Abstract
Mood depressive disorder is one of the most disabling chronic diseases with a high rate of everyday life disability that affects 350 million people around the world. Recent advances in neuroimaging have reported widespread structural abnormalities, suggesting a dysfunctional frontal-limbic circuit involved in the pathophysiological mechanisms of depression. However, a variety of different white matter regions has been implicated and is sought to suffer from lack of reproducibility of such categorical-based biomarkers. These inconsistent results might be attributed to various factors: actual categorical definition of depression as well as clinical phenotype variability. In this study, we 1/ examined WM changes in a large cohort (114 patients) compared to a healthy control group and 2/ sought to identify specific WM alterations in relation to specific depressive phenotypes such as anhedonia (i.e. lack of pleasure), anxiety and psychomotor retardation –three core symptoms involved in depression. Consistent with previous studies, reduced white matter was observed in the genu of the corpus callosum extending to the inferior fasciculus and posterior thalamic radiation, confirming a frontal-limbic circuit abnormality. Our analysis also reported other patterns of increased fractional anisotropy and axial diffusivity as well as decreased apparent diffusion coefficient and radial diffusivity in the splenium of the corpus callosum and posterior limb of the internal capsule. Moreover, a positive correlation between FA and anhedonia was found in the superior longitudinal fasciculus as well as a negative correlation in the cingulum. Then, the analysis of the anxiety and diffusion metric revealed that increased anxiety was associated with greater FA values in genu and splenium of corpus callosum, anterior corona radiata and posterior thalamic radiation. Finally, the motor retardation analysis showed a correlation between increased Widlöcher depressive retardation scale scores and reduced FA in the body and genu of the corpus callosum, fornix, and superior striatum. Through this twofold approach (categorical and phenotypic), this study has underlined the need to move forward to a symptom-based research area of biomarkers, which help to understand the pathophysiology of mood depressive disorders and to stratify precise phenotypes of depression with targeted therapeutic strategies.
Keywords: Diffusion-weighted imaging, Voxel-based analysis, Fractional anisotropy value, Depression, Categorical and phenotypic approach
Highlights
-
•
Mood depressive disorder is one of the most disabling chronic disease.
-
•
Past studies of diffusion analysis had found inconsistent results.
-
•
We analyzed white matter integrity in a large cohort of depressed patients.
-
•
We conducted both categorical and dimensional approaches.
-
•
In the future, these biomarkers could help to develop new therapeutic strategies.
1. Introduction
Mood Depressive Disorder (MDD) is one of the most disabling chronic diseases with a high rate of everyday life incapacity that affects 350 million people around the world (Smith, 2014). This pathology is considered underdiagnosed, without adequate therapeutic resources, which emphasizes the need to be a public health priority (Ferrari et al., 2013). One of the most important causes of misdiagnosis is the poor interrater reliability of actual classifications (Freedman et al., 2013). Mood Depressive Episode criterion of the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) (Edition, 2013) indeed encompass a wide range of clinical phenotypes of depression “from gravely disabled melancholic patients to many individuals in the general population who do not seek treatment” (Freedman et al., 2013). This suggests that categorical approach provided by DSM-5 is far from the level of neurosciences (Maj, 2014) and therefore could limit reproducibility of its findings in helping in diagnosis and therapeutics. In this context, despite the extensive therapy options available for depression, up to 80% of patients will suffer from a relapse (Gotlib and Hamilton, 2008). On the neurobiological side, this disease is characterized by a profound and persistent dysregulation of affect and mood (Fitzgerald, 2013), which coexists with additional disturbance including cognitive dysfunction, insomnia, fatigue and appetite disturbance (Drevets, 2001). Consequently, understanding the neural correlates underlying depression, and its phenotype variability, will optimize the diagnosis and treatment of individual depressed patients.
Over the last decades, recent advances in neuroimaging have greatly increased our knowledge of MDD, particularly its neural bases. Widespread structural abnormalities have been reported including regional tissue loss in the hippocampus, amygdala, basal ganglia, prefrontal cortex and anterior cingulate cortex (Arnone et al., 2013; Zou et al., 2010). These results suggested that a dysfunctional cortical-subcortical neural circuit is involved in the pathophysiology and psychopathology of depression (Chen et al., 2016; Korgaonkar et al., 2011; Sacchet et al., 2014). More particularly, an abnormal frontal-limbic network is considered as the locus of the dysfunction underlying mood-regulation in MDD (Chen et al., 2016; Korgaonkar et al., 2011; Sacchet et al., 2014). Structural connectivity analysis provides novel insights into network dysfunctions. White matter (WM) investigations have become a rapidly growing area of research in psychotic disorders including schizophrenia (Buchsbaum et al., 1998), bipolar disorder (Benedetti et al., 2015; Magioncalda et al., 2016) and depression (Chen et al., 2017). In this context, diffusion-weighted imaging (DWI), a noninvasive magnetic resonance imaging (MRI) technique based on the extent of water diffusion, enables the quantification of the fiber orientation and abnormalities of WM pathways within neural networks (Assaf and Pasternak, 2008; Taylor et al., 2004). The most common DWI measure is the fractional anisotropy (FA), an invariant property of DWI that reflects a nonspherical diffusion tensor with a preferential orientation.
Several studies (Chen et al., 2017; Korgaonkar et al., 2011) have revealed significant FA reductions in the body of the corpus callosum (CC) (Han et al., 2014; Korgaonkar et al., 2011), bilateral anterior limb of the internal capsule (ALIC) (Chen et al., 2016; Jia et al., 2010; Zou et al., 2008) and superior longitudinal fasciculus (SLF) (Ota et al., 2015) in MDD patients relative to healthy controls. However, existing knowledge about WM changes is limited due to the variety of different WM regions exhibited in these studies (Liao et al., 2013; Murphy and Frodl, 2011; Sexton et al., 2009). These inconsistent results might be attributed to various factors. The high heterogeneity of MDD individuals in terms of disease characteristics (i.e. symptoms and duration of the disease), could be considered a main cause of this variability (Chen et al., 2016). Previous studies have indeed demonstrated the influence of episodes or illness duration (de Diego-Adelino et al., 2014), illness severity (Henderson et al., 2013) and antidepressant drugs (Dusi et al., 2015; Taylor et al., 2014) on WM. The FA reduction was indeed associated with depression severity and illness duration in MDD patients in the genu of corpus callosum, anterior thalamic radiation, anterior cingulum, and sagittal striatum, which indicates that DWI may be of clinical value in measuring and tracking disability in MDD (Chen et al., 2016; Henderson et al., 2013). One additional source of heterogeneity could be the clinical phenotype (Fried, 2015), such as anhedonia (i.e. lack of pleasure), anxiety and psychomotor retardation –three core symptoms involved in depression. As mentioned above, DSM-5 diagnosis criteria include a wide panel of emotional, motivational and cognitive symptoms (Edition, 2013). Some of these dimensions can be negatively correlated such as motivation and anhedonia severity (Batail et al., 2017). Then, the high variability of diagnosis criteria could lead to poor diagnosis interrater reliability (Freedman et al., 2013). For the last two decades, neuroimaging studies in MDD have identified many putative biomarkers, based on actual disease classification, which remain inconsistent tools. Therefore, there is a need to find new imaging biomarkers which could potentially improve our understanding the pathophysiology of MDD (Young et al., 2016).
