Abstract
Protein kinase C (PKC), a multi-gene family, plays critical roles in signal transduction and cell regulation. Protein kinase C-eta (PKCη) is a unique member of the PKC family since its regulation is distinct from other PKC isozymes. PKCη was shown to regulate cell proliferation, differentiation and cell death. It was also shown to contribute to chemoresistance in several cancers. PKCη has been associated with several cancers, including renal cell carcinoma, glioblastoma, breast cancer, non-small cell lung cancer, and acute myeloid leukemia. However, mice lacking PKCη were more susceptible to tumor formation in a two-stage carcinogenesis model, and it is downregulated in hepatocellular carcinoma. Thus, the role of PKCη in cancer remains controversial. The purpose of this review article is to discuss how PKCη regulates various cellular processes that may contribute to its contrasting roles in cancer.
Keywords: protein kinase C, PKCη, cell proliferation, differentiation, senescence, apoptosis, drug resistance, tumor promotion, tumor suppression
1. Introduction
Intricate regulation of cellular signaling systems is critical for the proper functioning of cells. Consequently, a deregulation in signal transduction pathways can lead to many human diseases. Protein kinase C (PKC), a family of serine/threonine protein kinases, plays critical roles in signal transduction and cell regulation [1]. The identification of PKC as a receptor for tumor-promoting phorbol esters, which are potent activators of PKC and can substitute for the physiologic stimulator diacylglycerol (DAG) established a link between PKC and cancer [2].
PKC constitutes a multi-gene family that could be categorized into three groups based on their structural variations and biochemical properties [3]. The classical or conventional (c) PKCs (α, βI, βII, γ) require Ca2+ and DAG/phorbol esters for their activities. The novel (n) (δ, ε, η, θ) PKCs are insensitive to Ca2+ but respond to DAG/phorbol esters. The atypical (a) PKCs (ξ, ι) are insensitive to both Ca2+ and DAG/phorbol esters. While the physiological stimulator DAG causes transient activation of conventional and novel PKCs, the tumor-promoting phorbol esters cause persistent activation [3]. Activation of PKCs induces their translocation to the membrane followed by their degradation or downregulation [4].
The regulation of PKCη, a member of the novel PKC family, is unique [3]. Although PKCη is most closely related to PKCε [5,6], there are variations in the lipid-binding site [7]. It is the only PKC that is activated by cholesterol sulfate and sulfatide [8]. It is also resistant to translocation and downregulation when stimulated with phorbol esters or cholesterol sulfate [9,10]. In fact, we and others have shown that PKCη is upregulated by the tumor-promoting phorbol esters [11,12,13,14] as well as several structurally and functionally distinct PKC activators [13].
The expression of PKCη is also unique compared to other PKC isozymes [15,16]. It was isolated from cDNA libraries of mouse epidermis [5] and human keratinocytes [15]. PKCη mRNA was most abundant in lung tissues and was also detected in skin and heart tissues [15]. Contrary to other PKC isozymes, the expression of PKCη in the brain was low [15,16]. It was shown to be predominantly expressed in the epidermis of mouse skin and epithelia of the digestive and respiratory tracts, including the tongue, esophagus, forestomach, glandular stomach, intestine, colon, trachea and, bronchus [16].
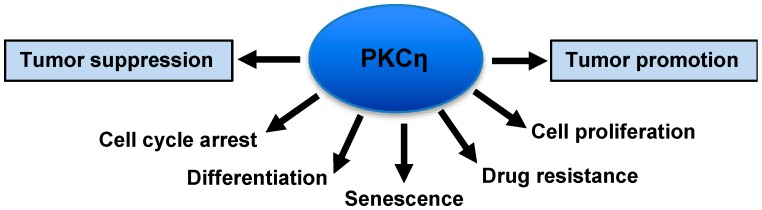
PKCη plays critical roles in cell proliferation, differentiation and cell death, as seen in Figure 1 [3,8,17]. There are, however, controversies regarding its role in tumor promotion versus tumor suppression. The present review article summarizes how PKCη regulates various cellular processes that may impact on its contrasting roles in cancer.
Figure 1.
Various functions of protein kinase C-eta (PKCη).
2. Regulation of Cell Proliferation
The most consistent function of PKCη is its ability to regulate cell cycle progression. The cell cycle is regulated primarily by the tumor suppressor protein Rb and cyclin-dependent kinases (CDK). While phosphorylation and inactivation of Rb by CDKs is needed to allow cell cycle progression, the inhibition of Rb phosphorylation by CDK inhibitors, such as p16, p21 and p27 causes cell cycle arrest [18].
Interestingly, both activation and inhibition of PKCη have been reported to cause cell cycle arrest (Table 1). Livneh et al. first reported that ectopic expression of PKCη in NIH3T3 cells inhibits Rb phosphorylation and induces CDK inhibitors p21 and p27, causing cell cycle arrest [19]. Overexpression of PKCη also inhibited cell growth in keratinocytes but in this study, PKCη overexpression had no effect on cell growth in either human or mouse fibroblasts [20]. On the other hand, Nomoto et al. showed that overexpression of PKCη in NIH3T3 cells induced anchorage-independent growth [21]. The reason for the distinct effects of PKCη overexpression on cell growth in NIH3T3 fibroblasts is not clear except different methods were used to monitor fibroblast cell growth. For example, while Livneh et al. [19] examined how PKCη affects cell cycle progression by analyzing different phases of the cell cycle by flow cytometric analysis, Ohba et al. [20] monitored cell growth by MTT assay, and Nomoto et al. [21] compared the colony forming ability of NIH3T3 cells transfected with either PKCη or a control vector in soft agar.
Table 1.
