Abstract
Capillary leak syndrome (CLS) is a rare disease with profound vascular leakage, which can be associated with a high mortality. There have been several reports on CLS as an adverse effect of anti-cancer agents and therapy, but the incidence of CLS according to the kinds of anti-cancer drugs has not been systemically evaluated. Thus, the aim of our study was to comprehensively meta-analyze the incidence of CLS by different types of cancer treatment or after bone marrow transplantation (BMT). We searched the literatures (inception to July 2018) and among 4612 articles, 62 clinical trials (studies) were eligible. We extracted the number of patients with CLS, total cancer patients, name of therapeutic agent and dose, and type of cancer. We performed a meta-analysis to estimate the summary effects with 95% confidence interval and between-study heterogeneity. The reported incidence of CLS was categorized by causative drugs and BMT. The largest number of studies reported on CLS incidence during interleukin-2 (IL-2) treatment (n = 18), which yielded a pooled incidence of 34.7% by overall estimation and 43.9% by meta-analysis. The second largest number of studies reported on anti-cluster of differentiation (anti-CD) agents (n = 13) (incidence of 33.9% by overall estimation and 35.6% by meta-analysis) or undergoing BMT (n = 7 (21.1% by overall estimation and 21.7% by meta-analysis). Also, anti-cancer agents, including IL-2 + imatinib mesylate (three studies) and anti-CD22 monoclinal antibodies (mAb) (four studies), showed a dose-dependent increase in the incidence of CLS. Our study is the first to provide an informative overview on the incidence rate of reported CLS patients as an adverse event of anti-cancer treatment. This meta-analysis can lead to a better understanding of CLS and assist physicians in identifying the presence of CLS early in the disease course to improve the outcome and optimize management.
Keywords: capillary leak syndrome, cancer, interleukin-2, anti-CD agents, bone marrow transplantation
1. Introduction
Capillary leak syndrome (CLS), also known as vascular leak syndrome (VLS), is a rare but fatal disease, and an idiopathic form of CLS was first reported by Clarkson in 1960 [1]. Patients with CLS show a profound increase of capillary permeability, which can result in the leakage of plasma with proteins out from capillaries, resulting in clinical features such as edema, hypotension, hypoalbuminemia, or hemoconcentration [2,3,4]. Most cases of CLS are classified as idiopathic forms, and its pathogenesis has not been elucidated yet. It may also develop as a secondary form, preceded by autoimmune diseases, infections, snakebites, and drugs [5]. Cancer and chemotherapy are also considered to be important causes of secondary CLS, but the underlying mechanisms remain mostly elusive [4,6]. CLS shows a high mortality rate, with one-year and five-year survival rates being 89% and 73%, respectively, in idiopathic forms [7]. If prophylactic treatment including intravenous immunoglobulin (IVIG) is provided, disease-specific mortality seems to decrease in idiopathic CLS [3,7,8]. However, there is no established treatment for secondary CLS, and supportive therapy with fluid management may be the most important element [5]. Currently, exact treatment guidelines for CLS do not exist [8,9,10,11]. Moreover, the capillary leak phenomenon can be similar between idiopathic CLS and secondary forms of CLS due to drugs, but the pathophysiology of them may be somewhat different.
CLS has also been reported as an adverse event in cancer patients receiving different types of anti-cancer treatments [3]. However, there has been a lack of awareness of CLS by oncologists due to the non-specific symptoms of this disease [3], and the incidence of CLS according to the different types of anti-cancer agents or therapy has not been systematically investigated.
Thus, in this study, we conducted a systematic review and meta-analysis to estimate the incidence proportion of CLS in cancer patients who received specific anti-cancer treatment or therapy, including bone marrow transplantation (BMT).
2. Methods
2.1. Literature Search Strategy and Study Selection
We followed the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for this systematic review (Supplementary Table S1). Two investigators (K.H.L. and I.R.L.) manually searched the literature (PubMed and EMBASE) to find original studies that reported cases of CLS as an adverse event in cancer patients who received specific cancer treatment or BMT. The search terms were: “(Capillary leak OR Vascular leak) AND (cancer OR carcinoma OR neoplasm OR tumor)”, and the date of the last search was 15 July 2018. If there was a discrepancy for the inclusion/exclusion of the respective article, it was discussed and resolved by consensus among three investigators (J.I.S., K.H.L., and I.R.L.). The full literature search strategy is presented in Figure 1.
Figure 1.
Flow chart of literature search. CLS: Capillary leak syndrome, G-CSF: Granulocyte colony-stimulating factor.
The eligibility criteria for inclusion were: studies on (1) CLS that was an adverse event of cancer treatment-related drugs; and (2) CLS that developed after BMT; and the exclusion criteria were: studies on (1) CLS that were caused by idiopathic forms, infection, or surgery; and (2) CLS attributed to cancer itself, or (3) missing raw data from the original study reporting on CLS as an adverse event of cancer treatment. Our initial search yielded 4612 articles, but we finally included 62 clinical trials (or studies) that met the inclusion criteria for this systematic review.
2.2. Data Extraction
For each eligible clinical trial (or study), we recorded the first author, publication year, journal name, period of study, country, total number of patients, number of patients who developed CLS, diagnosed cancer type, causative drugs, and the dose of drugs.
2.3. Analyses of Clinical Trials (or Studies)
The incidence of CLS for each study was estimated by calculating the ratio between the number of CLS patients and the total number of cancer patients who received the causative drug or BMT. The data for each study are presented in Table 1 [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. To estimate the incidence of CLS for the relevant groups, we presented the data with median (ranges) and also performed a meta-analysis to estimate the summary effects with proportion of CLS and 95% confidence interval (CI) using random-effect models [74,75]. Random effects meta-analysis provides the weighted average of the effect sizes of a group of studies with the assumption that individual studies are estimating different effects [76]. We evaluated the between-study heterogeneity using the I2 metric of inconsistency and P value of the χ2-based Cochran Q test. I2 is the ratio of the between-study variance over the sum of the within-study and between-study variances, and it ranges between 0–100%. I2 values of <25%, 25–50%, and >75% are usually judged to represent low, moderate (large), and high (very large) heterogeneity, respectively [77]. Since statistical tests for heterogeneity are not very powerful, a higher p value than usual (p < 0.10: significant heterogeneity) is used as the cut-off for clinical heterogeneity [78].
Table 1.
Summary profiles of clinical trials that reported capillary leak syndrome as an adverse event of anti-cancer drugs.
| Ref. No. | Author, Year of Publication | Period of Study | Country | Total Number | CLS | Incidence (%) | Diagnosis | Drug | Treatment Dose |
|---|---|---|---|---|---|---|---|---|---|
| IL-2 | |||||||||
| [12] | Atkins et al., 1999 | 1985–1993 | USA | 270 | 92 | 34.1 | Melanoma | IL-2 | 720,000 IU/kg every 8 h |
| [13] | Sparano et al., 1993 | 1988–1992 | USA | 44 | 40 | 90.9 | Melanoma | IL-2 | 6 × 106 IU/m2 every 8 h |
