Abstract
Calcium signaling, in addition to its numerous physiological roles, is also implicated in several pathological conditions including cancer. An increasing body of evidence suggest critical roles of calcium signaling in the promotion of different aspects of cancer, including cell proliferation, therapy resistance and metastatic-related processes. In many cases, this is associated with altered expression and/or activity of some calcium channels and pumps. Brain cancers have also been the subject of many of these studies. In addition to diverse roles of calcium signals in normal brain function, a number of proteins involved in calcium transport are implicated to have specific roles in some brain cancers including gliomas, medulloblastoma, neuroblastoma and meningioma. This review discusses research that has been conducted so far to understand diverse roles of Ca2+-transporting proteins in the progression of brain cancers, as well as any attempts to target these proteins towards a therapeutic approach for the control of brain cancers. Finally, some knowledge gaps in the field that may need to be further considered are also discussed.
Keywords: calcium signaling, brain cancers, therapeutic targeting
1. Introduction
Calcium (Ca2+) is a secondary messenger involved in a variety of cellular processes, including cell growth, apoptosis, differentiation, metabolism, muscle contraction, neuronal plasticity and gene transcription [1,2]. In addition to its diverse physiological roles, Ca2+ signaling is also implicated in various pathological conditions, including cancers. Diverse cellular processes critical for cancer progression are Ca2+ signal-dependent, including proliferation, angiogenesis, invasion and metastasis [3]. Recently, Ca2+ signaling has also been shown to promote growth and tumorigenesis of brain cancers. Although, most of these studies so far have been in glioblastomas, an increasing body of evidence suggest crucial role of Ca2+ signaling in other types of brain cancers too. While the calcium signal is ubiquitous, the proteins that control Ca2+ transport are not ubiquitously expressed. Indeed, altered expression and/or activity of specific Ca2+ channels and pumps have been shown in cancers. These proteins regulate highly specialized and selective processes which contribute to cancer initiation or progression [1,4]. At the same time, these proteins may not have vital roles in surrounding normal cells. Thus, altered expression or activity of these proteins in cancers can be therapeutically exploited to suppress a pathway that is critical for tumorigenesis. Such therapeutic approaches could be of vital importance in some brain cancer treatment where the current therapeutic options are inadequate or show debilitating side effects. This review discusses roles and therapeutic targeting of Ca2+ transporting proteins in brain cancers.
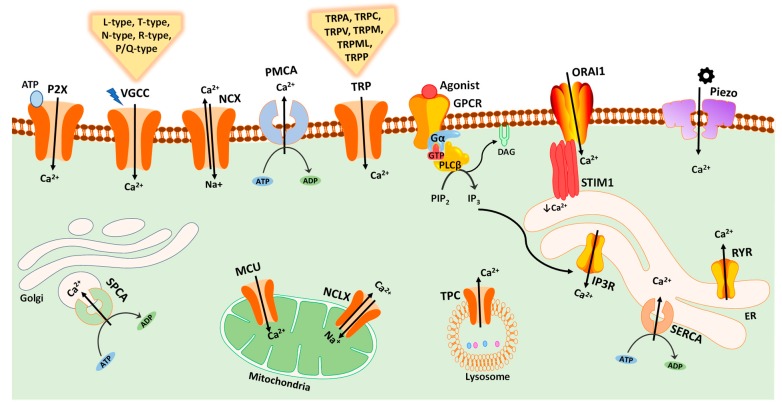
Intracellular Ca2+ signals generated by cells are specific in magnitude, time course and location and involve various components of calcium signaling toolkit including channels, pumps, exchangers, signaling proteins and its dependent effectors. Figure 1 shows an overview of the key components of Ca2+-transporting proteins, mediating Ca2+ transport in or out of cell, and into or out of intracellular organelles. Changes in Ca2+ signaling can induce modifications in cell physiology. Therefore, a firm control on Ca2+ levels within cells is essential [5].
Figure 1.
Schematic representation of major calcium channels, pumps, exchangers and sensors in mammalian cells. Ca2+ influx is mediated by plasma membrane channels including transient receptor potential (TRP) channels, voltage-gated calcium channels (VGCC), ligand-gated ionotropic P2X receptors, mechanosensitive Piezo channels, and store-operated Ca2+ entry pathway mediated by stromal interaction molecule 1 (STIM1) sensor and ORAI1 channels. Distribution of Ca2+ against a chemical gradient across cell compartments is regulated by Ca2+ pumps including the plasma membrane Ca2+-ATPase (PMCA), Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA), and Golgi network secretory pathway Ca2+/Mn2+-ATPase (SPCA). The endoplasmic reticulum (ER) Ca2+ channels include ryanodine receptor (RYR) and inositol 1,4,5-trisphosphate (IP3) receptor (IP3R); the latter is activated by IP3 ligand produced by the plasma membrane G protein-coupled receptor (GPCR) via Gaq and phospholipase C-β (PLCβ) proteins. Two-pore channels (TPC) regulate Ca2+ release from the endolysosomal system. Mitochondrial Ca2+ levels are controlled by mitochondrial calcium uniporter (MCU) complex, and mitochondrial Na+/Ca2+ exchanger (NCLX).
Normal cell functioning requires maintenance of varied distribution of Ca2+ against a chemical gradient across cell compartments which is carried out and regulated by energy driven Ca2+ pumps. These pumps change the affinity and availability of Ca2+ binding sites within cell compartments with variation in Ca2+ concentrations [6]. Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA), plasma membrane Ca2+-ATPase (PMCA) and Golgi network secretory pathway Ca2+/Mn2+-ATPase (SPCA) are the main pumps found in mammals. An upward or downregulated expression of Ca2+ pumps is observed in various cancers and is believed to promote differentiation and apoptosis resistance in tumor cells [6]. Ca2+ signals are mediated by inositol 1,4,5-trisphosphate (IP3) receptor and ryanodine receptors (RyRs) in sarco and endoplasmic reticulum. Voltage-gated Ca2+ channels (VGCC), transient receptor potential (TRP) ion channels, store-operated Ca2+ entry (SOCE) channels, and ligand-gated Ca2+ channels are involved in movement of Ca2+ ion across plasma membrane. For instance, TRP channels, also known as cellular sensors [7], consists of a number of cation channels that mediate cellular functions such as cell growth, apoptosis and motility [8]. Ligand-gated ionotropic P2X receptors are a class of purine receptors that regulate passage of Ca2+ into the cell in response to extracellular ATP [9]. Piezo channels are plasma membrane mechanosensitive channels that are stimulated by mechanical forces (Figure 1). A detailed description of components and complexity of Ca2+ signaling has been reviewed extensively elsewhere [2,10,11]. Intracellular Ca2+ can be derived from both internal (such as endoplasmic or sarcoplasmic reticulum) and external sources (such as via plasma membrane ion channels). Ca2+ produces signals in the form of waves and spikes. Spikes activate localized cellular processes, while Ca2+ waves occur when individual channels communicate with each other to produce global signals leading to activation of both intercellular (such as functioning of glial cells) and intracellular (such as gene transcription and cell proliferation) functions [12]. Ca2+ signals for longer periods are relayed via repetitive Ca2+ pulses known as oscillations. Both Ca2+ spikes and waves can produce oscillating signals triggering different processes depending upon their signal duration [12].
2. Calcium Signaling in Cancer
In recent years, studies elucidating the molecular mechanism involved in cancer have led to an enhanced understanding of various factors that play a role in disease progression and complexity [13]. In an attempt to simplify the complex biology underlying the disease, Hanahan and Weinberg in their review, “The hallmarks of cancer: the next generation”, introduced ten key characteristics of the neoplastic cells. These are maintenance of long term growth signaling, resistance to cell death, angiogenesis induction, metastasis instigation, growth suppressor evasion, uncontrolled proliferation potential, unstable genome and mutation, energy metabolism reprogramming, evasion of immune system and presence of tumor promoting inflammation [14]. Cancer pathology reveals genetic changes in the growth signaling that impair tightly regulated normal cell homeostasis leading to transformation of normal cells into malignant type with uncontrolled proliferative potential [15]. A growing body of evidence suggests that ion channels play a pivotal role in tumor biology and are involved in regulation of tumor cell characteristics such as uncontrolled growth, sustained angiogenesis, metastasis and resistance to apoptosis [16]. In some cases, this regulation occurs via the transmission of signals from the tumor microenvironment. For instance, the involvement of Ca2+ channels in regulation of epithelial to mesenchymal transition (EMT) [17], a process critical for cancer metastasis [18,19] and also contributes to drug resistance [20].