Function of PKCη in different cell/tumor types.
| Tissue/Tumor Type | Cell Line | Expression | Phenotype | Potential Mechanism | Reference |
|---|---|---|---|---|---|
| Fibroblasts | NIH3T3 | WT-PKCη | Cell cycle arrest, adipogenesis | ↑ p21, 27 & cyclin E; ↓ Rb phosphorylation | [19] |
| NIH3T3 | WT-PKCη | Anchorage-independent growth | [21] | ||
| NIH3T3 & NHF | WT-PKCη | No effect on cell growth | [20] | ||
| Keratinocytes | NHK | WT-PKCη | Growth arrest, terminal differentiation | ↑ p21 Phosphorylation; ↓ Cdk2, ↑ TGase 1 | [20,22] |
| NHK | WT-PKCη | Differentiation | ↑ Loricrin | [30] | |
| NHEK | WT-PKCη | Differentiation | Bidning and activation of RalA | [31] | |
| Mouse keratinocytes | WT-PKCη | Growth arrest, differentiation | ↓ EGFR, ↑Fyn activity | [32] | |
| PKCη-null | Delayed growth arrest & terminal differentiation | ↑ JNK/cJun, ↓ p27 | [26] | ||
| NHK | DN-PKCη | Inrease in UVB-induced apoptosis | ↓ UV-induced p38 MAPK activity | [33] | |
| Adenoid cystic carcinoma | ACC-2 & ACC-M | WT-PKCη | Suppressed cisplatin-induced apoptosis | ↓ p53/p21 | [34] |
| Breast cancer | MCF-7 | WT-PKCη | Increase in cell growth | ↑ cyclin D, -E and p21 | [24] |
| MCF-7, T47D | PKCη siRNA | Decreased clonogenic survival, induced senescence | ↑ p27 | [25] | |
| MCF-7 | PKCη shRNA | Decreased H2O2 and etoposide-induced senescence | ↓ p21, p27 & IL-6; ↑ IL-8 | [35] | |
| MCF-7 | WT-PKCη | Protects against TNF-induced apoptosis | [36] | ||
| MCF-7 | WT-PKCη | Protects against UVC- and CPT-induced apoptosis | ↓ JNK activity | [37] | |
| MCF-7 | WT-PKCη | Protects against CPT-induced apoptosis | ↑ NF-κB activity | [38] | |
| Glioblastoma | U-251 GBM | PKCη-KR | Decreased cell proliferation | ↓ Akt, mTOR activity | [28] |
| U-1242 MG | WT-PKCη | Icreased cell proliferation | ↑ ERK/Elk-1 activity | [39] | |
| U-1242 MG | WT-PKCη | Decrease in UV- and Υ irradiation-induced apoptosis | [40] | ||
| U-251 MG | PKCη-antisense | Sensitized to UV- and Υ irradiation-induced apoptosis | [40] | ||
| Leukemia | CML-derived KT1 | Peptide inhibitor | Suppression of IFN-dependent cell cycle arrest | [27] | |
| CD34+ progenitor | PKCη siRNA | Increase in clonogenic survival | [27] | ||
| CD34+ progenitor | CA-PKCη | Inhibition of cell growth, no effect on apoptosis | [27] | ||
| CML K562 | WT-PKCη | IM resistance | ↑ Raf/MEK/ERK signaling and CRAF | [41] | |
| CML stem cells | PKCη shRNA | Sensitized to IM | [41] | ||
| Lymphoma | IM-9, EBV+ B cells | PKCη siRNA | Cell cycle arrest | ↑ TAp73, p21, p38 MAPK; ↓ Cdks | [29] |
| IM9 | PKCη siRNA | Sensitized to BTZ and SRF | [29] | ||
| L428 | PKCη shRNA | Sensitized to doxorubicin- and CPT-induced apoptosis | [42] | ||
| Lung cancer | A549 | PKCη-antisense | Increased vincristine and paclitaxel-induced apoptosis | [43] | |
| Prostate cancer | PC3 | PKCη-antisense | Increased TRAIL-induced apoptosis | [44] | |
| Mesenchymal stem cells | hMSC | PKCη-C2 | chondrogenic differentiation | Increase in collagen type II | [45] |
Kashiwagi et al. provided a mechanistic explanation that association of PKCη with cyclin E/Cdk2/p21 complex causes phosphorylation of p21 at Ser146 site and dephosphorylation of Thr160 of Cdk2 resulting in inhibition of Cdk2 activity and G1 arrest in keratinocytes [8,22]. Subsequently, Livneh and co-workers confirmed that PKCη also forms complexes with cyclin E/Cdk2 in NIH3T3 and MCF-7 cells, and this complex formation was most prominent in serum-starved cells and could be visualized in the perinuclear region [23]. However, PKCη overexpression had opposite effects on cell growth in NIH3T3 versus MCF-7 cells. In contrast to NIH3T3 cells where PKCη overexpression was shown to inhibit cell growth [19], induced expression of PKCη in MCF-7 cells promoted cell growth [24]. Overexpression of PKCη caused an increase in both p21 and p27 in NIH3T3 cells [19] but p27 was not altered in MCF-7 cells [24]. Thus, an increase in p27 may be required for cell growth inhibition in MCF-7 cells. Consistent with this notion, we recently observed that knockdown of PKCη decreased clonogenic survival of breast cancer MCF-7 and T47D cells and this was associated with an increase in p27 [25]. Hara et al. showed that p27 mRNA was downregulated in PKCη-null keratinocytes grown in 3D organotypic culture [26]. While an increase in p27 was associated with cell growth inhibition in both keratinocytes [26] and breast cancer cells [25], depletion of PKCη caused a decrease in p27 in keratinocytes [26] but an increase in p27 in breast cancer cells [25]. It is not clear why PKCη had opposite effects on p27 in keratinocytes versus breast cancer cells, however, it reinforces the notion that PKCη functions in a context-dependent manner.
An increase in PKCη was shown to be responsible for the anti-leukemic effects of IFNα in chronic myeloid leukemia cells [27]. PKCη overexpression caused cell cycle arrest in normal and leukemic human myeloid cells but had no effect on erythroid progenitor cells [27]. PKCη was shown to promote cell proliferation in glioblastoma cells by acting upstream of Akt and mTOR signaling pathways [28]. Knockdown of PKCη inhibited cell cycle progression in B lymphoma cells, suggesting a growth-promoting effect of PKCη [29]. Thus, the function of PKCη on cell proliferation varies significantly with cell types (Table 1).
