Abstract
Background. Sacubitril/valsartan in heart failure (HF) with reduced ejection fraction (HFrEF) was shown to be superior to enalapril in reducing the risk of death and hospitalization for HF. Our aim was to evaluate the cardiopulmonary effects of sacubitril/valsartan in patients with HFrEF. Methods. We conducted an observational study. Ninety-nine ambulatory patients with HFrEF underwent serial cardiopulmonary exercise tests (CPET) after initiation of sacubitril/valsartan in addition to recommended therapy. Results. At baseline, 37% of patients had New York Heart Association (NYHA) class III. After a median follow-up of 6.2 months (range 3–14.9 months) systolic blood pressure decreased from 117 ± 14 to 101 ± 12 mmHg (p < 0.0001), left ventricular ejection fraction (LVEF) increased from 27 ± 6 to 29.7 ± 7% (p < 0.0001), peak oxygen consumption (VO2) improved from 14.6 ± 3.3 (% of predicted = 53.8 ± 14.1) to 17.2 ± 4.7 mL/kg/min (% of predicted = 64.7 ± 17.8) (p < 0.0001), minute ventilation/carbon dioxide production relationship (VE/VCO2 Slope) decreased from 34.1 ± 6.3 to 31.7 ± 6.1 (p = 0.006), VO2 at anaerobic threshold increased from 11.3 ± 2.6 to 12.6 ± 3.5 mL/kg/min (p = 0.007), oxygen pulse increased from 11.5 ± 3.0 to 13.4 ± 4.3 mL/kg/min (p < 0.0001), and ∆VO2/∆Work increased from 9.2 ± 1.5 to 10.1 ± 1.8 mL/min/watt (p = 0.0002). Conclusion. Sacubitril/valsartan improved exercise tolerance, LVEF, peak VO2, and ventilatory efficiency at 6.2 months follow-up. Further studies are necessary to better clarify underlying mechanisms of this functional improvement.
Keywords: heart failure, sacubitril/valsartan, cardiopulmonary test, exercise tolerance
1. Introduction
Combining renin-angiotensin-aldosterone system blockade with natriuretic peptide system enhancement may deliver functional benefits to patients with heart failure (HF) with reduced ejection fraction (HFrEF). In the PARADIGM-HF study, angiotensin receptor/neprilysin inhibitor sacubitril/valsartan was shown to be superior to enalapril in reducing the risk of death and hospitalization for HF [1]. However, little is known about the effects of sacubitril/valsartan on cardiopulmonary function. Recent studies showed an improvement in exercise tolerance at 6-min walk test (6-MWT) after initiation of sacubitril/valsartan in patients with HFrEF [2,3,4]. In this clinical setting, only one study demonstrated an increase in peak oxygen consumption (VO2) after initiation of sacubitril/valsartan, but it was limited by a small sample size (16 patients) and a very short-term follow-up (1 month) [5].
Cardiopulmonary exercise test (CPET) is a valuable tool in HFrEF, allowing accurate assessment of patients’ functional capacity and providing prognostically relevant parameters (e.g., peak VO2 and minute ventilation/carbon dioxide production relationship [VE/VCO2 slope]) [6,7,8,9,10].
In this study, we sought to evaluate the effects of sacubitril/valsartan on prognostically significant CPET parameters in a larger population of HFrEF patients and with a longer follow-up.
2. Materials and Methods
2.1. Patient Selection and Study Design
This prospective, observational study was approved by the Institutional Research Review Boards of the Cardiovascular Rehabilitation Unit of Buccheri La Ferla Fatebenefratelli Hospital and of the Department for the Treatment and Study of Cardiothoracic Diseases and Cardiothoracic Transplantation IRCCS-ISMETT, Palermo, Italy. All patients provided informed consent. This study complies with the principles of the Declaration of Helsinki and national regulations. Sacubitril/valsartan was administered to patients with HFrEF, on top of guidelines recommended therapy [11]. Patients were included in the study in accordance with the Italian reimbursement criteria for sacubitril/valsartan: 1. symptomatic HF defined as New York Heart Association (NYHA) class II–IV, 2. left ventricular ejection fraction below 35%, as measured using echocardiography, 3. previous treatment with an individual optimal dose of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for at least 6 months, 4. systolic arterial blood pressure ≥100 mmHg, 5. serum K+ level <5.4 mEq/L, 6. estimated glomerular filtration rate >30 mL/min/1.73 m2, 7. absence of severe liver insufficiency (Child-Pugh C), and 8. no history of angioedema.
In accordance with European Society of Cardiology Prevention guidelines, patients were encouraged to have a minimum of 2.5 h a week of moderate intensity aerobic activity, in multiple bouts each lasting ≥10 min, 5 days a week [12]. Patients were not engaged in exercise-based cardiac rehabilitation, and physical activity was prescribed according to patient’s age, past habits, comorbidities, preferences, and goals.