In tumor cells, altered Ca2+ movement often involves aberrant expressions, cellular localization and activity of Ca2+-transporting proteins contributing towards tumor specific characteristics such as unlimited growth, metastasis and resistance to apoptosis [3,4]. Since Ca2+ signals are vital for normal cell function, alteration in these signals may lead to modified calcium codes that can trigger tumorigenic behavior. Ca2+ pulses serve as “calcium codes” differing in magnitude, time and frequency. Prolonged Ca2+ pulses are involved in cell division process, angiogenesis, differentiation and genomic instability in cancer cells [21].
Brain Cancers
Brain cancers are neoplasms that originate within the brain and spinal cord mainly from neurons or glial cells (astrocytes, oligodendrocytes and ependymal cells), and occasionally from pituitary gland, meninges, lymphatic tissue and cranial nerves [22]. These tumors exhibit with varying degrees of malignancy and are associated with high mortality and morbidity in both children and adults [22,23].
Medulloblastomas (MBs), ependymomas and high-grade gliomas are the most prevalent tumors affecting children and adults [23]. Medulloblastoma, arising from immature neuronal precursors of cerebellum, affects about 25% of pediatric brain cancer patients [24]. For treatment, patients are divided into standard and high-risk groups. High-risk patients have residual tumor mass of >1.5 cm2 showing metastasis, whereas low risk patients have residual tumor with the size of < 1.5 cm2 without metastasis. Current therapies for medulloblastomas include surgery, radiotherapy and chemotherapy. The use of chemotherapeutic agents as an adjuvant has led to improved results and radiation dose reduction [25].
Ependymomas, accounting for approximately 10% of pediatric brain tumors, originate from ependymal cells that form the lining of the spinal cord and ventricles of the brain [25]. Recent studies indicate that ependymomas have varied genetic aberrations and transcriptional profiles depending upon their location within the central nervous system. Surgery and irradiation remain the mainstay of treatment, as chemotherapy shows limited efficacy in most patients [25,26].
In adults, the most common type of brain tumor is high-grade gliomas. It includes glioblastoma and anaplastic astrocytoma [27]. Current treatment options include maximal surgical resectioning, followed by radiation and adjuvant temozolomide chemotherapy. For anaplastic gliomas, the standard treatment is surgery and radiotherapy, as the efficacy of chemotherapy remains uncertain [27].
Despite improving the survival rate of patients, current therapies pose serious complications that impact quality of life in patients. Traumatic injuries such as damage to blood vessels and ischemia are common problems associated with surgical resectioning, while radiation and chemotherapy cause neurocognitive deficits, cerebral necrosis, peripheral neuropathy, myelopathy and secondary neoplasms [28]. Current neoplastic treatments not only cause neurological complications, but also act as precursor to problems such as tumor relapse [29]. Lack of specific targeting of tumor cells by standard care therapies results in damage to normal cells leading to systemic toxicity. Furthermore, their inability to kill cancer stem cells (CSCs), that possess self-renewal properties, leads to appearance of secondary tumors [30]. CSCs are rare population of tumor cells that have the ability to multiply and differentiate via both symmetrical and asymmetrical cell division. These cells play a key role in initiation, growth, maintenance, metastasis and survival of cancers. It is, therefore, essential to develop treatments that specifically target and eliminate CSCs with minimal damage to normal cells for more effective clinical outcomes [31]. Recent studies have indicated the involvement of calcium channels in controlling a number of functions important in CSCs such as cell volume, progression, metastasis and angiogenesis and therefore, deregulated calcium signaling could serve as a target for cancer therapy [32].
3. Calcium Signaling in the Brain
Ca2+ channels play crucial role in nervous system development. Short-term rise in Ca2+ levels, known as calcium transients, are believed to play an important role in mediating various neuronal development stages such as growth, migration, differentiation, survival and formation of neuronal network by maintaining different influx patterns [33].
In brain, key functions of neurons are regulated by flow of Ca2+ between the intracellular compartments and across plasma membrane in brain. The movement of Ca2+ is triggered in response to stimulus such as membrane depolarization, intracellular Ca2+ store depletion, mechanical stretch, extracellular agonists and intracellular messengers [34].
The influx of Ca2+ leads to induction of a number of Ca2+ dependent processes such as neurotransmitter release, neuronal excitability, synaptic plasticity, memory storage and gene expression [35,36,37]. This diversity in neural functions mediated by Ca2+ signal is a result of distinctive signals that differ in their scale, temporal and spatial properties [34]. Specific changes in the Ca2+ concentration results in distinct function modification in the same neuron types depending upon the short, medium and long duration and distance travelled by the signal [34].
Ca2+ regulated functions involve a set of ion channels, exchangers and pumps present in plasma membrane, mitochondria, endoplasmic reticulum, golgi apparatus and nucleus, collectively known as Ca2+ toolkit. These Ca2+-transporting proteins together with G protein-coupled receptors, Ca2+ binding proteins, and related transcriptional networks mediate functions important for brain physiology [38].
4. Ca2+-Transporting Proteins in the Progression of Brain Cancers
The following section summarizes the specific roles of Ca2+-transporting proteins in brain cancers. Most of these studies are conducted in glioma cells, with some research focusing on medulloblastoma, neuroblastoma and meningioma cells. Table 1 summarizes studies showcasing specific roles of Ca2+-transporting proteins in the progression of brain cancers. It should be noted that in this review, we only discuss studies that show direct association of Ca2+ transporters and not the ones based on other ion transporters that are Ca2+ dependent. This is, exemplified, by the role of calcium-activated potassium channel KCa3.1 in glioma cell migration, invasion and temozolomide resistance [39].
Table 1.
Ca2+-transporting proteins with demonstrated specific roles in brain cancers.
| Cancer | Channel/Regulator | Model | Targeting Approach | Role of Channel/Regulator | References |
|---|---|---|---|---|---|
| Glioma | STIM1, ORAI1 | Primary GB cells | siRNA | Regulates proliferation (only ORAI1) and invasion | [41] |
| ORAI1 | U251, C6 cells | siRNA, antagonist | Regulates cell proliferation and apoptosis | [42] | |
| Cav3.1, Cav3.2 | U251, U87 and T98G cells | siRNA, antagonist | Regulate apoptosis and proliferation, and sensitize cells to ionizing radiation | [47] | |
| Cav3.2 | GB primary stem cells, Xenografted mice | siRNA, antagonist | Promotes proliferation and stemness. Sensitizes cells to TMZ * | [49] | |
| TRPC1 | D54MG cells, flank tumor | shRNA, antagonist | Promotes cell proliferation and cytokinesis, as well as tumor size | [52] | |
| D54MG cells | shRNA, antagonist | Promotes cell motility | [53] | ||
| TRPC6 | U251, U87, C6 cells, Xenografts | shRNA, DNC6 * antagonist | Promotes cell growth, clonogenicity, and G2/M transition | [54] | |
| U251 cells | shRNA, DNC6 * antagonist | Induces HIF-1α accumulation and glucose uptake | [57] | ||
| U373, HMEC-1 cells | siRNA | Promotes NFAT activation, cell proliferation and angiogenesis | [58] | ||
| TRPM2 | A172 cells | Overexpression | Induces cell death | [59] | |
| TRPM7 | A172 cells | siRNA | Promotes proliferation and migration | [60] | |
| TRPM8 | DBTRG cells | Agonist, antagonist | Promotes cell migration | [61,62] | |
| TRPV1 | U373 cells | Agonist | Induces apoptosis | [63] | |
| TRPV2 | U87MG, MZC cells | Agonist | Increases chemosensitivity | [65] | |
| U87MG, MZC cells | siRNA, overexpression | Negatively regulates proliferation and resistance to cell death | [66] | ||
| GB primary stem cells | Antagonist, siRNA, overexpression in xenograft | Promotes differentiation and inhibits proliferation | [67] | ||
| IP3R3 | U178, U87, T98G cells, Organotypic, Xenograft | Antagonist | Regulates invasion and migration | [69] | |
| P2X7R | C6 cells | Agonist | Promotes migration and inflammation | [71] | |
| C6 cells, Xenograft | Antagonist, shRNA | Negatively regulates cell proliferation, tumor growth and angiogenesis | [72] | ||
| M059J, GL261 cells | Agonist, antagonist, siRNA | Promotes cell radiosensitivity | [73,74] | ||
| 1321N1 cells | Agonist | Promotes ERK1/2 activation | [75] | ||
| MB | TRPC4 | DAOY, ONS76 cells, Organotypic | Agonist, antagonist, overexpression | Promotes cell motility | [76] |
| Neuroblastoma | Cav3.1, Cav3.2 | N1E-115 cells | siRNA, antagonist overexpression | Promotes cell proliferation | [77] |
| Cav3.2 | NG108-15 cells | siRNA | Promotes cell differentiation | [78] | |
| TRPM2 | SH-SY5Y cells, Xenograft | Overexpression, antagonist | Regulates cell death/viability | [79,80] | |
| P2X7R | N2a cells | Antagonist | Promotes cell proliferation | [81] | |
| Meningioma | L-type channels | IOMM-Lee cells, xenograft | Antagonist | Promotes apoptosis and cell-cycle arrest | [85,86] |
* DNC6, Expression of the dominant-negative mutant TRPC6; GB, glioblastoma; TMZ, temozolomide; MB, medulloblastoma.
