 U/mL) | |||
| Normal | 87.5 (2.3) | 95.4 (2.3) | 0.004 ** |
| Elevated | 75.9 (6.0) | 82.6 (7.1) | |
| Response to CT | |||
| Yes | 82.1 (3.8) | 91.8 (3.9) | 0.001 ** |
| No | 47.0 (12.4) | 57.1 (18.7) | |
| Serum MUC2 level | |||
| Elevated (>median) | 83.7 (4.1) | 89.5 (5,0) | 0.65 |
| Normal (<median) | 85.2 (2.8) | 93.5 (2.8) |
** p < 0.05. Abbreviations, LDH: lactate dehydrogenase; ULN: upper limit of normal; CT: chemotherapy.
Figure 3.
Kaplan–Meier survival curves in breast cancer patients according to serum MUC2 levels (p = 0.65). OS: overall survival.
4. Discussion
In our study, we aimed to evaluate serum MUC2 levels in patients with breast cancer and age and sex-matched healthy controls. According to our results, serum MUC2 levels are higher in the BC patients than the control group. As far as we know, this is the first serum study of MUC2 in the literature so far.
MUC2 expression is absent in the ductal epithelium of the healthy breast [13]. MUC2 is expressed in pre-malignant breast lesions like ductal carcinoma in situ (DCIS), and is upregulated in lobular carcinoma in situ (LCIS). Both membrane-bound (MUC1, MUC3, MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6) are upregulated in ductal adenocarcinoma of the breast. Some studies uncovered several unique roles of mucins, which include modulation in proliferative, invasive and metastatic potential of tumour cells, throughout the progression of breast cancer.
There are only a few studies that evaluated the clinical implication of MUC2 expression in breast cancer tissue. Walsh et al. reported that BC patients whose tumours were positive for MUC2 had a significantly shorter survival compared to those with MUC2 non-expressing tumours (49 vs. 75 months) [14]. However, Rakha et al. found that there is no significant prognostic association in survival analysis with MUC2 expression in BC [15]. Do et al. also reported that there is no significant prognostic association in survival analysis with MUC2 expression in BC regarding both overall survival (OS) and disease-free survival (DFS) [16]. In our study, serum MUC2 levels were not associated with survival (p = 0.65) (Figure 3).
Rakha et al. reported a significant inverse association between MUC2 expression and ER status [15]. Patel et al. found MUC2 negative tumours were associated with a higher histological grade [17]. On the contrary, Ohashi et al. found no clinical implication regarding MUC2 expression in BC [18]. We also did not notice any prognostic or clinical implication other than a possible diagnostic role of serum MUC2 levels.
The limitations of our study were that it was a single-centre, observational study, and other mucin levels were not evaluated. We did not consider the effects of radiotherapy, hormone therapy, or targeted therapies; these factors may have been affected our results.
5. Conclusions
We discovered that serum MUC2 levels play a diagnostic role, although we did not find any predictive and prognostic role in survival in BC patients. For use as a diagnostic or screening tool, serum levels of MUC2 should be studied on a larger scale.
