Abstract
KCNMA1 is a gene located at 10q22 that encodes the pore-forming α-subunit of the large-conductance Ca2+-activated K+ channel. KCNMA1 is down-regulated in gastric carcinoma tumors, through hypermethylation of its promoter. In the present study, we have evaluated the expression levels of KCNMA1 both in a mouse model of Colorectal Cancer (CRC) and in human CRC samples. Additionally, epigenetic mechanisms of KCNMA1 gene regulation were investigated. We observed a significant down-regulation of KCNMA1 both in a human and mouse model of CRC. No differences in KCNMA1 levels were, however, observed at different TNM stages. We also wanted to determine whether the modulation in KCNMA1 was dependent on epigenetic mechanisms. A statistically significant inverse correlation between KCNMA1 expression and mir-17-5p levels was observed in patients with CRC. Furthermore, in the tumor samples, we found a significant hypermethylation of the promoter, in the loci cg24113782 and cg25655799, compared to healthy tissue. Overall, our data suggest the possible use of KCNMA1 as a therapeutic target in the early stages of CRC.
Keywords: KCNMA1, Colorectal Cancer, epigenetics, DNA methylation, mir-17-5p, mir-31, mir-211
1. Introduction
KCNMA1 is a gene encoding the Calcium-Activated Potassium Channel Subunit Alpha-1, also known as the Large Conductance Calcium-Activated Potassium Channel, Subfamily M, Alpha Member 1 (KCa1.1), which represents high voltage-activated channel conductance for potassium ions [1]. KCNMA1 is located at chromosome 10 (10q22.3) and chromosome 14 in the human and murine genome, respectively. The channel consists of four subunits that self-assemble to form homo-tetramers and is located in the endoplasmic reticulum, in the Golgi apparatus, and in the cellular plasma membrane [1]. The channel is involved in different tumor processes, from cell proliferation to apoptosis, and response to hypoxia and to chemotherapeutic agents. Growing evidence indicates that K+ channels may be involved in the oncogenesis process. Amplification of the KCNMA1 gene was observed in 16% of advanced prostate tumors [2] and an up-regulation of its expression was observed in breast carcinoma [3,4,5], prostate cancer [6], glioblastoma [7], and cervical cancers [8]. In contrast, KCNMA1 is down-regulated or silenced in primary cells and in gastric carcinoma cell lines (MGC-803, BGC-823, MKN-82, SGC-7901), through hyper-methylation of its promoter, in particular, the CpG island, cg24113782 [9]. A role for miRNAs, in particular mir-17-5p, mir-31, and mir-211, in the regulation of KCNMA1 expression has also been determined in different tumors, including ovarian cancer [10], pleural mesothelioma [11], and cutaneous melanoma [12].
The primary aim of this study was to define the modulation of the KCNMA1 gene, both in a mouse model of colorectal cancer (CRC) and in human CRC samples. To this aim, we have used the well-established model of CRC, induced by the administration of dextran sodium sulfate (DSS)/azoxymethane (AOM), which rapidly recapitulates the aberrant crypt foci-adenoma-carcinoma sequence that occurs in human CRC [13,14]. The secondary aim of the study was to determine whether the modulation of this gene may correlate with the disease stage or the Overall Survival (OS) of the patients. Finally, we have evaluated the potential epigenetic mechanisms involved in the regulation of KCNMA1 expression in CRC.
2. Results
2.1. KCNMA1 Expression Is Modulated in Preclinical Models of Ulcerative Colitis (UC) and UC-Associated CRC
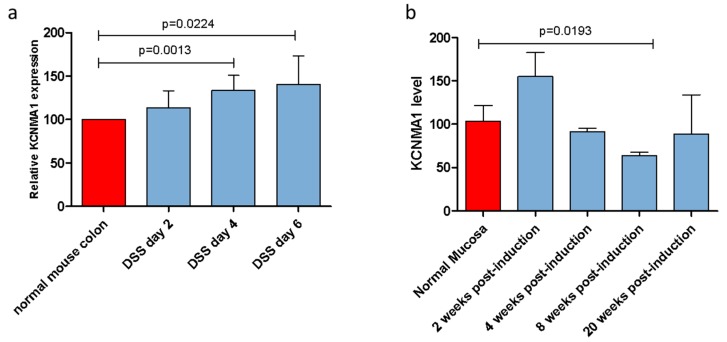
In the UC model induced by DSS administration, a significant increase in KCNMA1 levels was observed in the inflamed colonic mucosa, starting from day 4 (p = 0.0013) up to day 6 post-induction (p = 0.0224) (Figure 1a). Similarly, a 49.4% increase in KCNMA1 levels was observed in the second week after the DSS/AOM administration in the colorectal model (Figure 1b). In contrast, an important reduction of KCNMA1 expression levels in the colorectal mucosa can be observed starting from the fourth week of DSS/AOM administration, reaching statistical significance in the mucosa with high degree dysplasia (eighth week) (p = 0.01923).
Figure 1.
KCNMA1 is modulated in murine models of ulcerative colitis (UC) and colorectal cancer. (a) Transcriptional levels of KCNMA1 in a murine model of UC induced by the administration of 3% dextran sodium sulfate (DSS) in drinking water were determined by Real-Time PCR; (b) expression of KCNMA1 in the DSS/azoxymethane model was determined by Real-Time PCR at different time points (0, 2, 4, 8, and 20 weeks from disease induction) (n = 5–6 animals per group). The expression level is presented as percentage of increase as compared to control (normal mucosa) group, which was arbitrarily set to 100. ANOVA followed by Bonferroni post hoc test was used to assess statistical differences among groups.
At the 20th week post-induction, there was a trend toward lower levels of KCNMA1 levels in samples from diseased animals as compared to those observed in the healthy mucosa of the controls (Figure 1b).