4.1. Calcium Signaling in Glioma Cells
4.1.1. Store-Operated Ca2+ Entry (SOCE) in Glioma Cells
SOCE is a process of Ca2+ influx to refill the endoplasmic reticulum’s (ER) Ca2+ after depletion. This process is initiated when the reduced levels of ER Ca2+ are sensed by STIM1 leading to its oligomerization and interaction with ORAI1 Ca2+ channel on the plasma membrane consequently resulting in the opening of the channel and influx of Ca2+ [40]. In primary human glioblastoma cells, higher levels of SOCE and ORAI1 expression have been observed in comparison to non-malignant human primary astrocytes [41]. While small interfering RNA (siRNA)-mediated silencing of ORAI1 (but not STIM1) marginally reduces cell proliferation, silencing of both ORAI1 and STIM1 dramatically reduces invasion of glioblastoma cells [41]. In another study, pharmacological inhibition of SOCE and ORAI1 silencing, led to decrease in cell proliferation and induction of apoptosis in glioblastoma cells [42].
4.1.2. Voltage-Gated Calcium Channels (VGCCs) in Glioma Cells
In neurons, Ca2+ influx is regulated by VGCCs. Opening of these channels results in high intracellular Ca2+ levels that activate processes such as neurotransmitter release, neuron growth and gene expression [43]. VGCCs are divided into three main categories: high voltage activated channels (including P/Q-type, N-type and L-type), intermediate voltage activated R-type channels, and low voltage activated T-type channels (CaV3 channels) [44]. T-type Ca2+ channels control processes such as cell proliferation and differentiation by regulating Ca2+ levels at low voltage through creation of resting inward calcium current. Aberrant expression of T-type Ca2+ channels is observed in diverse tumor types [45]. In human glioblastoma cells, mibefradil, a T-type Ca2+ channel blocker with a weak L-type channel inhibiting activity [46], and siRNA-mediated downregulation of CACNA1G (CaV.3.1) and CACNA1H (CaV3.2) reduces cell proliferation, induces apoptotic cell death, and sensitizes cells to ionizing radiation via AKT/mTORC2 axis [47]. In another study, treatment with mibefradil was shown to enhance the efficacy of subsequently administered temozolomide in human glioblastoma xenograft lines grown in immunodeficient mice [48]. More recently, small hairpin RNA (shRNA)-mediated silencing of CaV3.2 or mibefradil treatment was shown to inhibit the proliferation, survival and stemness features of glioblastoma stem-like cells (GSC) as well as sensitize them to temozolomide chemotherapy [49]. This regulation was shown to be mediated by inhibition of pro-survival AKT/mTOR pathway, promotion of proapoptotic survivin and BAX pathways, and modulation of the expression of number of oncogenes and tumor suppressor genes [49]. Mibefradil, taken orally, suppressed growth of GSC-derived xenografts, increased survival and enhanced temozolomide sensitivity [49].
4.1.3. TRP Channels in Glioma Cells
TRP channels are expressed in a number of tissues where they control diverse cellular functions such as cell growth, proliferation, differentiation, migration and apoptosis [50]. TRP channels are activated by both chemical and physical stimuli as well as through changes in cell microenvironment [50]. Number of studies have shown the important roles of TRP channels in glioma progression. In D54MG glioblastoma cells, treatment of cells with SKF96365 (a non-selective TRP-canonical (TRPC) channel blocker) led to inhibition of cell proliferation and cytokinesis [51]. In another study, shRNA mediated suppression of TRPC1 or treatment with SKF96365 in D54MG glioma cells, resulted in inhibition of cell proliferation and incomplete cell division [52]. This study also showed that shRNA suppression of TRPC1 in a flank tumor model, reduced tumor size [52]. Further studies demonstrated that motility of D54MG cells stimulated by epidermal growth factor (EGF), is regulated by TRPC1 channels, in a manner that is dependent on lipid raft proteins [53]. Another TRPC member, TRPC6, was shown to have a crucial role in cell growth, clonogenic ability and G2/M phase cell cycle transition, and its inhibition promoted antiproliferative effects of ionizing radiation [54].
Calcium signaling regulates hypoxia-associated pathways, and several Ca2+-transporting proteins are shown to interplay with hypoxia-inducible factor-1 (HIF-1), which is master regulator of hypoxia transcriptional responses [55,56]. In glioma cells, TRPC6 is activated by hypoxia, where it contributes to hypoxic elevation of intracellular Ca2+. Inhibition of TRPC6, by shRNA or expression of its dominant-negative mutant (DNC6), abolished hypoxia-induced HIF-1α protein accumulation in U251 human glioblastoma cells via promotion of HIF-1α hydroxylation [57]. Hypoxic activation of TRPC6 was also shown to contribute to cell metabolism via regulation of glucose transporter 1 (GLUT1) expression and glucose uptake [57]. In another study, hypoxic upregulation of TRPC6 expression (via Notch signaling), resulted in activation of nuclear factor of activated T-cells (NFAT), proliferation, invasion and angiogenesis of glioblastoma cells [58].
A number of TRP-melastatin (TRPM) channels have been implicated in glioblastoma progression: reactive oxygen species (ROS)-activated TRPM2 channel induces cell death in A172 human glioblastoma cells [59]; TRPM7 channel promotes proliferation and migration of A172 cells possibly through activation of JAK2/STAT3 and Notch signaling pathways [60]; and TRPM8 channel, via activation of the large-conductance Ca2+-activated K+ membrane ion channels (BK channels), regulates migration of DBTRG glioblastoma cells [61,62].
TRP-vanilloid (TRPV) channels also have important functions in gliomas. Activation of TRPV1 by capsaicin, leads to Ca2+ influx and induction of apoptosis in U373 human glioblastoma astrocytoma cells via p38 mitogen-activated protein kinases (MAPK) activation [63]. Interestingly, neural precursor cells, in an antitumorigenic response, migrate to high-grade astrocytoma cells and activate highly-expressed TRPV1 channels on these cells by releasing endovanilloids. This activation results in cancer cell death via activating transcription factor-3 (ATF3)-dependent ER stress pathway [64]. Cannabidiol-induced TRPV2 channel potentiates chemosensitivity of carmustine, temozolomide and doxorubicin in U87MG and MZC cells [65]. TRPV2 was shown in another study, to negatively regulate glioma cell proliferation and survival, and resistance to extracellular signal-regulated kinase (ERK) dependent Fas-induced apoptotic cell death [66]. Another study in in vitro and in vivo models showed that TRPV2 enhances glioblastoma stem-like cells (GSCs) differentiation toward a more mature glial phenotype, and suppresses their proliferation [67].