| [14] | Tarhini et al., 2007 | 2000–2003 | USA | 26 | 7 | 26.9 | Melanoma | IL-2 | 600,000 IU/kg every 8 h for up to 14 doses for 2 cycles |
| [15] | Talpur et al., 2012 | 2003–2008 | USA | 8 | 6 | 75.0 | Cutaneous peripheral T-cell lymphoma | IL-2 | Dose level 18 μg/kg |
| [16] | Gallagher et al, 2007 | 2006 | Israel | 14 | 14* | 100.0 | Melanoma, renal cell carcinoma | IL-2 | Dose level 8–14 μg/kg |
| [17] | Shusterman et al., 2010 | NA | USA | 39 | 12 | 30.8 | Neuroblastoma | IL-2 | Dose level 12 mg/m2 |
| [18] | Shaughnessy et al., 2005 | NA | USA | 2 | 1 | 50.0 | Non-Hodgkin lymphoma, Hodgkin disease, acute leukemia, myelodysplastic syndrome, chronic myelogenous leukemia, multiple myeloma, aplastic anemia | IL-2 | Dose level 9.0 μg/kg |
| [18] | Shaughnessy et al., 2005 | NA | USA | 20 | 2 | 10.0 | Non-Hodgkin lymphoma, Hodgkin disease, acute leukemia, myelodysplastic syndrome, chronic myelogenous leukemia, multiple myeloma, aplastic anemia | IL-2 | Dose level 4.5 μg/kg |
| [19] | Frankel et al., 2003 | NA | USA | 18 | 2 | 11.1 | Chronic lymphocytic leukemia | IL-2 | Dose level 9 or 18 μg/kg |
| [20] | Duvic et al., 2002 | NA | USA | 71 | 18 | 25.4 | Cutaneous T-cell lymphoma | IL-2 | Dose level 9 or 18 μg/kg |
| [21] | Foss et al., 2001 | NA | USA | 15 | 2 | 13.3 | Cutaneous T-cell lymphoma | IL-2 | Dose level 9 or 18 μg/kg |
| [22] | Sievers et al., 2000 | NA | USA | 60 | 7 | 11.7 | Acute myelogenous leukemia | IL-2 | 9,000,000 IU/m2 for 4 days and 16,000,000 IU/m2 for 10 days |
| [23] | Duvic et al., 1998 | NA | USA | 4 | 1 | 25.0 | Cutaneous T cell lymphoma | IL-2 | Dose level 9 or 18 μg/kg |
| [24] | Meehan et al., 1997 | 1993–1995 | USA | 57 | 3 | 5.3 | Breast cancer | IL-2 | MTD 6 × 106 IU/m2/day |
| [25] | Chang et al., 1993 | NA | Japan | 20 | 15 | 75.0 | Melanoma, renal cell cancer | IL-2 | Using vaccine-primed lymph node cell with IL-2 (180,000 IU/kg) |
| [26] | van Haelst Pisani C et al., 1991 |
NA | France | 5 | 4 | 80.0 | Melanoma, renal cell cancer | IL-2 | Human recombinant IL-2 3 × 10⁶ IU/m²/24 h for 4 or 5 days |
| [27] | Philip et al., 1989 | 1987–1988 | France | 20 | 8 | 40.0 | Renal cell cancer | IL-2 | IL-2 3 × 10⁶ IU/m² with lymphapheresis(17), IL-2 3 × 10 ⁶IU/m²(3) |
| [28] | Carey et al., 1997 | NA | UK | 10 | 10* | 100.0 | Malignant melanoma, renal cell cancer | IL-2 | Using 3 × 10⁶ IU/m²/day for 5 days |
| IL-2 with other agents | |||||||||
| [16] | Gallagher et al., 2007 | 2006 | Israel | 4 | 4 | 100.0 | Renal cell carcinoma | IL-2 + bevacizumab |
IL-2 dose level 9–14 μg/kg |
| [29] | Pautier et al., 2013 | NA | France | 3 | 0 | 0.0 | Melanoma, ovarian adenocarcinoma, Merkel-cell carcinoma, gastrointestinal stromal tumor, rectal adenocarcinoma, cervical adenocarcinoma | IL-2 + imatinib mesylate |
IL-2: 3,000,000 IU/day, imatinib mesylate 400 mg/day |
| [29] | Pautier et al., 2013 | NA | France | 11 | 1 | 9.0 | Melanoma, ovarian adenocarcinoma, Merkel-cell carcinoma, gastrointestinal stromal tumor, rectal adenocarcinoma, cervical adenocarcinoma | IL-2 + imatinib mesylate |
IL-2: 6,000,000 IU/day, imatinib mesylate 400 mg/day |
| [29] | Pautier et al., 2013 | NA | France | 3 | 1 | 33.3 | Melanoma, ovarian adenocarcinoma, Merkel-cell carcinoma, gastrointestinal stromal tumor, rectal adenocarcinoma, cervical adenocarcinoma | IL-2 + imatinib mesylate |
IL-2: 9,000,000 IU/day, imatinib mesylate 400 mg/day |
| [30] | O’Brien et al., 2006 | NA | Ireland | 10 | 0 | 0.0 | Melanoma | IL-2 + taurolidine |
IL-2 72 MIU/m2 for 120 h Taurolidine 2% w/v via continuous infusion |
| [31] | Pichert et al., 1991 | 1988–1989 | Switzerland | 14 | 14* | 100.0 | Renal cell carcinoma, melanoma | IL-2 + IFN-alfa 2a |
IL-2 3 MIU/m2 for 4 days IFN-alpha 6 MIU/m2 for 2 days (1, 4 day) |
| [13] | Sparano et al., 1993 | 1988–1992 | USA | 41 | 33 | 80.5 | Melanoma | IL-2 + IFN-alfa |
IL-2 4.5 × 106 IU/m2 per dose IFN-alpha 2 4.5 × 106 IU/m2 |
| [32] | Gilman et al., 2009 | 1997–2002 | USA | 19 | 3 | 15.8 | Neuroblastoma | IL-2 + ch14.18 | Ch14.18 20 and 40 mg/m2/day IL-2 4.5 × 106 IU/m2/day |
| [33] | Meehan et al., 2010 | NA | USA | 12 | 2 | 16.7 | Multiple myeloma, non-Hodgkin lymphoma |
IL-2 + GM-CSF + G-CSF | IL-2 6 × 105–1.5 × 106 IU/m2 G-CSF 5 μg/kg GM-CSF 7.5 μg/kg |
| [34] | Yu et al., 2010 | 2001–2009 | USA | 226 | 51 | 22.6 | Neuroblastoma | IL-2 + GM-CSF + anti-GD2 + isotretionoin |
IL-2 3.0 × 106 IU/m2 (week 1), 4.5 × 106 IU/m2 (week 2) GM-CSF 250 μg/m2 isotretionoin 160mg/m2 |
| [35] | Hamblin et al., 1993 | 1988–1989 | UK | 16 | 1 | 6.3 | Metastatic colorectal cancer | IL-2 + 5-FU | IL-2 18 × 10 IU/m2/day over 120 h 5FU 600 mg/m2 |
| [36] | Savage et al., 1997 | NA | UK | 24 | 6 | 25.0 | Metastatic renal cancer | IL-2 + 5-FU | IL-2 9 × 106 IU 5-FU 200 mg/m2 |
| IL-1 with other agents | |||||||||
| [37] | Smith et al., 1993 | 1990–1992 | USA | 15 | 6 | 40.0 | Colon cancer, melanoma, renal cell cancer, lung cancer, pancreatic cancer, liposarcoma, adenocarcinoma with unknown primary site | IL-1 alpha + carboplatin | IL-1 alpha 0.03, 0.1, 0.3 μg/kg carboplatin 800 mg/m2 |
| [38] | Worth et al., 1997 | 1994 | USA | 9 | 4 | 44.4 | Osteosarcoma | IL-1 alpha + etoposide | IL-1 alpha 0.1 μg/kg etoposide 100 mg/m2 |
| IL-4 | |||||||||
| [39] | Sosman et al., 1994 | NA | USA | 17 | 2 | 11.8 | Renal cell carcinoma, melanoma, colon carcinoma, cholangiocarcinoma | IL-4+IL-2 | IL-4 40–600 μg /m2/day IL-2 11.2 MIU/m2/day |
| GM-CSF | |||||||||
| [40] | Gorin et al., 1992 | 1988–1990 | France | 44 | 3 | 6.8 | Non-Hodgkin lymphoma | GM-CSF | Dose level 250 μg/m2 |
| [41] | Liberati et al., 1991 | NA | Italy | 14 | 1 | 7.1 | Non-Hodgkin lymphoma | GM-CSF | Dose level 5 μg/kg |
| [42] | Steward et al., 1989 | NA | USA &UK | 20 | 3 | 15.0 | Metastatic solid tumors | GM-CSF | Using dose 0.3, 1.0, 3.0, 10, 30, and 60 μg/kg/day Dose level 32 μg /kg |
| Gemcitabine | |||||||||
| [43] | Jidar et al., 2009 | NA | France | 23 | 1 | 4.3 | Cutaneous T-cell lymphoma | Gemcitabine | Using dose 700–1000 mg/m2 |
| [44] | Kurosaki et al., 2009 | 2003–2006 | Japan | 27 | 1 | 3.7 | Pancreatic cancer | Gemcitabine | Dose level 1000 mg/m2 biweekly |
| [45] | Dumontet et al., 2001 | 1988–2000 | France | 36 | 1 | 2.8 | Non-Hodgkin lymphoma | Gemcitabine | Dose level 1 g/m2 |
| SS1P | |||||||||
| [46] | Kreitman et al., 2009 | NA | USA | 24 | 13 | 54.2 | Peritoneal mesothelioma, pleural mesothelioma, pleural–peritoneal mesothelioma, ovarian carcinoma, pancreatic carcinoma | SS1P | Dose level 4–25 μg/kg |
| [47] | Hassan et al., 2007 | 2000–2006 | USA | 34 | 2 | 5.9 | Peritoneal mesothelioma, pleural mesothelioma, pleural–peritoneal mesothelioma, ovarian carcinoma, pancreatic carcinoma | SS1P | Dose level 18 or 25 μg/kg |
| Anti-CD agents | |||||||||
| [48] | Sausville et al., 1995 | NA | USA | 11 | 4 | 36.4 | B-cell lymphoma | Anti-CD22 | Dose level 28.8 mg/m2 MTD 19.2 mg/m2 |
| [49] | Vitetta et al., 1991 | NA | USA | 15 | 15* | 100.0 | B-cell lymphoma | Anti-CD22 | Using dose 12.5, 25, 50, 75, 100 mg/m2 |
| [50] | Wayne et al., 2014 | NA | USA | 7 | 2 | 28.6 | Acute lymphoblastic leukemia | Anti-CD22 | Dose level 30 μg/kg |
| [51] | Amlot et al., 1993 | NA | USA | 26 | 3 | 11.5 | B-cell lymphoma | Anti-CD22 | Using Maximal single dose 2.5–13.9 mg/m2 |
| [52] | Stathis et al., 2014 | NA | Switzerland | 5 | 1 | 20.0 | Non-Hodgkin lymphoma | Anti-CD22 + temsirolimus | Using dose Anti-CD22 0.8 mg/m2 + temsirolimus 15 mg/day, Anti-CD22 0.8 mg/m2 + temsirolimus 10 mg/day |
| [53] | Schindler et al., 2011 | NA | USA | 17 | 1 | 5.9 | B-cell acute lymphoblastic leukemia | Anti-CD19 + anti-CD22 | Dose level 8 mg/m2 |
| [54] | Bachanova et al., 2015 | NA | USA | 25 | 7 | 28.0 | Pre-B acute lymphoblastic leukemia, chronic lymphocytic leukemia, Non-Hodgkin lymphoma | Anti-CD19 + anti-CD22 | Dose level 40–60 μg/kg |