In this context of research on biomarkers, the research domain criteria (RDoC) program have been created. This initiative was proposed by the American National Institute of Mental Health to develop “new ways of classifying mental disorder based on behavioral dimensions and neurobiological measures” (NIMH, 2008). This approach promises to increase our understanding of basic network-level abnormalities and their relation to psychopathology (Insel et al., 2010). Then, this new research framework based on dimension could help a better understanding of phenotypical variability described in depression. This disease, as currently defined, spans two of the RDoC domains: the loss construct within the negative valence systems domain and various reward constructs within the positive valence systems domain. Our study focuses specifically on these two dimensions, anxiety and anhedonia by analyzing theirs relations with diffusion metrics (Cuthbert and Insel, 2013; Insel et al., 2010). Then, although RDoC program does not propose a behavioral domain, the relationship with psychomotor retardation was investigated because of its central role in depression and discriminative potential for different phenotypes (melancholic subtype, bipolar/unipolar and consequences on treatment) (Bennabi et al., 2013).
In this paper, we proposed a twofold approach (categorical and phenotypical), in order to show that a phenotypic approach allows to identify more reliable knowledge on pathophysiology underlying depression than categorical one. Therefore, the first aim of this study was to examine WM changes in a large cohort of MDD patients to address more consistent and replicable WM microarchitecture abnormalities than those found in previous studies. The second aim of our study was to identify specific WM alterations in relation to specific dimensions of depression such as anhedonia, anxiety and psychomotor retardation.
2. Materials and methods
2.1. Participants
One hundred and fourteen depressed patients were recruited from routine care units in the psychiatric university hospital of Rennes between November 2014 and January 2017 and were enrolled in a naturalistic prospective cohort study. The study was approved by an ethic committee and is registered in www.clinicaltrial.gov (NCT02286024), written informed consents were obtained from all subjects.
The study was proposed to patients suffering from a Mood Depressive Episode (MDE) under DSM-5 criteria with or without personal history of MDD (unipolar or bipolar subtype). Exclusion criteria included other Axis-I disorders (except for obsessive-compulsive disorder and four anxious comorbidities such as posttraumatic stress disorder, social phobia, generalized anxiety disorder or panic disorder), and psychotic symptoms, which were explored using the Mini-International Neuropsychiatric Interview (Lecrubier et al., 1997). Patients with severe chronic physical illness were not included. Other exclusion criteria were potential safety contraindications for MRI (pacemakers, metal implants, pregnancy, and lactation), diagnosed neurodegenerative disorders (e.g. Parkinson's disease, Alzheimer's disease, Huntington's disease), a history of significant head injury, or diagnosed dementia (according to DSM-5 criteria).
Depressed patients underwent clinical interview and examination, including routine neuropsychological testing and MRI. For each subject, demographic data, comorbidities and medication, as well as clinical variables were collected (see Table 1). A composite measure of medication load for each patient was assessed using a previously established method (Sackeim, 2001), taking into account the number of medication class prescribed to the patients as well as each dosage.
Table 1.
Demographic and clinical characteristics of the MDD group.
| MDD group (n = 114) |
|||
|---|---|---|---|
| Mean | SD | Range | |
| Sociodemographic variables | |||
| Age (years) | 48.2 | 15.3 | 18–77 |
| Gender (M/F) | 43M/71F | – | – |
| Education (years) | 12.2 | 3.6 | 6–23 |
| Duration of illness (years) | 15.1 | 13.9 | 0–60 |
| Number of episodes | 4.4 | 4.5 | 0–30 |
| Duration of episode (weeks) | 30,0 | 37,8 | 0–170 |
| Number of suicidal attempts | 1,1 | 2,0 | 0–10 |
| Diagnosis (UP/BP) | 68UP/32BP | ||
| Anxious coborbidities | 61.3% | ||
| Medication | |||
| Medication load | 3,1 | 1,2 | 0–7 |
| Antidepressant | 74,6% | – | – |
| Mood stabilizer | 35,1% | – | – |
| Antipsychotic | 14,0% | – | – |
| Benzodiazepine | 49,1% | – | – |
| Clinical variables | |||
| WDRS | 21,4 | 9,0 | 2–43 |
| MADRS | 27,1 | 5,9 | 15–43 |
| STAI-YA | 57 | 13.3 | 28–0 |
| SHAPS | 5,5 | 4,0 | 0–14 |
| AES | 40,3 | 8,9 | 24–69 |
| YMRS | 1.6 | 1.7 | 0–7 |
All results except gender and medications are given as mean, standard deviation (SD) and the range. The percentage of patients having used the prescribed medicines: antidepressant, mood stabilizer, antipsychotic and benzodiazepine as well as the medication load has been reported. The MDD group is divided into unipolar (UP) and bipolar (BP) subtypes. The percentage of patients with anxious comorbidities including posttraumatic stress disorder, social phobia, generalized anxiety disorder and panic disorder has been measured. WDRS: Widlöcher Depressive Retardation Scale; MADRS: Montgomery-Åsberg Depression Rating Scale; STAI: State-Trait Anxiety Inventory A; SHAPS: Snaith Hamilton Pleasure Scale; AES: Apathy Evaluation Scale; YMRS: Young Mania Rating Scale.
The control group was composed of 65 healthy volunteers, who previously participated in neuroimaging studies. The two groups were matched in terms of age (CTL: 48.6 ± 11.8, MDD: 48.3 ± 15.4; Student t-test p-value = 0.5) and gender (CTL: 17M/48F, MDD: 43M/71F; Chi-square p = 0.5).
2.2. Clinical assessment
Patients were assessed by a single structured clinical interview by a trained psychiatrist. Anxious comorbidities were retrieved using the Mini-International Neuropsychiatric Interview (Lecrubier et al., 1997). Depression severity was assessed using Montgomery and Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979). Manic symptoms were retrieved using the Young Mania Rating Scale (YMRS) (Young et al., 1978). Additionally, the Widlöcher Depressive Retardation Scale (WDRS) (Widlöcher, 1983) was used to estimate psychomotor retardation. State anxiety was measured using State Trait Anxiety Inventory A (STAI-YA) (Spielberger et al., 1970). Then, the anhedonia and apathy scores were assessed by Snaith Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995) and Apathy Evaluation Scale (AES) (Marin et al., 1991), respectively.
2.3. MRI acquisition
Patients and healthy subjects were scanned on a 3 T whole body Siemens MR scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. The 3D T1-weighted image was acquired covering the whole brain (176 sagittal slices) with TR = 1.9 s, TE = 2.26 ms, flip angle = 9°, in-plane resolution = 2 mm × 2 mm, FOV = 256 mm × 256 mm and thickness/gap = 1.0/0 mm).
DWI data were gathered on 60 slices using an interleaved slice acquisition, slice thickness of 2 mm, no gap, in-plane resolution = 1 mm × 1 mm and in a 256 mm × 256 mm field of view. The acquisition matrix was 128 × 128, the reconstruction matrix was 128 × 128, using 30 directions and a b-value of 1000 s/mm2. TR/TE = 11,000/99 ms, flip angle was 90°, pixel bandwidth was 1698 Hz, and the imaging frequency was 128 MHz.