2.2. KCNMA1 Levels Are Reduced in Human CRC
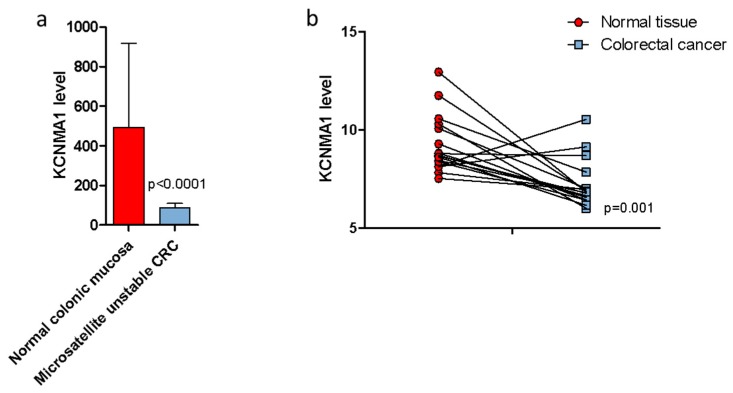
In order to confirm the results obtained in the DSS/AOM murine model, the microarray datasets GSE24514 and GSE32323 were analyzed. As shown in Figure 2, significantly lower levels of KCNMA1 (p < 0.001) were observed in patients with colorectal adenocarcinoma bearing microsatellite instability (MSI) as compared to healthy patients (Figure 2a), and in neoplastic tissue as compared to healthy mucosa (Figure 2b).
Figure 2.
KCNMA1 expression is modulated in colorectal cancer (CRC). (a) KCNMA1 levels were determined in colonic mucosa of healthy subjects (n = 15) and in patients with microsatellite unstable CRC (n = 34); (b) and on neoplastic and normal tissue of each patient (n = 17). Unpaired (a) and paired (b) two-tailed Student’s T test was applied to assess statistical differences between groups.
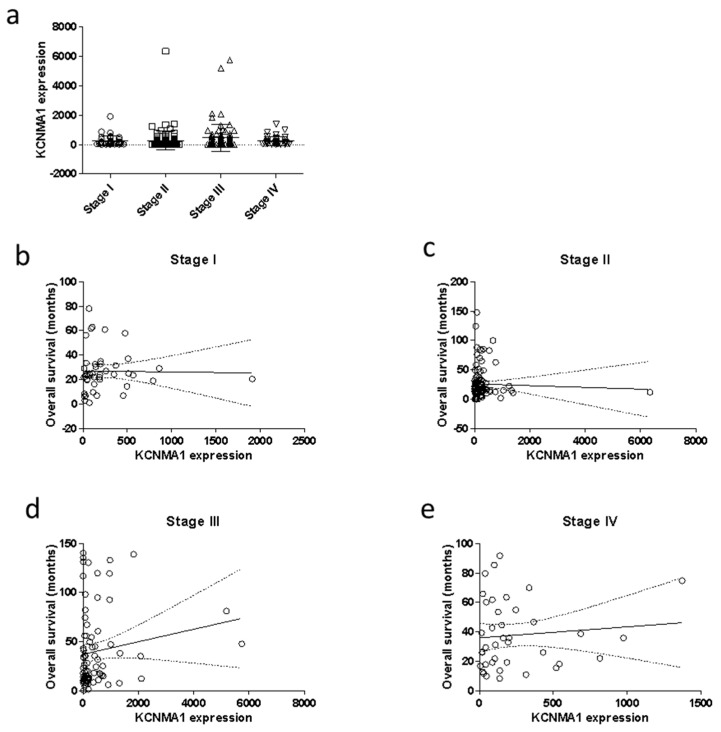
No correlation was found between the transcriptional levels of KCNMA1 and the tumor stage (Figure 3a). We have also performed a correlation analysis between KCNMA1 and the OS, stratifying the patients’ population for stage of the disease. As shown in Figure 3, there is no significant correlation between KCNMA1 and survival, although a trend of correlation (p = 0.1839) can be observed in Stage III patients. (Figure 3d).
Figure 3.
Modulation of the transcriptional levels of KCNMA1 in relation to TNM stage and overall survival (OS). Data regarding the levels of KCNMA1 in patients (n = 282) with CRC were obtained from the TGCA database, through the cBioPortal for Cancer Genomics (http://www.cbioportal.org/) website. Statistical differences among groups were evaluated by applying ANOVA test. The correlation between KCNMA1 levels and OS was calculated by using the non-parametric Spearman’s test. (a) KCNMA1 expression levels in CRC samples at different disease stages; (b) correlation between KCNMA1 expression and OS in samples of CRC at stage I; (c) correlation between KCNMA1 expression and OS in samples of CRC at stage II; (d) correlation between KCNMA1 expression and OS in samples of CRC at stage III; (e) correlation between KCNMA1 expression and OS in samples of CRC at stage IV;
Analysis of the genetic mutations in the DNA-sequenced cohort of CRC patients from the TGCA database revealed that only 3.54% of patients with CRC bears a mutation in the KCNMA1 gene (http://www.cbioportal.org/)), including one case of in-frame deletion and nine cases of missense mutations, as shown in Table 1. These results therefore suggest that the altered levels of KCNMA1 gene expression in patients with CRC are not determined by the systematic presence of genetic mutations.
Table 1.
Mutations of KCNMA1 in colorectal cancer patients.