| [55] | Schnell et al., 2003 | NA | Germany | 27 | 3 | 11.1 | Hodgkin lymphoma | Anti-CD25 | Dose level 15–20 mg/m2 |
| [56] | Schnell et al., 2000 | NA | Germany | 18 | 18* | 100.0 | Hodgkin lymphoma | Anti-CD25 | Dose level 15 mg/m2/cycle |
| [57] | Engert et al., 1997 | NA | Germany | 15 | 1 | 6.7 | Hodgkin lymphoma | Anti-CD25 | Dose level 5 mg/m2(3), 10 mg/m2(3), 15 mg/m2(6), 20 mg/m2(3) |
| [58] | Schnell et al., 2002 | NA | Germany | 17 | 3 | 17.6 | Hodgkin lymphoma, Non-Hodgkin lymphoma | Anti-CD30 | Dose level 7.5 mg/m2(1), 10 mg/m2(2) MTD 5 mg/m2 |
| [59] | Stone et al., 1996 | NA | USA | 23 | 16 | 69.6 | Non-Hodgkin lymphoma | Anti-CD19 + IgG-HD37-dgA |
MTD 19.2 mg/m2 |
| [60] | Uckun et al., 1999 | 1996–1998 | USA | 15 | 1 | 6.7 | Acute lymphoblastic leukemia, chronic lymphocytic leukemia | CD19 receptor directed tyrosine kinase inhibitor B43-Genistein | Dose level 0.1 mg/kg |
| Other agents | |||||||||
| [61] | Baluna et al., 1996 | NA | USA | 56 | 12 | 21.4 | Non-Hodgkin lymphoma | Ricin A chain-containing immunotoxin | Using IgG-HD37-RTA continuous infusion 9.6–19.2 mg/m2(2), bolus infusion range 2–24 mg/m2(2) IgG-RFB4-RTA continuous infusion 9.6–28.8 mg/m2(4), bolus infusion 23–48 mg/m2(2) Fab’-RFB4-RTA bolus infusion 25–100 mg/m2(2) |
| [62] | Borghaei et al., 2009 | NA | USA | 39 | 6 | 15.4 | NSCLC, pancreatic cancer | ABR-217620 | Dose level 20 μg/kg |
| [63] | Hochhauser et al., 2009 | 2004–2006 | UK | 16 | 10 | 62.5 | Ampulla of vater cancer, cholangiocarcinoma, colorectal cancer, lung cancer, esophagus cancer, pancreatic cancer, sarcoma, malignant melanoma, stomach cancer | Pyrrolobenzodiazepine | Using dose 15–240 μg/m2 |
| [64] | Posey et al., 2002 | NA | USA | 46 | 1 | 2.2 | Colorectal cancer, pancreatic cancer, ovarian cancer, breast cancer, lung cancer, prostate cancer, head and neck cancer, stomach cancer, endometrial cancer, thyroid cancer, unknown primary lesion | SGN-10 (or BR96 sFv-PE40) |
Dose level > or = 0.384 mg/m2 |
| [65] | Elias et al., 2001 | NA | USA | 5 | 4 | 80.0 | Breast cancer | Paclitaxel | Dose level 150 mg/m2 |
| [66] | Grossbard et al., 1993 | 1990–1991 | USA | 12 | 5 | 41.7 | Non-Hodgkin lymphoma | Anti-B4-bR | Using dose 20, 40, 50 μg/kg/day for 7 days MTD 40 μg/kg |
| [67] | Pazdur et al., 1991 | NA | USA | 17 | 6 | 35.3 | Metastatic cancer | FK973 | Using dose 30 mg/m2(2), 45 mg/m2(4) |
| [68] | Barrett et al., 1982 | 1980–1981 | UK | 36 | 4 | 11.1 | Acute myeloid leukemia, acute lymphoblastic leukemia, aplastic anemia, mucopolysaccharidosis, metachromic leukodystrophy | Dihydro benzoxazine | Using dose 12.5 mg/kg(10), 500 g/m2(26) |
| [69] | Zwaan et al., 2014 | NA | Multicenter in Europe† | 36 | 3 | 8.3 | Acute myeloid leukemia | Cyclosporine | Using dose plasma concentration <100 μg/L |
| [69] | Zwaan et al., 2014 | NA | Multicenter in Europe† | 29 | 1 | 3.4 | Acute myeloid leukemia | Clofarabine + cytarabine + liposomal daunorubicin |
Clofarabine 20, 30, 40 mg/m2 Ara-C 2 g/m2/day dauorubicin 40–60 mg/m2 |
NA: not available (information was not included in the case series article), CLS: capillary leak syndrome, Using dose: drug dose that was administered to patients, Dose level: serum drug level when the patients show toxicity, DLT: dose limited toxicity, IL: Interleukin, w/v: weight/volume percentage, ch14.18: a chimeric human/murine anti-GD2 antibody, MIU: million international units, GVHD: graft-versus-host disease, INF: interferon, GM-CSF: granulocyte-macrophage colony-stimulating factor, G-CSF: granulocyte-colony stimulating factor, 5-FU: 5-fluorouracil, SS1P: recombinant anti-mesothelin immunotoxin, CD: cluster of differentiation, MTD: maximum tolerated dose, NSCLC: Non small cell lung cancer, ABR-217620: naptumomab estafenatox, SGN-10: a single-chain immunotoxin, Anti-B4-bR: B-cell restricted immunotoxin anti-B4-blocked ricin, FK973: novel, substituted dihydro benzoxazine structurally similar to mitomycin, USA: United States of America, UK: United Kindom; *All study patients developed capillary leak syndrome after receiving anti-cancer agents. There were no capillary leak syndrome features before treatment. † Study population was collected from multiple centers in Europe: Netherlands, Austria, Germany, France, the Czech Republic, and the United Kingdom.
2.4. Statistical Analysis
To meta-analyze the incidence of CLS according to the causative anti-cancer drugs or after BMT, the summary effects with 95% CI and the between-study heterogeneity were analyzed by using MedCalc version 15.8 software (MedCalc Software, Ostend, Belgium).
3. Results
There were 62 clinical trials (or studies) that reported on the incidence of CLS in patients receiving anti-cancer treatments or after BMT. Most of these studies were clinical trials in which the incidence of CLS was reported as an adverse event of anti-cancer treatment (Table 1) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. Among these, six studies reported on CLS associated with BMT with or without other agents (Table 2) [40,42,70,71,72,73]. The results of meta-analyses on the incidence of CLS induced by various drugs in cancer patients are summarized in Table 3 and Supplementary Figure S1a–n.
Table 2.
Summary profiles of clinical studies that reported capillary leak syndrome related to bone marrow transplantation.
| Ref. No. | Author, Year | Total Number |
CLS | Incidence (%) |
Diagnosis | Hypothesis or Risk Factors |
|---|---|---|---|---|---|---|
| Only BMT related | ||||||
| [70] | Cahill, et al., 1996 | 55 | 29 | 52.7 | Both allogeneic and autologous transplant recipients | Pivotal contribution by circulating leukocytes |
| [71] | Nurnberger, et al., 1993 | 12 | 4 | 33.3 | Acute lymphoblastic leukemia Aplastic anemia Fanconi’s anemia Neuroblastoma Ewing’s sarcoma Lymphoepithelial carcinoma |
C1 Inhibitor activity decreased to 0.60-fold to 0.80-fold Elevated C4d concentrations (up to 2.4 mg/dL, upper normal threshold value: 0.9) |
| [72] | Nurnberger, et al., 1997 | 96 | 20 | 20.8 | Acute lymphoblastic leukemia Acute myeloblastic leukemia Chronic myeloblastic leukemia Severe aplastic anemia Ewing tumors Rhabdomyosarcoma Neuroblastoma Lymphoepithelioma |
Receiving G-CSF or GM-CSF* GVHD prophylaxis : MTX plus cyclosporine A Allogeneic-related BMT, solid tumor Unrelated BMT, hematologic disease Patients with high-risk pretreatment |
| [40] | Gorin et al., 1992 | 44 | 3 | 6.8 | Non-Hodgkin’s lymphoma | BMT after using GM-CSF* ( Dose level 250 μg/m2) |
| [42] | Steward et al., 1989 | 20 | 3 | 15.0 | Metastatic solid tumors | BMT after using GM-CSF* (Using dose 0.3, 1.0, 3.0, 10, 30, and 60 μg/kg/day, dose level 32 μg/kg) |
| [72] | Nurnberger, et al., 1997 | 142 | 22 | 15.5 | Acute lymphoblastic leukemia Chronic myelomonocytic leukemia Severe aplastic anemia Fanconi’s anemia Non-Hodgkin’s lymphoma, Ewing tumor Neuroblastoma Rhabdomyosarcoma Wiskott–Aldrich syndrome |
BMT after using G-CSF* Low levels of C1 esterase inhibitor† |
| [73] | Salat et al, 1995 | 48 | 7 | 14.6 | Acute lymphoblastic leukemia Acute myeloblastic leukemia Chronic myeloblastic leukemia Hodgkin’s lymphoma Non-Hodgkin’s lymphoma Severe aplastic anemia Multiple myeloma |
Elevation of terminal complement complex (TCC) levels Elevation of functional Cl-esterase inhibitor (CI-INH) Elevation of Cl-inhibitor antigen (CI-INH antigen) |
CLS: capillary leak syndome, GM-CSF: granulocyte-macrophage colony-stimulating factor, G-CSF: granulocyte-colony stimulating factor, GVHD: Graft-versus-host disease, MTX: methotrexate, BMT: bone marrow transplantation; * These patients initially received bone marrow transplantation, and then received GM-CSF to correct neutropenia; † To correct this status, 15 severe CLS patients were treated with C1 INH concentrate using a cumulative dose of 180 units/kg in this article.