2.4. Image processing
Preprocessing of diffusion images was mainly performed using the open source medical image processing toolbox Anima (https://github.com/Inria-Visages/Anima-Public/wiki). Diffusion images were corrected for eddy current-induced image distortion using a block-matching distortion correction method ensuring an opposite symmetric transformation (Hedouin et al., 2017). Then, rigid realignment was then performed to compensate for subject motion and ensure voxel-to-voxel correspondence across gradients. Then, denoising step using blockwise non-local means filtering was applied (Coupé et al., 2008). Skull stripping was performed using an atlas-registration-based method. More precisely, the structural image of each patient was transformed to the atlas image, using the linear and non-linear block-matching algorithms (Commowick et al., 2012a; Ourselin et al., 2000). Then, the atlas intracranial brain mask was inversely transformed by applying the inverse of two calculated transformation matrix and multiplied with the original structural image of the patient. Diffusion tensor images were then estimated using a log-Euclidean estimation method proposed by Fillard et al. (Fillard et al., 2007), from which diffusion scalar maps were calculated.
For statistical comparisons, the diffusion metric maps were transformed into the Montreal Neurological Institute (MNI) template space, using a two-step co-registration method. First, the individual FA image was co-registered with the structural image with 1 mm isotropic resolution, using a rigid transformation following by a non-rigid registration, using the block-matching algorithm (Commowick et al., 2012b; Ourselin et al., 2000). Then the structural image was non-linearly transformed to the MNI template space, using the block-matching (Commowick et al., 2012b; Ourselin et al., 2000). Then, we applied those transformations to the other diffusion metric maps (ADC, AD and RD maps) to normalize them to the MNI space.
Next, a cross-subject mean FA image was computed to generate a WM tract skeleton, which was thresholded to FA >0.3 to confine the statistical analysis within the major WM tracts (Du et al., 2017). Then, this map was used to mask the FA, apparent diffusion coefficient (ADC), axial diffusivity (AD) and radial diffusivity (RD) maps. The preprocessing pipeline is available online on github https://github.com/Inria-Visages/Anima-Scripts-Public (in diffusion/animaDiffusionImagePreprocessing.py).
2.5. Statistical analysis
For each diffusion metric map (FA, ADC, AD and RD), independent 2-sample t-tests were also performed on a voxel-by-voxel basis via AFNI's 3dttest++ (p < 0.001) between MDD and CTL, with age, gender and duration of disease as nuisance covariates. To correct for multiple comparisons, Monte Carlo simulations via AFNI's 3dClustSim command (Forman et al., 1995) were applied to obtain a corrected significance level of p < 0.05. This correction for multiple comparisons was achieved by setting a minimum cluster size of 214 voxels.
To investigate the relationship between diffusion metrics and clinical variables (WDRS, STAI-YA and SHAPS) in the MDD group, a voxel-wise linear regression model was performed in MDD group. The normality of the clinical variable distribution was before verified using Shapiro-Wilk test. Minimum cluster size after correction for multiple comparisons at p < .05 was achieved by setting a minimum cluster size of 234 voxels with a voxelwise alpha level of p < .001.
3. Results
3.1. Demographics and clinical measures
Demographic and clinical variables of the MDD group is summarized in Table 1. Women, middle-aged predominantly represented the patient population. They have severe characteristics of disease with a long mean duration of illness (range: 25% < 3 years and 17% > 21 years) and episode (range: 50% ≥ 14 episodes). A large majority of patients suffered from recurrent depressive episodes (50% of the patients had more that 3 episodes). Actual depressive episode was moderately intense (MADRS total score = 27.4 ± 5.9). Patients did not show any significant manic symptomatology (YMRS total score = 1.6 ± 1.7). Moreover, 78% of this cohort suffered from high anxiety (36 ≤ STAI-YA ≤ 65) and 27% from very high anxiety higher than 65 as well as high rate of anxious comorbidities. All patients were under treatment (Table 1).). A significant correlation between medication load and disease duration was observed (r = 0.23 and p-value = 0.0072), in the cohort.
3.2. Whole brain structural alteration data: differences between groups
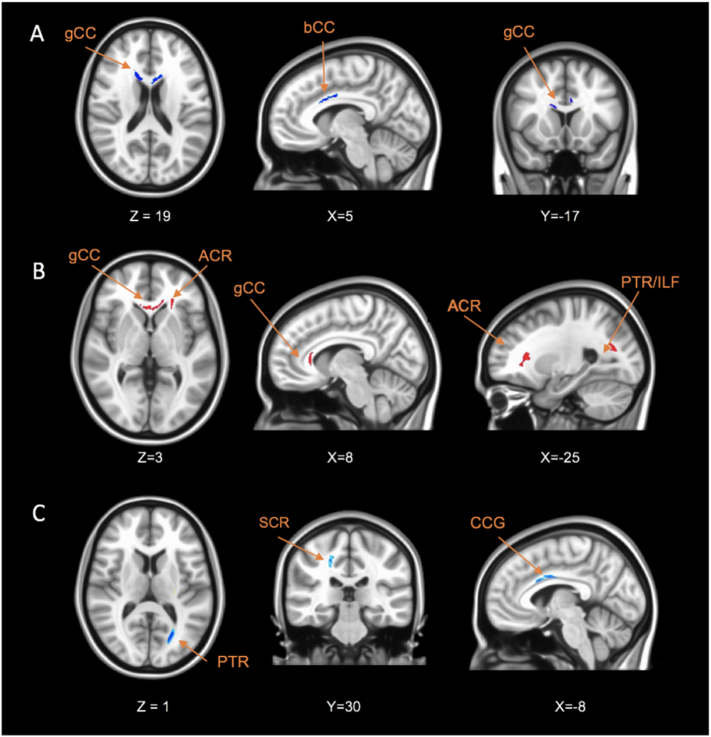
Compared with CTL, the MDD group had lower FA in the cingulum, the inferior longitudinal fasciculus (ILF), posterior thalamic radiation (PTR), cingulum and the genu of CC (see Fig. 1). However, the MDD group had greater FA in the posterior limb of the internal capsule (PLIC) and splenium of CC. The maximum FA differences in genu of CC and splenium of CC were −33% and +11%, respectively. The identified WM regions and the maximum of the t-values for the clusters (corrected for age, gender and disease duration) are summarized in Table 2 (more detail information is reported in Table A.1 in Appendix A).
Fig. 1.
Axial, coronal and sagittal brain slices showing significant differences of the FA values between MDD and CTL groups. Voxels with negative t-values (MDD < Control) are shown in blue and positive values (MDD > Control) with red. The acronym gCC indicates the genu of corpus callosum (CC); sCC, splenium of CC; ACR, anterior of the corona radiata; PTR, posterior thalamic radiation; ILF, inferior longitudinal fasciculus; PLIC, posterior Limb of the internal capsule; CCG, cingulum. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Brain areas with significant FA differences between the MDD and CTL groups.
| Inter-group comparisons |
MDD vs.CTL |
|||||
|---|---|---|---|---|---|---|
| Cerebral regions | Tracts | Right or Left | MNI coordinates | t-value | cluster size | p-value |
| Anterior cingulate | gCC | R/L | [±11,35,5] | −7.41 | 6959 | <10−6 |
| Cingulum gyrus | CCG | R/L | [±11,-25,33] | −9.36 | 36,859 | <10−6 |
| Insula | ILF | R/L | [±6,-16,31] | −3.39 | 4338 | <10−6 |
| Thalamus | PTR | R/L | [±27, −28,7] | −10.15 | 4109 | <10−6 |
| Parahippocampal gyrus | PLIC | R/L | [±15,18,-16] | 7.01 | 451 | <10−6 |
| Posterior cingulate | SCC | R/L | [−8,38,7] | 3.91 | 398 | 0.0004 |
The t-value and the p-value are the maximum statistics of the cluster and the corresponding p-value. Their coordinates are reported in the Montreal Neurological Institute (MNI) template. The cluster size is given in mm3. The acronym gCC and sCC indicates the genu and splenium of the corpus callosum; ILF, Inferior Longitudinal Fasciculus; PTR, Posterior Thalamic Radiation; PLIC, Posterior Limb of the Internal Capsule; CCG, cingulum.
