Objective
NELL-1 is an osteogenic protein first discovered to control ossification of the cranium. NELL-1 exists in at least two isoforms. The full-length NELL-1 contains 810 amino acid (aa) (NELL-1810), the N-terminal-truncated NELL-1 isoform contains 570 aa (NELL-1570). The differences in cellular effects between NELL-1 isoforms are not well understood. Methods: Here, BMSC were derived from adult or aged mice, followed by overexpression of NELL-1810 or NELL-1570. Cell morphology, proliferation, and gene expression were examined. Results/Conclusions: Overall, the proliferative effect of NELL-1570 was age dependent, showing prominent induction in adult but not aged mice.
Keywords: Mesenchymal stem cell, Nell-1, BMSC, Osteogenesis
1. Introduction
NELL-1 is a large, secreted protein first examined in the context of human craniofacial skeletal development, where NELL-1 was noted to be expressed in areas of ossification and its overexpression associated with human craniosynostosis (CS).1 Since that time, transgenic Nell-1 overexpressing mice have been observed to recapitulate a craniosynostosis phenotype.2 Conversely, Nell-1 deficient mice exhibit a perinatal lethal phenotype, with cranial and vertebral bone defects and reduced mineralization.3 In adult, Nell-1 haploinsufficiency results in low bone mineral density (BMD) which is accentuated with age, associated with reduced osteoblastic differentiation, and increased bone fragility.4 NELL-1 exerts regulatory effects on a broad array of mesenchymal cell types, including mesenchymal progenitor/stem cells (MPC/MSCs),4, 5, 6, 7 pre-osteoblasts,4,8 odontoblasts,9 and chondroblasts.10, 11, 12 NELL-1 binds to the heterodimer Integrin α3β113 as well as cell surface Cntnap4 (Contactin-associated protein-like 4),14 resulting in intracellular signaling changes including focal adhesion kinase (FAK),15 mitogen activated protein kinase (MAPK),16, 17, 18 and Wnt/β-catenin signaling activation.4,19
NELL-1 exists in at least two isoforms. While the full-length NELL-1 contains 810 amino acid (aa) residues (referred to as NELL-1810), the N-terminal-truncated NELL-1 isoform contains 570 aa residues (referred to as NELL-1570). Previously, we observed that NELL-1570 significantly stimulated MSC proliferation in multiple MSC-like populations such as murine C3H10T1/2 MSC cell line, mouse primary MSCs, and human perivascular MSC. In contrast, NELL-1810 demonstrated only limited stimulation of MSC proliferation.7 In vivo, NELL-1570 induced significant calvarial defect regeneration accompanied by increased cell proliferation. Nevertheless, the differences in cellular effects between NELL-1810 and NELL-1570 are only partially understood. Moreover, the age-dependent effects of NELL-1 isoforms on MSC are entirely unknown.
2. Methods
2.1. Bone marrow mesenchymal stem cell (BMSC) cell isolation
Mouse bone marrow mesenchymal stem cells (BMSC) were harvested from 4-month-old (adult) and 14-month-old (aged) C57BL/6 mice by flushing the femoral marrow cavities and harvesting adherent cells on standard culture-treated plates according to prior reports.4 Passage 5 cells or less were used for all assays. Cells were cultured at 37 °C in a humidified atmosphere containing 95% air and 5% CO2. The expansion of cells was performed in DMEM, 10% fetal bovine serum (FBS), 1% penicillin/streptomycin. Medium was changed every 3 d unless otherwise noted.