Table 3.
Meta-analyses on the incidence of capillary leak syndrome induced by various anti-cancer drugs or after BMT in cancer patients.
| Causative Drugs | Numberof Studies | Total Number of Patients | Number of CLS | Incidence of CLS (Overall) | Incidence of CLS by Meta-Analysis (95%CI) | Heterogeneity I2 (p Value) | Incidence of CLS Median (Ranges) |
|---|---|---|---|---|---|---|---|
| IL-2 | 18 | 703 | 244 | 34.7% | 43.9% (29.5–58.9) | 92.6% (p < 0.0001) | 32.4% (5.3–100) |
| IL-2 with other agents | 13 | 405 | 118 | 29.1% | 32.0% (15.6–51.1) | 91.1% (p < 0.0001) | 16.7% (0–100) |
| IL-2 + IFN-alpha 2a | 2 | 55 | 47 | 85.5% | 90.4% (64.1–100) | 80.0% (p = 0.0255) | 90.3% (80.5–100) |
| IL-2 + imatinib mesylate | 3 | 17 | 2 | 11.8% | 15.0% (3.1–33.4) | 0% (p = 0.4889) | 9.0% (0–33.3) |
| IL-2 + bevacizumab | 1 | 4 | 4 | 100.0% | - | - | - |
| IL-2 + 5-FU | 2 | 40 | 7 | 17.5% | 17.1% (3.7–37.4) | 56.1% (p = 0.1312) | 33.3% (6.3–25.0) |
| IL-1 with other agents | 2 | 24 | 10 | 41.7% | 42.3% (24.3–61.4) | 0% (p = 0.8266) | 42.2% (40–44.4) |
| IL-4 (+IL-2) | 1 | 17 | 2 | 11.8% | - | - | - |
| GM-CSF | 3 | 78 | 7 | 9.0% | 10.1% (4.6–17.6) | 0% (p = 0.5802) | 7.1% (6.8–15.0) |
| Gemcitabine | 3 | 86 | 3 | 3.5% | 4.9% (1.4–10.3) | 0% (p = 0.9273) | 3.7% (2.8–4.3) |
| SS1P | 2 | 58 | 15 | 25.9% | 26.9 (0.00–78.6) | 94.5% (p < 0.0001) | 30.1 (5.9–54.2) |
| Anti-CD agents | 13 | 221 | 75 | 33.9% | 35.6% (16.1–60.0) | 91.8% (p < 0.0001) | 20.0% (5.9–100) |
| Anti-CD22 | 4 | 59 | 24 | 40.7% | 48.1% (6.3–91.7) | 93.7 (p < 0.0001) | 44.1% (11.5–100) |
| Anti-CD19 + anti-CD22 | 2 | 42 | 8 | 19.0% | 17.8% (2.7–42.2) | 69.6% (p = 0.0699) | 17.0% (5.9–28.0) |
| Anti-CD25 | 3 | 60 | 22 | 36.7% | 42.2% (0.02–98.0) | 97.0% (p < 0.0001) | 11.1% (6.7–100) |
| BMT | 7 | 417 | 88 | 21.1% | 21.7% (12.2–33.1) | 83.9% (p < 0.0001) | 15.5% (6.8–52.7) |
| Only BMT-related | 3 | 163 | 53 | 32.5% | 35.5% (14.7–59.6) | 87.5% (p = 0.0003) | 33.3% (20.8–52.7) |
| BMT with other agents | 4 | 254 | 35 | 13.8% | 14.2% (10.2–18.7) | 0% (p = 0.5001) | 14.8% (6.8–15.5) |
CLS: capillary leak syndome, IL: interleukin, GM-CSF: granulocyte-macrophage colony-stimulating factor, 5-FU: 5-fluorouracil, SS1P: recombinant anti-mesothelin immunotoxin, CD: cluster of differentiation, BMT: bone marrow transplant.
There were 18 studies that reported on the incidence of CLS associated with the use of interleukin-2 (IL-2), which ranged from 5.3% to 100%. The incidence of CLS by IL-2 was 34.7% by overall estimation and 43.9% by meta-analysis. Although varying treatment doses were used, no correlations were found between the dose of IL-2 and the overall incidence of CLS. IL-2 was used in combination with other agents in several studies. These included combinations with bevacizumab (one study), imatinib mesylate (one study, three dose-related results), taurolidine (one study), interferon (IFN)-alpha (two studies), chimeric human/murine anti-GD2 ch14.18 monoclonal antibody (mAb) (one study), granulocyte-macrophage colony-stimulating factor (GM-CSF) + granulocyte colony-stimulating factor (G-CSF) (one study), GM-CSF + anti-GD2 mAb + isotretinoin (one study) and 5-fluorouracil (5-FU) (two studies). The incidence of CLS in patients treated with IL-2 with other agents was 29.1% by overall estimation and 32.0% by meta-analysis. We found that the highest incidence of CLS (80.5% and 100%) was observed when IL-2 was combined with IFN-alpha. In the IL-2 + imatinib mesylate group, there was a dose-related increase in the incidence of CLS (0% → 9% → 33.3%). The incidence of CLS in patients who received IL-2 + bevacizumab (IL-2 dose: 9 μg/kg) was 100%. In cases with concomitant IL-2 + 5-FU treatment, the incidence of CLS varied from 6.3% to 25.0%, resulting in 17.5% by overall estimation and 17.1% by meta-analysis.
Two studies reported on the incidence of CLS associated with the use of IL-1 in combination with carboplatin (one study, 40% CLS incidence) or etoposide (one study, 44.4%). Three studies reported on the incidence of CLS associated with the use of GM-CSF, which ranged from 6.8% to 15.0%. The incidence of CLS in patients treated with GM-CSF was low (9.0%) by overall estimation and 10.1% by meta-analysis. The incidence of CLS by GM-CSF was 9.0% by overall estimation and 10.1% by meta-analysis.
Three studies reported on the incidence of CLS associated with the use of gemcitabine, which was very low (2.8–4.3%). The incidence of CLS caused by gemcitabine was 3.5% by overall estimation and 4.9% by meta-analysis. There were two studies that reported on the incidence of CLS associated with the use of SS1P (recombinant anti-mesothelin immunotoxin), which was 5.9% and 54.2%, and showed no dose-response.
Thirteen studies reported on the incidence of CLS associated with the use of various kinds of anti-cluster of differentiation (CD) agents, which ranged from 5.9% to 100%. The incidence of CLS by various kinds of anti-CD agents was 33.9% by overall estimation and 35.6% by meta-analysis. There were four studies that reported on the incidence of CLS associated with the use of anti-CD22 mAb, which ranged from 11.5% to 100%. The incidence of CLS by various kinds of anti-CD22 mAb was 40.7% by overall estimation and 48.1% by meta-analysis. It appeared that there was an increasing incidence of CLS with an increasing treatment dose of anti-CD22 mAb. The addition of anti-CD19 mAb to anti-CD22 mAb treatment did not result in a further increase in the incidence of CLS. Three studies reported on the incidence of CLS associated with the use of anti-CD25, which ranged from 6.7% to 100%. The incidence of CLS by various kinds of anti-CD25 was 36.7% by overall estimation and 42.2% by meta-analysis.
There were single studies reporting on other drugs associated with CLS in cancer patients, with an incidence of CLS ranging from 3.4% to 80%. The incidence of CLS was high with the use of pyrrolobenzodiazepine (one study, 62.5%), paclitaxel (one study, 80.0%), and moderate with the use of anti-B4-bR (B-cell restricted immunotoxin anti-B4-blocked ricin) (one study, 41.7%), FK973 (novel, substituted dihydro benzoxazine structurally similar to mitomycin) (one study, 35.3%), and low with the use of SGN-10 (a single-chain immunotoxin) (one study, 2.2%), clofarabine + cytarabine + liposomal daunorubicin (one study, 3.4%), cyclosporine (one study, 8.3%), dihydro benzoxazine (one study, 11.1%), ABR-217620 (naptumomab estafenatox) (one study, 15.4%), and ricin A chain-containing immunotoxin (one study, 21.4%).