For these significant regions, the results of two-sample t-test were also reported in the Table 3 for the other diffusion metrics (ADC, AD and RD). Of the 6 clusters examined, all of them showed significant RD differences after FDR correction, whereas only 3 and 5 regions showed significant differences for ADC and AD, respectively.
Table 3.
Statistic results of diffusion metric (FA, ADC, AD and RD) between the MDD and control groups.
| Inter-group comparison |
MDD vs.CTL |
||||
|---|---|---|---|---|---|
| Cerebral regions | Tracts | FA | ADC | AD | RD |
| Anterior cingulate | gCC | ↘ | ↗ | ↘ | ↗ |
| Cingulum gyrus | Cingulum | ↘ | ↗ | NS | ↗ |
| Insula | ILF | ↘ | ↗ | ↘ | ↗ |
| Thalamus | PTR | ↘ | ↗ | ↘ | ↗ |
| Parahippocampal gyrus | PLIC | ↗ | ↘ | ↘ | ↘ |
| Posterior cingulate | sCC | ↗ | ↘ | ↘ | ↘ |
Up and down arrows indicate significant increased and decreased of the diffusion metric (FA, ADC, AD and RD) in MDD group compared to healthy control group obtained with two-sample t-test (p < 0.05). Only the arrows in bold front correspond to changes, which survived after FDR correction (p ≤ 0.05). The acronym NS means no significant difference was found. gCC and sCC: genu and splenium of the Corpus Callosum, ILF: Inferior Longitudinal Fasciculus, PTR: Posterior Thalamic Radiation, PLIC: Posterior Limb of the Internal Capsule.
3.3. Correlation of structural connectivity and clinical scores
Based on the previous results showing similar spatial distribution for all the diffusion metrics, the correlation analyses were restricted to FA values. Moreover, this metric is more likely to capture microstructure-induced diffusion abnormalities that than ADC, AD and RD (Westin et al., 2002).
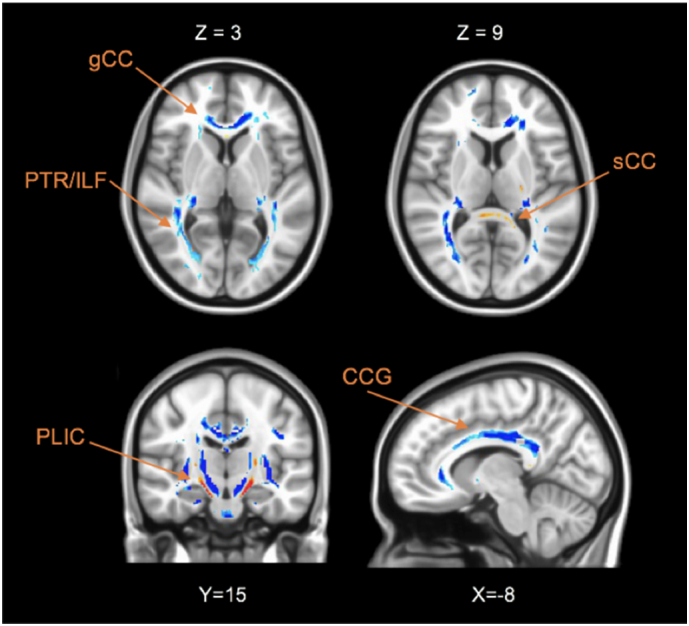
Analyses revealed eight clusters, as shown in Table 4 and Fig. 2. As the psychomotor retardation increased (i.e. WRDS values increased), the FA value decreased in the body and the genu of the CC, the superior corona radiata, the superior striatum (see Fig. 2.A.). Additionally, as displayed in Fig. 2.B., a positive correlation was found between the FA values and anxiety, measured by STAI-YA, in the genu and splenium of the CC, anterior corona radiata and posterior thalamic radiation. However, anxiety was negatively correlated with FA values in the body of CC. Similarly, a negatively correlation was found between the FA values and anhedonia with cingulum, genu of CC and posterior thalamic radiation (see Fig. 2.C.). Furthermore, increased anhedonia (i.e. SHAPS values increased) was associated with greater FA values in superior longitudinal fasciculus.
Table 4.
Voxel-wise correlations between FA and clinical variables.
| Cerebral regions | Tracts | Right or Left | MNI coordinates | r-coef | Cluster size | p-value |
|---|---|---|---|---|---|---|
| Correlation with WDRS | ||||||
| Anterior cingulate | gCC/bCC | R | [14,2,27] | −0.40 | 676 | 0.0001 |
| L | [−4,10,29] | −0.29 | 476 | 0.0002 | ||
| Correlation with STAI-YA | ||||||
| Anterior cingulate | gCC | R/L | [1, 27, 5] | 0.35 | 863 | 0.0001 |
| Insula | ACR | L | [−24, 29, 10] | 0.30 | 554 | 0.002 |
| Precuneus | PTR | L | [−26,-69, 19] | 0.35 | 534 | 0.0001 |
| Correlation with SHAPS | ||||||
| Cingulate gyrus | SCR/CCG | R | [23,−31,44] | −3,36 | 233 | 0.001 |
| L | [−4,1,32] | −3,36 | 240 | 0.001 | ||
| Posterior cingulate | PTR | L | [−22,-69,10] | -3,49 | 342 | 0.0005 |
The r-coef corresponds to the maximum correlation of the cluster and their coordinates are reported in the MNI template. The cluster size is given in mm3. WDRS: Widlöcher Depressive Retardation Scale; MADRS: Montgom ery-Åsberg Depression Rating Scale; STAI-YA: State-Trait Anxiety Inventory; SHAPS: Snaith Hamilton Pleasure Scale, gCC, bCC and sCC: genu, body and splenium of the Corpus Callosum, ACR and SCR: anterior and superior corona radiata, SS: sagittal stratum, PLIC: posterior limb of the internal capsule, PTR: posterior thalamic radiation, SLF: superior longitudinal fasciculus, PTR: posterior thalamic radiation.
Fig. 2.
Axial, coronal and sagittal brain slices showing significant correlation between FA values and clinical variables: (A) WDRS, (B) STAI-YA and (C) SHAPS within the MDD group. Voxels with negative correlation are shown in blue and positive correlation with red. The acronym gCC indicates the genu of corpus callosum (CC); bCC, body of CC; ACR, anterior of the corona radiata; PTR, posterior thalamic radiation; ILF, inferior longitudinal fasciculus; SCR, superior, superior corona radiata; CCG, cingulum. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
4.1. Anatomic connectivity abnormalities in depression
In this large depression cohort, analyses revealed widespread white matter abnormalities in the MDD group compared to CTL. As previously reported in many studies (Chen et al., 2016; Duffy et al., 2014; Henderson et al., 2013; Korgaonkar et al., 2011; Olvet et al., 2016), reduced WM FA (as well as decreased AD and increased ADC/RD) was observed in the genu of the CC extending to the ILF and PTR in MDD patients relative to healthy controls. Decreased FA and AD were observed in the cingulum combined with a normal or slightly increased RD and ADC. This pattern may represent decreased myelination (Korgaonkar et al., 2011); however, it could also result from an altered WM microstructure in the affected WM tracts (e.g., axonal degeneration (Bennett et al., 2010)).