| Sample ID | Cancer Type | Protein Change | Mutation Type | Copy # |
|---|---|---|---|---|
| TCGA-AA-3831-01 | Colon Adenocarcinoma | S60del | IF del | Diploid |
| TCGA-AA-3524-01 | Colon Adenocarcinoma | T905M | Missense | Diploid |
| TCGA-AA-A01V-01 | Colon Adenocarcinoma | R909W | Missense | Diploid |
| TCGA-AA-A00E-01 | Colon Adenocarcinoma | S580P | Missense | Diploid |
| TCGA-AA-A00L-01 | Colon Adenocarcinoma | A381T | Missense | Diploid |
| TCGA-AA-3984-01 | Colon Adenocarcinoma | S1190P | Missense | Diploid |
| TCGA-AA-A00N-01 | Mucinous Adenocarcinoma of the... | K1153T | Missense | Diploid |
| TCGA-AA-A00N-01 | Mucinous Adenocarcinoma of the... | N801S | Missense | Diploid |
| TCGA-AA-A010-01 | Colon Adenocarcinoma | L1236F | Missense | Diploid |
| TCGA-AA-A010-01 | Colon Adenocarcinoma | E824D | Missense | Diploid |
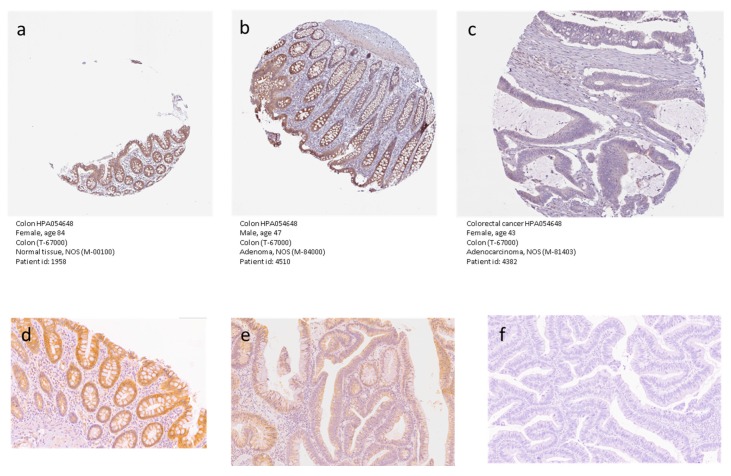
In order to validate the data from the preclinical models and the bioinformatic analysis, we first consulted the freely accessible database, The Human Protein Atlas (https://www.proteinatlas.org/). According to the database, endothelial cells of the normal colon samples show a low staining intensity for KCNMA1, while the glandular and the peripheral nerve/ganglion cells have a medium staining positivity for the HPA054648 antibody staining. As compared to normal colon samples, lower levels of KCNMA1 expression were observed in CRC samples, which showed a high staining intensity in zero out of 12 samples, medium intensity in one out of 12 samples, low intensity in six out of 12 samples, and no positivity in five out of 12 samples (https://www.proteinatlas.org/). Representative pictures of normal and CRC samples are presented in Figure 4. We have also validated the data obtained from The Human Protein Atlas by performing immunohistochemical analysis in three samples comprising normal colonic mucosa (a), tubule-villous adenoma with low-grade dysplasia (b), and adenocarcinoma (c). Protein expression was present in normal epithelium, reduced in dysplastic epithelium, and completely lost in neoplastic epithelium.
Figure 4.
Immunohistochemical analysis for KCNMA1 protein expression in samples of normal colonic mucosa (a), adenoma (b), and adenocarcinoma (c). The images obtained from The Human Protein Atlas database (https://www.proteinatlas.org/pathology). Immunohistochemical analysis for KCNMA1 protein expression in samples of normal colonic mucosa (d), tubule-villous adenoma with low-grade dysplasia (e), and adenocarcinoma (f). Magnification for all images: 20×.
2.3. MiRNA Analysis and Methylation Profile of KCNMA1
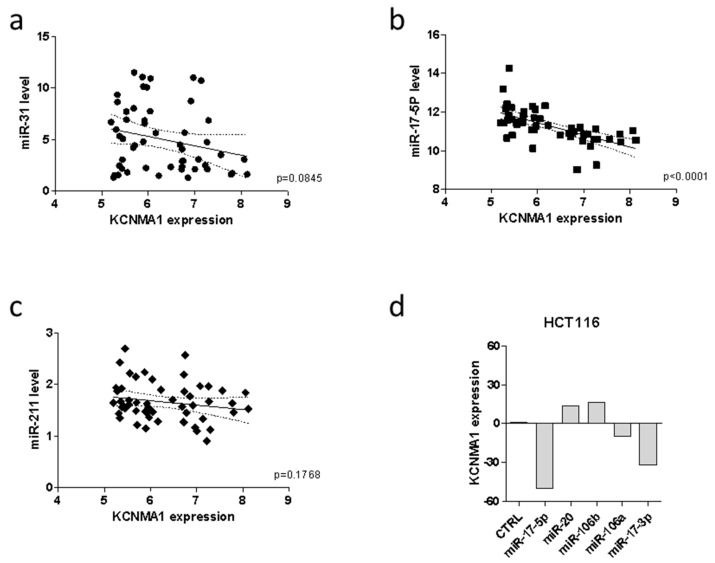
Previous reports have shown that epigenetic mechanisms are directly involved in the regulation of KCNMA1 expression in a variety of tumors. To evaluate whether KCNMA1 modulation in CRC could be associated with specific miRNA profiles, the GSE35834 microarray dataset was interrogated. A statistically significant inverse correlation was observed between KCNMA1 expression and mir-17-5p (p < 0.0001), while only a trend of inverse correlation was observed for mir-31 (p = 0.0845) in patients with CRC (Figure 5a,b). No correlation was instead, observed between KCNMA1 expression and mir-211 (Figure 5c).
Figure 5.
MiRNAs regulating KCNMA1 expression. Correlation between KCNMA1 expression levels and miR-31 (a), miR-17-5p (b), and miR-211 (c) in CRC patients (n = 31), as determined in the GSE35834 dataset; (d) modulation of KCNMA1 expression upon transfection of HCT116 cells with miRNAs belonging to the miR-106b family, as determined in the GSE14831 dataset.
According to the correlation analysis, as determined in the GSE14831 dataset, transfection of the HCT116 colon cancer cell line with miR-17-5p was associated with a marked downregulation of KCNMA1 expression (Figure 5d).