There were seven studies reporting the incidence of CLS associated with BMT with or without other agents, which ranged from 6.8% to 52.7%. The incidence of CLS associated with BMT was 21.1% by overall estimation and 21.7% by meta-analysis (Table 2 and Table 3, Supplementary Figure S1o).
4. Discussion
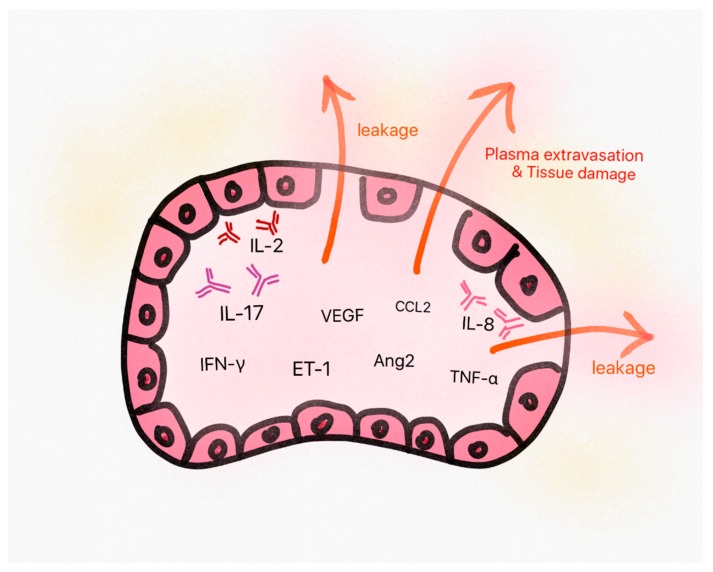
CLS is an important medical condition that is characterized by the escape of blood plasma into the interstitial space, resulting in edema, hypoalbuminemia, hemoconcentration, and low blood pressure [2]. The pathogenesis of secondary CLS due to anti-cancer treatment is not well-known, but there are several studies supporting the role of pathogenic molecules of idiopathic CLS including multiple cytokines, angiopoietin-2, and vascular endothelial growth factor (VEGF) [5,10,79,80], although the pathophysiology of idiopathic and secondary CLS may be somewhat different, because CLS by anti-cancer drugs could also develop due to a direct toxicity to the capillary system. These molecules are mostly related to an increase in the permeability of vascular endothelial cells leading to vascular leakage. Especially, multiple animal studies suggest that IL-2 causes the acute injury of normal tissues by enhancing neutrophil adhesion and generating reactive oxygen intermediates, proteases, and pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF alpha), which can cause a vascular leakage [81,82]. The proposed pathogenesis of CLS is demonstrated in Figure 2.
Figure 2.
The proposed pathogenesis of capillary leak syndrome (CLS). Some pathogenic molecules in CLS show increased levels in sera, which triggers endothelial cell damage and plasma leakage from vessels. This is supposed to eventually result in the classic triad of symptoms (hypoalbuminemia, hemoconcentration, and hypotension) and normal tissue damages. VEGF: vascular endothelial growth factor, IL: Interleukin, TNF: Tumor necrosis factor, IFN: Interferon, ET: endothelin, CCL: chemokine ligand, Ang2: angiopoietin-2.
Evaluating the incidence of CLS is challenging because the clinical presentations of CLS are non-specific, and it is expected that cases have been misdiagnosed in the past. Recently, CLS has been increasingly diagnosed due to increased awareness of the disease [4]. CLS due to anti-cancer drugs has been sporadically reported in the literature, and it has recently been registered in VigiBase (http://www.vigiaccess.org/), the World Health Organization global Individual Case Safety Report (ICSR) database, which contains reports of suspected adverse drug reactions (ADRs) collected by national drug authorities in more than 130 countries between 1967 and February 2018 [10]. However, it did not report the incidence rate of CLS for patients treated with anti-cancer drugs, and our study firstly reported the incidence of CLS according to the drugs or after BMT by meta-analysis.
Due to the lack of an overall understanding of CLS as an adverse effect of anti-cancer treatment, we carried out a systematic analysis of published clinical trials (or studies) to evaluate the incidence of anti-cancer treatment-related CLS. Through calculating the number of CLS among total patients reported in clinical trials (or studies), we were able to estimate the pooled incidence of CLS when patients were treated with several anti-cancer treatment-related drugs and after BMT. The most studied drug was IL-2, which was used as a cancer immunotherapy, and the incidence of CLS was 34.7% by overall estimation, suggesting that it may be a common adverse effect, and that the phenomenon of CLS has been underestimated in cancer patients in the past. In addition, the incidence of CLS in cancer patients differed according to the specific drug or drug combinations that were used and ranged from 5.3% to 100.0%. Therefore, our analysis shows important results that oncologists should be aware of. However, these studies did not report on the treatment strategies or clinical outcome of CLS, because most studies reported CLS as an adverse event of the drug. The clinical and laboratory data, treatment modalities, and mortality rate of patients and contributing factors leading to mortality of CLS in cancer are well analyzed in our recent systematic review of sporadic case reports [4].
We also found that BMT may be an important risk factor for CLS in cancer patients. The incidence of CLS associated with BMT with or without other agents ranged from 6.8% to 52.7%. The pathophysiology of CLS in BMT-related CLS has not been fully studied, but some hypotheses on the contributing factors have been suggested such as pivotal contribution by circulating leukocytes, decreased C1 esterase inhibitor activity, elevated C4d concentrations, the use of G-CSF or GM-CSF, and elevation of terminal complement complex (TCC) levels [70,71,72,73]. Future studies in this area may shed light on the pathophysiology of CLS associated with BMT and trigger the development of novel therapeutic approaches.
Besides IL-2 and BMT, we identified several potential causative drugs of CLS. The overall estimation of CLS incidence by causative drugs varied from 3.5% (gemcitabine, three studies) to 100% (IL-2 + bevacizumab, one study). Studies with IL-2 + bevacizumab (one study, 100%) and IL-2 + IFN-alpha 2a (two studies, overall estimation 85.5%, meta-analysis 90.4%) showed relatively high CLS incidence proportions, while studies with gemcitabine (three studies, overall estimation 3.5%, meta-analysis 4.9%) and GM-CSF (three studies, overall estimation 9.0%, meta-analysis 10.1%) showed low incidence. Likewise, anti-cancer agents, including IL-2 + imatinib mesylate (three studies) and anti-CD22 mAb (four studies) showed a dose-dependent increase in the incidence of CLS. Considering the small number of studies, it is difficult to state whether there are dose-related trends for these agents. Further studies should be performed to clarify this relationship in order to establish comprehensive therapeutic guidelines, taking CLS as an adverse effect into account.
Several limitations of this study should be considered. First, the studies that we included were not placebo-controlled trials with a control arm that would allow defining how much of the CLS was attributable to treatment rather than the type and severity of the treated condition. Since the included individual studies just reported the number of CLS as an adverse event of anti-cancer drugs, the prognosis and long-term outcome of CLS could not be addressed. Therefore, further clinical trials or observational studies should attempt to address the prospective associations between CLS and anti-cancer treatment. Second, coexisting conditions were not considered in our study. For example, we extracted the name of the causative drug and its dose, but other effects such as drug combination or cumulative effects may have affected the outcomes. Also, there might be other causes for CLS besides anti-cancer treatment, so potential confounders should be acknowledged.
5. Conclusions
Our study is the first systematic analysis of the incidence of CLS in cancer patients treated with various anti-cancer agents and therapy. The incidence of CLS due to IL-2 (18 studies) was 34.7% by overall estimation and 43.9% by meta-analysis, and the corresponding figures for BMT were 21.1% and 21.7%, respectively CLS was also reported in cases receiving other agents. Our study results highlight the need for inclusion of the risk of development of CLS in the choice of treatment and preparation of the appropriate management for cancer patients in anticipation of this syndrome. Thus, we recommend that physicians and oncologists should be aware of secondary CLS in cancer patients during anti-cancer treatment, and encourage careful observation to prevent CLS or enable timely management when CLS develops.
Supplementary Materials
The following are available online at http://www.mdpi.com/2077-0383/8/2/143/s1, Table S1: Checklist summarizing compliance with PRISMA guidelines, Figure S1: Forest plot of meta-analysis to estimate the incidence of capillary leak syndrome according to various anti-cancer treatments.