3. Regulation of Differentiation
Most of the earlier studies associated growth inhibitory effects of PKCη with its ability to trigger differentiation partly because PKCη was most abundant in epithelial tissues [46], was expressed during epidermal differentiation [47] and was shown to be localized in differentiating or differentiated epithelial cells [16]. Moreover, cholesterol sulfate, a metabolite of cholesterol generated during squamous differentiation caused marked stimulation of PKCη activity [48]. PKCη levels were increased both at the soluble and particulate fractions of primary mouse keratinocytes during calcium-induced differentiation [49].
Several studies investigated potential mechanisms by which PKCη triggers keratinocyte differentiation. Ohba et al. [20] demonstrated that overexpression of PKCη in human and mouse keratinocytes but not in fibroblasts enhanced the expression and activity of transglutaminase 1, a key enzyme involved in squamous cell differentiation. PKCη was shown to associate with and activate the Src kinase family member Fyn, which is required for normal keratinocyte differentiation [32]. Overexpression of Fyn enhanced the expression of CDK inhibitors p21 and p27, induced the differentiation marker transglutaminase, and suppressed the growth of keratinocytes but had no effect in dermal fibroblasts [32]. These results provided an explanation why PKCη induced differentiation in keratinocytes but not in fibroblasts. PKCη also increased JunD-mediated transcription of loricrin, which is expressed at the late stage of keratinocyte differentiation [30]. The small G protein RalA was shown to interact with PKCη resulting in the activation of RalA and the induction of keratinocyte differentiation [31]. Hara et al. [26] demonstrated that growth arrest and terminal differentiation were delayed in PKCη-null keratinocytes and this was associated with downregulation of p27 mRNA via c-Jun N-terminal kinase (JNK) signaling. Re-expression of PKCη or suppression of JNK/c-Jun signaling caused upregulation of p27 mRNA resulting in cell cycle arrest and terminal differentiation [26]. Overexpression of the C2 domain of PKCη increased the expression of collagen type II, and led to chondrogenic differentiation in mesenchymal stem cells [45]. Thus, an association between inhibition of cell proliferation and differentiation was established.
4. Regulation of Apoptosis
Apoptosis is a physiologic form of cell death required to maintain tissue homeostasis. Lack of cell death by apoptosis can lead to cancer. In addition, since many cancer chemotherapeutic drugs kill cancer cells by inducing apoptosis, a defect in apoptotic signaling pathways can contribute to chemoresistance. There are two major pathways of cell death: the extrinsic or receptor-initiated pathway and the intrinsic or mitochondrial pathway. The extrinsic pathway is triggered by binding of ligands to the tumor necrosis factor-α (TNF) receptor superfamily, whereas cytotoxic chemotherapeutic drugs primarily utilize the intrinsic pathway. While activation of caspases induces apoptosis, pro- and anti-apoptotic Bcl-2 family members regulate apoptosis.
We observed that several different PKC activators protected against TNF-induced apoptosis whereas the PKC-specific inhibitor bisindolylmaleimide II enhanced apoptosis in breast cancer MCF-7 cells [11]. Since PKCη is the only PKC isozyme upregulated by PKC activators and downregulated by the PKC inhibitor bisindolylmaleimide II, this study implicated PKCη in protecting against TNF-induced apoptosis [4]. Beck et al. showed that there was a correlation between the upregulation of multiple drug resistance-associated genes and PKCη expression in specimens derived from sixty-four primary breast cancer patients, implicating PKCη in anticancer drug resistance [50]. While these results are correlative, we showed that ectopic expression of PKCη in MCF-7 cells protected against TNF-induced apoptosis [36]. Subsequently, it was shown that PKCη protects against apoptosis induced by UV and gamma irradiation in glioblastoma cell lines [40]. Downregulation of PKCη by anti-sense oligonucleotides (ODN) enhanced vincristine- and paclitaxel-induced apoptosis in A549 lung cancer cells [43] and TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in prostate cancer PC3 cells [44]. PKCη levels were higher in Hodgkin’s lymphoma (HL)-derived L428 cells that are resistant to doxorubicin and camptothecin (CPT) compared to drug-sensitive KMH2 cells, and knockdown of PKCη by siRNA in L428 cells sensitized them to these drugs [42]. In addition, PKCη suppressed and shR-PKCη promoted cisplatin-induced apoptosis in adenoid cystic carcinoma (ACC) cells [34]. These results suggest that PKCη may confer resistance to anticancer therapy.
Several studies explored the mechanisms by which PKCη regulates apoptosis. Overexpression of wild-type PKCη inhibited and dominant-negative PKCη enhanced UVB-induced apoptosis in normal human keratinocytes (NHK) [33]. UV-induced activation of p38 MAP kinase suppressed caspase-3 activity in NHK, and this was blocked by dominant-negative PKCη, suggesting that PKCη negatively regulates UV-induced apoptosis in NHK cells via the p38 MAP kinase pathway [33]. In MCF-7 breast cancer cells, PKCη was shown to protect against DNA damaging agents, such as UVC irradiation and anticancer drug CPT by suppressing JNK activity [37]. PKCη also protected against CPT via activation of NF-κB, leading to the induction of anti-apoptotic Bcl-2 in MCF-7 cells [38].
Akt, mTOR and mitogen-activated protein kinase (MAPK) pathways are known to promote cell survival. There are, however, controversies regarding how PKCη regulates the Akt signaling pathway. While Aeder et al. reported that PKCη activates both Akt and mTOR pathways in glioblastoma cells [28], Shahaf et al. reported that PKCη negatively regulates Akt in MCF-7 cells [51]. In the latter study, Akt activation was monitored in response to IGF-1 or insulin stimulation [51].