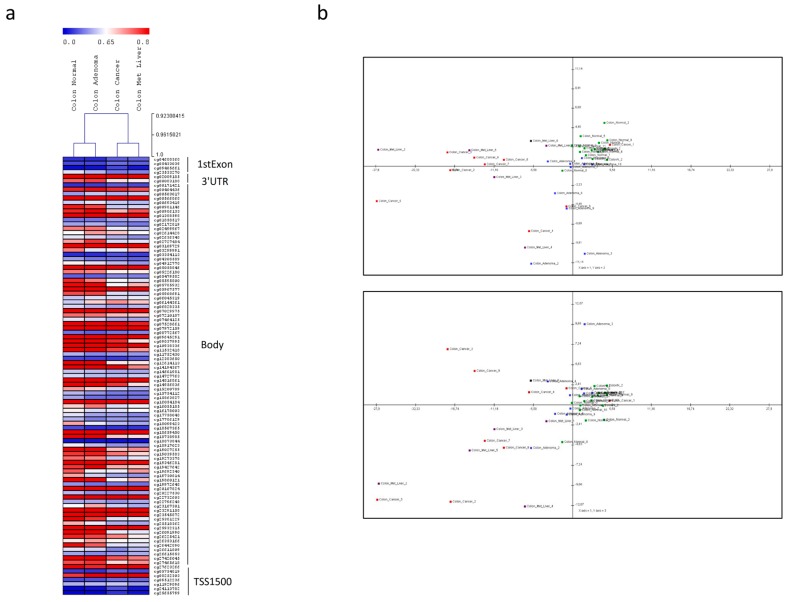
The analysis of the methylation profile (Figure 6a) and the Principal Components Analysis (PCA) (Figure 6b, Table A1) show that the DNA methylation pattern of the KCNMA1 gene allows to differentiate the normal colonic mucosa from adenoma and from adenocarcinoma, with and without liver metastases. However, as shown by PCA analysis, a high variance in the KCNMA1-associated methylation pattern can be observed among adenocarcinoma samples (Figure 6b).
Figure 6.
Methylation profiles of colorectal cancer samples as determined in the GSE53051 microarray dataset. (a) Methylation pattern of the KCNMA1 gene; (b) Principal Component Analysis (PCA) of normal colon samples (n = 18, green samples), colon adenoma (n = 10, blue samples), colorectal adenocarcinoma (n = 9, red samples), and colorectal liver metastases (n = 6, violet samples). PCA graph was constructed using the first and second components (top panel) and the first and third components (bottom panel) for the KCNMA1-associated beta values.
Interestingly, on the contrary to what can be observed in the promoter, numerous loci located in the body of the gene are significantly hypomethylated in the colon adenocarcinoma as compared to healthy tissue (Figure 6a).
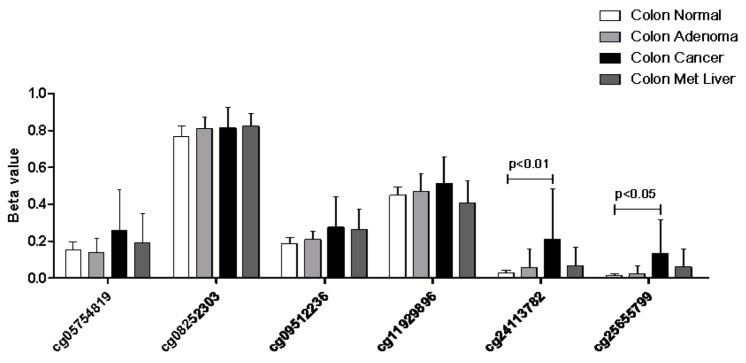
When analyzing the promoter-associated methylation patterns, we found a significant hypermethylation of the cg24113782 and cg25655799 loci as compared to healthy tissue (Figure 7).
Figure 7.
Methylation profiles of colorectal cancer samples as determined in the GSE53051 microarray dataset. Methylation patterns of the KCNMA1 promoter are expressed as Beta values.
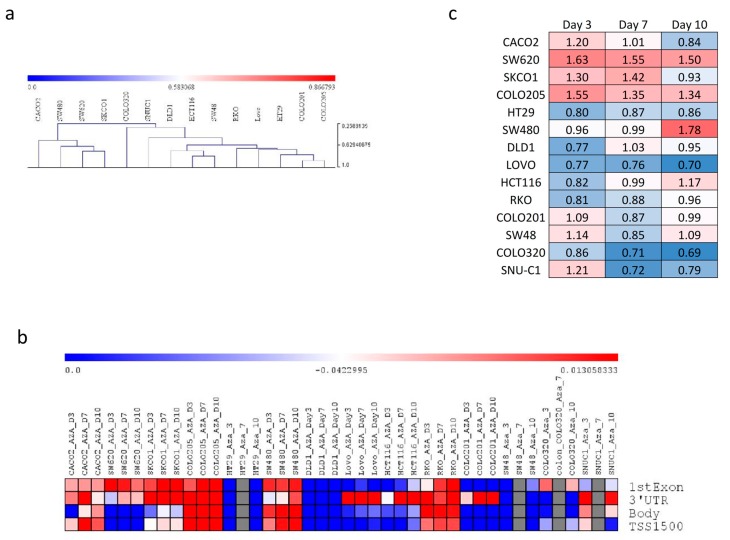
In accordance with the high variability in the methylation patterns of the KCNMA1 gene observed in primary human samples, marked differences in KCNMA1 gene methylation were observed in established colon cancer cell lines (Figure 8a,b). Accordingly, treatment with the DNA-methyltransferase inhibitor 5-azacitidine (AZA) induced a variable degree of hypomethylation in the KCNMA1 gene promoter and body (Figure 8b). Therefore, treatment with AZA was associated with an increase in KCNMA1 expression in some colon cancer cell lines (i.e., SW620, SKCO1, COLO205, and SW480) (Figure 8c ), while a lack of effect, or even a downregulation of KCNMA1, was instead observed for other cells lines, such as LOVO and COLO320 (Figure 8c).
Figure 8.
In vitro effect of the hypomethylating agent 5-azacitidine (AZA) on the expression of KCNMA1 in 14 human colon cancer cell lines. (a) Hierarchical clustering was performed on the KCNMA1-associated methylation levels, as obtained from the GSE57342 dataset; (b) heatmap showing the modulation of KCNMA1 gene methylation upon AZA treatment of 14 human colon cancer cell lines, as obtained from the GSE57342 dataset. The mean beta value is presented; (c) heatmap showing the modulation of KCNMA1 upon AZA treatment of 14 human colon cancer cell lines, as obtained from the GSE57341 dataset. The fold change variation respective to the untreated cells is presented.