4.1.4. IP3 Receptors in Glioma Cells
IP3 receptors (IP3R) convert external stimulus to intracellular Ca2+ signals of diverse spatial and temporal patterns such as waves and oscillations [68]. In glioblastoma cells, pharmacological inhibition of IP3R3 by caffeine, reduces cell invasion and migration, and increases survival of subject animals [69].
4.1.5. P2X Receptors in Glioma Cells
Members of the P2X receptors have been shown to play roles in different cancer types [70]. In gliomas, several studies have implicated the role of P2X7 receptors. In rat C6 glioma cells, activation of P2X7 receptors promoted cell migration and expression of pro-inflammatory factors [71]. In another study in C6 cells, P2X7 receptor suppression via use of antagonist or shRNA, promoted epidermal growth factor receptor (EGFR) signaling and growth of cells in in vitro, and tumor growth and angiogenesis in rat-transplanted in vivo models [72]. Intriguingly, P2X7 receptor activation in radiosensitive M059J cells resulted in enhanced cell death [73]. Indeed, P2X7 receptor was later proposed as a predictor gene for glioma patient radiosensitivity and survival probability [74]. In 1321N1 astrocytoma cells, P2X7 receptor was shown to activate ERK1/2 phosphorylation via activation of proline-rich tyrosine kinase 2 (Pyk2), c-Src, phosphatidylinositol 3′-kinase (PI3K), and protein kinase C (PKC) [75].
4.2. Calcium Signaling in Medulloblastoma Cells
In medulloblastoma cells, proton-sensing ovarian cancer G protein-coupled receptor 1 (OGR1) promotes the expression of TRPC4 channels in transformed granule cells (DAOY cells). TRPC4 channels in these cells (but not in normal cerebellar granule precursor cells), enhances cell motility in wound healing and transwell migration assays [76].
4.3. Calcium Signaling in Neuroblastoma Cells
In mouse N1E-115 neuroblastoma cells, using plasmid overexpression, siRNA-mediated silencing or mibefradil treatment, it was shown that CaV3.1 and CaV3.2 promote cell proliferation [77]. CaV3.2 also stimulates differentiation of NG108-15 cells via an autocrine mechanism of facilitating extracellular secretion of differentiation-promoting factors [78].
Full-length and short isoforms of the TRPM2 channels (TRPM2-L and TRPM2-S respectively) are shown to be upregulated in neuroblastoma tissues compared to adrenal glands [79]. SH-SY5Y neuroblastoma cells stably expressing TRPM2-L showed enhanced protection against cell death induced by oxidative stress via increased forkhead box transcription factor 3a (FOXO3a) and superoxide dismutase 2 (SOD2) levels, while cells expressing TRPM2-S showed enhanced levels of ROS and reduced cell viability [79,80]. TRPM2-S expressing SH-SY5Y xenografts showed reduced HIF-1/2α levels and their target proteins, as well as reduced tumor growth [80]. Pharmacological inhibition of TRPM2-L or expression of TRPM2-S enhanced sensitivity of cells to doxorubicin [80].
P2X7 receptors, in addition to glioma cells that was discussed above, plays role in neuroblastoma cells too. In neuroblastoma cells lacking trophic support (serum-deprived), P2X7 receptor expression is enhanced via EGFR and PI3K/AKT pathway, where it facilitates cell proliferation [81].
Retinoic acid-mediated differentiation of neuroblastic (N-type) SH-SY5Y cells was shown to be associated with downregulation of SOCE and expression of STIM1 and ORAI1 proteins [82]. Similarly, in another study, an increase in Ca2+ efflux and expression of PMCA2, PMCA3 and PMCA4 was observed in differentiated IMR-32 neuroblastoma cells compared to undifferentiated cells [83]. In both these studies, however, it was not discussed if SOCE or PMCA pumps are involved in the induction of differentiation or these altered activity/expression are consequences of differentiation.
Treatment of neuroblastoma cells with chemotherapeutic agents, cisplatin and topotecan, increased intracellular Ca2+ levels over time. Furthermore, expression of S100A6, IP3R1, IP3R3, RYR1, RYR3 were altered upon treatment with cisplatin and topotecan, and pharmacological modulators of Ca2+-transporting proteins in combination with these two agents enhanced cytotoxicity [84]. Further studies are needed to determine the specific roles of these Ca2+-transporting proteins in neuroblastoma progression.
4.4. Calcium Signaling in Meningioma Cells
VGCCs are shown to promote meningioma cells. The addition of diltiazem and verapamil (mainly L-type calcium channel blockers) to meningioma chemotherapy drug hydroxyurea (HU), and the antiprogesterone, mifepristone (RU486), enhanced cell growth inhibition through induction of apoptosis and G1 cell-cycle arrest in vitro. This approach also decreased tumor size in meningioma subcutaneous mouse flank tumors through suppression of proliferation and microvascular density [85,86].
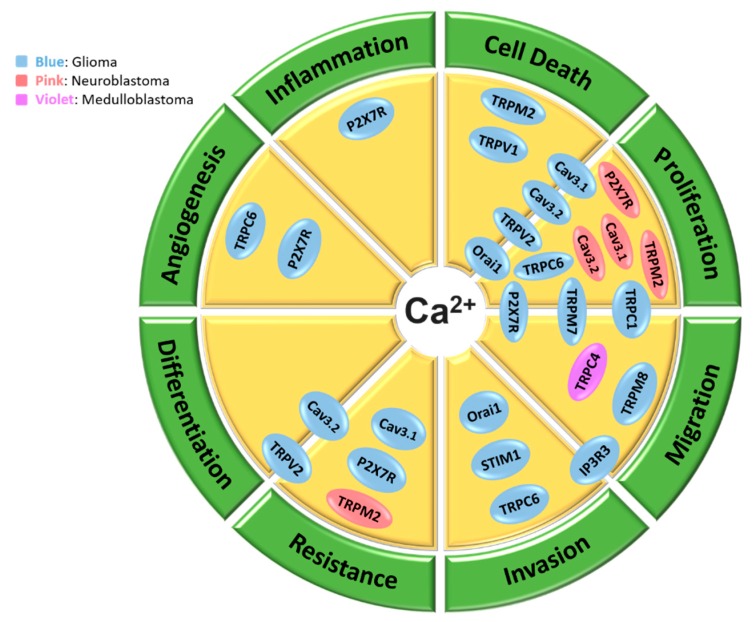
Figure 2 compiles different processes of brain cancer progression assessed in these studies into distinct categories of cellular processes and places each Ca2+ channel/regulator into its relevant process where it has shown involvement. These processes include proliferation, migration, invasion, therapy resistance/therapy sensitivity, differentiation, angiogenesis, inflammation and cell death. Many of these processes are involved in cancer hallmarks.
Figure 2.
Schematic representation of biological processes that are promoted by Ca2+ signaling proteins in brain cancer cells. Several Ca2+ channels are shown to contribute to various pro-tumor processes in glioma cells (blue color), neuroblastoma cells (pink color) and medulloblastoma cells (violet color). These processes include proliferation, migration, invasion, therapy resistance/therapy sensitivity, differentiation, angiogenesis, inflammation and cell death. Proteins that are positioned on the borders of two processes, contribute to both processes.
5. Targeting Calcium Signaling Pathways as a Therapeutic Approach for Brain Cancers
In the previous section, we discussed studies that have shown important roles of Ca2+-transporting proteins in the promotion of different aspects of brain cancer progression. In this section, we discuss studies that are more translational and are conducted in in vivo preclinical models or in clinical trials.