Author Contributions
Conceptualization, S.B. and S.K.; Data curation, S.B. and M.S.; Formal analysis, A.Z., M.Z.U., and S.K.; Methodology, S.B.; Supervision, S.K.; Validation, M.S., M.Z.U., and E.B.D.; Visualization, S.K.; Writing—original draft, E.B.D. and S.K.; Writing—review and editing, A.Z.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.De Santis C., Ma J., Bryan L., Jemal A. Breast cancer statistics 2013. CA Cancer J. Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B.O., Yip C.H., Smith R.A., Shyyan R., Sener S.F., Eniu A., Carlson R.W., Azavedo E., Harford J. Guideline implementation for breast healthcare in low-income and middle income countries: Overview of the Breast Health Global Initiative Global Summit 2007. Cancer. 2008;113:2221–2224. doi: 10.1002/cncr.23844. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistic 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Shah R., Rosso K., Nothanson S.D. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J. Clin. Oncol. 2014;5:283–298. doi: 10.5306/wjco.v5.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anders C.K., Johnson R., Litton J., Phillips M., Bieyer A. Breast cancer before age 40 years. Semin. Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhanisha S.S., Guruvayoorappan C., Drishya S., Abeesh P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 2018;122:98–122. doi: 10.1016/j.critrevonc.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Kufe D. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gum J.R., Jr., Hicks J.W., Toribara N.W., Siddiki B., Kim Y.S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 9.Chang S.K., Dohrman A.F., Basbaum C.B., Ho S.B., Tsuda T., Toribara N.W., Gum J.R., Kim Y.S. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology. 1994;107:28–36. doi: 10.1016/0016-5085(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 10.Adsay N.V., Merati K., Nassar H., Shia J., Sarkar F., Pierson C.R., Cheng J.D., Visscher D.W., Hruban R.H., Klimstra D.S. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: Coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am. J. Surg. Pathol. 2003;27:571–578. doi: 10.1097/00000478-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Osunkoya A.O., Adsay N.V., Cohen C., Epstein J.I., Smith S.L. MUC2 expression in primary mucinous and nonmucinous adenocarcinoma of the prostate: An analysis of 50 cases on radical prostatectomy. Mod. Pathol. 2008;21:789–794. doi: 10.1038/modpathol.2008.47. [DOI] [PubMed] [Google Scholar]
- 12.Matsukita S., Nomoto M., Kitajima S., Tanaka S., Goto M., Irimura T., Kim Y.S., Sato E., Yonezawa S. Expression of mucins (MUC1, MUC2, MUC5AC and MUC6) in mucinous carcinoma of the breast: Comparison with invasive ductal carcinoma. Histopathology. 2003;42:26–36. doi: 10.1046/j.1365-2559.2003.01530.x. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay P., Chakraborty S., Ponnusamy M.P., Lakshmanan I., Jain M., Batra S.K. Mucins in the pathogenesis of breast cancer: Implications in diagnosis, prognosis and therapy. Biochim. Biophys. Acta. 2011;1815:224–240. doi: 10.1016/j.bbcan.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh M.D., McGuckin M.A., Devine P.L., Hohn B.G., Wright R.G. Expression of MUC2 epithelial mucin in breast carcinoma. J. Clin. Pathol. 1993;46:922–925. doi: 10.1136/jcp.46.10.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakha E.A., Boyce R.W., Abd El-Rehim D., Kurien T., Green A.R., Paish E.C., Robertson J.F., Ellis I.O. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod. Pathol. 2005;18:1295–1304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 16.Do S.I., Kim K., Kim D.H., Chae S.W., Park Y.L., Park C.H., Sohn J.H. Associations between the Expression of Mucins (MUC1, MUC2, MUC5AC, and MUC6) and Clinicopathologic Parameters of Human Breast Ductal Carcinomas. J. Breast Cancer. 2013;16:152–158. doi: 10.4048/jbc.2013.16.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel D.S., Khandeparkar S.G.S., Joshi A.R., Kulkarni M.M., Dhande B., Lengare P., Phegade L.A., Narkhede K. Immunohistochemical Study of MUC1, MUC2 and MUC5AC Expression in Primary Breast Carcinoma. J. Clin. Diagn. Res. 2017;11:EC30–EC34. doi: 10.7860/JCDR/2017/26533.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohashi R., Hayama A., Yanagihara K., Yamashita K., Sakatani T., Takei H., Naito Z. Prognostic significance of mucin expression profiles in breast carcinoma with signet ring cells: A clinicopathological study. Diagn. Pathol. 2016;11:131. doi: 10.1186/s13000-016-0584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daemen A., Manning G. HER2 is not a cancer subtype but rather a pan-cancer event and is highly enriched in AR-driven breast tumors. Breast Cancer Res. 2018;20:8. doi: 10.1186/s13058-018-0933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]