2.2. Viral transfection
Generation and transfection of MSC were performed based on our prior studies.7 The coding sequences of NELL-1810 and NELL-1570 were obtained from our previously isolated NELL-1810 clone, as described,1 using polymerase chain reaction (PCR). The ends with appropriate restriction sites were subsequently generated using PCR. The resulting gene fragments were inserted into the pFG12 plasmid20 to substitute for the GFP gene. These inserts were controlled by the ubiquitin C promoter. These generated transduction plasmids were used to cotransfect 293T cells with plasmids containing the lentiviral packaging proteins and envelope proteins, as described previously.20 Viruses from 293T cell cultures were collected and concentrated by ultracentrifugation at 17,000 rpm for 60 min at 4 °C, using the SW32 rotor of a Beckman centrifuge Beckman Coulter (Brea, CA). The lentiviral GFP vector, FG12, was titrated by transduction of 293T cells with limited dilutions to determine the tissue culture infective dose of 50% chance (TCID50) and by measuring the viral p24 protein contents in preparations. Titers of other viral vectors with the p24 content in viral preparations were used to determine titers, with FG12 as the reference. Generally, 1 pg of p24 content in a viral preparation was equivalent to 5–20 TCID50 units.
Transfection of 293T cells to generate lentiviral vectors was performed by calcium precipitation using a kit purchased from Promega (Madison, WI). Transfection of BMSC was accomplished using Lipofectamine from Invitrogen (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Lentiviral transduction was performed by addition of lentiviral vectors to cell cultures at a multiplicity of infection (MOI) of 10:1. Three hours prior to transduction, the medium was changed to either serum-free or specific medium (depending on the requirements of the particular experiments). Immediately before transduction, the culture medium was replaced with serum-free medium. Three hours post-transduction, the cell cultures were washed to remove virus, and fresh medium was added.
2.3. Cell proliferation
Cells were seeded in 96 well plates at a density of 1000 cells per well and allowed to adhere overnight. BMSC from either 4 or 14-month-old animals were cultured in DMEM +10% FBS +1% Pen Strep with each lentiviral treatment group for 2 d followed by MTS assay per the manufacturer's instructions (Promega, Madison, WI).
2.4. Ribonucleic acid (RNA) isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Gene expression was assayed by quantitative RT-PCR, based on our previous methods.4,19 Primers are in Supplemental Table 1. Briefly, total RNA was extracted using RNEasy Kit (Qiagen, Santa Clarita, CA). 1 μg of total RNA from each sample was subjected to first-strand complementary deoxyribonucleic acid (cDNA) synthesis using the SuperScript III Reverse-Transcriptase Kit (Life Technologies) to a final volume of 20 μL The reverse transcription reaction was performed at 65 °C for 5 min, followed by 50 °C for 50 min and 85 °C for 5 min. For qRT-PCR, the reaction was performed using 2 × SYBR green RT-PCR master mix and an ABI PRISM 7300 qRT-PCR system instrument (Applied Biosystems, Foster City, CA). qRT-PCR was performed using 96 well optical plates at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, and at 60 °C for 60 s. The relative quantification of gene expression was performed using a Comparative CT method according to the manufacturer's protocol and was normalized to the expression levels of the housekeeping gene in each sample.
2.5. Statistical analysis
All results were expressed as mean ± standard deviation (SD). Statistical analyses were performed using the SPSS16.0 software. All data were normally distributed. Student's t-test was used for two-group comparisons, and one-way ANOVA test was used for comparisons of 3 or more groups, followed by Tukey's post hoc test. Differences were considered significant when P < 0.05.
3. Results
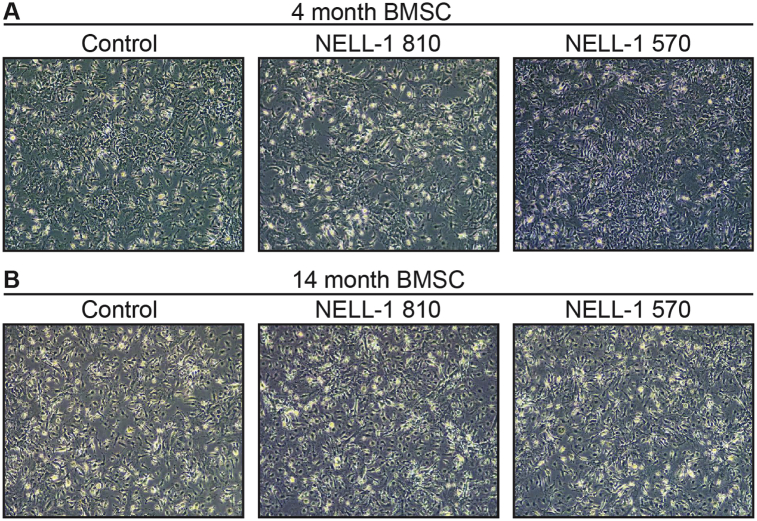
3.1. NELL-1 isoforms do not induce a change in BMSC morphology
In order to investigate the age-dependent effects of NELL-1 isoforms, BMSC were first isolated from adult (4 mo) and aged (14 mo) old male mice. Lentiviral mediated overexpression of NELL-1 isoforms was next performed (NELL-1810 or NELL-1570) or lentiviral control (FG12). Overexpression of Nell-1 gene transcripts was verified as per prior studies.7 Overall, no significant difference in cell morphology was seen with BMSC harvested from adult or aged BMSC. As well, no significant change in cell morphology was observed among BMSC across any treatment conditions (Fig. 1).