Author Contributions
J.I.S. and J.H.O. designed the study. J.H.O., I.R.L., K.H.L., J.W.S. and J.I.S. collected the data and J.I.S. did the analysis. G.H.J., J.I.S., M.E., D.W.K., H.J.v.d.V., A.K., O.A.R., B.S., M.S., N.V., E.D., A.K. and J.R. wrote the first draft of the manuscript and gave critical comments on manuscript draft. All authors had full access to all the study data. All authors reviewed, wrote and approved the final version. The corresponding author had final responsibility for the decision to submit for publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Clarkson B., Thompson D., Horwith M., Luckey E.H. Cyclical edema and shock due to increased capillary permeability. Am. J. Med. 1960;29:193–216. doi: 10.1016/0002-9343(60)90018-8. [DOI] [PubMed] [Google Scholar]
- 2.Druey K.M., Greipp P.R. Narrative review: The systemic capillary leak syndrome. Ann. Intern. Med. 2010;153:90–98. doi: 10.7326/0003-4819-153-2-201007200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eo T.S., Chun K.J., Hong S.J., Kim J.Y., Lee I.R., Lee K.H., Eisenhut M., Kronbichler A., Shin J.I. Clinical Presentation, Management, and Prognostic Factors of Idiopathic Systemic Capillary Leak Syndrome: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2018;6:609–618. doi: 10.1016/j.jaip.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Shin J.I., Lee K.H., Lee I.R., Oh J.H., Kim D.W., Shin J.W., Eo T.S., Kronbichler A., Eisenhut M., van der Vliet H.J. Systemic Capillary Leak Syndrome (Clarkson Syndrome) in Cancer Patients: A Systematic Review. J. Clin. Med. 2018;7:418. doi: 10.3390/jcm7110418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddall E., Khatri M., Radhakrishnan J. Capillary leak syndrome: Etiologies, pathophysiology, and management. Kidney Int. 2017;92:37–46. doi: 10.1016/j.kint.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Duron L., Delestre F., Amoura Z., Arnaud L. Idiopathic and secondary capillary leak syndromes: A systematic review of the literature. Rev. Med. Interne. 2015;36:386–394. doi: 10.1016/j.revmed.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Gousseff M., Arnaud L., Lambert M., Hot A., Hamidou M., Duhaut P., Papo T., Soubrier M., Ruivard M., Malizia G., et al. The systemic capillary leak syndrome: A case series of 28 patients from a European registry. Ann. Intern. Med. 2011;154:464–471. doi: 10.7326/0003-4819-154-7-201104050-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lambert M., Launay D., Hachulla E., Morell-Dubois S., Soland V., Queyrel V., Fourrier F., Hatron P.Y. High-dose intravenous immunoglobulins dramatically reverse systemic capillary leak syndrome. Crit. Care Med. 2008;36:2184–2187. doi: 10.1097/CCM.0b013e31817d7c71. [DOI] [PubMed] [Google Scholar]
- 9.Marra A.M., Gigante A., Rosato E. Intravenous immunoglobulin in systemic capillary leak syndrome: A case report and review of literature. Expert Rev. Clin. Immunol. 2014;10:349–352. doi: 10.1586/1744666X.2014.882771. [DOI] [PubMed] [Google Scholar]
- 10.Mertz P., Lebrun-Vignes B., Salem J.E., Arnaud L. Characterizing drug-induced capillary leak syndromes using the World Health Organization VigiBase. J. Allergy Clin. Immunol. 2019;143:433–436. doi: 10.1016/j.jaci.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Shin J.I., Lee J.S. Beneficial effect of intravenous immunoglobulins on systemic capillary leak syndrome in patients with monoclonal gammopathy. Crit. Care Med. 2009;37:795. doi: 10.1097/CCM.0b013e3181959c3d. [DOI] [PubMed] [Google Scholar]
- 12.Atkins M.B., Lotze M.T., Dutcher J.P., Fisher R.I., Weiss G., Margolin K., Abrams J., Sznol M., Parkinson D., Hawkins M., et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 13.Sparano J.A., Fisher R.I., Sunderland M., Margolin K., Ernest M.L., Sznol M., Atkins M.B., Dutcher J.P., Micetich K.C., Weiss G.R., et al. Randomized phase III trial of treatment with high-dose interleukin-2 either alone or in combination with interferon alfa-2a in patients with advanced melanoma. J. Clin. Oncol. 1993;11:1969–1977. doi: 10.1200/JCO.1993.11.10.1969. [DOI] [PubMed] [Google Scholar]
- 14.Tarhini A.A., Kirkwood J.M., Gooding W.E., Cai C., Agarwala S.S. Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. J. Clin. Oncol. 2007;25:3802–3807. doi: 10.1200/JCO.2006.10.2822. [DOI] [PubMed] [Google Scholar]
- 15.Talpur R., Duvic M. Pilot study of denileukin diftitox alternate dosing regimen in patients with cutaneous peripheral T-cell lymphomas. Clin. Lymphoma Myeloma Leuk. 2012;12:180–185. doi: 10.1016/j.clml.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher D.C., Bhatt R.S., Parikh S.M., Patel P., Seery V., McDermott D.F., Atkins M.B., Sukhatme V.P. Angiopoietin 2 is a potential mediator of high-dose interleukin 2-induced vascular leak. Clin. Cancer Res. 2007;13:2115–2120. doi: 10.1158/1078-0432.CCR-06-2509. [DOI] [PubMed] [Google Scholar]
- 17.Shusterman S., London W.B., Gillies S.D., Hank J.A., Voss S.D., Seeger R.C., Reynolds C.P., Kimball J., Albertini M.R., Wagner B., et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: A Children’s Oncology Group (COG) phase II study. J. Clin. Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaughnessy P.J., Bachier C., Grimley M., Freytes C.O., Callander N.S., Essell J.H., Flomenberg N., Selby G., Lemaistre C.F. Denileukin diftitox for the treatment of steroid-resistant acute graft-versus-host disease. Biol. Blood Marrow. Transplant. 2005;11:188–193. doi: 10.1016/j.bbmt.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Frankel A.E., Fleming D.R., Hall P.D., Powell B.L., Black J.H., Leftwich C., Gartenhaus R. A phase II study of DT fusion protein denileukin diftitox in patients with fludarabine-refractory chronic lymphocytic leukemia. Clin. Cancer Res. 2003;9:3555–3561. [PubMed] [Google Scholar]
- 20.Duvic M., Kuzel T.M., Olsen E.A., Martin A.G., Foss F.M., Kim Y.H., Heald P.W., Bacha P., Nichols J., Liepa A. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ONTAK) Clin. Lymphoma. 2002;2:222–228. doi: 10.3816/CLM.2002.n.003. [DOI] [PubMed] [Google Scholar]
- 21.Foss F.M., Bacha P., Osann K.E., Demierre M.F., Bell T., Kuzel T. Biological correlates of acute hypersensitivity events with DAB(389)IL-2 (denileukin diftitox, ONTAK) in cutaneous T-cell lymphoma: Decreased frequency and severity with steroid premedication. Clin. Lymphoma. 2001;1:298–302. doi: 10.3816/CLM.2001.n.005. [DOI] [PubMed] [Google Scholar]
- 22.Sievers E.L., Lange B.J., Sondel P.M., Krailo M.D., Gan J., Tjoa T., Liu-Mares W., Feig S.A. Children’s cancer group trials of interleukin-2 therapy to prevent relapse of acute myelogenous leukemia. Cancer J. Sci. Am. 2000;6(Suppl. 1):S39–S44. [PubMed] [Google Scholar]
- 23.Duvic M., Cather J., Maize J., Frankel A.E. DAB389IL2 diphtheria fusion toxin produces clinical responses in tumor stage cutaneous T cell lymphoma. Am. J. Hematol. 1998;58:87–90. doi: 10.1002/(SICI)1096-8652(199805)58:1<87::AID-AJH18>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Meehan K.R., Verma U.N., Cahill R., Frankel S., Areman E.M., Sacher R.A., Foelber R., Rajagopal C., Gehan E.A., Lippman M.E., et al. Interleukin-2-activated hematopoietic stem cell transplantation for breast cancer: Investigation of dose level with clinical correlates. Bone Marrow. Transplant. 1997;20:643–651. doi: 10.1038/sj.bmt.1700954. [DOI] [PubMed] [Google Scholar]
- 25.Chang A.E., Yoshizawa H., Sakai K., Cameron M.J., Sondak V.K., Shu S. Clinical observations on adoptive immunotherapy with vaccine-primed T-lymphocytes secondarily sensitized to tumor in vitro. Cancer Res. 1993;53:1043–1050. [PubMed] [Google Scholar]
- 26.Van Haelst Pisani C., Kovach J.S., Kita H., Leiferman K.M., Gleich G.J., Silver J.E., Dennin R., Abrams J.S. Administration of interleukin-2 (IL-2) results in increased plasma concentrations of IL-5 and eosinophilia in patients with cancer. Blood. 1991;78:1538–1544. [PubMed] [Google Scholar]