The CC is the largest WM pathway providing for information transfer between the two hemispheres. The genu is the most rostral region of the CC connecting between prefrontal brain regions. The most caudal region, the splenium, connects association areas of the parietal and temporal lobes (anterior splenium) and occipital lobes (posterior splenium). In addition, the body is the midsection between the genu and the splenium associating the motor areas (Jiang et al., 2017). In general, the corpus callosum changes structurally throughout life, but most dramatically during childhood and adolescence (Ballmaier et al., 2008). Moreover, patients with disruption in axonal myelination exhibit executive deficits as well as cognitive dysfunction. Therefore, this tract constitutes key components of the frontotemporal and frontolimbic connections whose dysfunction is thought to underlie many of the emotional, cognitive and behavioral deficits associated with depression (Bracht et al., 2015; Drevets, 2001). However, the splenium is less vulnerable to damage than the genu and body of the CC, which are both late-myelinating (Ballmaier et al., 2008). Moreover, reduced callosal thickness was found in this area only in late-onset depression compared to control, reflecting a more widespread compromise in corticocortical connectivity (Ballmaier et al., 2008).
The cingulum bundle lies within the cingulate gyrus and is an important association pathway linking prefrontal and parahippocampal regions. To date, there is no conclusive evidence for cingulum bundle microstructural alterations in MDD groups. No changes in FA were demonstrated in adults (Carballedo et al., 2012; Zhang et al., 2012). However, reductions of FA in this region were reported in treatment-resistant MDD (de Diego-Adelino et al., 2014). The structural brain tissue markers (FA and MD) in the middle-anterior and middle-posterior cingulum bundle was associated with executive functioning and divided attention (Schermuly et al., 2010). The PTR joins the occipital and posterior parietal cortex with the posterior thalamus, including the pulvinar, and includes the optic radiation from the lateral geniculate body. A previous meta-analytic study that compared 231 MDD patients with 261 healthy participants found reduced FA in posterior thalamic radiation (Liao et al., 2013). This decrease in thalamus may be related to the role of the thalamus in motivation circuit, contributing to the motivational deficits associated with depression (Henderson et al., 2013) or to dysfunctional facial recognition in patients with MDD, as shown by other functional MRI studies (Liao et al., 2013). The ILF connects the anterior part of temporal and occipital lobes, and could be related to emotion and visual processing information deficits of depression (de Diego-Adelino et al., 2014).
From a clinical perspective, our findings provide robust evidence that the reduction of white-matter connectivity in the interhemispheric connections and fronto-limbic neuronal circuits may lead to a better understanding of the pathophysiology of MDD. These results are consistent with an overall hypothesis that depression involves a disconnection of prefrontal, striatal, and limbic emotional areas.
However, contrary to others studies, this analysis also reported other patterns with an increase of FA and AD as well as decreased ADC and RD in the PLIC and sCC in patients with depression. These findings in this large MDD cohort contrast with the previous results obtained with small and heterogeneous samples of patients suffering from depression. Previous studies reported inconsistent results concerning the sCC, varying from reduced WM connectivity (Ota et al., 2015; Reppermund et al., 2014) to no effect. The white matter abnormality in the sCC and PLIC could potentially be associated with anxiety (Kim et al., 2017). As described in the Section 3.1 and Section 4.2, patients of this cohort indeed suffered from high anxiety.
4.2. Phenotypic approach
The former categorical approach has shown WM abnormalities in accordance with some evidence the literature (Chen et al., 2016; Duffy et al., 2014; Henderson et al., 2013; Korgaonkar et al., 2011; Olvet et al., 2016). Nevertheless, these widespread abnormalities can suffer from lack of reproducibility and specificity when studying such biomarker between depressed samples and healthy subjects (Dong et al., 2018; Olvet et al., 2016). In line with this, some authors have emphasized the need to work on dimensional characteristics within depressed samples in order to better link behavior and biological measures. This issue has been drawn as a good way to take into account clinical variability in depressive disorder (Olvet et al., 2016).
4.2.1. Anhedonia
Consistent with previous studies, we found decreased FA as anhedonia increased in the cingulum. Indeed, Bracht et al. suggested that this tract could be a biomarker of vulnerability to depression (Bracht et al., 2015). Thus, the FA value in the cingulum bundle was also described negatively correlated with trait anhedonia (Keedwell et al., 2012). In addition, the same abnormality was described in women with a family history of depression (Keedwell et al., 2012), or in first depressed un-medicated patients (Zhang et al., 2012). Altogether, these results suggested that cingulum WM alteration is involved in the pathophysiological mechanism of depression and is probably linked with reward sensitivity dimension.
Increased FA related to increased anhedonia was also found in SLF. Some studies reported the role of this tract in linking aspects of default mode network and cognitive control and its consequence on executive functioning (Jenkins et al., 2016). Moreover, patients suffering from depression could exhibit a deficit in executive functioning and related with a deficit in programing actions relative to hedonic valence. However, based on these arguments, the opposite correlation between FA and SHAPS was found in SLF. This result may represent a compensatory neuroplasticity or selective neurodegeneration mechanism (Jenkins et al., 2016; Mole et al., 2016).
Other significant decreased FA were found associated with decreased anhedonia in PTR. This result echoes the literature that pointed out the involvement of thalamic cortical projections in MDD (Hermesdorf et al., 2017, Korgaonkar et al., 2011). Previous studies have found that such WM alterations were particularly significant in melancholic MDD (Korgaonkar et al., 2011). Our study is the first that links anhedonia and PTR. Moreover, decreased FA was related with increased anhedonia in gCC. The WM abnormalities in this region were linked with the severity of depression (Chen et al., 2016). This tract is involved in decision making, reward processing (i.e. anhedonia) and emotional regulation (Chen et al., 2016). The strucutral alteration in this area leads to disturbances in cognitive functioning such as memory, executive functions and emotions (Chen et al., 2016). Taken together, our result can lead to the hypothesis that WM alteration in gCC and PTR participates to the severity of depression through one specific phenotype involved in emotion regulation, executive functioning, reward, and decision-making such as anhedonia.
4.2.2. Anxiety
To our knowledge, no study has correlated patient anxiety with white matter diffusion metrics in depressed patients. Most of the studies have chosen a categorical approach in comparing MDD with or without anxious comorbidity (Canu et al., 2015; Delaparte et al., 2017). Despite a large sample, no significant connectivity abnormality has been linked with anxiety (Delaparte et al., 2017).
In this study, the phenotypic analysis of anxiety revealed a relationship between anxiety and FA values in genu and splenium of CC, ACR and PTR. Kim et al. also reported a positive correlation between anxiety and FA values in these three tracts (Kim et al., 2017). Moreover, a meta-analysis has identified that patients suffering from emotional disorders, such as post-traumatic stress disorders had decreased FA in superior corona radiata and anterior thalamic radiation compared with healthy controls (Jenkins et al., 2016).