Table 2 summarizes clinical trial studies that are completed or currently underway in brain cancers using modulators of Ca2+-transporting proteins and outlines the outcomes of these studies. Perhaps one of the most important studies, is recent completion of phase II clinical trial of Mipsagargin (also known as G-202) for recurrent or progressive glioblastoma (NCT02067156), as well as for prostate cancer [87]. Mipsagargin which is a SERCA pump inhibitor, is an analogue of thapsigargin. Mipsagargin is a non-toxic prodrug that is activated through binding to and cleavage by prostate-specific membrane antigen (PSMA) that is rich in many cancers [87]. Mibefradil, has been also at the center of attention for clinical trials. Indeed, two clinical trials of phase I were recently completed for assessment of mibefradil in combination with temozolomide (NCT01480050, [88]) or hypofractionated radiation (NCT02202993, [89]) for recurrent glioblastoma. These treatments were well-tolerated in patients and showed some promising responses in selective of patients suggesting that these treatments warrant further investigation [88,89]. A phase I clinical trial was also recently completed for assessment of carboxyamidotriazole orotate (CTO), an inhibitor of non-voltage dependent calcium channels (blocking both the Ca2+ influx and release from intracellular stores), for recurrent and newly diagnosed glioblastoma and other anaplastic gliomas (NCT01107522, [90,91]). This study showed safe co-administration of CTO with temoxolomide or chemoradiation, favorable brain penetration and promising signals of activity in patients [90,91]. A Phase II study focusing on combination of verapamil and hydroxyurea (HU) for refractory meningiomas showed no effect of HU or verapamil on tumor recurrence and progression-free survival (PFS) [92].
Table 2.
Modulators of Ca2+-regulating proteins in clinical trials for treatment of brain cancers.
| Intervention | Channel/Pump Targeted | Disease | Clinical Phase | Study End Year | Results | NCT # | References |
|---|---|---|---|---|---|---|---|
| Verapamil +Hydroxyurea (HU) | L-type channels | Refractory Meningiomas | II | 2015 | No effect of HU or verapamil on tumor recurrence and PFS | 00706810 | [92] |
| Mipsagargin | SERCA pump | Recurrent or progressive GB | II | 2017 | Favorable tolerability and pharmacokinetic profile | 02067156 | [87] |
| Mibefradil +Temozolomide | T-type channels | Recurrent Glioma | I | 2017 | Well tolerated and promising responses in patients | 01480050 | [88] |
| Mibefradil +Hypofractionated radiation | T-type channels | Recurrent GB | I | 2017 | Safe co-administration, effective brain penetration, and promising local control signals in some patients | 02202993 | [89] |
| CTO +Temozolomide or chemoradiation | Non-voltage channels | GB and other anaplastic gliomas | I | Still active | Safe co-administration, favorable brain penetration, and promising signals of activity | 01107522 | [90,91] |
GB, glioblastoma; PFS, progression-free survival; CTO, Carboxyamidotriazole orotate.
Apart from these clinical studies, there have also been some exciting pre-clinical studies in in vivo models with translational potential. In a recent study, pharmacological targeting of T-type Ca2+ channels with niguldipine and mibefradil, induced selective cell death of glioma-initiating cells and enhanced host survival in an orthotopic mouse model of human glioma [93]. In another study, trifluoperazine (TFP), a well-known antipsychotic, inhibited proliferation (via induction of cell death), migration and invasion of glioblastoma cells in vitro, and tumor growth in in vivo xenograft mouse model. This effect was shown to be via direct binding of TFP to the Ca2+-binding protein, calmodulin subtype 2 (CaM2), leading to dissociation of CaM2 from IP3R and subsequent extensive and irreversible Ca2+ release from endoplasmic reticulum by IP3R1 and IP3R2. Interestingly, this study also showed that TFP has less toxic effects on neural stem cells compared to glioblastoma cells [94]. Bradykinin, a neuropeptide of the vasculature, was shown to induce Ca2+ release from internal stores, which ultimately led to increase of glioma invasion. This was shown to be mediated by inducing amoeboid migration via contraction of the cytoskeleton and activation of Ca2+-dependent K+ and Cl− channels [95]. As also discussed in previous section, another recent study demonstrated novel roles of CaV3.2 in glioblastoma stem-like cells and further supported the use of mibefradil in combination with temozolomide for glioblastoma therapy [49]. Furthermore, in vitro studies in glioblastoma cells demonstrated enhanced radiosensitivity with TRPC6 inhibition [54], while activation of TRPV2 channel increased chemosensitivity of cells [65]. P2X7 receptor activation also led to an increase in radiosensitivity of glioblastoma cells both in vitro [73] and in vivo [74].
Altogether, these studies showcase promising approaches of therapeutic targeting Ca2+ signaling pathways for the control of brain cancers. It is interesting to note that the therapeutic targeting of plasma membrane Ca2+ channels in brain cancers has been focused on the use of blockers of these channels and no study has used channel activators to induce cytosolic Ca2+ overload and subsequent cell death, as was recently shown in breast cancer cells through activation of TRPV4 channel [96].
6. Conclusions and Perspectives
As discussed throughout the paper, a number of studies suggest involvement of Ca2+-transporting proteins in different aspects of brain cancer progression. Interestingly, all the proteins that were identified in these studies were Ca2+ channels of different classes (except from STIM1 that is an ER Ca2+ sensor). These Ca2+ channels included T-type voltage-gated channels, number of TRP channels, ORAI1, IP3R3 and P2X7R. As shown in Figure 2 many of the cellular processes that are regulated by these proteins are involved in cancer hallmarks. It should be noted that these studies identify roles of Ca2+ signaling and specific transporting proteins in the progression of cancer, however it would be interesting and critical to also assess if calcium signaling and its regulating proteins are involved in the initiation of brain cancers. Furthermore, many of these studies are conducted in in vitro models of cancer cells (in most cases continuous cell lines). While these in vitro continuous cell line models provide very important information, these studies need to be also conducted in primary brain cancer cells and in vivo models to have a better understanding of potential translational impact. It is also imperative that in addition to cancer cells, the effect of targeting identified proteins be assessed in normal brain cells, highly sensitive cells that when damaged can result in debilitating permanent side-effects.
As was shown, majority of the research conducted so far in the field of calcium signaling in brain cancer has been undertaken in glioma cells with most of it in the past few years, underlining that there remain many unanswered research questions in this emerging field. In particular, role of Ca2+-transporting proteins in transmitting the signals from the brain tumor microenvironment to cancer cells, is an area of research that requires further investigation.
Ion channels are reported to represent 18% of all FDA-approved small-molecule drug targets [97]. Given the extensively long and expensive process of drug development, assessment of these existing drugs in brain cancers, alone or in conjunction with current therapies, may provide the opportunity of rapid drug re-purposing for brain cancer therapy. In light of the extensive roles of calcium signaling in normal function of cells, the challenge lies in targeting calcium signaling proteins without causing major side effects. Current advancements in several fields can assist in achievement of this goal, including advancement in development of more selective and less toxic compounds, integrative genomics and transcriptomics studies providing gene maps of brain cancers, and novel drug delivery and activation approaches. These will significantly contribute towards safe and effective therapeutic targeting of Ca2+-regulating proteins in coming years.