Fig. 1.
BMSC cell morphology after NELL-1810 or NELL-1570 overexpression. BMSC harvested from male C57BL/6 mice of two ages, including (A) 4-month-old (adult) and (B) 14-month-old (aged) mice. Lentiviral overexpression of NELL-1 isoforms was performed, including NELL-1810 and NELL-1570. In all cases, control cells were treated with a lentivirus expressing GFP (FG12). Representative cytomorphology is shown, which showed no significant difference across ages or treatment groups.
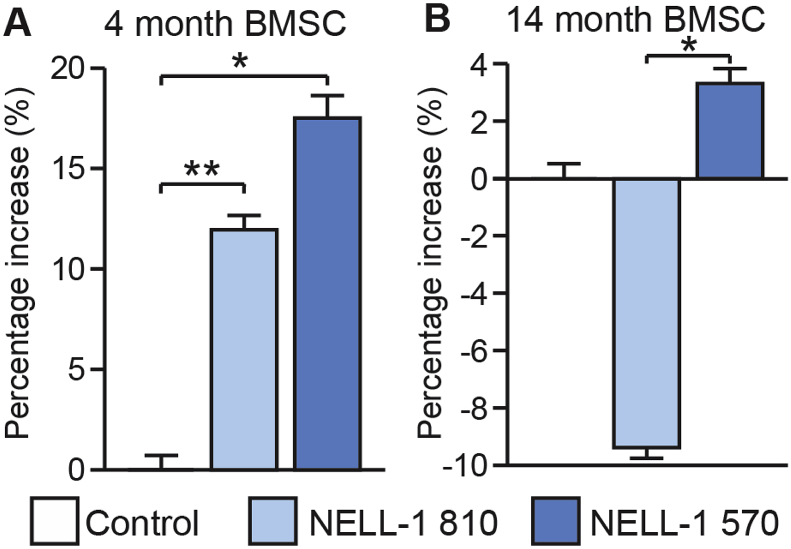
3.2. NELL-1 isoform induced BMSC proliferation is age dependent
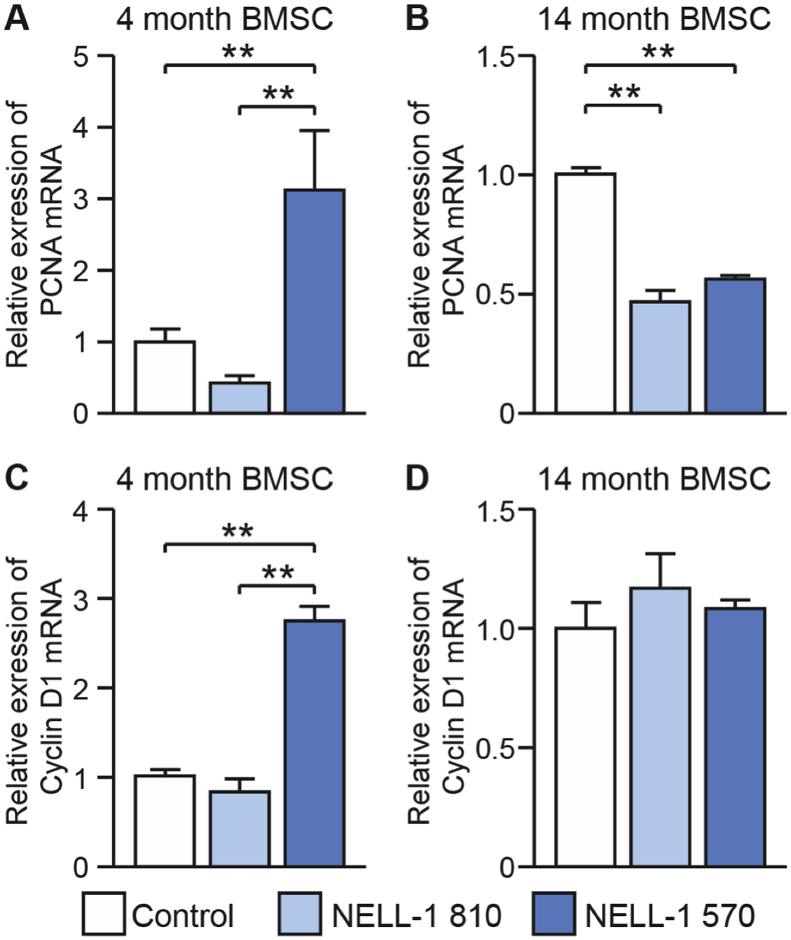
Next, we inquired as to age dependent effects of NELL-1 isoforms on BMSC proliferation (Fig. 2). Among adult (4 mo) BMSC, overexpression of either NELL-1 isoform demonstrated a significant increase in cellular proliferation, as assessed by MTS assays (Fig. 2A). In agreement with prior observations, NELL-1570 showed a greater degree of a mitogenic effect in comparison to full length NELL-1. Interestingly among aged (14 mo) BMSC, results were quite different (Fig. 2B). Here, overexpression of NELL-1810 resulted in a slight reduction in cell proliferation in comparison to FG12 control. Moreover, a slight increase in proliferation was observed among NELL-1570 overexpressing aged BMSC. However, the mitogenic effect of NELL-1570 was much more prominent among adult rather than aged BMSC (∼17% versus ∼3.5% increase). Gene expression of PCNA and Cyclin D1 was next assessed among adult and aged BMSC under each treatment condition (Fig. 3). Gene expression of PCNA and Cyclin D1 were