3. Discussion
In the present study, we have studied the expression of KCNMA1 in preclinical and human CRC and found that KCNMA1 is significantly downregulated in CRC, independently of the cancer stage. In addition, we propose that this downregulation is, at least partially, controlled by diverse epigenetic mechanisms, in particular DNA methylation and the miRNA, miR-17-5p. Our data are in line with observations from Ma G et al. [9] in gastric cancer, where it was found that the gene plays a tumor suppressor role. The lack of significant upregulation of KCNMA1 upon treatment of colon cancer cells with DAC can be explained in several ways. First of all, as shown in the heatmap presented in Figure 6, there is an opposite pattern of methylation in the promoter and body of the KCNMA1 gene. Indeed, while hypermethylation can be observed in the promoter, a general trend of hypomethylation can be observed in the gene body. Additionally, PCA analysis of the KCNMA1-associated methylation revealed a high variance among colon cancer samples, which is reflected by the high variability of methylation observed in the 14 colon cancer cell lines analyzed. Accordingly, a strong variability in KCNMA1 modulation is observed upon treatment of these cell lines with a DNA methyl-transferases inhibitor.
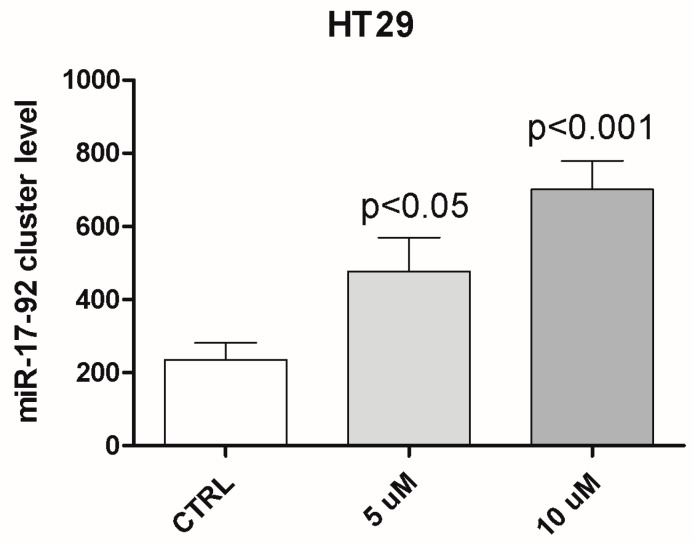
Furthermore, additional mechanisms of gene expression regulation can take place; in particular, the role of miRNAs seems to be relevant, as shown in the paper. Interestingly, as shown in Appendix A Figure A1, DAC treatment of HT29 cells determines an upregulation of the miR-17-92 cluster host gene. This apparently contradictory result further supports the complex regulation of KCNMA1 expression in CRC.
Despite this, several other mechanisms underlying KCNMA1 expression are likely to be involved: for instance, histone modification (acetylation, methylation, sumoylation of the different core proteins); factors affecting RNA stability; transcription factors (e.g., at the gene enhancer GH10J077636 only, there seems to be putative transcription binding sites for 42 transcription factors: YY1, GLIS2, ZIC2, SP7, ZBTB8A, PRDM10, POLR2A, CTBP1, CTCF, ZEB2, PATZ1, ZNF524, BACH1, YBX1, REST, SCRT2, ZBTB20, EZH2, DPF2, ZNF561, ZBTB10, ZBTB26, IKZF1, NR2F1, OSR2, SMARCA5, ZBTB17, ZNF366, ZFHX2, RB1, ZNF335, ZNF777, GATA3, ZNF513, YY2, SP3, ZNF217, ZNF2, ZNF785 and EGR2, as shown in the GeneCards database, https://www.genecards.org/cgi-bin/carddisp.pl?gene=KCNMA1).
Our observation of the transcriptomic downregulation of KCNMA1 has also been confirmed by immunohistochemistry data from The Human Protein Atlas database and from immunohistochemistry analysis of the CRC samples available in the repository of the Department of Medical and Surgical Sciences and Advanced Technology “G.F. Ingrassia”, at the University of Catania.
In epithelial cells of the gastrointestinal tract, KCNMA1 is implicated in a variety of cellular functions, e.g., electrolyte and substrate transport, cell volume regulation, cell migration, wound healing, proliferation, apoptosis, and carcinogenesis. Colonic electrolyte transport appears to be affected in the premalignant colon, which may contribute to the irregularities in defecation, often observed in patients with human colonic cancer. However, dysplastic transformation is associated with depolarization of the crypt epithelial cells, possibly indicating a change in K+ channel regulation or expression. However, the biological role of KCNMA1 in the gastrointestinal tract is still to be deciphered. Ma and collaborators have previously shown that the overexpression of KCNMA1 in the human gastric cancer cell lines, MGC803 and BGC823, was associated with a significant reduction in their invasion and migration properties and inhibited the tumor growth in a xenograft murine model [8]. They have also shown that KCNMA1 over-expression increased the numbers of both early apoptotic cells and late apoptotic cells in transfected MGC803 and BGC823 cells compared with control cells. In addition, they have shown an inverse correlation between KCNMA1 and the PTK2 gene, which is involved in FAK apoptosis pathways. Accordingly, KCNMA1 overexpression was not sufficient to inhibit the migration and invasion of gastric cancer cells after PTK2 knockdown [9].
On the other hand, in the metastatic breast cancer cell lines MCF7, MDA-MB-231, and MDA-MB-361, KCNMA1 promotes cell invasiveness and transendothelial migration, which could be attenuated by siRNA knockdown or inhibition with Iberiotoxin [5]. Further studies are therefore needed for a full understanding of the involvement of this gene in the development and progression of colon cancer.