Funding
The project was funded by internal sources of the Division of Pharmacy, School of Medicine, University of Tasmania, Australia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Prevarskaya N., Ouadid-Ahidouch H., Skryma R., Shuba Y. Remodelling of Ca2+ transport in cancer: How it contributes to cancer hallmarks? Philos. Trans. R. Soc. B. 2014;369 doi: 10.1098/rstb.2013.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Monteith G.R., Prevarskaya N., Roberts-Thomson S.J. The calcium-cancer signalling nexus. Nat. Rev. Cancer. 2017;17:367–380. doi: 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- 4.Monteith G.R., Davis F.M., Roberts-Thomson S.J. Calcium channels and pumps in cancer: Changes and consequences. J. Biol. Chem. 2012;287:31666–31673. doi: 10.1074/jbc.R112.343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roderick H.L., Cook S.J. Ca2+ signalling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 6.Prevarskaya N., Skryma R., Shuba Y. Targeting Ca(2)(+) transport in cancer: Close reality or long perspective? Expert Opin. Ther. Targets. 2013;17:225–241. doi: 10.1517/14728222.2013.741594. [DOI] [PubMed] [Google Scholar]
- 7.Fels B., Bulk E., Petho Z., Schwab A. The Role of TRP Channels in the Metastatic Cascade. Pharmaceuticals. 2018;11:48. doi: 10.3390/ph11020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapovalov G., Ritaine A., Skryma R., Prevarskaya N. Role of TRP ion channels in cancer and tumorigenesis. Semin. Immunopathol. 2016;38:357–369. doi: 10.1007/s00281-015-0525-1. [DOI] [PubMed] [Google Scholar]
- 9.Azimi I., Beilby H., Davis F.M., Marcial D.L., Kenny P.A., Thompson E.W., Roberts-Thomson S.J., Monteith G.R. Altered purinergic receptor-Ca2+ signaling associated with hypoxia-induced epithelial-mesenchymal transition in breast cancer cells. Mol. Oncol. 2016;10:166–178. doi: 10.1016/j.molonc.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carafoli E. Calcium signaling: A tale for all seasons. Proc. Natl. Acad. Sci. USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azimi I., Roberts-Thomson S.J., Monteith G.R. Calcium influx pathways in breast cancer: Opportunities for pharmacological intervention. Br. J. Pharmacol. 2014;171:945–960. doi: 10.1111/bph.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berridge M.J., Bootman M.D., Lipp P. Calcium—A life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 13.Arcangeli A., Crociani O., Lastraioli E., Masi A., Pillozzi S., Becchetti A. Targeting ion channels in cancer: A novel frontier in antineoplastic therapy. Curr. Med. Chem. 2009;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Prevarskaya N., Skryma R., Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010;16:107–121. doi: 10.1016/j.molmed.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Cuddapah V.A., Sontheimer H. Ion channels and transporters in cancer. 2. Ion channels and the control of cancer cell migration. Am. J. Physiol. Cell Physiol. 2011;301:C541–C549. doi: 10.1152/ajpcell.00102.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azimi I., Monteith G.R. Plasma membrane ion channels and epithelial to mesenchymal transition in cancer cells. Endocr. Relat. Cancer. 2016;23:R517–R525. doi: 10.1530/ERC-16-0334. [DOI] [PubMed] [Google Scholar]
- 18.Thiery J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 19.Davis F.M., Azimi I., Faville R.A., Peters A.A., Jalink K., Putney J.W., Jr., Goodhill G.J., Thompson E.W., Roberts-Thomson S.J., Monteith G.R. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33:2307–2316. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A., Settleman J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkash J., Asotra K. Calcium wave signaling in cancer cells. Life Sci. 2010;87:587–595. doi: 10.1016/j.lfs.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheirollahi M., Dashti S., Khalaj Z., Nazemroaia F., Mahzouni P. Brain tumors: Special characters for research and banking. Adv. Biomed. Res. 2015;4:4. doi: 10.4103/2277-9175.148261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Robles P., Fiest K.M., Frolkis A.D., Pringsheim T., Atta C., St Germaine-Smith C., Day L., Lam D., Jette N. The worldwide incidence and prevalence of primary brain tumors: A systematic review and meta-analysis. Neuro Oncol. 2015;17:776–783. doi: 10.1093/neuonc/nou283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huse J.T., Holland E.C. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer. 2010;10:319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 25.Karajannis M., Allen J.C., Newcomb E.W. Treatment of pediatric brain tumors. J. Cell. Physiol. 2008;217:584–589. doi: 10.1002/jcp.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gajjar A., Pfister S.M., Taylor M.D., Gilbertson R.J. Molecular insights into pediatric brain tumors have the potential to transform therapy. Clin. Cancer Res. 2014;20:5630–5640. doi: 10.1158/1078-0432.CCR-14-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theeler B.J., Groves M.D. High-grade gliomas. Curr. Treat. Options Neurol. 2011;13:386–399. doi: 10.1007/s11940-011-0130-0. [DOI] [PubMed] [Google Scholar]
- 28.Lu Lee E., Westcarth L. Neurotoxicity associated with cancer therapy. J. Adv. Pract. Oncol. 2012;3:11–21. doi: 10.6004/jadpro.2012.3.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keime-Guibert F., Napolitano M., Delattre J.Y. Neurological complications of radiotherapy and chemotherapy. J. Neurol. 1998;245:695–708. doi: 10.1007/s004150050271. [DOI] [PubMed] [Google Scholar]
- 30.Padma V.V. An overview of targeted cancer therapy. Biomedicine. 2015;5:19. doi: 10.7603/s40681-015-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dragu D.L., Necula L.G., Bleotu C., Diaconu C.C., Chivu-Economescu M. Therapies targeting cancer stem cells: Current trends and future challenges. World J. Stem Cells. 2015;7:1185–1201. doi: 10.4252/wjsc.v7.i9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrone F.B., Gehring M.P., Nicoletti N.F. Calcium Channels and Associated Receptors in Malignant Brain Tumor Therapy. Mol. Pharmacol. 2016;90:403–409. doi: 10.1124/mol.116.103770. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg S.S., Spitzer N.C. Calcium signaling in neuronal development. Cold Spring Harb. Perspect. Biol. 2011;3:a004259. doi: 10.1101/cshperspect.a004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zundorf G., Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011;14:1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgoyne R.D., Haynes L.P. Understanding the physiological roles of the neuronal calcium sensor proteins. Mol. Brain. 2012;5:2. doi: 10.1186/1756-6606-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marambaud P., Dreses-Werringloer U., Vingtdeux V. Calcium signaling in neurodegeneration. Mol. Neurodegener. 2009;4:20. doi: 10.1186/1750-1326-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikoletopoulou V., Tavernarakis N. Calcium homeostasis in aging neurons. Front Genet. 2012;3:200. doi: 10.3389/fgene.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brini M., Cali T., Ottolini D., Carafoli E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Alessandro G., Grimaldi A., Chece G., Porzia A., Esposito V., Santoro A., Salvati M., Mainiero F., Ragozzino D., Di Angelantonio S., et al. KCa3.1 channel inhibition sensitizes malignant gliomas to temozolomide treatment. Oncotarget. 2016;7:30781–30796. doi: 10.18632/oncotarget.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azimi I., Bong A.H., Poo G.X.H., Armitage K., Lok D., Roberts-Thomson S.J., Monteith G.R. Pharmacological inhibition of store-operated calcium entry in MDA-MB-468 basal A breast cancer cells: Consequences on calcium signalling, cell migration and proliferation. Cell. Mol. Life Sci. 2018;75:4525–4537. doi: 10.1007/s00018-018-2904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motiani R.K., Hyzinski-Garcia M.C., Zhang X., Henkel M.M., Abdullaev I.F., Kuo Y.H., Matrougui K., Mongin A.A., Trebak M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013;465:1249–1260. doi: 10.1007/s00424-013-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H., Hughes J.D., Rollins S., Chen B., Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp. Mol. Pathol. 