increased among NELL-1570 overexpressing BMSC (Fig. 3A,C). Consistent with our prior observations, these increases were not observed among aged BMSC (Fig. 3B,D). Overall, the mitogenic effects of NELL-1570 are age dependent, and are observed in adult but not aged mouse BMSC.
Fig. 2.
NELL-1 isoform induced cell proliferation is age dependent. Using BMSC derived from 4-month or 14-month-old animals, lentiviral overexpression of NELL-1 isoforms was performed, including NELL-1810 and NELL-1570. In all cases, control cells were treated with a lentivirus expressing GFP (FG12). MTS assay performed after 48 h. Percentage change shown. (A) Adult mouse-derived BMSC show a significant increase in cell proliferation, especially with NELL-1570 overexpression. (B) Aged mouse-derived BMSC show a blunted, non-significant increase with NELL-1570 overexpression. Among this age group, NELL-1810 overexpression reduced cell proliferation. *P < 0.05; **P < 0.01.
Fig. 3.
NELL-1 isoform induced changes in proliferation associated gene expression is age dependent. Using BMSC derived from 4 month or 14 month old animals, lentiviral overexpression of NELL-1 isoforms was performed, including NELL-1810 and NELL-1570. In all cases, control cells were treated with a lentivirus expressing GFP (FG12). Gene expression analysis by qRT-PCR among BMSC derived from adult (4 months) and aged (14 months) mice of proliferation associated genes. Among adult animal BMSC, a mitogenic effect is observed with NELL-1570 (A,C). In contrast, no difference among aged animal BMSC was observed in PCNA or CyclinD1 expression (B,D). **P < 0.01.