Another important finding of the study was the down-regulation of this gene observed in patients with colorectal adenocarcinoma with MSI as compared to healthy patients. The possibility of having a potential biomarker of MSI could represent an important step towards immunological targeting therapy, regardless of tumor histology and location. The MSI represents a prognostic and predictive factor in CRC: patients with a high MSI have a better prognosis with a good response to immunotherapy compared to those without MSI [15,16,17].
4. Materials and Methods
4.1. Animals
C57/BL6 male mice (Envigo, San Pietro al Natisone, UD, Italy) weighing between 20–22 g were housed within a limited access rodent facility and kept in groups of a maximum of five mice, in polycarbonate isolator cages with a filter top and external air supply and free access to food and water. Animal care was in compliance with local regulations on the protection of animals used for experimental and other scientific purposes (Directive 86/609/EEC, enforced by the Italian D.L. No. 116 of 27 January 1992).
4.2. Murine Models of UC and UC-Associated Cancer
The transcriptional levels of KCNMA1 were initially determined in a preclinical model of UC, which was induced in the C57/BL6 mouse by the administration of 3% DSS in drinking water. The colon samples were collected at day 0, 2, 4, and 6 post-induction (n = 5–6 mice per group), for the analysis of KCNMA1 expression. The well-established DSS/AOM model was used for UC-associated cancer. Briefly, a single dose of AOM (10 mg/kg) was administered intra-peritoneally to day 1, followed by three cycles of DSS in drinking water (cycle 1: 2%, days 8~14, cycle 2: 1.5%, day 29~33; cycle 3: 1.5%, day 50~54). Mucosa samples were collected at 2, 4, 8, and 20 weeks.
4.3. RNA Isolation and Real-Time RT-PCR
Total RNA was extracted from samples using TRIzol reagent (Life Technologies, Monza, Italy). Two micrograms of RNA were retro-transcribed, and cDNA was used for the determination of KCNMA1 by real-time RT-PCR using the FastStart SYBR Green Master (Roche, Monza, Italy). Primer sequences were in house-designed or obtained from the PrimerBank database (http://pga.mgh.harvard.edu/primerbank/).
4.4. Analysis of Transcription Profiles of KCNMA1 in Patients with CRC
The transcriptional levels of KCNMA1 in CRC samples were evaluated in the two microarray datasets, GSE24514 and GSE32323, obtained from the Gene Expression Omnibus database (GEO; https://www.ncbi.nlm.nih.gov/gds). GSE24514 included expression data from 34 microsatellite-instable CRC samples and 15 normal colonic mucosal samples. MSI samples were of both sporadic and HNPCC (Hereditary Non-Polyposis Colorectal Cancer) origin and included ordinary and mucinous adenocarcinomas. Additionally, the samples consisted of right and left colons, as well as the rectum [15]. GSE24514 was generated using the Affymetrix Human Genome U133A Array and data were analyzed by MAS5.0 and normalized using the quantile normalization procedure. The GSE32323 included data from 17 pairs of cancer and non-cancerous tissues from CRC patients. Clinical and demographic data of the patients included in the study are available in the relative publication [16]. GSE32323 was generated using the Affymetrix Human Genome U133 Plus 2.0 Array and data were normalized by robust multichip analysis (RMA).
Data regarding the levels of KCNMA1 in patients with CRC were obtained from the TGCA database, through the cBioPortal for Cancer Genomics (http://www.cbioportal.org/) website. RNA-seq data were processed and normalized using RSEM to generate TPM (transcripts per million). Overall, this study comprised RNA-seq data from 282 CRC samples. Forty samples consisted of mucinous adenocarcinoma of the colon and rectum. None of the patients received neoadjuvant therapy prior to resection. Identification of genetic aberrations in the KCNMA1 genes has been performed on a cohort of 70 DNA-sequenced CRC patients, as available in the TGCA database.
4.5. Immunohistochemistry Analysis of KCNMA1 Expression in CRC Samples
Modulation of KCNMA1 expression in CRC was validated using data obtained from the Human Protein Atlas database (https://www.proteinatlas.org/pathology) and from immunohistochemical staining of a small number of CRC samples available in the repository of the Department of Medical and Surgical Sciences and Advanced Technology “G.F. Ingrassia”, University of Catania. Immunohistochemistry analyses were performed using the standard streptavidin–biotin labeling technique using the LSAB kit (Dako, Glostrup, Denmark) with appropriate positive and negative controls. Sections derived from paraffin embedded specimens, obtained from biopsies of three patients with normal colonic mucosa, three patients with tubule-villous adenomas, and three patients with invasive adenocarcinomas, were deparaffinized in xylene for 15 min, rehydrated, and treated with 3% H2O2 for 10 min to block endogenous peroxidase activity. Briefly, deparaffinized sections were incubated with anti-KCNMA1 antibodies (HPA054648, Sigma-Aldrich, Milan, Italy) (dilution: 1:400). Microwave pretreatment was crucial to enhancing the staining in all samples examined. Bound antibody was revealed by incubation with 3,3-diaminobenzidine (Sigma-Aldrich, St.Louis, MO, USA) in 0.01% H2O2 for 5 min at room temperature. Sections were then counterstained with hematoxylin, dehydrated, and mounted. Negative controls involving the omission of the primary antibody were included.
4.6. Analysis of Epigenetic Regulation of KCNMA1 Expression in CRC
The association between KCNMA1 expression and the levels of the miRNAs, mir-17-5p, mir-31, and mir-211 was determined for samples of patients with colorectal adenocarcinoma from the GSE35834, available in the GEO databank. The dataset included data from 31 primitive colorectal cancer from patients who underwent surgery at the University of Padova [18]. The Affymetrix Multispecies miRNA-1 Array and Affymetrix Human Exon 1.0 ST Array were used to generate the dataset. For both platforms, raw data were preprocessed using the RMA algorithm. For the miRNA-1 Array, only the human probes were considered for the normalization and summarization.
The DNA methylation patterns of the KCNMA1 gene were obtained from the GSE53051 microarray dataset. The dataset contains the methylation profiles of normal colon samples (n = 18), colon adenoma (n = 10), colorectal adenocarcinoma (n = 9), and CRC with liver metastases (n = 6) [19]. The platform used for the dataset was the Illumina Infinium 450k Human Methylation Beadchip.