Development of resistance to the BCR-ABL inhibitor imatinib mesylate (IM) is a significant problem in the treatment of chronic myelogenous leukemia (CML). Upregulation of PRKCH, the gene encoding PKCη, was identified as one mechanism contributing to IM resistance independent of any mutation in BCR-ABL [41]. PRKCH was elevated in IM-resistant CML patient samples and CML stem cells [41]. The mechanism by which PKCη contributed to IM resistance involved activation of the RAF/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signaling via phosphorylation/activation of CRAF [41]. We have recently shown that knockdown of PKCη in breast cancer cells led to the downregulation of the anti-apoptotic Bcl-2 family protein Mcl-1 via the ubiquitin proteasome-mediated pathway [52]. Knockdown of PKCη inhibited ERK1/2 phosphorylation but knockdown of ERK1, but not ERK2, decreased Mcl-1 levels in MCF-7 cells. Moreover, overexpression of ERK1 rescued the effect of PKCη knockdown on Mcl-1 downregulation [52]. These results suggest that PKCη functions upstream of ERK1 in MCF-7 breast cancer cells.
5. Regulation of Senescence
Cellular senescence is defined as a permanent arrest of proliferative cells that are metabolically active [53]. The consequences of senescence could be beneficial or detrimental depending on the cellular context, the nature of the stimulus and the state of senescence [54,55,56]. Senescence can cause tumor suppression by inducing permanent cell cycle arrest and by recruiting immune systems to clear senescent cells [57,58]. However, senescent cells can also contribute to tumor progression and relapse. Senescence-associated secretory phenotype (SASP), which is associated with the secretion of growth factors, pro-inflammatory cytokines, chemokines, and matrix remodeling enzymes, could facilitate tumor growth under certain cellular contexts [57,59].
Zurgil et al. reported that PKCη promotes senescence in MCF-7 breast cancer cells in response to oxidative stress and etoposide-induced DNA damage [35]. In contrast, we found that knockdown of PKCη induced senescence in breast cancer MCF-7 and T47D cells [25]. The apparent discordant results could be partly explained by the differences in experimental design. In the study by Zurgil et al., high concentrations of H2O2 (150 µM) or etoposide (400 µM) caused a substantial increase in senescence, which was attenuated by PKCη knockdown [35]. In fact, knockdown of PKCη by itself caused a modest but significant increase in cellular senescence [35], and this was consistent with our results [25]. shRNA-mediated knockdown of PKCη had little effect on p27 and p21 but attenuated the increase in p21 and p27 by etoposide [35]. In addition, PKCη knockdown increased IL-6 secretion but suppressed IL-8 secretion [35]. It is not clear why PKCη had opposite effects on these pro-inflammatory cytokines both of which are associated with SASP. We found that silencing of PKCη by siRNA caused a substantial increase in p27 in both MCF-7 and T47D cells [25]. Moreover, silencing of p27 attenuated senescence induced by PKCη knockdown [25], suggesting upregulation of p27 as one mechanism contributing to the induction of senescence caused by PKCη deficiency.
6. Tumor Suppression by PKCη
Canzian et al. first reported that PKCη is decreased by 5- to 10-fold in murine lung tumors compared to normal murine lung [60], suggesting that a decrease in PKCη may be associated with lung carcinogenesis. A clue to the tumor suppressive role of PKCη came from the observation that cholesterol sulfate, which acts as a second messenger of PKCη and induced squamous differentiation, inhibited skin carcinogenesis when applied prior to tumor-promoting phorbol ester TPA. This suggests that PKCη inhibits the promotional phase of skin carcinogenesis [61]. Further evidence regarding the tumor suppressive role of PKCη came from the observation that PKCη-knockout mice were more sensitive to tumor formation in a two-stage carcinogenesis model compared to wild-type mice [62]. The ability of PKCη to inhibit tumor promotion was associated with its ability to induce differentiation in keratinocytes [8].
The possible tumor suppressive role of PKCη was also investigated by analyzing human tissue samples. PKCη mRNA was significantly lower in colon tumors compared to normal mucosa samples [63]. PKCη expression was decreased in locally invasive breast tumor tissues compared to the surrounding normal epithelium, suggesting that PKCη is decreased during later stages of transformation [64]. Overexpression of PKCη was shown to exert anti-leukemic responses in chronic myeloid leukemia cells by inhibiting cell cycle progression of myeloid progenitor growth via type 1 interferon receptor [27]. PKCη expression was decreased in 82% of hepatocellular carcinoma (HCC) tissues compared to adjacent normal tissues and was associated with poorer long-term survival of HCC patients [65].
7. Tumor Promotion by PKCη
Overexpression of PKCη in NIH3T3 cells was shown to induce anchorage-independent growth [21], suggesting that PKCη may also contribute to tumor progression. A correlation between PKCη expression and tumor progression was noted in renal cell carcinoma (RCC) [66]. PKCη was shown to promote proliferation of malignant astrocytoma and glioblastoma but not non-malignant astrocytes [28,39]. PKCη levels were high in EBV-transformed B cells and EBV-positive B cells, such as Raji, Daudi and IM-9 cells but not in normal B cells [29]. We have shown that PKCη levels were increased with the aggressiveness of breast cancer in the progressive MCF-10A series, and knockdown of PKCη attenuated breast cancer cell growth [25,67]. Increased PKCη expression was associated with poor prognosis in non-small cell lung cancer patients [68]. It has been reported that the microRNA (miRNA) miR-24-3p functions as a tumor suppressor in human lacrimal adenoid cystic carcinoma (LACC) via the p53/p21 pathway by downregulating PKCη, and overexpression of PKCη rescued the tumor suppressive function of miR-24-3p by downregulating p53 [34]. PKCη levels were higher in LACC tissues compared to adjacent non-tumor tissues and overexpression of miR-24-3p decreased PKCη mRNA and protein levels [34]. PKCη contributed to the malignant phenotype of ACC cells by enhancing cell proliferation, migration and invasion and by inhibiting apoptosis [34]. The PRKCH gene was highly expressed in hematopoietic stem cells (HSC) and leukemia stem cells (LSC) [69]. PKCη expression was also associated with poor prognosis in acute myeloid leukemia (AML) patients, although it was not required for the development of AML [69].
8. Conclusions
The function of PKCη varies significantly with cell types (Table 1). Several laboratories showed that PKCη induced terminal differentiation in keratinocytes [8,20,26,31,32] and PKCη-null mice were more susceptible to tumor formation [61,62]. In contrast, there were controversies regarding the role of PKCη in NIH3T3 fibroblasts [19,20,21]. The transcriptional regulation of PKCη [70] as well as the regulation of downstream signaling of PKCη [32] appear to be distinct in keratinocytes versus fibroblasts.