2011;91:753–760. doi: 10.1016/j.yexmp.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Simms B.A., Zamponi G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron. 2014;82:24–45. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Li L., Li D.P., Chen S.R., Chen J., Hu H., Pan H.L. Potentiation of high voltage-activated calcium channels by 4-aminopyridine depends on subunit composition. Mol. Pharmacol. 2014;86:760–772. doi: 10.1124/mol.114.095505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoni G., Santoni M., Nabissi M. Functional role of T-type calcium channels in tumour growth and progression: Prospective in cancer therapy. Br. J. Pharmacol. 2012;166:1244–1246. doi: 10.1111/j.1476-5381.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abernethy D.R. Pharmacologic and pharmacokinetic profile of mibefradil, a T- and L-type calcium channel antagonist. Am. J. Cardiol. 1997;80:4C–11C. doi: 10.1016/S0002-9149(97)00564-X. [DOI] [PubMed] [Google Scholar]
- 47.Valerie N.C., Dziegielewska B., Hosing A.S., Augustin E., Gray L.S., Brautigan D.L., Larner J.M., Dziegielewski J. Inhibition of T-type calcium channels disrupts Akt signaling and promotes apoptosis in glioblastoma cells. Biochem. Pharmacol. 2013;85:888–897. doi: 10.1016/j.bcp.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Keir S.T., Friedman H.S., Reardon D.A., Bigner D.D., Gray L.A. Mibefradil, a novel therapy for glioblastoma multiforme: Cell cycle synchronization and interlaced therapy in a murine model. J. Neurooncol. 2013;111:97–102. doi: 10.1007/s11060-012-0995-0. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Cruickshanks N., Yuan F., Wang B., Pahuski M., Wulfkuhle J., Gallagher I., Koeppel A.F., Hatef S., Papanicolas C., et al. Targetable T-type Calcium Channels Drive Glioblastoma. Cancer Res. 2017;77:3479–3490. doi: 10.1158/0008-5472.CAN-16-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J., Luan Y., Yu R., Zhang Z., Zhang J., Wang W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci. Trends. 2014;8:1–10. doi: 10.5582/bst.8.1. [DOI] [PubMed] [Google Scholar]
- 51.Bomben V.C., Sontheimer H.W. Inhibition of transient receptor potential canonical channels impairs cytokinesis in human malignant gliomas. Cell Prolif. 2008;41:98–121. doi: 10.1111/j.1365-2184.2007.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bomben V.C., Sontheimer H. Disruption of transient receptor potential canonical channel 1 causes incomplete cytokinesis and slows the growth of human malignant gliomas. Glia. 2010;58:1145–1156. doi: 10.1002/glia.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bomben V.C., Turner K.L., Barclay T.T., Sontheimer H. Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas. J. Cell. Physiol. 2011;226:1879–1888. doi: 10.1002/jcp.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding X., He Z., Zhou K., Cheng J., Yao H., Lu D., Cai R., Jin Y., Dong B., Xu Y., et al. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J. Natl. Cancer Inst. 2010;102:1052–1068. doi: 10.1093/jnci/djq217. [DOI] [PubMed] [Google Scholar]
- 55.Azimi I. The interplay between HIF-1 and calcium signalling in cancer. Int. J. Biochem. Cell Biol. 2018;97:73–77. doi: 10.1016/j.biocel.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Azimi I., Milevskiy M.J.G., Kaemmerer E., Turner D., Yapa K.T.D.S., Brown M.A., Thompson E.W., Roberts-Thomson S.J., Monteith G.R. TRPC1 is a differential regulator of hypoxia-mediated events and Akt signalling in PTEN-deficient breast cancer cells. J. Cell Sci. 2017;130:2292–2305. doi: 10.1242/jcs.196659. [DOI] [PubMed] [Google Scholar]
- 57.Li S., Wang J., Wei Y., Liu Y., Ding X., Dong B., Xu Y., Wang Y. Crucial role of TRPC6 in maintaining the stability of HIF-1alpha in glioma cells under hypoxia. J. Cell Sci. 2015;128:3317–3329. doi: 10.1242/jcs.173161. [DOI] [PubMed] [Google Scholar]
- 58.Chigurupati S., Venkataraman R., Barrera D., Naganathan A., Madan M., Paul L., Pattisapu J.V., Kyriazis G.A., Sugaya K., Bushnev S., et al. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res. 2010;70:418–427. doi: 10.1158/0008-5472.CAN-09-2654. [DOI] [PubMed] [Google Scholar]
- 59.Ishii M., Oyama A., Hagiwara T., Miyazaki A., Mori Y., Kiuchi Y., Shimizu S. Facilitation of H2O2-induced A172 human glioblastoma cell death by insertion of oxidative stress-sensitive TRPM2 channels. Anticancer Res. 2007;27:3987–3992. [PubMed] [Google Scholar]
- 60.Liu M., Inoue K., Leng T., Guo S., Xiong Z.G. TRPM7 channels regulate glioma stem cell through STAT3 and Notch signaling pathways. Cell. Signal. 2014;26:2773–2781. doi: 10.1016/j.cellsig.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wondergem R., Ecay T.W., Mahieu F., Owsianik G., Nilius B. HGF/SF and menthol increase human glioblastoma cell calcium and migration. Biochem. Biophys. Res. Commun. 2008;372:210–215. doi: 10.1016/j.bbrc.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 62.Wondergem R., Bartley J.W. Menthol increases human glioblastoma intracellular Ca2+, BK channel activity and cell migration. J. Biomed. Sci. 2009;16:90. doi: 10.1186/1423-0127-16-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amantini C., Mosca M., Nabissi M., Lucciarini R., Caprodossi S., Arcella A., Giangaspero F., Santoni G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 64.Stock K., Kumar J., Synowitz M., Petrosino S., Imperatore R., Smith E.S., Wend P., Purfurst B., Nuber U.A., Gurok U., et al. Neural precursor cells induce cell death of high-grade astrocytomas through stimulation of TRPV1. Nat. Med. 2012;18:1232–1238. doi: 10.1038/nm.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nabissi M., Morelli M.B., Santoni M., Santoni G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2013;34:48–57. doi: 10.1093/carcin/bgs328. [DOI] [PubMed] [Google Scholar]
- 66.Nabissi M., Morelli M.B., Amantini C., Farfariello V., Ricci-Vitiani L., Caprodossi S., Arcella A., Santoni M., Giangaspero F., De Maria R., et al. TRPV2 channel negatively controls glioma cell proliferation and resistance to Fas-induced apoptosis in ERK-dependent manner. Carcinogenesis. 2010;31:794–803. doi: 10.1093/carcin/bgq019. [DOI] [PubMed] [Google Scholar]
- 67.Morelli M.B., Nabissi M., Amantini C., Farfariello V., Ricci-Vitiani L., di Martino S., Pallini R., Larocca L.M., Caprodossi S., Santoni M., et al. The transient receptor potential vanilloid-2 cation channel impairs glioblastoma stem-like cell proliferation and promotes differentiation. Int. J. Cancer. 2012;131:E1067–E1077. doi: 10.1002/ijc.27588. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida Y., Imai S. Structure and function of inositol 1,4,5-trisphosphate receptor. Jpn. J. Pharmacol. 1997;74:125–137. doi: 10.1254/jjp.74.125. [DOI] [PubMed] [Google Scholar]
- 69.Kang S.S., Han K.S., Ku B.M., Lee Y.K., Hong J., Shin H.Y., Almonte A.G., Woo D.H., Brat D.J., Hwang E.M., et al. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res. 2010;70:1173–1183. doi: 10.1158/0008-5472.CAN-09-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adinolfi E., Capece M., Amoroso F., De Marchi E., Franceschini A. Emerging roles of P2X receptors in cancer. Curr. Med. Chem. 2015;22:878–890. doi: 10.2174/0929867321666141012172913. [DOI] [PubMed] [Google Scholar]