Exclusion criteria were: 1. hospitalization for HF within 90 days before ambulatory evaluation, 2. myocardial revascularization within 180 days before ambulatory evaluation, 3. concomitant initiation of cardiac resynchronization therapy and/or percutaneous mitral valve treatment during study follow-up or in the previous 6 months, 4. congenital heart disease, and 5. inability to perform CPET.
Sacubitril/valsartan was administered according to established guidelines [11]. Up-titration was performed every 4 weeks, if tolerated by the patient. Changes in the dosage of diuretics were allowed during the study follow-up if deemed necessary. N-terminal pro-brain natriuretic peptides (NT-proBNP) serum levels were detected at baseline and at 3, 6, and 12 months.
2.2. CPET Protocol
Baseline CPET was performed before starting administration of sacubitril/valsartan. Serial CPETs were performed at 3, 6, and 12 months. All CPETs were performed on a cycle ergometer at 60 rpm. A ramp protocol was systematically performed: work load started at 10 watts for 2 min (warm-up) and increased by 10 watts every 60 s. Breath by breath analysis of expiratory O2, CO2, and expired volumes was performed using the Vmax® 2900 metabolic cart (SensorMedics, Yorba Linda, CA, USA). Heart rate, 12 lead ECG, and oximetry (with pulse oximeter) were monitored continuously. Patients were encouraged to exercise until they felt unable to continue because of dyspnea or fatigue. The respiratory exchange ratio (RER) is the ratio between the amount of CO2 produced in metabolism and O2 used, representing a measure of exercise effort with RER > 1.05–1.10 indicating maximal effort [10]. Anaerobic threshold (i.e., the point during exercise when a switch from aerobic to anaerobic metabolism occurs) was measured using the V-slope analysis from the plot of carbon dioxide production (VCO2) versus VO2 and confirmed using ventilatory equivalents and end-tidal pressures of CO2 and O2. The rate at which VO2 increased per watt of work (∆VO2/∆Work) was calculated for the progressively increasing exercise period, beginning 1 min after work rate started to increase. ∆VO2/∆Work slope and VO2 at anaerobic threshold (AT-VO2) were used as a measure of muscle efficiency. The relationship between minute ventilation and carbon dioxide production (VE/VCO2 slope) was used as a measure of ventilatory efficiency and was calculated from 1 min after the beginning of loaded exercise up to the end of the isocapnic buffering period. Reported values of VO2, ventilation, and tidal volume at peak exercise are the averages over the 30 s in which the examined event occurred. Percent predicted VO2 represents the achieved peak VO2 adjusted for age, weight, and height and expressed as a percentage. We measured percent predicted VO2 using the equations by Wasserman and Hansen [13].
2.3. Statistical Analyses
Statistical analysis was performed using SAS JMP 9 software package. Continuous variables are described as mean±standard deviation, or as median and interquartile (IQ) range, in case of non-normal distribution. Categorical variables are expressed as number (percentages). Baseline and follow-up CPET parameters were compared using a Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables, respectively. Changes from baseline were tested using a paired t-test or McNemar test, as appropriate. A p-value <0.05 was considered statistically significant. Nominal logistic regression was conducted to assess correlations between exercise tolerance, VO2, and VE/VCO2. A 6% increase of VO2 from baseline was used as a cut-off to individuate a significant improvement in VO2, according to current literature on this topic [10].
3. Results
3.1. Patients Characteristics
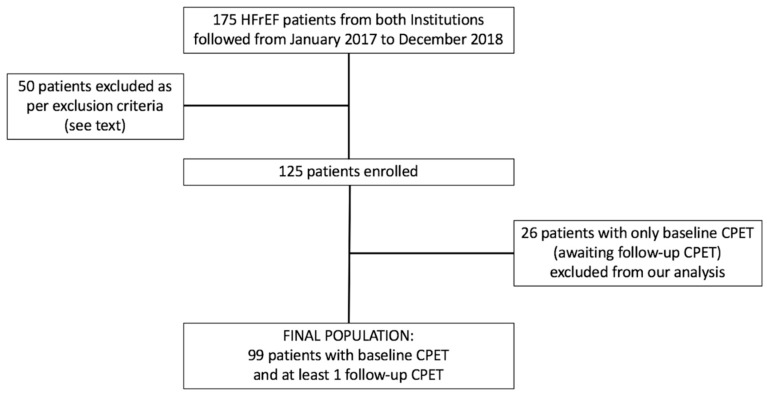
At present, a total of 125 patients have been enrolled and have undergone at least basal CPET. The final population for this study consisted of 99 patients for whom at least 1 follow up CPET was available (Figure 1). Baseline characteristics are listed in Table 1. Mean age was 58.7 ± 9.3 years, 86% were males, 51% had ischemic heart disease, 63% were on NYHA class II, 37% were on NYHA class III, and 17% were on atrial fibrillation. Mean left ventricular ejection fraction (LVEF) was 27 ± 6%. The starting dose of sacubitril/valsartan was 24/26 mg in 69% of patients.