In order to investigate the effect of the hypomethylating agent AZA on the expression and methylation levels of KCNMA1, the GSE57341 and GSE57342 datasets were interrogated [20]. These datasets include whole-genome expression and methylation data of 14 human colon cancer cells lines, treated with either 0 or 0.5 μM of AZA at different time points (day 3, 7, and 10).
4.7. Statistical Analysis
Statistical differences between groups were evaluated by applying either the ANOVA or two-tailed Student’s T test. The correlation between KCNMA1 levels and OS was calculated using the non-parametric Spearman’s test. Values of p < 0.05 were considered statistically significant.
5. Conclusions
The characterization of the expression levels of KCNMA1 and the mechanisms underlying its modulation could help in defining its role in the CRC. Overall, our data suggest the possible use of KCNMA1 as a biomarker in the early stages of CRC and as a possible therapeutic target.
Appendix A
Figure A1.
DAC treatment of HT29 cells determines an upregulation of the miR-17-92 cluster host gene.
Table A1.
PCA coordinates for the samples in GSE53051.
| Sample | X | Y | Z |
|---|---|---|---|
| Colon_Normal_1 | 1.735467 | 1.358078 | −1.74759 |
| Colon_Normal_2 | 4.342013 | 5.111035 | −1.65102 |
| Colon_Normal_3 | −0.25661 | 2.49674 | 0.359764 |
| Colon_Normal_4 | 1.501905 | 2.161722 | 0.570626 |
| Colon_Normal_5 | 0.961287 | 3.563164 | 0.071522 |
| Colon_Normal_6 | 3.428178 | 1.750228 | 1.24613 |
| Colon_Normal_7 | 3.580552 | 2.792617 | 1.531011 |
| Colon_Normal_8 | −1.62841 | −0.42259 | −4.3392 |
| Colon_Normal_9 | 4.737718 | 3.108791 | 1.336153 |
| Colon_Normal_10 | 0.88916 | 1.758846 | −0.51744 |
| Colon_Normal_11 | 0.849407 | 2.382122 | 1.14948 |
| Colon_Normal_12 | 2.52812 | 1.950024 | 0.647312 |
| ColonN_2 | 2.949582 | 2.091265 | 2.387948 |
| ColonN_2 | 4.124992 | 1.94098 | 0.049728 |
| ColonN_2 | 4.724679 | 0.837779 | 2.424594 |
| ColonN_2 | 4.442956 | 2.109996 | 1.577544 |
| ColonN_2 | 2.655884 | 0.930409 | 1.088807 |
| Colon_Adenoma_1 | 1.481603 | 0.340708 | 1.481484 |
| Colon_Adenoma_2 | −6.04664 | −11.1386 | −5.05503 |
| Colon_Adenoma_3 | 1.561727 | −9.99486 | 9.808415 |
| Colon_Adenoma_4 | −3.68738 | 0.668282 | 2.941357 |
| Colon_Adenoma_5 | −0.52092 | 0.373219 | 1.291463 |
| Colon_Adenoma_6 | −2.653 | −3.03014 | −0.95898 |
| Colon_Adenoma_7 | −0.44732 | −0.00646 | −0.31317 |
| Colon_Adenoma_8 | 1.141704 | 1.037116 | 2.029349 |
| Colon_Adenoma_9 | −1.0016 | −4.7615 | −1.04647 |
| Colon_Adenoma_10 | 2.62769 | 0.301795 | 1.581635 |
| Colon_Cancer_1 | 5.112652 | 2.56774 | 0.636602 |
| Colon_Cancer_2 | −17.4902 | −0.3567 | −11.5103 |
| Colon_Cancer_3 | −17.9117 | 1.695159 | 6.819301 |
| Colon_Cancer_4 | −6.336 | −7.3712 | 1.640066 |
| Colon_Cancer_5 | −27.9047 | −3.92621 | −11.265 |
| Colon_Cancer_6 | −1.03183 | −4.53172 | 2.102261 |
| Colon_Cancer_7 | −12.6164 | 0.340978 | −4.18775 |
| Colon_Cancer_8 | −9.90718 | 0.857501 | −5.01775 |
| Colon_Cancer_9 | −14.0838 | 1.098967 | 4.202911 |
| Colon_Met_Liver_1 | −3.8611 | 2.457655 | −1.83603 |
| Colon_Met_Liver_2 | −27.6862 | 2.011681 | −9.32142 |
| Colon_Met_Liver_3 | −11.2724 | −1.14682 | −2.78756 |
| Colon_Met_Liver_4 | −6.92045 | −9.26846 | −12.0695 |
| Colon_Met_Liver_5 | −14.7814 | 1.954306 | −5.35754 |
| Colon_Met_Liver_6 | −6.07612 | 3.006146 | 3.031533 |
Author Contributions
Conceptualization, F.N., S.P., and A.P.; Methodology, G.L.D., G.L.G., and S.P.; Validation, G.M., L.S. and S.M.; Formal Analysis, M.S.B., P.F. and K.M.; Investigation, M.S.B. and S.M.; Resources, F.N. and S.P.; Data Curation, K.M. and L.S.; Writing—original draft preparation, M.S.B., P.F. and A.P.; Writing—review and editing, G.M., F.N. and S.P.; Visualization, P.F. and K.M.; Supervision, P.F. and K.M.; Project administration, G.L.D., G.L.G. and S.P.; Funding acquisition, G.L.D., G.L.G. and S.P.