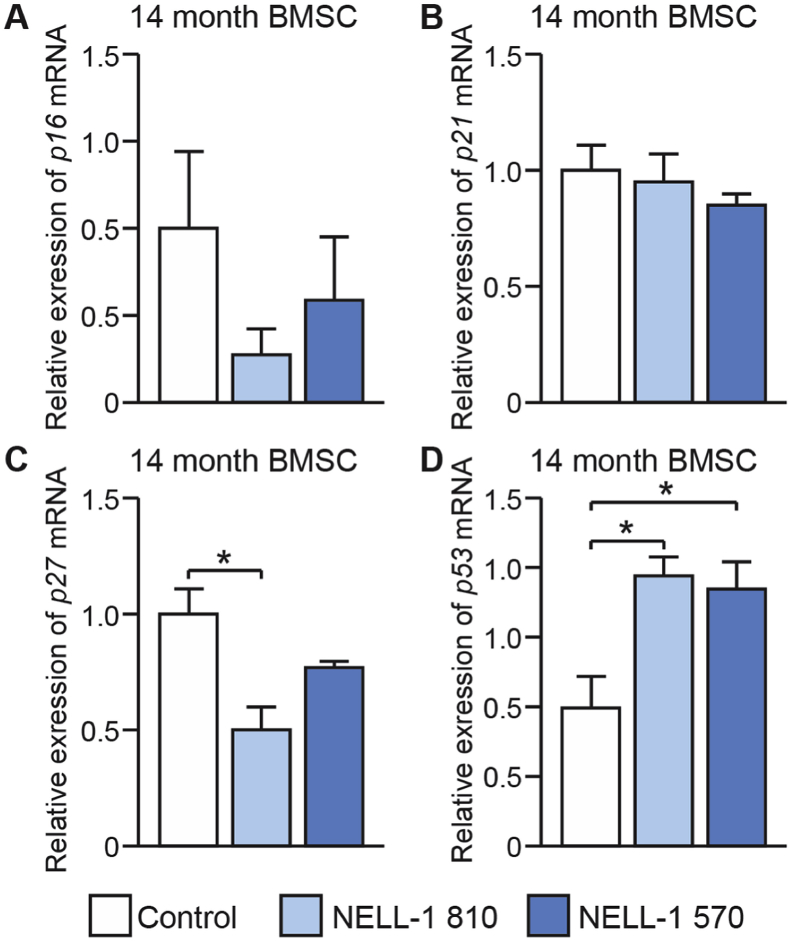
3.3. NELL-1 isoform induced changes in senescence associated gene expression among aged BMSC
Finally, we assayed whether in aged BMSC the loss of a mitogenic effect of NELL-1 isoforms was associated with cellular senescence. Here, gene markers were assayed by qRT-PCR after overexpression of either NELL-1 isoform (Fig. 4). Expression of p16 and p27 were non-significantly reduced with overexpression of either isoform (Fig. 4A,C). No change in p21 expression was observed (Fig. 4B), while both isoforms results in a significant increase in p53 gene transcripts (Fig. 4D). Overall, a consistent induction of cellular senescence associated genes was not seen with overexpression of either NELL-1 isoform.
Fig. 4.
NELL-1 isoforms do not significantly induce cellular senescence associated gene expression among aged BMSC. Using BMSC derived from 14-month-old animals, lentiviral overexpression of NELL-1 isoforms was performed, including NELL-1810 and NELL-1570. In all cases, control cells were treated with a lentivirus expressing GFP (FG12). Gene expression analysis by qRT-PCR among BMSC derived from aged (14 months) mice. With the exception of p53 induction (D), no significant increase was observed across gene markers of senescence, including (A) p16, (B) p21, or (C) p27. Similar findings were observed among adult-derived BMSC. *P < 0.05.
4. Discussion
In summary, we report that the mitogenic effects of NELL-1570 are age dependent, and are observed in adult but not aged mouse BMSC. Overall, a consistent induction of cellular senescence associated genes was not seen with overexpression of either NELL-1 isoform. Overall, these studies provide further insight into the age-dependent and isoform specific effects of the osteoinductive protein NELL-1.