- 27.Philip T., Mercatello A., Negrier S., Philip I., Rebattu P., Kaemmerlin P., Gaspard M., Tognier E., Combaret V., Bijmann J.T., et al. Interleukin-2 with and without LAK cells in metastatic renal cell carcinoma: The Lyon first-year experience in 20 patients. Cancer Treat. Rev. 1989;16(Suppl. A):91–104. doi: 10.1016/0305-7372(89)90028-5. [DOI] [PubMed] [Google Scholar]
- 28.Carey P.D., Wakefield C.H., Guillou P.J. Neutrophil activation, vascular leak toxicity, and cytolysis during interleukin-2 infusion in human cancer. Surgery. 1997;122:918–926. doi: 10.1016/S0039-6060(97)90333-0. [DOI] [PubMed] [Google Scholar]
- 29.Pautier P., Locher C., Robert C., Deroussent A., Flament C., Le Cesne A., Rey A., Bahleda R., Ribrag V., Soria J.C., et al. Phase I clinical trial combining imatinib mesylate and IL-2 in refractory cancer patients: IL-2 interferes with the pharmacokinetics of imatinib mesylate. Oncoimmunology. 2013;2:e23079. doi: 10.4161/onci.23079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien G.C., Cahill R.A., Bouchier-Hayes D.J., Redmond H.P. Co-immunotherapy with interleukin-2 and taurolidine for progressive metastatic melanoma. Ir. J. Med. Sci. 2006;175:10–14. doi: 10.1007/BF03168992. [DOI] [PubMed] [Google Scholar]
- 31.Pichert G., Jost L.M., Fierz W., Stahel R.A. Clinical and immune modulatory effects of alternative weekly interleukin-2 and interferon alfa-2a in patients with advanced renal cell carcinoma and melanoma. Br. J. Cancer. 1991;63:287–292. doi: 10.1038/bjc.1991.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilman A.L., Ozkaynak M.F., Matthay K.K., Krailo M., Yu A.L., Gan J., Sternberg A., Hank J.A., Seeger R., Reaman G.H., et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: A report from the Children’s Oncology Group. J. Clin. Oncol. 2009;27:85–91. doi: 10.1200/JCO.2006.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meehan K.R., Talebian L., Wu J., Hill J.M., Szczepiorkowski Z.M., Sentman C.L., Ernstoff M.S. Immune mobilization of autologous blood progenitor cells: Direct influence on the cellular subsets collected. Cytotherapy. 2010;12:1013–1021. doi: 10.3109/14653249.2010.515580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu A.L., Gilman A.L., Ozkaynak M.F., London W.B., Kreissman S.G., Chen H.X., Smith M., Anderson B., Villablanca J.G., Matthay K.K., et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamblin T.J., Sadullah S., Williamson P., Stevenson J., Oskam R., Palmer P., Franks C.R. A phase-III study of recombinant interleukin 2 and 5-fluorouracil chemotherapy in patients with metastatic colorectal cancer. Br. J. Cancer. 1993;68:1186–1189. doi: 10.1038/bjc.1993.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savage P., Costelna D., Moore J., Gore M.E. A phase II study of continuous infusional 5-fluorouracil (5-FU) and subcutaneous interleukin-2 (IL-2) in metastatic renal cancer. Eur. J. Cancer. 1997;33:1149–1151. doi: 10.1016/S0959-8049(96)00515-1. [DOI] [PubMed] [Google Scholar]
- 37.Smith J.W., Longo D.L., Alvord W.G., Janik J.E., Sharfman W.H., Gause B.L., Curti B.D., Creekmore S.P., Holmlund J.T., Fenton R.G., et al. The effects of treatment with interleukin-1 alpha on platelet recovery after high-dose carboplatin. N. Engl. J. Med. 1993;328:756–761. doi: 10.1056/NEJM199303183281103. [DOI] [PubMed] [Google Scholar]
- 38.Worth L.L., Jaffe N., Benjamin R.S., Papadopoulos N.E., Patel S., Raymond A.K., Jia S.F., Rodriguez C., Gano J., Gianan M.A., et al. Phase II study of recombinant interleukin 1alpha and etoposide in patients with relapsed osteosarcoma. Clin. Cancer Res. 1997;3:1721–1729. [PubMed] [Google Scholar]
- 39.Sosman J.A., Fisher S.G., Kefer C., Fisher R.I., Ellis T.M. A phase I trial of continuous infusion interleukin-4 (IL-4) alone and following interleukin-2 (IL-2) in cancer patients. Ann. Oncol. 1994;5:447–452. doi: 10.1093/oxfordjournals.annonc.a058878. [DOI] [PubMed] [Google Scholar]
- 40.Gorin N.C., Coiffier B., Hayat M., Fouillard L., Kuentz M., Flesch M., Colombat P., Boivin P., Slavin S., Philip T. Recombinant human granulocyte-macrophage colony-stimulating factor after high-dose chemotherapy and autologous bone marrow transplantation with unpurged and purged marrow in non-Hodgkin’s lymphoma: A double-blind placebo-controlled trial. Blood. 1992;80:1149–1157. [PubMed] [Google Scholar]
- 41.Liberati A.M., Cinieri S., Schippa M., Di Clemente F., Filippo S., Grignani F. GM-CSF: Clinical trials in non-Hodgkin’s lymphoma patients with chemotherapy induced leucopenia. Leukemia. 1991;5:119–122. [PubMed] [Google Scholar]
- 42.Steward W.P., Scarffe J.H., Austin R., Bonnem E., Thatcher N., Morgenstern G., Crowther D. Recombinant human granulocyte macrophage colony stimulating factor (rhGM-CSF) given as daily short infusions--a phase I dose-toxicity study. Br. J. Cancer. 1989;59:142–145. doi: 10.1038/bjc.1989.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jidar K., Ingen-Housz-Oro S., Beylot-Barry M., Paul C., Chaoui D., Sigal-Grinberg M., Morel P., Dubertret L., Bachelez H. Gemcitabine treatment in cutaneous T-cell lymphoma: A multicentre study of 23 cases. Br. J. Dermatol. 2009;161:660–663. doi: 10.1111/j.1365-2133.2009.09230.x. [DOI] [PubMed] [Google Scholar]
- 44.Kurosaki I., Kawachi Y., Nihei K., Tsuchiya Y., Aono T., Yokoyama N., Shimizu T., Hatakeyama K. Liver perfusion chemotherapy with 5-Fluorouracil followed by systemic gemcitabine administration for resected pancreatic cancer: Preliminary results of a prospective phase 2 study. Pancreas. 2009;38:161–167. doi: 10.1097/MPA.0b013e31818815f7. [DOI] [PubMed] [Google Scholar]
- 45.Dumontet C., Morschhauser F., Solal-Celigny P., Bouafia F., Bourgeois E., Thieblemont C., Leleu X., Hequet O., Salles G., Coiffier B. Gemcitabine as a single agent in the treatment of relapsed or refractory low-grade non-Hodgkin’s lymphoma. Br. J. Haematol. 2001;113:772–778. doi: 10.1046/j.1365-2141.2001.02795.x. [DOI] [PubMed] [Google Scholar]
- 46.Kreitman R.J., Hassan R., Fitzgerald D.J., Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin. Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan R., Bullock S., Premkumar A., Kreitman R.J., Kindler H., Willingham M.C., Pastan I. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin. Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 48.Sausville E.A., Headlee D., Stetler-Stevenson M., Jaffe E.S., Solomon D., Figg W.D., Herdt J., Kopp W.C., Rager H., Steinberg S.M., et al. Continuous infusion of the anti-CD22 immunotoxin IgG-RFB4-SMPT-dgA in patients with B-cell lymphoma: A phase I study. Blood. 1995;85:3457–3465. [PubMed] [Google Scholar]
- 49.Vitetta E.S., Stone M., Amlot P., Fay J., May R., Till M., Newman J., Clark P., Collins R., Cunningham D., et al. Phase I immunotoxin trial in patients with B-cell lymphoma. Cancer Res. 1991;51:4052–4058. [PubMed] [Google Scholar]
- 50.Wayne A.S., Shah N.N., Bhojwani D., Silverman L.B., Whitlock J.A., Stetler-Stevenson M., Sun W., Liang M., Yang J., Kreitman R.J., et al. Phase 1 study of the anti-CD22 immunotoxin moxetumomab pasudotox for childhood acute lymphoblastic leukemia. Blood. 2017;130:1620–1627. doi: 10.1182/blood-2017-02-749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amlot P.L., Stone M.J., Cunningham D., Fay J., Newman J., Collins R., May R., McCarthy M., Richardson J., Ghetie V., et al. A phase I study of an anti-CD22-deglycosylated ricin A chain immunotoxin in the treatment of B-cell lymphomas resistant to conventional therapy. Blood. 1993;82:2624–2633. [PubMed] [Google Scholar]
- 52.Stathis A., Freedman A.S., Flinn I.W., Maddocks K.J., Weitman S., Berdeja J.G., Mejia A.V., Zucca E., Green R., Romanelli A., et al. A Phase I Study of IMGN529, an Antibody-Drug Conjugate (ADC) Targeting CD37, in Adult Patients with Relapsed or Refractory B-Cell Non-Hodgkin’s Lymphoma (NHL) Blood. 2014;124:1760. [Google Scholar]