Funding
This study was supported by research funds #150.84.504 of the Department of Medical and Surgical Sciences and Advanced Technology “G.F. Ingrassia”, University of Catania.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Ge L., Hoa N.T., Wilson Z., Arismendi-Morillo G., Kong X.-T., Tajhya R.B., Beeton C., Jadus M.R. Big Potassium (BK) ion channels in biology, disease and possible targets for cancer immunotherapy. Int. Immunopharmacol. 2014;22:427–443. doi: 10.1016/j.intimp.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloch M., Ousingsawat J., Simon R., Schraml P., Gasser T.C., Mihatsch M.J., Kunzelmann K., Bubendorf L. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene. 2007;26:2525–2534. doi: 10.1038/sj.onc.1210036. [DOI] [PubMed] [Google Scholar]
- 3.Xie M., Zhou L., Chen X., Gainey L.O., Xiao J., Nanes M.S., Hou A., You S., Chen Q. Progesterone and Src Family Inhibitor PP1 Synergistically Inhibit Cell Migration and Invasion of Human Basal Phenotype Breast Cancer Cells. Biomed. Res. Int. 2015;2015:1–14. doi: 10.1155/2015/426429. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Oeggerli M., Tian Y., Ruiz C., Wijker B., Sauter G., Obermann E., Güth U., Zlobec I., Sausbier M., Kunzelmann K., et al. Role of KCNMA1 in Breast Cancer. PLoS ONE. 2012;7:e41664. doi: 10.1371/journal.pone.0041664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khaitan D., Sankpal U.T., Weksler B., Meister E.A., Romero I.A., Couraud P.-O., Ningaraj N.S. Role of KCNMA1gene in breast cancer invasion and metastasis to brain. BMC Cancer. 2009;9:258. doi: 10.1186/1471-2407-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du C., Zheng Z., Li D., Chen L., Li N., Yi X., Yang Y., Guo F., Liu W., Xie X., et al. BKCa promotes growth and metastasis of prostate cancer through facilitating the coupling between αvβ3 integrin and FAK. Oncotarget. 2016;7:40174–40188. doi: 10.18632/oncotarget.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bury M., Girault A., Mégalizzi V., Spiegl-Kreinecker S., Mathieu V., Berger W., Evidente A., Kornienko A., Gailly P., Vandier C., et al. Ophiobolin A induces paraptosis-like cell death in human glioblastoma cells by decreasing BKCa channel activity. Cell Death Dis. 2013;4:e561. doi: 10.1038/cddis.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramírez A., Vera E., Gamboa-Domínguez A., Lambert P., Gariglio P., Camacho J. Calcium-activated potassium channels as potential early markers of human cervical cancer. Oncol. Lett. 2018;15:7249–7254. doi: 10.3892/ol.2018.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma G., Liu H., Hua Q., Wang M., Du M., Lin Y., Ge Y., Gong W., Zhao Q., Qiang F., et al. KCNMA1 cooperating with PTK2 is a novel tumor suppressor in gastric cancer and is associated with disease outcome. Mol. Cancer. 2017;16:46. doi: 10.1186/s12943-017-0613-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel P., Pink R.C., Caley D.P., Currie J.M.S., Brooks S.A., Carter D.R.F. Over-expression of miR-31 or loss of KCNMA1 leads to increased cisplatin resistance in ovarian cancer cells. Tumor Biol. 2016;37:2565–2573. doi: 10.1007/s13277-015-4081-z. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y.Y., Wright C.M., Kirschner M.B., Williams M., Sarun K.H., Sytnyk V., Leshchynska I., Edelman J.J., Vallely M.P., McCaughan B.C., et al. KCa1.1, a calcium-activated potassium channel subunit alpha 1, is targeted by miR-17-5p and modulates cell migration in malignant pleural mesothelioma. Mol. Cancer. 2016;15:44. doi: 10.1186/s12943-016-0529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazar J., DeYoung K., Khaitan D., Meister E., Almodovar A., Goydos J., Ray A., Perera R.J. The Regulation of miRNA-211 Expression and Its Role in Melanoma Cell Invasiveness. PLoS ONE. 2010;5:e13779. doi: 10.1371/journal.pone.0013779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Robertis M., Massi E., Poeta M.L., Carotti S., Morini S., Cecchetelli L., Signori E., Fazio V.M. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka T. Development of an Inflammation-Associated Colorectal Cancer Model and Its Application for Research on Carcinogenesis and Chemoprevention. Int. J. Inflam. 2012;2012:1–16. doi: 10.1155/2012/658786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alhopuro P., Sammalkorpi H., Niittymäki I., Biström M., Raitila A., Saharinen J., Nousiainen K., Lehtonen H.J., Heliövaara E., Puhakka J., et al. Candidate driver genes in microsatellite-unstable colorectal cancer. Int. J. Cancer. 2012;130:1558–1566. doi: 10.1002/ijc.26167. [DOI] [PubMed] [Google Scholar]
- 16.Khamas A., Ishikawa T., Shimokawa K., Mogushi K., Iida S., Ishiguro M., Mizushima H., Tanaka H., Uetake H., Sugihara K. Screening for epigenetically masked genes in colorectal cancer Using 5-Aza-2′-deoxycytidine, microarray and gene expression profile. Cancer Genom. Proteom. 2012;9:67–75. [PubMed] [Google Scholar]
- 17.Boland C.R., Goel A. Microsatellite Instability in Colorectal Cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pizzini S., Bisognin A., Mandruzzato S., Biasiolo M., Facciolli A., Perilli L., Rossi E., Esposito G., Rugge M., Pilati P., et al. Impact of microRNAs on regulatory networks and pathways in human colorectal carcinogenesis and development of metastasis. BMC Genom. 2013;14:589. doi: 10.1186/1471-2164-14-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timp W., Bravo H.C., McDonald O.G., Goggins M., Umbricht C., Zeiger M., Feinberg A.P., Irizarry R.A. Large hypomethylated blocks as a universal defining epigenetic alteration in human solid tumors. Genome Med. 2014;6:61. doi: 10.1186/s13073-014-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Chiappinelli K.B., Guzzetta A.A., Easwaran H., Yen R.-W.C., Vatapalli R., Topper M.J., Luo J., Connolly R.M., Azad N.S., et al. Immune regulation by low doses of the DNA methyltransferase inhibitor 5-azacitidine in common human epithelial cancers. Oncotarget. 2014;5:587–598. doi: 10.18632/oncotarget.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]