In our recent study, we observed that Nell-1 signaling increases Sca-1+ MSC numbers across multiple orthopaedic models, including MSC culture, murine long bone injection, murine systemic administration, and non-human primate spinal fusion.21 In this study, the long isoform of NELL-1 was used only. Overall, it is not clear the extent to which this expansion in Sca-1+ MSC numbers has a clear correlate to the increase in cell proliferation observed in cell culture in the current studies. First, the effects of NELL-1570 on Sca-1+ cell subsets has not been directly tested. Second, the exact identity of bone marrow resident Sca-1+ MPCs is still poorly understood. It is unclear if the Sca-1+CD31-CD45- stromal population are bona fide MSC, their committed osteoblastic progeny, or a combination of the two. Importantly, the mitogenic effects of NELL-1 protein are modest and it is unclear if this alone underlies the significant changes in Sca-1+ cell numbers observed in our prior study.21
Overall, NELL-1 possesses several theoretical benefits as a potential bone-forming therapy. NELL-1 has documented tumor suppressive properties and its expression is lost in several malignant epithelial tumors.22,23 Although NELL-1 is expressed among many skeletal tumors, its expression does not correlate with benign versus malignant tumor types.24,25 Finally, NELL-1 has an excellent safety profile. Mice with constitutive Nell-1 overexpression have a normal lifespan without increased development of tumors.2 Similarly, five-day intravenous testing of recombinant NELL-1 showed essentially no pathologic changes.4 Nevertheless, Nell-1 signaling has known functions in an wide variety of developmental processes, including vascular26 and neural development.27 With this in mind, a more thorough study of these potential off-target effects must be considered.
Conflicts of interest
K.T. and A.W.J. are inventors of NELL-1-related patents. K.T. is a founder and board members of Bone Biologics Inc./Bone Biologic Corp., which sublicenses NELL-1 patents from the UC Regents, which also hold equity in the company.
Acknowledgments
The present work was supported by the NIH/NIAMS (R01 AR070773, K08 AR068316), NIH NIDCR (R21 DE027922), Department of Defense (W81XWH-18-1-0121, W81XWH-18-1-0336), American Cancer Society (Research Scholar Grant, RSG-18-027-01-CSM), the Maryland Stem Cell Research Foundation, and the Musculoskeletal Transplant Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or Department of Defense.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2019.02.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ting K., Vastardis H., Mulliken J.B. Human NELL-1 expressed in unilateral coronal synostosis. J Bone Miner Res. 1999;14:80–89. doi: 10.1359/jbmr.1999.14.1.80. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X., Kuroda S., Carpenter D. Craniosynostosis in transgenic mice overexpressing Nell-1. J Clin Invest. 2002;110:861–870. doi: 10.1172/JCI15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai J., Shannon M.E., Johnson M.D. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum Mol Genet. 2006;15:1329–1341. doi: 10.1093/hmg/ddl053. [DOI] [PubMed] [Google Scholar]
- 4.James A.W., Shen J., Zhang X. NELL-1 in the treatment of osteoporotic bone loss. Nat Commun. 2015;6:7362. doi: 10.1038/ncomms8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C.S., Zhang X., Peault B. Accelerated chondrogenic differentiation of human perivascular stem cells with NELL-1. Tissue Eng. 2016;22:272–285. doi: 10.1089/ten.tea.2015.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S., Zhang X., Shen J. Brief report: human perivascular stem cells and nel-like protein-1 synergistically enhance spinal fusion in osteoporotic rats. Stem Cell. 2015;33:3158–3163. doi: 10.1002/stem.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang S., Shen J., Liu Y. Proliferation and osteogenic differentiation of mesenchymal stem cells induced by a short isoform of NELL-1. Stem Cell. 2015;33:904–915. doi: 10.1002/stem.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan C.M., Zhang X., James A.W. NELL-1 increases pre-osteoblast mineralization using both phosphate transporter Pit 1 and Pit 2. Biochem Biophys Res Commun. 2012;422:351–357. doi: 10.1016/j.bbrc.2012.04.077. [DOI] [PubMed] [Google Scholar]