- 53.Schindler J., Gajavelli S., Ravandi F., Shen Y., Parekh S., Braunchweig I., Barta S., Ghetie V., Vitetta E., Verma A. A phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia. Br. J. Haematol. 2011;154:471–476. doi: 10.1111/j.1365-2141.2011.08762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bachanova V., Frankel A.E., Cao Q., Lewis D., Grzywacz B., Verneris M.R., Ustun C., Lazaryan A., McClune B., Warlick E.D., et al. Phase I study of a bispecific ligand-directed toxin targeting CD22 and CD19 (DT2219) for refractory B-cell malignancies. Clin. Cancer Res. 2015;21:1267–1272. doi: 10.1158/1078-0432.CCR-14-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schnell R., Borchmann P., Staak J.O., Schindler J., Ghetie V., Vitetta E.S., Engert A. Clinical evaluation of ricin A-chain immunotoxins in patients with Hodgkin’s lymphoma. Ann. Oncol. 2003;14:729–736. doi: 10.1093/annonc/mdg209. [DOI] [PubMed] [Google Scholar]
- 56.Schnell R., Vitetta E., Schindler J., Borchmann P., Barth S., Ghetie V., Hell K., Drillich S., Diehl V., Engert A. Treatment of refractory Hodgkin’s lymphoma patients with an anti-CD25 ricin A-chain immunotoxin. Leukemia. 2000;14:129–135. doi: 10.1038/sj.leu.2401626. [DOI] [PubMed] [Google Scholar]
- 57.Engert A., Diehl V., Schnell R., Radszuhn A., Hatwig M.T., Drillich S., Schon G., Bohlen H., Tesch H., Hansmann M.L., et al. A phase-I study of an anti-CD25 ricin A-chain immunotoxin (RFT5-SMPT-dgA) in patients with refractory Hodgkin’s lymphoma. Blood. 1997;89:403–410. [PubMed] [Google Scholar]
- 58.Schnell R., Staak O., Borchmann P., Schwartz C., Matthey B., Hansen H., Schindler J., Ghetie V., Vitetta E.S., Diehl V., et al. A Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma. Clin. Cancer Res. 2002;8:1779–1786. [PubMed] [Google Scholar]
- 59.Stone M.J., Sausville E.A., Fay J.W., Headlee D., Collins R.H., Figg W.D., Stetler-Stevenson M., Jain V., Jaffe E.S., Solomon D., et al. A phase I study of bolus versus continuous infusion of the anti-CD19 immunotoxin, IgG-HD37-dgA, in patients with B-cell lymphoma. Blood. 1996;88:1188–1197. [PubMed] [Google Scholar]
- 60.Uckun F.M., Messinger Y., Chen C.L., O’Neill K., Myers D.E., Goldman F., Hurvitz C., Casper J.T., Levine A. Treatment of therapy-refractory B-lineage acute lymphoblastic leukemia with an apoptosis-inducing CD19-directed tyrosine kinase inhibitor. Clin. Cancer Res. 1999;5:3906–3913. [PubMed] [Google Scholar]
- 61.Baluna R., Sausville E.A., Stone M.J., Stetler-Stevenson M.A., Uhr J.W., Vitetta E.S. Decreases in levels of serum fibronectin predict the severity of vascular leak syndrome in patients treated with ricin A chain-containing immunotoxins. Clin. Cancer Res. 1996;2:1705–1712. [PubMed] [Google Scholar]
- 62.Borghaei H., Alpaugh K., Hedlund G., Forsberg G., Langer C., Rogatko A., Hawkins R., Dueland S., Lassen U., Cohen R.B. Phase I dose escalation, pharmacokinetic and pharmacodynamic study of naptumomab estafenatox alone in patients with advanced cancer and with docetaxel in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2009;27:4116–4123. doi: 10.1200/JCO.2008.20.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hochhauser D., Meyer T., Spanswick V.J., Wu J., Clingen P.H., Loadman P., Cobb M., Gumbrell L., Begent R.H., Hartley J.A., et al. Phase I study of sequence-selective minor groove DNA binding agent SJG-136 in patients with advanced solid tumors. Clin. Cancer Res. 2009;15:2140–2147. doi: 10.1158/1078-0432.CCR-08-1315. [DOI] [PubMed] [Google Scholar]
- 64.Posey J.A., Khazaeli M.B., Bookman M.A., Nowrouzi A., Grizzle W.E., Thornton J., Carey D.E., Lorenz J.M., Sing A.P., Siegall C.B., et al. A phase I trial of the single-chain immunotoxin SGN-10 (BR96 sFv-PE40) in patients with advanced solid tumors. Clin. Cancer Res. 2002;8:3092–3099. [PubMed] [Google Scholar]
- 65.Elias A.D., Richardson P., Avigan D., Ibrahim J., Joyce R., Demetri G., Levine J., Warren D., Arthur T., Reich E., et al. A short course of induction chemotherapy followed by two cycles of high-dose chemotherapy with stem cell rescue for chemotherapy naive metastatic breast cancer. Bone Marrow. Transplant. 2001;27:269–278. doi: 10.1038/sj.bmt.1702780. [DOI] [PubMed] [Google Scholar]
- 66.Grossbard M.L., Gribben J.G., Freedman A.S., Lambert J.M., Kinsella J., Rabinowe S.N., Eliseo L., Taylor J.A., Blattler W.A., Epstein C.L., et al. Adjuvant immunotoxin therapy with anti-B4-blocked ricin after autologous bone marrow transplantation for patients with B-cell non-Hodgkin’s lymphoma. Blood. 1993;81:2263–2271. [PubMed] [Google Scholar]
- 67.Pazdur R., Ho D.H., Daugherty K., Bradner W.T., Krakoff I.H., Raber M.N. Phase I trial of FK973: Description of a delayed vascular leak syndrome. Investig. New Drugs. 1991;9:377–382. doi: 10.1007/BF00183587. [DOI] [PubMed] [Google Scholar]
- 68.Barrett A.J., Kendra J.R., Lucas C.F., Joss D.V., Joshi R., Pendharkar P., Hugh-Jones K. Cyclosporin A as prophylaxis against graft-versus-host disease in 36 patients. Br. Med. J. (Clin. Res. Ed.) 1982;285:162–166. doi: 10.1136/bmj.285.6336.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zwaan C.M., Dworzak M., Klingebiel T., Rössig C., Leverger G., Stary J., Bont E.S.D., Ramnarain S., Bertrand Y., Brethon B., Strahm B., et al. Clofarabine in Combination with High-Dose Cytarabine and Liposomal Daunorubicin in Pediatric AML: Results of a Phase 1B Combination Study By the ITCC Consortium. Blood. 2014;124:989. [Google Scholar]
- 70.Cahill R.A., Spitzer T.R., Mazumder A. Marrow engraftment and clinical manifestations of capillary leak syndrome. Bone Marrow. Transplant. 1996;18:177–184. [PubMed] [Google Scholar]
- 71.Nurnberger W., Michelmann I., Petrik K., Holthausen S., Willers R., Lauermann G., Eisele B., Delvos U., Burdach S., Gobel U. Activity of C1 esterase inhibitor in patients with vascular leak syndrome after bone marrow transplantation. Ann. Hematol. 1993;67:17–21. doi: 10.1007/BF01709661. [DOI] [PubMed] [Google Scholar]
- 72.Nurnberger W., Heying R., Burdach S., Gobel U. C1 esterase inhibitor concentrate for capillary leakage syndrome following bone marrow transplantation. Ann. Hematol. 1997;75:95–101. doi: 10.1007/s002770050321. [DOI] [PubMed] [Google Scholar]
- 73.Salat C., Holler E., Schleuning M., Eisele B., Reinhardt B., Kolb H., Pihusch R., Domrath R., Hiller E. Levels of the terminal complement complex, C3a-desArg and C1-inhibitor in adult patients with capillary leak syndrome following bone marrow transplantation. Ann. Hematol. 1995;71:271–274. doi: 10.1007/BF01697978. [DOI] [PubMed] [Google Scholar]
- 74.DerSimonian R., Laird N. Meta-analysis in clinical trials. Controlled Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 75.Lau J., Ioannidis J.P., Schmid C.H. Quantitative synthesis in systematic reviews. Ann. Intern. Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 76.Ioannidis J.P., Tarone R., McLaughlin J.K. The false-positive to false-negative ratio in epidemiologic studies. Epidemiology. 2011;22:450–456. doi: 10.1097/EDE.0b013e31821b506e. [DOI] [PubMed] [Google Scholar]
- 77.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. Ed.) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334:94–96. doi: 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie Z., Chan E., Yin Y., Ghosh C.C., Wisch L., Nelson C., Young M., Parikh S.M., Druey K.M. Inflammatory Markers of the Systemic Capillary Leak Syndrome (Clarkson Disease) J. Clin. Cell. Immunol. 2014;5:1000213. doi: 10.4172/2155-9899.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie Z., Ghosh C.C., Patel R., Iwaki S., Gaskins D., Nelson C., Jones N., Greipp P.R., Parikh S.M., Druey K.M. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome) Blood. 2012;119:4321–4332. doi: 10.1182/blood-2011-08-375816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lentsch A.B., Miller F.N., Edwards M.J. Mechanisms of leukocyte-mediated tissue injury induced by interleukin-2. Cancer Immunol. Immunother. 1999;47:243–248. doi: 10.1007/s002620050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H., Song S., Kou G., Li B., Zhang D., Hou S., Qian W., Dai J., Tian L., Zhao J., et al. Treatment of hepatocellular carcinoma in a mouse xenograft model with an immunotoxin which is engineered to eliminate vascular leak syndrome. Cancer Immunol. Immunother. 2007;56:1775–1783. doi: 10.1007/s00262-007-0321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.