- 71.Wei W., Ryu J.K., Choi H.B., McLarnon J.G. Expression and function of the P2X(7) receptor in rat C6 glioma cells. Cancer Lett. 2008;260:79–87. doi: 10.1016/j.canlet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 72.Fang J., Chen X., Zhang L., Chen J., Liang Y., Li X., Xiang J., Wang L., Guo G., Zhang B., et al. P2X7R suppression promotes glioma growth through epidermal growth factor receptor signal pathway. Int. J. Biochem. Cell Biol. 2013;45:1109–1120. doi: 10.1016/j.biocel.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Gehring M.P., Pereira T.C., Zanin R.F., Borges M.C., Braga Filho A., Battastini A.M., Bogo M.R., Lenz G., Campos M.M., Morrone F.B. P2X7 receptor activation leads to increased cell death in a radiosensitive human glioma cell line. Purinergic Signal. 2012;8:729–739. doi: 10.1007/s11302-012-9319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gehring M.P., Kipper F., Nicoletti N.F., Sperotto N.D., Zanin R., Tamajusuku A.S., Flores D.G., Meurer L., Roesler R., Filho A.B., et al. P2X7 receptor as predictor gene for glioma radiosensitivity and median survival. Int. J. Biochem. Cell Biol. 2015;68:92–100. doi: 10.1016/j.biocel.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Gendron F.P., Neary J.T., Theiss P.M., Sun G.Y., Gonzalez F.A., Weisman G.A. Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am. J. Physiol. Cell Physiol. 2003;284:C571–C581. doi: 10.1152/ajpcell.00286.2002. [DOI] [PubMed] [Google Scholar]
- 76.Wei W.C., Huang W.C., Lin Y.P., Becker E.B.E., Ansorge O., Flockerzi V., Conti D., Cenacchi G., Glitsch M.D. Functional expression of calcium-permeable canonical transient receptor potential 4-containing channels promotes migration of medulloblastoma cells. J. Physiol. 2017;595:5525–5544. doi: 10.1113/JP274659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panner A., Cribbs L.L., Zainelli G.M., Origitano T.C., Singh S., Wurster R.D. Variation of T-type calcium channel protein expression affects cell division of cultured tumor cells. Cell Calcium. 2005;37:105–119. doi: 10.1016/j.ceca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Chemin J., Nargeot J., Lory P. Ca(v)3.2 calcium channels control an autocrine mechanism that promotes neuroblastoma cell differentiation. Neuroreport. 2004;15:671–675. doi: 10.1097/00001756-200403220-00019. [DOI] [PubMed] [Google Scholar]
- 79.Chen S.J., Zhang W., Tong Q., Conrad K., Hirschler-Laszkiewicz I., Bayerl M., Kim J.K., Cheung J.Y., Miller B.A. Role of TRPM2 in cell proliferation and susceptibility to oxidative stress. Am. J. Physiol. Cell Physiol. 2013;304:C548–C560. doi: 10.1152/ajpcell.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen S.J., Hoffman N.E., Shanmughapriya S., Bao L., Keefer K., Conrad K., Merali S., Takahashi Y., Abraham T., Hirschler-Laszkiewicz I., et al. A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2alpha. J. Biol. Chem. 2014;289:36284–36302. doi: 10.1074/jbc.M114.620922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomez-Villafuertes R., Garcia-Huerta P., Diaz-Hernandez J.I., Miras-Portugal M.T. PI3K/Akt signaling pathway triggers P2X7 receptor expression as a pro-survival factor of neuroblastoma cells under limiting growth conditions. Sci. Rep. 2015;5:18417. doi: 10.1038/srep18417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bell N., Hann V., Redfern C.P., Cheek T.R. Store-operated Ca(2+) entry in proliferating and retinoic acid-differentiated N- and S-type neuroblastoma cells. Biochim. Biophys. Acta. 2013;1833:643–651. doi: 10.1016/j.bbamcr.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Usachev Y.M., Toutenhoofd S.L., Goellner G.M., Strehler E.E., Thayer S.A. Differentiation induces up-regulation of plasma membrane Ca(2+)-ATPase and concomitant increase in Ca(2+) efflux in human neuroblastoma cell line IMR-32. J. Neurochem. 2001;76:1756–1765. doi: 10.1046/j.1471-4159.2001.00169.x. [DOI] [PubMed] [Google Scholar]
- 84.Florea A.M., Varghese E., McCallum J.E., Mahgoub S., Helmy I., Varghese S., Gopinath N., Sass S., Theis F.J., Reifenberger G., et al. Calcium-regulatory proteins as modulators of chemotherapy in human neuroblastoma. Oncotarget. 2017;8:22876–22893. doi: 10.18632/oncotarget.15283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ragel B.T., Gillespie D.L., Kushnir V., Polevaya N., Kelly D., Jensen R.L. Calcium channel antagonists augment hydroxyurea- and ru486-induced inhibition of meningioma growth in vivo and in vitro. Neurosurgery. 2006;59:1109–1121. doi: 10.1227/01.NEU.0000245597.46581.FB. [DOI] [PubMed] [Google Scholar]
- 86.Ragel B.T., Couldwell W.T., Wurster R.D., Jensen R.L. Chronic suppressive therapy with calcium channel antagonists for refractory meningiomas. Neurosurg. Focus. 2007;23:E10. doi: 10.3171/FOC-07/10/E10. [DOI] [PubMed] [Google Scholar]
- 87.Mahalingam D., Wilding G., Denmeade S., Sarantopoulas J., Cosgrove D., Cetnar J., Azad N., Bruce J., Kurman M., Allgood V.E., et al. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: Results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br. J. Cancer. 2016;114:986–994. doi: 10.1038/bjc.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holdhoff M., Ye X., Supko J.G., Nabors L.B., Desai A.S., Walbert T., Lesser G.J., Read W.L., Lieberman F.S., Lodge M.A., et al. Timed sequential therapy of the selective T-type calcium channel blocker mibefradil and temozolomide in patients with recurrent high-grade gliomas. Neuro. Oncol. 2017;19:845–852. doi: 10.1093/neuonc/nox020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lester-Coll N.H., Kluytenaar J., Pavlik K.F., Yu J.B., Contessa J.N., Moliterno J., Piepmeier J., Becker K.P., Baehring J., Huttner A.J., et al. Mibefradil dihydrochloride with hypofractionated radiation for recurrent glioblastoma: Preliminary results of a phase I dose expansion trial. Int. J. Radiat. Oncol. Bio. Phys. 2016;96:S93. doi: 10.1016/j.ijrobp.2016.06.233. [DOI] [Google Scholar]
- 90.Omuro A., Beal K., McNeill K., Young R.J., Thomas A., Lin X., Terziev R., Kaley T.J., DeAngelis L.M., Daras M., et al. Multicenter Phase IB Trial of Carboxyamidotriazole Orotate and Temozolomide for Recurrent and Newly Diagnosed Glioblastoma and Other Anaplastic Gliomas. J. Clin. Oncol. 2018;36:1702–1709. doi: 10.1200/JCO.2017.76.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Das M. Carboxyamidotriazole orotate in glioblastoma. Lancet Oncol. 2018 doi: 10.1016/S1470-2045(18)30347-4. [DOI] [PubMed] [Google Scholar]
- 92.Karsy M., Hoang N., Barth T., Burt L., Dunson W., Gillespie D.L., Jensen R.L. Combined Hydroxyurea and Verapamil in the Clinical Treatment of Refractory Meningioma: Human and Orthotopic Xenograft Studies. World Neurosurg. 2016;86:210–219. doi: 10.1016/j.wneu.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 93.Niklasson M., Maddalo G., Sramkova Z., Mutlu E., Wee S., Sekyrova P., Schmidt L., Fritz N., Dehnisch I., Kyriatzis G., et al. Membrane-Depolarizing Channel Blockers Induce Selective Glioma Cell Death by Impairing Nutrient Transport and Unfolded Protein/Amino Acid Responses. Cancer Res. 2017;77:1741–1752. doi: 10.1158/0008-5472.CAN-16-2274. [DOI] [PubMed] [Google Scholar]
- 94.Kang S., Hong J., Lee J.M., Moon H.E., Jeon B., Choi J., Yoon N.A., Paek S.H., Roh E.J., Lee C.J., et al. Trifluoperazine, a Well-Known Antipsychotic, Inhibits Glioblastoma Invasion by Binding to Calmodulin and Disinhibiting Calcium Release Channel IP3R. Mol. Cancer Ther. 2017;16:217–227. doi: 10.1158/1535-7163.MCT-16-0169-T. [DOI] [PubMed] [Google Scholar]
- 95.Seifert S., Sontheimer H. Bradykinin enhances invasion of malignant glioma into the brain parenchyma by inducing cells to undergo amoeboid migration. J. Physiol. 2014;592:5109–5127. doi: 10.1113/jphysiol.2014.274498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters A.A., Jamaludin S.Y.N., Yapa K., Chalmers S., Wiegmans A.P., Lim H.F., Milevskiy M.J.G., Azimi I., Davis F.M., Northwood K.S., et al. Oncosis and apoptosis induction by activation of an overexpressed ion channel in breast cancer cells. Oncogene. 2017;36:6490–6500. doi: 10.1038/onc.2017.234. [DOI] [PubMed] [Google Scholar]
- 97.Santos R., Ursu O., Gaulton A., Bento A.P., Donadi R.S., Bologa C.G., Karlsson A., Al-Lazikani B., Hersey A., Oprea T.I., et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017;16:19–34. doi: 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]