Figure 1.
Flow chart of the study design. ARNI: angiotensin receptor-neprilysin inhibitor; CPET: cardiopulmonary exercise test; HFrEF: heart failure with reduced ejection fraction.
Table 1.
Patient characteristics at baseline (n = 99).
| Demographics | |
|---|---|
| Age, year, mean ± SD | 58.7 ± 9.3 |
| Female sex, no. (%) | 14 (14) |
| SBP, mmHg, mean ± SD | 117 ± 14 |
| DBP, mmHg, mean ± SD | 72 ± 10 |
| Heart rate, beats/min, mean ± SD | 67 ± 11 |
| Body mass index, kg/m2, mean ± SD | 28.1 ± 4.2 |
| Medical History | |
| Hypertension, no. (%) | 51 (51) |
| Diabetes, no. (%) | 34 (34) |
| Atrial fibrillation, no. (%) | 17 (17) |
| COPD, no. (%) | 10 (10) |
| eGFR, mL/min/1.73m2, mean ± SD | 67.8 ± 23.7 |
| Nt-pro-BNP, median (IQ range) | 1200 (446–2120) |
| LVEF (%), mean ± SD | 27 ± 6 |
| LVEDV, mL, mean ± SD | 218 ± 57 |
| LVESV, mL, mean ± SD | 153 ± 56 |
| Ischemic cardiomyopathy, no. (%) | 51 (51) |
| Non-ischemic cardiomyopathy, no. (%) | 48 (49) |
| NYHA functional class II, no. (%) | 62 (63) |
| NYHA functional class III, no. (%) | 37 (37) |
| NYHA functional class IV, no. (%) | 0 (0) |
| Medical Therapy | |
| Furosemide, no. (%) | 88 (89) |
| Furosemide dosage, mean ± SD | 102 ± 105 |
| Antialdosterone, no. (%) | 87 (88) |
| ACE-inhibitors, no. (%) | 62 (63) |
| ARBs, no. (%) | 25 (25) |
| Beta-blockers, no. (%) | 93 (94) |
| Ivabradine, no. (%) | 20 (20) |
| Digoxin, no. (%) | 7 (7) |
| Implantable cardioverter defibrillator, no. (%) | 76 (77) |
| Cardiac resynchronization therapy, no. (%) | 22 (22) |
ACE: angiotensin-converting enzyme; ARB: angiotensin receptor inhibitor; COPD: chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR: estimated glomerular filtration rate (as assessed by MDRD formula); IQ: inter-quartile; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; Nt-pro-BNP: N-terminal pro–B-type natriuretic peptide; NYHA: New York Heart Association; SBP: systolic blood pressure; SD: standard deviation.
At a median follow-up of 6.2 months (range 3–14.9 months), 28%, 38%, and 34% of the patients were on 24/26 mg, 49/51 mg, and 97/103 mg of sacubitril/valsartan, respectively.
Patients characteristics in the sacubitril/valsartan low and high doses cohorts are reported in Table 2.
Table 2.
Patients characteristics in the sacubitril/valsartan low and high doses cohorts.