Caution should be exercised in interpreting the function of PKCη in various systems. Some of the studies that implicated PKCη in tumor promotion versus tumor suppression are correlative, and the number of tumor samples analyzed to definitively assess the function of PKCη may not be adequate. In studies involving genetic manipulation of PKCη, it is important to consider the methods used to manipulate PKCη, the selection pressure and the extent of PKCη knockdown/overexpression, all of which may affect the outcome of the results. The specificity of the antibodies used to detect total and phosphorylated PKCη should also be carefully determined.
It is also important to recognize the complexity of the cellular signaling systems. For example, a decrease in cell proliferation may also promote epithelial-to-mesenchymal transition and is associated with increased malignancy [71]. This may explain why overexpression of PKCη inhibited cell growth [19] but enhanced anchorage-independent growth [21] in NIH3T3 cells. Similarly, a decrease in cell growth is often associated with an increase in cell death by apoptosis. However, inhibition of cell proliferation may also restrict the ability of conventional chemotherapeutic drugs that target actively proliferating cells to kill cancer cells. This is consistent with our observation that the induction of senescence caused by PKCη knockdown was associated with a decrease in doxorubicin-induced apoptosis [25]. In addition, while overexpression of PKCη inhibited cell growth in NHK [8,20,26,31,32], PKCη overexpression inhibited UVB-induced apoptosis in these cells [33].
PKCη interacts with several signaling pathways and the status of these pathways will influence the function of PKCη. The ability of PKCη to regulate cellular senescence may also contribute to its contrasting roles in cancer since depending on the cellular context, cellular senescence may promote or suppress cancer. Thus, a thorough understanding of how PKCη regulates various cellular processes is essential prior to exploiting this enigmatic PKC family member for cancer therapy.
Acknowledgments
The author wishes to thank Deepanwita Pal for critical reading of the manuscript.
Abbreviations
| Abbrev. | Definition |
| ACC | adenoid cystic carcinoma |
| AML | acute myelocytic leukemia |
| Bcl-2 | B-cell lymphoma 2 |
| Btz | bortezomib |
| CDK | cyclin-dependent kinase |
| CML | chronic myelogenous leukemia |
| CPT | camptothecin |
| DAG | diacylglycerol |
| DN | dominant-negative |
| EGFR | epidermal growth factor receptor |
| ERK | extracellular signal-regulated kinase |
| GBM | glioblastoma multiforme |
| HSC | hematopoietic stem cells |
| IFN | Interferon-α |
| IM | imatinib mesylate |
| JNK | c-Jun N-terminal kinase |
| LSC | leukemia stem cells |
| MAPK | mitogen-activated protein kinase |
| Mcl-1 | myeloid cell leukemia 1 |
| mTOR | mechanistic target of rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | nuclear factor-κB |
| NHEK | normal human epidermal keratinocytes |
| NHF | normal human fibroblasts |
| NHK | normal human keratinocytes |
| ODN | oligonucleotide |
| PKC | protein kinase C |
| SASP | senescence-associated secretory phenotype |
| SRF | Sorafenib |
| TNF | tumor necrosis factor-α |
| TGase 1 | transglutaminase 1 |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| TRAIL | TNF-related apoptosis-inducing ligand |
| WT | wild-type |
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg P.M. Protein kinase C as the receptor for the phorbol ester tumor promoters: Sixth Rhoads memorial award lecture. Cancer Res. 1988;48:1–8. [PubMed] [Google Scholar]
- 3.Pal D., Basu A. The unique protein kinase Ceta: Implications for breast cancer (review) Int. J. Oncol. 2014;45:493–498. doi: 10.3892/ijo.2014.2443. [DOI] [PubMed] [Google Scholar]
- 4.Basu A. The potential of protein kinase C as a target for anticancer treatment. Pharmacol. Ther. 1993;59:257–280. doi: 10.1016/0163-7258(93)90070-T. [DOI] [PubMed] [Google Scholar]
- 5.Osada S., Mizuno K., Saido T.C., Akita Y., Suzuki K., Kuroki T., Ohno S. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J. Biol. Chem. 1990;265:22434–22440. [PubMed] [Google Scholar]
- 6.Kazanietz M.G., Areces L.B., Bahador A., Mischak H., Goodnight J., Mushinski J.F., Blumberg P.M. Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol. Pharmacol. 1993;44:298–307. [PubMed] [Google Scholar]
- 7.Littler D.R., Walker J.R., She Y.M., Finerty P.J., Jr., Newman E.M., Dhe-Paganon S. Structure of human protein kinase C eta (PKCeta) C2 domain and identification of phosphorylation sites. Biochem. Biophys. Res. Commun. 2006;349:1182–1189. doi: 10.1016/j.bbrc.2006.08.160. [DOI] [PubMed] [Google Scholar]
- 8.Kashiwagi M., Ohba M., Chida K., Kuroki T. Protein kinase C eta (PKC eta): Its involvement in keratinocyte differentiation. J. Biochem. 2002;132:853–857. doi: 10.1093/oxfordjournals.jbchem.a003297. [DOI] [PubMed] [Google Scholar]
- 9.Murakami A., Chida K., Suzuki Y., Kikuchi H., Imajoh-Ohmi S., Kuroki T. Absence of down-regulation and translocation of the eta isoform of protein kinase C in normal human keratinocytes. J. Investig. Dermatol. 1996;106:790–794. doi: 10.1111/1523-1747.ep12346391. [DOI] [PubMed] [Google Scholar]