4.2.3. Psychomotor retardation
Our phenotypic analysis with motor retardation revealed a correlation between increased WRDS scores and reduced FA in body and genu of CC, superior corona radiata and superior striatum. To date, only two studies had explored the relationship between diffusion metrics and motor retardation in depression, using a behavioral metric called actimetry. First, a positive association was found between activity level and structural alterations of cortico-cortical white matter in motor pathways, such the connection between the rostral anterior cingulate cortex and pre-Supplementary Motor Area (SMA) and between the dorsolateral prefrontal cortex and pre-SMA in MDD (Bracht et al., 2012). The authors attributed this result to a relation between these circuits and movement initiation. Then, the second study has pointed out a negative link between FA and left parahippocampal gyrus (Walther et al., 2012), suggesting a plausible link between cerebral alteration in this region and impaired motor planning. Consistent with these results, we exhibited the implication of parahippocampal gyrus and the cingulate. Furthermore, we found that striatal pathway could also play a role in psychomotor retardation. This circuit was also reported in (Bennabi et al., 2013), showing a link between this behavior impairment and dopamine as well as noradrenergic transmission in basal ganglia such as striatum and caudate nucleus.
To sum up, this phenotypic approach pointed out abnormal structural connectivity that have never been demonstrated in depression. One critical finding concerns anxiety which has been linked with higher connectivity in areas such as corpus callosum, anterior corona radiata and posterior thalamic radiation whereas categorical approach for depression have shown strictly the opposite findings for posterior thalamic radiation and genu of corpus callosum. These results support the stratification of patients suffering from depression according to these core dimensions. The phenotypic approach appeared to be a useful way to complete the categorical approach in a better definition of the variability of different clinical profiles. This study is in line with dimensional approach suggested by Olvet et al. (2016) as it allowed a better understanding of critical mechanism involved in pathophysiology of depression and could help to develop more reliable imaging biomarkers.
5. Limitations
The depression cohort is heterogeneous with regard to demographic and clinical parameters such as age and gender of subjects, age of onset of depression, bipolar disorder, potentially increasing variability. However, the patient heterogeneity is also a strength of this study because it disrupts coincidental associations.
Another investigation will be needed to determine whether diffusion changes are associated with poorer neurocognitive performance. Further studies should include neurocognitive tests in order to refine clinical phenotype such as executive functioning, memory, attention, information treatment speed, and emotional patterns, and therefore permit a better accuracy of biomarkers.
The clinical variables, SHAPS, WRDS and STAI-YA, were not reported for the CTL group. Further studies could evaluate their effects on WM organization using a cohort including control subject and patients.
All patients were under psychotropic medication. There is some evidence that psychotropic treatment could lead to decreased FA in schizophrenic patients after medication (Wang et al., 2013). Moreover, in the context of depression, several studies reported that brain changes of white matter are associated with the disease process rather than being effects of medication (Bracht et al., 2015).
In the future, we will extend this work by assessing more robust information of white matter microstructure provided by multi-compartment models and multi-shell diffusion MRI data.
Finally, it is important to note that this study cannot be included in a RDoC program. In fact, it does not strictly emphasize the recommendations such as translational perspective (normal to pathologic, from basic science to behavioral science), or the use of reliable and valid measures of fundamental components (Cuthbert and Insel, 2013). Nevertheless, this study addresses the utility of a deconstruction of actual phenotype of depression from a simple disease-based biomarker to a more specific, symptom-based, severity-related, biomarker.
6. Conclusions
Our study aimed to conduct both categorical and dimensional approach in the study of anatomical connectivity biomarkers in MDD.
First, our results highlight the well-known WM abnormalities in a large cohort of currently depressed patients and compared to controls. The frontolimbic disconnection hypothesis has been replicated as well as large decreased connectivity in widespread brain areas (corpus callosum extending to the inferior fasciculus and posterior thalamic radiation). In addition to previous studies with smaller samples, our results reveal other patterns of increased FA in posterior limb of internal capsule and splenium of corpus callosum.
On the other side, the phenotypic approach has identified some specific patterns related to anxiety, anhedonia and psychomotor retardation – three core symptoms of depression. This latter approach highlighted that within a depressive population, there is a wide variability of anatomic connectivity depending on the severity of each core dimension. This study pointed out that such an approach probably allows more specific and accurate biomarkers than categorical ones, which emphasized widespread abnormalities. Ours brings some evidence for the development of more symptom-based approach within depressed samples.
In the future, a dimensional approach, in addition to the categorical one, could help stratifying different depressive phenotypes, better understanding the pathophysiology characterizing core dimensions, and consequently propose a robust framework for developing targeted therapeutic strategies.
Acknowledgments
MRI data acquisition was supported by the Neurinfo MRI research facility from the University of Rennes I. Neurinfo is granted by the European Union (FEDER), the French State, the Brittany Council, Rennes Metropole, Inria, Inserm and the University Hospital of Rennes. This work has been funded by Institut des Neurosciences Cliniques de Rennes (INCR). The authors thank Mr. Stéphane Brousse and Mr. Jacques Soulabaille for their involvement in the conduct of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101710.