- 9.Santamaría A., Juárez S., Reche A. Low-molecular-weight heparin, bemiparin, in the outpatient treatment and secondary prophylaxis of venous thromboembolism in standard clinical practice: the ESFERA Study. Int J Clin Pract. 2006;60:518–525. doi: 10.1111/j.1368-5031.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen W., Zhang X., Siu R.K. Nfatc2 is a primary response gene of Nell-1 regulating chondrogenesis in ATDC5 cells. J Bone Miner Res. 2011;26:1230–1241. doi: 10.1002/jbmr.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M., Siu R.K., Ting K., Wu B.M. Effect of Nell-1 delivery on chondrocyte proliferation and cartilaginous extracellular matrix deposition. Tissue Eng. 2010;16:1791–1800. doi: 10.1089/ten.TEA.2009.0384. [DOI] [PubMed] [Google Scholar]
- 12.Siu R.K., Zara J.N., Hou Y. NELL-1 promotes cartilage regeneration in an in vivo rabbit model. Tissue Eng. 2012;18:252–261. doi: 10.1089/ten.tea.2011.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasebe A., Nakamura Y., Tashima H. The C-terminal region of NELL1 mediates osteoblastic cell adhesion through integrin alpha3beta1. FEBS Lett. 2012;586:2500–2506. doi: 10.1016/j.febslet.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Li C., Zheng Z., Ha P. Neurexin superfamily cell membrane receptor contactin-associated protein like-4 (Cntnap4) is involved in neural EGFL-like 1 (Nell-1)-Responsive osteogenesis. J Bone Miner Res. 2018 Oct;33(10):1813–1825. doi: 10.1002/jbmr.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen J., James A.W., Chung J. NELL-1 promotes cell adhesion and differentiation via Integrinbeta1. J Cell Biochem. 2012;113:3620–3628. doi: 10.1002/jcb.24253. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Ting K., Bessette C.M. Nell-1, a key functional mediator of Runx2, partially rescues calvarial defects in Runx2(+/-) mice. J Bone Miner Res. 2011;26:777–791. doi: 10.1002/jbmr.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen F., Walder B., James A.W. NELL-1-dependent mineralisation of Saos-2 human osteosarcoma cells is mediated via c-Jun N-terminal kinase pathway activation. Int Orthop. 2012;36:2181–2187. doi: 10.1007/s00264-012-1590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bokui N., Otani T., Igarashi K. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 2008;582:365–371. doi: 10.1016/j.febslet.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen J., James A.W., Zhang X. Novel Wnt regulator NEL-like molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. Am J Pathol. 2016;186:419–434. doi: 10.1016/j.ajpath.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin X.F., An D.S., Chen I.S., Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci U S A. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James A.W., Shen J., Tsuei R. NELL-1 induces Sca-1+ mesenchymal progenitor cell expansion in models of bone maintenance and repair. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Z., Mori Y., Yang J. Hypermethylation of the nel-like 1 gene is a common and early event and is associated with poor prognosis in early-stage esophageal adenocarcinoma. Oncogene. 2007;26:6332–6340. doi: 10.1038/sj.onc.1210461. [DOI] [PubMed] [Google Scholar]
- 23.Mori Y., Cai K., Cheng Y. A genome-wide search identifies epigenetic silencing of somatostatin, tachykinin-1, and 5 other genes in colon cancer. Gastroenterology. 2006;131:797–808. doi: 10.1053/j.gastro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Shen J., LaChaud G., Shrestha S. NELL-1 expression in tumors of cartilage. J Orthop. 2015;12:S223–S229. doi: 10.1016/j.jor.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J., LaChaud G., Khadarian K. NELL-1 expression in benign and malignant bone tumors. Biochem Biophys Res Commun. 2015;460:368–374. doi: 10.1016/j.bbrc.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 26.Askarinam A., James A.W., Zara J.N. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng. 2013;19:1386–1397. doi: 10.1089/ten.tea.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura R., Nakamoto C., Obama H., Durward E., Nakamoto M. Structure-function analysis of Nel, a thrombospondin-1-like glycoprotein involved in neural development and functions. J Biol Chem. 2012;287:3282–3291. doi: 10.1074/jbc.M111.281485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.