- 10.Chida K., Sagara H., Suzuki Y., Murakami A., Osada S., Ohno S., Hirosawa K., Kuroki T. The eta isoform of protein kinase C is localized on rough endoplasmic reticulum. Mol. Cell. Biol. 1994;14:3782–3790. doi: 10.1128/MCB.14.6.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu A. The involvement of novel protein kinase C isozymes in influencing sensitivity of breast cancer MCF-7 cells to tumor necrosis factor-alpha. Mol. Pharmacol. 1998;53:105–111. doi: 10.1124/mol.53.1.105. [DOI] [PubMed] [Google Scholar]
- 12.Chen C.C., Wang J.K., Chen W.C. TPA induces translocation but not down-regulation of new PKC isoform eta in macrophages, MDCK cells and astrocytes. FEBS Lett. 1997;412:30–34. doi: 10.1016/S0014-5793(97)00697-2. [DOI] [PubMed] [Google Scholar]
- 13.Pal D., Outram S.P., Basu A. Novel regulation of protein kinase C-eta. Biochem. Biophys. Res. Commun. 2012;425:836–841. doi: 10.1016/j.bbrc.2012.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resnick M.S., Luo X., Vinton E.G., Sando J.J. Selective up-regulation of protein kinase C eta in phorbol ester-sensitive versus -resistant EL4 mouse thymoma cells. Cancer Res. 1997;57:2209–2215. [PubMed] [Google Scholar]
- 15.Bacher N., Zisman Y., Berent E., Livneh E. Isolation and characterization of PKC-L, a new member of the protein kinase C-related gene family specifically expressed in lung, skin, and heart. Mol. Cell. Biol. 1991;11:126–133. doi: 10.1128/MCB.11.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osada S., Hashimoto Y., Nomura S., Kohno Y., Chida K., Tajima O., Kubo K., Akimoto K., Koizumi H., Kitamura Y., et al. Predominant expression of nPKC eta, a Ca(2+)-independent isoform of protein kinase C in epithelial tissues, in association with epithelial differentiation. Cell Growth Differ. 1993;4:167–175. [PubMed] [Google Scholar]
- 17.Zurgil U., Ben-Ari A., Rotem-Dai N., Karp G., Krasnitsky E., Frost S.A., Livneh E. PKCeta is an anti-apoptotic kinase that predicts poor prognosis in breast and lung cancer. Biochem. Soc. Trans. 2014;42:1519–1523. doi: 10.1042/BST20140182. [DOI] [PubMed] [Google Scholar]
- 18.Maddika S., Ande S.R., Panigrahi S., Paranjothy T., Weglarczyk K., Zuse A., Eshraghi M., Manda K.D., Wiechec E., Los M. Cell survival, cell death and cell cycle pathways are interconnected: Implications for cancer therapy. Drug Resist. Update. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Livneh E., Shimon T., Bechor E., Doki Y., Schieren I., Weinstein I.B. Linking protein kinase C to the cell cycle: Ectopic expression of PKC eta in NIH3T3 cells alters the expression of cyclins and Cdk inhibitors and induces adipogenesis. Oncogene. 1996;12:1545–1555. [PubMed] [Google Scholar]
- 20.Ohba M., Ishino K., Kashiwagi M., Kawabe S., Chida K., Huh N.H., Kuroki T. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol. Cell. Biol. 1998;18:5199–5207. doi: 10.1128/MCB.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nomoto S., Watanabe Y., Ninomiya-Tsuji J., Yang L.X., Nagai Y., Kiuchi K., Hagiwara M., Hidaka H., Matsumoto K., Irie K. Functional analyses of mammalian protein kinase C isozymes in budding yeast and mammalian fibroblasts. Genes Cells. 1997;2:601–614. doi: 10.1046/j.1365-2443.1997.1470346.x. [DOI] [PubMed] [Google Scholar]
- 22.Kashiwagi M., Ohba M., Watanabe H., Ishino K., Kasahara K., Sanai Y., Taya Y., Kuroki T. PKCeta associates with cyclin E/cdk2/p21 complex, phosphorylates p21 and inhibits cdk2 kinase in keratinocytes. Oncogene. 2000;19:6334–6341. doi: 10.1038/sj.onc.1204028. [DOI] [PubMed] [Google Scholar]
- 23.Shtutman M., Hershko T., Maissel A., Fima E., Livneh E. PKCeta associates with cyclin E/Cdk2 complex in serum-starved MCF-7 and NIH-3T3 cells. Exp. Cell Res. 2003;286:22–29. doi: 10.1016/S0014-4827(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 24.Fima E., Shtutman M., Libros P., Missel A., Shahaf G., Kahana G., Livneh E. PKCeta enhances cell cycle progression, the expression of G1 cyclins and p21 in MCF-7 cells. Oncogene. 2001;20:6794–6804. doi: 10.1038/sj.onc.1204885. [DOI] [PubMed] [Google Scholar]
- 25.Basu A., Pal D., Blaydes R. Differential effects of protein kinase C-eta on apoptosis versus senescence. Cell. Signal. 2018;55:1–7. doi: 10.1016/j.cellsig.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Hara T., Miyazaki M., Hakuno F., Takahashi S., Chida K. PKCeta promotes a proliferation to differentiation switch in keratinocytes via upregulation of p27Kip1 mRNA through suppression of JNK/c-Jun signaling under stress conditions. Cell Death Dis. 2011;2:e157. doi: 10.1038/cddis.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redig A.J., Sassano A., Majchrzak-Kita B., Katsoulidis E., Liu H., Altman J.K., Fish E.N., Wickrema A., Platanias L.C. Activation of protein kinase Cη by type I interferons. J. Biol. Chem. 2009;284:10301–10314. doi: 10.1074/jbc.M807254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aeder S.E., Martin P.M., Soh J.W., Hussaini I.M. PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene. 2004;23:9062–9069. doi: 10.1038/sj.onc.1208093. [DOI] [PubMed] [Google Scholar]