Appendix A. Supplementary data
Supplementary material
References
- Arnone D., McKie S., Elliott R., Juhasz G., Thomas E., Downey D., Williams S., Deakin J., Anderson I. State-dependent changes in hippocampal grey matter in depression. Mol. Psychiatry. 2013;18:1265–1272. doi: 10.1038/mp.2012.150. [DOI] [PubMed] [Google Scholar]
- Assaf Y., Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J. Mol. Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Ballmaier M., Kumar A., Elderkin-Thompson V., Narr K.L., Luders E., Thompson P.M., Hojatkashani C., Pham D., Heinz A., Toga A.W. Mapping callosal morphology in early-and late-onset elderly depression: an index of distinct changes in cortical connectivity. Neuropsychopharmacology. 2008;33:1528. doi: 10.1038/sj.npp.1301538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batail J., Palaric J., Guillery M., Gadoullet J., Sauleau P., Le Jeune F., Vérin M., Robert G.…Drapier D. Apathy and depression: which clinical specificities? Personal. Med. Psychiatry. 2017;7:21–26. [Google Scholar]
- Benedetti F., Bollettini I., Poletti S., Locatelli C., Lorenzi C., Pirovano A., Smeraldi E., Colombo C. White matter microstructure in bipolar disorder is influenced by the serotonin transporter gene polymorphism 5-HTTLPR. Genes Brain Behav. 2015;14:238–250. doi: 10.1111/gbb.12206. [DOI] [PubMed] [Google Scholar]
- Bennabi D., Vandel P., Papaxanthis C., Pozzo T., Haffen E. Psychomotor retardation in depression: a systematic review of diagnostic, pathophysiologic, and therapeutic implications. Biomed. Res. Int. 2013;2013 doi: 10.1155/2013/158746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett I.J., Madden D.J., Vaidya C.J., Howard D.V., Howard J.H. Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Hum. Brain Mapp. 2010;31:378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Federspiel A., Schnell S., Horn H., Höfle O., Wiest R., Dierks T., Strik W., Müller T.J., Walther S. Cortico-cortical white matter motor pathway microstructure is related to psychomotor retardation in major depressive disorder. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T., Linden D., Keedwell P. A review of white matter microstructure alterations of pathways of the reward circuit in depression. J. Affect. Disord. 2015;187:45–53. doi: 10.1016/j.jad.2015.06.041. [DOI] [PubMed] [Google Scholar]
- Buchsbaum M.S., Tang C.Y., Peled S., Gudbjartsson H., Lu D., Hazlett E.A., Downhill J., Haznedar M., Fallon J.H., Atlas S.W. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Canu E., Kostić M., Agosta F., Munjiza A., Ferraro P.M., Pesic D., Copetti M., Peljto A., Tosevski D.L., Filippi M. Brain structural abnormalities in patients with major depression with or without generalized anxiety disorder comorbidity. J. Neurol. 2015;262:1255–1265. doi: 10.1007/s00415-015-7701-z. [DOI] [PubMed] [Google Scholar]
- Carballedo A., Amico F., Ugwu I., Fagan A., Fahey C., Morris D., Meaney J., Leemans A., Frodl T. Reduced fractional anisotropy in the uncinate fasciculus in patients with major depression carrying the met-allele of the Val66Met brain-derived neurotrophic factor genotype. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159:537–548. doi: 10.1002/ajmg.b.32060. [DOI] [PubMed] [Google Scholar]
- Chen G., Hu X., Li L., Huang X., Lui S., Kuang W., Ai H., Bi F., Gu Z., Gong Q. Disorganization of white matter architecture in major depressive disorder: a meta-analysis of diffusion tensor imaging with tract-based spatial statistics. Sci. Rep. 2016;6 doi: 10.1038/srep21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Guo Y., Zhu H., Kuang W., Bi F., Ai H., Gu Z., Huang X., Lui S., Gong Q. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: a voxel-based meta-analysis of diffusion tensor imaging. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;76:179–187. doi: 10.1016/j.pnpbp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Commowick O., Wiest-Daesslé N., Prima S. Automated diffeomorphic registration of anatomical structures with rigid parts: application to dynamic cervical MRI. Med. Image Comput. Comput. Assist. Intervent.–MICCAI. 2012;2012:163–170. doi: 10.1007/978-3-642-33418-4_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commowick O., Wiest-Daesslé N., Prima S. Biomedical Imaging (ISBI), 2012 9th IEEE International Symposium on. IEEE; 2012. Block-ing strategies for rigid registration of multimodal medical images; pp. 700–703. [Google Scholar]
- Coupé P., Yger P., Prima S., Hellier P., Kervrann C., Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging. 2008;27:425–441. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B.N., Insel T.R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaparte L., Yeh F.-C., Adams P., Malchow A., Trivedi M.H., Oquendo M.A., Deckersbach T., Ogden T., Pizzagalli D.A., Fava M. A comparison of structural connectivity in anxious depression versus non-anxious depression. J. Psychiatr. Res. 2017;89:38–47. doi: 10.1016/j.jpsychires.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Diego-Adelino J., Pires P., Gomez-Anson B., Serra-Blasco M., Vives-Gilabert Y., Puigdemont D., Martin-Blanco A., Alvarez E., Perez V., Portella M. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol. Med. 2014;44:1171–1182. doi: 10.1017/S003329171300158X. [DOI] [PubMed] [Google Scholar]
- Dong D., Wang Y., Chang X., Chen X., Chang X., Luo C., Yao D. Common and diagnosis-specific fractional anisotropy of white matter in schizophrenia, bipolar disorder, and major depressive disorder: evidence from comparative voxel-based meta-analysis. Schizophr. Res. 2018;193:456–458. doi: 10.1016/j.schres.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Du X., Liu L., Yang Y., Qi X., Gao P., Zhang Y., Zhang Q. Diffusion tensor imaging of the structural integrity of white matter correlates with impulsivity in adolescents with internet gaming disorder. Brain Behav. 2017;7(8) doi: 10.1002/brb3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S.L., Paradise M., Hickie I.B., Lewis S.J., Naismith S.L., Lagopoulos J. Cognitive impairment with and without depression history: an analysis of white matter microstructure. J. Psychiatr. Neurosci. 2014;39:135. doi: 10.1503/jpn.130079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusi N., Barlati S., Vita A., Brambilla P. Brain structural effects of antidepressant treatment in major depression. Curr. Neuropharmacol. 2015;13:458–465. doi: 10.2174/1570159X1304150831121909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Pub; 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) [Google Scholar]
- Ferrari A.J., Charlson F.J., Norman R.E., Patten S.B., Freedman G., Murray C.J., Vos T., Whiteford H.A. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillard P., Pennec X., Arsigny V., Ayache N. Clinical DT-MRI estimation, smoothing, and fiber tracking with log-Euclidean metrics. IEEE Trans. Med. Imaging. 2007;26:1472–1482. doi: 10.1109/TMI.2007.899173. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.J. Gray colored glasses: is major depression partially a sensory perceptual disorder? J. Affect. Disord. 2013;151:418–422. doi: 10.1016/j.jad.2013.06.045. [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Freedman R., Lewis D.A., Michels R., Pine D.S., Schultz S.K., Tamminga C.A., Gabbard G.O., Gau S.S.-F., Javitt D.C., Oquendo M.A. The initial field trials of DSM-5: new blooms and old thorns. Am. Psychiatric Assoc. 2013;170(1):1–5. doi: 10.1176/appi.ajp.2012.12091189. [DOI] [PubMed] [Google Scholar]
- Fried E.I. Problematic assumptions have slowed down depression research: why symptoms, not syndromes are the way forward. Front. Psychol. 2015;6:309. doi: 10.3389/fpsyg.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Hamilton J.P. Neuroimaging and depression: current status and unresolved issues. Curr. Dir. Psychol. Sci. 2008;17:159–163. [Google Scholar]
- Han K.-M., Choi S., Jung J., Na K.-S., Yoon H.-K., Lee M.-S., Ham B.-J. Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J. Affect. Disord. 2014;155:42–48. doi: 10.1016/j.jad.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Hedouin R., Commowick O., Bannier E., Scherrer B., Taquet M., Warfield S.K., Barillot C. Block-matching distortion correction of echo-planar images with opposite phase encoding directions. IEEE Trans. Med. Imaging. 2017;36:1106–1115. doi: 10.1109/TMI.2016.2646920. [DOI] [PubMed] [Google Scholar]
- Henderson S.E., Johnson A.R., Vallejo A.I., Katz L., Wong E., Gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front. Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermesdorf M., Berger K., Szentkirályi A., Schwindt W., Dannlowski U., Wersching H. Reduced fractional anisotropy in patients with major depressive disorder and associations with vascular stiffness. NeuroImage: Clin. 2017;14:151–155. doi: 10.1016/j.nicl.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. Psychiatric Assoc. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jenkins L.M., Barba A., Campbell M., Lamar M., Shankman S.A., Leow A.D., Ajilore O., Langenecker S.A. Shared white matter alterations across emotional disorders: a voxel-based meta-analysis of fractional anisotropy. NeuroImage: Clin. 2016;12:1022–1034. doi: 10.1016/j.nicl.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Huang X., Wu Q., Zhang T., Lui S., Zhang J., Amatya N., Kuang W., Chan R.C., Kemp G.J. High-field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am. J. Psychiatr. 2010;167:1381–1390. doi: 10.1176/appi.ajp.2010.09101513. [DOI] [PubMed] [Google Scholar]
- Jiang J., Zhao Y.-J., Hu X.-Y., Du M.-Y., Chen Z.-Q., Wu M., Li K.-M., Zhu H.-Y., Kumar P., Gong Q.-Y. Microstructural brain abnormalities in medication-free patients with major depressive disorder: a systematic review and meta-analysis of diffusion tensor imaging. J. Psychiatry Neurosci. 2017;42:150. doi: 10.1503/jpn.150341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell P.A., Chapman R., Christiansen K., Richardson H., Evans J., Jones D.K. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol. Psychiatry. 2012;72:296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Kim M.-K., Kim B., Choi T.K., Lee S.-H. White matter correlates of anxiety sensitivity in panic disorder. J. Affect. Disord. 2017;207:148–156. doi: 10.1016/j.jad.2016.08.043. [DOI] [PubMed] [Google Scholar]
- Korgaonkar M.S., Grieve S.M., Koslow S.H., Gabrieli J.D., Gordon E., Williams L.M. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum. Brain Mapp. 2011;32:2161–2171. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y., Sheehan D.V., Weiller E., Amorim P., Bonora I., Sheehan K.H., Janavs J., Dunbar G.C. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur. Psychiatry. 1997;12:224–231. [Google Scholar]
- Liao Y., Huang X., Wu Q., Yang C., Kuang W., Du M., Lui S., Yue Q., Chan R.C., Kemp G.J. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J. Psychiatry Neurosci. 2013;38:49. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magioncalda P., Martino M., Conio B., Piaggio N., Teodorescu R., Escelsior A., Marozzi V., Rocchi G., Roccatagliata L., Northoff G. Patterns of microstructural white matter abnormalities and their impact on cognitive dysfunction in the various phases of type I bipolar disorder. J. Affect. Disord. 2016;193:39–50. doi: 10.1016/j.jad.2015.12.050. [DOI] [PubMed] [Google Scholar]
- Maj M. Keeping an open attitude towards the RDoC project. World Psychiatry. 2014;13:1–3. doi: 10.1002/wps.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin R.S., Biedrzycki R.C., Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Mole J.P., Subramanian L., Bracht T., Morris H., Metzler-Baddeley C., Linden D.E. Increased fractional anisotropy in the motor tracts of Parkinson's disease suggests compensatory neuroplasticity or selective neurodegeneration. Eur. Radiol. 2016;26:3327–3335. doi: 10.1007/s00330-015-4178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murphy M.L., Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol. Mood Anxiety Disord. 2011;1:3. doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH . National Institute of Mental Health; 2008. The National Institute of Mental Health strategic plan. pp. (NIH Publication No. 08-6368) [Google Scholar]
- Olvet D.M., Delaparte L., Yeh F.C., DeLorenzo C., McGrath P.J., Weissman M.M., Adams P., Fava M., Deckersbach T., McInnis M.G. A comprehensive examination of white matter tracts and connectometry in major depressive disorder. Depress. Anxiety. 2016;33:56–65. doi: 10.1002/da.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M., Noda T., Sato N., Hattori K., Hori H., Sasayama D., Teraishi T., Nagashima A., Obu S., Higuchi T. White matter abnormalities in major depressive disorder with melancholic and atypical features: a diffusion tensor imaging study. Psychiatry Clin. Neurosci. 2015;69:360–368. doi: 10.1111/pcn.12255. [DOI] [PubMed] [Google Scholar]
- Ourselin S., Roche A., Prima S., Ayache N. MICCAI. Springer; 2000. Block matching: A general framework to improve robustness of rigid registration of medical images; pp. 557–566. [Google Scholar]
- Reppermund S., Zhuang L., Wen W., Slavin M.J., Trollor J.N., Brodaty H., Sachdev P.S. White matter integrity and late-life depression in community-dwelling individuals: diffusion tensor imaging study using tract-based spatial statistics. Br. J. Psychiatry. 2014;205:315–320. doi: 10.1192/bjp.bp.113.142109. [DOI] [PubMed] [Google Scholar]
- Sacchet M.D., Prasad G., Foland-Ross L.C., Joshi S.H., Hamilton J.P., Thompson P.M., Gotlib I.H. Structural abnormality of the corticospinal tract in major depressive disorder. Biol. Mood Anxiety Disord. 2014;4:8. doi: 10.1186/2045-5380-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim H.A. The definition and meaning of treatment-resistant depression. J. Clin. Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Schermuly I., Fellgiebel A., Wagner S., Yakushev I., Stoeter P., Schmitt R.e., emsp14 al, Knickenberg R., Bleichner F., Beutel M. Association between cingulum bundle structure and cognitive performance: an observational study in major depression. Eur. Psychiatry. 2010;25:355–360. doi: 10.1016/j.eurpsy.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Sexton C.E., Mackay C.E., Ebmeier K.P. A systematic review of diffusion tensor imaging studies in affective disorders. Biol. Psychiatry. 2009;66:814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Smith K. Mental health: a world of depression. Nat. News. 2014;515:180. doi: 10.1038/515180a. [DOI] [PubMed] [Google Scholar]
- Snaith R., Hamilton M., Morley S., Humayan A., Hargreaves D., Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br. J. Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. 1970. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Taylor W.D., Hsu E., Krishnan K.R.R., MacFall J.R. Diffusion tensor imaging: background, potential, and utility in psychiatric research. Biol. Psychiatry. 2004;55:201–207. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Taylor W.D., Kudra K., Zhao Z., Steffens D.C., MacFall J.R. Cingulum bundle white matter lesions influence antidepressant response in late-life depression: a pilot study. J. Affect. Disord. 2014;162:8–11. doi: 10.1016/j.jad.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S., Hügli S., Höfle O., Federspiel A., Horn H., Bracht T., Wiest R., Strik W., Müller T.J. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol. Dis. 2012;47:13–19. doi: 10.1016/j.nbd.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Wang Q., Cheung C., Deng W., Li M., Huang C., Ma X., Gong Q. White-matter microstructure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychol. Med. 2013;43(11):2301–2309. doi: 10.1017/S0033291713000238. [DOI] [PubMed] [Google Scholar]
- Widlöcher D.J. Psychomotor retardation: clinical, theoretical, and psychometric aspects. Psychiatr. Clin. N. Am. 1983;6(1):27–40. [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Young J.J., Silber T., Bruno D., Galatzer-Levy I.R., Pomara N., Marmar C.R. Is there progress? An overview of selecting biomarker candidates for major depressive disorder. Front. Psychiatry. 2016;7:72. doi: 10.3389/fpsyt.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Leow A., Ajilore O., Lamar M., Yang S., Joseph J., Medina J., Zhan L., Kumar A. Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology. 2012;37:959. doi: 10.1038/npp.2011.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K., Huang X., Li T., Gong Q., Li Z., Ou-yang L., Deng W., Chen Q., Li C., Ding Y. Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. J. Psychiatry Neurosci. 2008;33:525. [PMC free article] [PubMed] [Google Scholar]
- Zou K., Deng W., Li T., Zhang B., Jiang L., Huang C., Sun X., Sun X. Changes of brain morphometry in first-episode, drug-naïve, non–late-life adult patients with major depression: an optimized voxel-based morphometry study. Biol. Psychiatry. 2010;67:186–188. doi: 10.1016/j.biopsych.2009.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material