- 29.Park G.B., Choi Y., Kim Y.S., Lee H.K., Kim D., Hur D.Y. Silencing of PKCeta induces cycle arrest of EBV(+) B lymphoma cells by upregulating expression of p38-MAPK/TAp73/GADD45alpha and increases susceptibility to chemotherapeutic agents. Cancer Lett. 2014;350:5–14. doi: 10.1016/j.canlet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Kamioka N., Akahane T., Kohno Y., Kuroki T., Iijima M., Honma I., Ohba M. Protein kinase C delta and eta differently regulate the expression of loricrin and Jun family proteins in human keratinocytes. Biochem. Biophys. Res. Commun. 2010;394:106–111. doi: 10.1016/j.bbrc.2010.02.125. [DOI] [PubMed] [Google Scholar]
- 31.Shirai Y., Morioka S., Sakuma M., Yoshino K., Otsuji C., Sakai N., Kashiwagi K., Chida K., Shirakawa R., Horiuchi H., et al. Direct binding of RalA to PKCeta and its crucial role in morphological change during keratinocyte differentiation. Mol. Biol. Cell. 2011;22:1340–1352. doi: 10.1091/mbc.e10-09-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabodi S., Calautti E., Talora C., Kuroki T., Stein P.L., Dotto G.P. A PKC-eta/Fyn-dependent pathway leading to keratinocyte growth arrest and differentiation. Mol. Cell. 2000;6:1121–1129. doi: 10.1016/S1097-2765(00)00110-6. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura M., Tanaka N., Kuroki T., Ichihashi M., Ohba M. The eta isoform of protein kinase C inhibits UV-induced activation of caspase-3 in normal human keratinocytes. Biochem. Biophys. Res. Commun. 2003;303:350–356. doi: 10.1016/S0006-291X(03)00345-0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M.X., Zhang J., Zhang H., Tang H. miR-24-3p Suppresses Malignant Behavior of Lacrimal Adenoid Cystic Carcinoma by Targeting PRKCH to Regulate p53/p21 Pathway. PLoS ONE. 2016;11:e0158433. doi: 10.1371/journal.pone.0158433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Zurgil U., Ben-Ari A., Atias K., Isakov N., Apte R., Livneh E. PKCeta promotes senescence induced by oxidative stress and chemotherapy. Cell Death Dis. 2014;5:e1531. doi: 10.1038/cddis.2014.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akkaraju G.R., Basu A. Overexpression of protein kinase C-eta attenuates caspase activation and tumor necrosis factor-alpha-induced cell death. Biochem. Biophys. Res. Commun. 2000;279:103–107. doi: 10.1006/bbrc.2000.3903. [DOI] [PubMed] [Google Scholar]
- 37.Rotem-Dai N., Oberkovitz G., Abu-Ghanem S., Livneh E. PKCeta confers protection against apoptosis by inhibiting the pro-apoptotic JNK activity in MCF-7 cells. Exp. Cell Res. 2009;315:2616–2623. doi: 10.1016/j.yexcr.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Raveh-Amit H., Hai N., Rotem-Dai N., Shahaf G., Gopas J., Livneh E. Protein kinase Ceta activates NF-kappaB in response to camptothecin-induced DNA damage. Biochem. Biophys. Res. Commun. 2011;412:313–317. doi: 10.1016/j.bbrc.2011.07.090. [DOI] [PubMed] [Google Scholar]
- 39.Uht R.M., Amos S., Martin P.M., Riggan A.E., Hussaini I.M. The protein kinase C-eta isoform induces proliferation in glioblastoma cell lines through an ERK/Elk-1 pathway. Oncogene. 2007;26:2885–2893. doi: 10.1038/sj.onc.1210090. [DOI] [PubMed] [Google Scholar]
- 40.Hussaini I.M., Carpenter J.E., Redpath G.T., Sando J.J., Shaffrey M.E., Vandenberg S.R. Protein kinase C-eta regulates resistance to UV- and gamma-irradiation-induced apoptosis in glioblastoma cells by preventing caspase-9 activation. Neuro Oncol. 2002;4:9–21. doi: 10.1093/neuonc/4.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma L., Shan Y., Bai R., Xue L., Eide C.A., Ou J., Zhu L.J., Hutchinson L., Cerny J., Khoury H.J., et al. A therapeutically targetable mechanism of BCR-ABL-independent imatinib resistance in chronic myeloid leukemia. Sci. Transl. Med. 2014;6:252ra121. doi: 10.1126/scitranslmed.3009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Ghanem S., Oberkovitz G., Benharroch D., Gopas J., Livneh E. PKCeta expression contributes to the resistance of Hodgkin’s lymphoma cell lines to apoptosis. Cancer Biol. Ther. 2007;6:1375–1380. doi: 10.4161/cbt.6.9.4527. [DOI] [PubMed] [Google Scholar]
- 43.Sonnemann J., Gekeler V., Ahlbrecht K., Brischwein K., Liu C., Bader P., Muller C., Niethammer D., Beck J.F. Down-regulation of protein kinase Ceta by antisense oligonucleotides sensitises A549 lung cancer cells to vincristine and paclitaxel. Cancer Lett. 2004;209:177–185. doi: 10.1016/j.canlet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Sonnemann J., Gekeler V., Sagrauske A., Muller C., Hofmann H.P., Beck J.F. Down-regulation of protein kinase Ceta potentiates the cytotoxic effects of exogenous tumor necrosis factor-related apoptosis-inducing ligand in PC-3 prostate cancer cells. Mol. Cancer Ther. 2004;3:773–781. [PubMed] [Google Scholar]
- 45.Ku B.M., Yune Y.P., Lee E.S., Hah Y.S., Park J.Y., Jeong J.Y., Lee D.H., Cho G.J., Choi W.S., Kang S.S. PKCeta Regulates the TGFbeta3-induced Chondevrepogenic Differentiation of Human Mesenchymal Stem Cell. Dev. Reprod. 2013;17:299–309. doi: 10.12717/DR.2013.17.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashimoto Y., Osada S., Ohno S., Kuroki T. A Ca(2+)-independent protein kinase C, nPKC eta: Its structure, distribution and possible function. Tohoku J. Exp. Med. 1992;168:275–278. doi: 10.1620/tjem.168.275. [DOI] [PubMed] [Google Scholar]
- 47.Koizumi H., Kohno Y., Osada S., Ohno S., Ohkawara A., Kuroki T. Differentiation-associated localization of nPKC eta, a Ca(++)-independent protein kinase C, in normal human skin and skin diseases. J. Investig. Dermatol. 1993;101:858–863. doi: 10.1111/1523-1747.ep12371707. [DOI] [PubMed] [Google Scholar]
- 48.Ikuta T., Chida K., Tajima O., Matsuura Y., Iwamori M., Ueda Y., Mizuno K., Ohno S., Kuroki T. Cholesterol sulfate, a novel activator for the eta isoform of protein kinase C. Cell Growth Differ. 1994;5:943–947. [PubMed] [Google Scholar]
- 49.Denning M.F., Dlugosz A.A., Williams E.K., Szallasi Z., Blumberg P.M., Yuspa S.H. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995;6:149–157. [PubMed] [Google Scholar]
- 50.Beck J., Bohnet B., Brugger D., Bader P., Dietl J., Scheper R.J., Kandolf R., Liu C., Niethammer D., Gekeler V. Multiple gene expression analysis reveals distinct differences between G2 and G3 stage breast cancers, and correlations of PKC eta with MDR1, MRP and LRP gene expression. Br. J. Cancer. 1998;77:87–91. doi: 10.1038/bjc.1998.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahaf G., Rotem-Dai N., Koifman G., Raveh-Amit H., Frost S.A., Livneh E. PKCeta is a negative regulator of AKT inhibiting the IGF-I induced proliferation. Exp. Cell Res. 2012;318:789–799. doi: 10.1016/j.yexcr.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 52.Pal D., Basu A. Protein kinase C-eta regulates Mcl-1 level via ERK1. Cell Signal. 2017;40:166–171. doi: 10.1016/j.cellsig.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Lee S., Schmitt C.A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 2019;21:94–101. doi: 10.1038/s41556-018-0249-2. [DOI] [PubMed] [Google Scholar]
- 54.Childs B.G., Durik M., Baker D.J., van Deursen J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee C.S., Baek J., Han S.Y. The Role of Kinase Modulators in Cellular Senescence for Use in Cancer Treatment. Molecules. 2017;22:1411. doi: 10.3390/molecules22091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lujambio A. To clear, or not to clear (senescent cells)? That is the question. Bioessays. 2016;38(Suppl. 1):S56–S64. doi: 10.1002/bies.201670910. [DOI] [PubMed] [Google Scholar]
- 57.Campisi J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Childs B.G., Baker D.J., Kirkland J.L., Campisi J., van Deursen J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014;15:1139–1153. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carnero A. Markers of cellular senescence. Methods Mol. Biol. 2013;965:63–81. doi: 10.1007/978-1-62703-239-1_4. [DOI] [PubMed] [Google Scholar]
- 60.Canzian F., Gariboldi M., Manenti G., De Gregorio L., Osada S., Ohno S., Dragani T.A., Pierotti M.A. Expression in lung tumors and genetic mapping of the novel murine protein kinase C eta. Mol. Carcinog. 1994;9:111–113. doi: 10.1002/mc.2940090302. [DOI] [PubMed] [Google Scholar]
- 61.Chida K., Murakami A., Tagawa T., Ikuta T., Kuroki T. Cholesterol sulfate, a second messenger for the eta isoform of protein kinase C, inhibits promotional phase in mouse skin carcinogenesis. Cancer Res. 1995;55:4865–4869. [PubMed] [Google Scholar]
- 62.Chida K., Hara T., Hirai T., Konishi C., Nakamura K., Nakao K., Aiba A., Katsuki M., Kuroki T. Disruption of protein kinase Ceta results in impairment of wound healing and enhancement of tumor formation in mouse skin carcinogenesis. Cancer Res. 2003;63:2404–2408. [PubMed] [Google Scholar]
- 63.Doi S., Goldstein D., Hug H., Weinstein I.B. Expression of multiple isoforms of protein kinase C in normal human colon mucosa and colon tumors and decreased levels of protein kinase C beta and eta mRNAs in the tumors. Mol. Carcinog. 1994;11:197–203. doi: 10.1002/mc.2940110405. [DOI] [PubMed] [Google Scholar]
- 64.Masso-Welch P.A., Winston J.S., Edge S., Darcy K.M., Asch H., Vaughan M.M., Ip M.M. Altered expression and localization of PKC eta in human breast tumors. Breast Cancer Res. Treat. 2001;68:211–223. doi: 10.1023/A:1012265703669. [DOI] [PubMed] [Google Scholar]
- 65.Lu H.C., Chou F.P., Yeh K.T., Chang Y.S., Hsu N.C., Chang J.G. Analysing the expression of protein kinase C eta in human hepatocellular carcinoma. Pathology. 2009;41:626–629. doi: 10.3109/00313020903273076. [DOI] [PubMed] [Google Scholar]
- 66.Brenner W., Farber G., Herget T., Wiesner C., Hengstler J.G., Thuroff J.W. Protein kinase C eta is associated with progression of renal cell carcinoma (RCC) Anticancer Res. 2003;23:4001–4006. [PubMed] [Google Scholar]
- 67.Pal D., Outram S.P., Basu A. Upregulation of PKCeta by PKCepsilon and PDK1 involves two distinct mechanisms and promotes breast cancer cell survival. Biochim. Biophys. Acta. 2013;1830:4040–4045. doi: 10.1016/j.bbagen.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krasnitsky E., Baumfeld Y., Freedman J., Sion-Vardy N., Ariad S., Novack V., Livneh E. PKCeta is a novel prognostic marker in non-small cell lung cancer. Anticancer Res. 2012;32:1507–1513. [PubMed] [Google Scholar]
- 69.Porter S.N., Magee J.A. PRKCH regulates hematopoietic stem cell function and predicts poor prognosis in acute myeloid leukemia. Exp. Hematol. 2017 doi: 10.1016/j.exphem.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quan T., Fisher G.J. Cloning and characterization of the human protein kinase C-eta promoter. J. Biol. Chem. 1999;274:28566–28574. doi: 10.1074/jbc.274.40.28566. [DOI] [PubMed] [Google Scholar]
- 71.Evdokimova V., Tognon C., Ng T., Sorensen P.H. Reduced proliferation and enhanced migration: Two sides of the same coin? Molecular mechanisms of metastatic progression by YB-1. Cell Cycle. 2009;8:2901–2906. doi: 10.4161/cc.8.18.9537. [DOI] [PubMed] [Google Scholar]