| Sacubitril/Valsartan 24/26 mg 28 pts |
Sacubitril/Valsartan 97/103 mg 34 pts |
P value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age, year, mean ± SD | 57.8 ± 10.8 | 57.4 ± 8.6 | 0.87 |
| Female sex, no. (%) | 7 (25) | 2 (6) | 0.06 |
| Ischemic cardiomyopathy, no. (%) | 14 (50) | 18 (53) | 0.99 |
| NYHA II, no. (%) | 14 (50) | 27 (79) | 0.018 |
| NYHA III, no. (%) | 14 (50) | 7 (21) | 0.018 |
| Diabetes, no. (%) | 7 (25) | 10 (29) | 0.77 |
| Atrial fibrillation, no. (%) | 6 (21) | 2 (6) | 0.12 |
| eGFR (MDRD), ml/min/1.73m2, mean ± SD | 63.3 ± 21.6 | 72.6 ± 16.7 | 0.07 |
| Furosemide dose, mean ± SD | 108 ± 126 | 63 ± 95 | 0.03 |
| Implantable cardioverter defibrillator, no. (%) | 22 (78) | 24 (70) | 0.56 |
| Cardiac resynchronization therapy, no. (%) | 8 (28) | 8 (23) | 0.77 |
| SBP, NT-pro-BNP, EDV, ESV, and LVEF (Baseline and Follow-up Data) | |||
| SBP, mmHg, mean ± SD (Baseline) | 114.3 ± 12.1 | 120.5 ± 14.7 | 0.07 |
| SBP, mmHg, mean ± SD (Follow-up) | 96 ± 11 | 105 ± 12 | 0.004 |
| Nt-pro-BNP, median (IQ range) (Baseline) | 1623.5 (477–2947) | 815 (358–1929) | 0.013 |
| Nt-pro-BNP, median (IQ range) (Follow-up) | 1065 (376–1739) | 394.5 (195–952) | 0.01 |
| LVEDV, ml, mean±SD (Baseline) | 208 ± 54 | 222 ± 55 | 0.31 |
| LVEDV, ml, mean±SD (Follow-up) | 209 ± 56 | 209 ± 59 | 0.98 |
| LVESV, ml, mean±SD (Baseline) | 147 ± 57 | 161 ± 48 | 0.29 |
| LVESV, ml, mean±SD (Follow-up) | 146 ± 57 | 143 ± 50 | 0.89 |
| LVEF (%), mean ± SD (Baseline) | 28.1 ± 5.7 | 28.3 ± 5.1 | 0.88 |
| LVEF (%), mean ± SD (Follow-up) | 28.6 ± 6.3 | 32.3 ± 6.6 | 0.026 |
eGFR: estimated glomerular filtration rate (as assessed by MDRD formula); IQ: inter-quartile; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; Nt-pro-BNP: N-terminal pro–B-type natriuretic peptide; NYHA: New York Heart Association; SBP: systolic blood pressure; SD: standard deviation.
3.2. CPET, NT-ProBNP, and Left Ventricular Function
Baseline and follow-up CPET results are shown in Table 3. At baseline, most patients were classified as Weber Class C and Ventilatory Class II [14]; at follow-up, we observed a 17% increase in peak VO2 (∆= +2.6 mL/kg/min, p < 0.0001), a 10.9% increase in percent predicted VO2 (p < 0.0001), and a 16% increase in O2 pulse (∆= +1.9 mL/beat; p < 0.001), and an improvement in ventilatory response with a 7% reduction in VE/VCO2 slope (∆= −2.4; p = 0.006). AT-VO2 increased from 11.3 ± 2.6 to 12.6 ± 3.5 mL/kg/min (p = 0.007); moreover, a 9% increase in ∆VO2/∆Work slope (∆= +0.9 mL/beat; p = 0.0002) and a 25% increase in exercise tolerance (∆= +18 watt; p < 0.0001) were obtained. At follow-up, systolic blood pressure significantly decreased from 117 ± 14 to 101 ± 12 mmHg (p < 0.0001) and 51 patients had a flat systolic blood pressure response during exercise (51% at follow-up versus 34% at baseline, p = 0.021). Of note, this did not lead to sacubitril/valsartan discontinuation in any patient.
Table 3.
Cardiopulmonary exercise test parameters (n = 99).
| Baseline | Follow-up | p value | |
|---|---|---|---|
| Peak VO2, mL/kg/min, mean ± SD | 14.6 ± 3.3 | 17.2 ± 4.7 | <0.0001 |
| Predicted peak VO2, %, mean ± SD | 53.8 ± 14.1 | 64.7 ± 17.8 | <0.0001 |
| VE/VCO2 slope, mean ± SD | 34.1 ± 6.3 | 31.7 ± 6.1 | 0.006 |
| VE/VCO2 slope≥ 34, no. (%) | 46 (46) | 33 (33) | 0.08 |
| Peak RER, mean ± SD | 1.12 ± 0.09 | 1.13 ± 0.09 | 0.45 |
| Watt (Peak), mean ± SD | 70 ± 22 | 88 ± 29 | <0.0001 |
| AT VO2, mL/kg/min, mean ± SD | 11.3 ± 2.6 | 12.6 ± 3.5 | 0.007 |
| Predicted AT VO2, %, mean ± SD | 42.3 ± 11.5 | 47.2 ± 12.5 | 0.009 |
| AT undetectable, no. (%), mean ± SD | 16 (16) | 9 (9) | 0.19 |
| O2pulse (ml/beat) | 11.5 ± 3.0 | 13.4 ± 4.3 | 0.0007 |
| ∆VO2/∆work, mL/min/watt, mean ± SD | 9.2 ± 1.5 | 10.1 ± 1.8 | 0.0002 |
| Peak ventilation, L/min, mean ± SD | 48.7 ± 12.7 | 59.3 ± 18.9 | <0.0001 |
| Peak tidal volume, L, mean ± SD | 1.57 ± 0.43 | 1.75 ± 0.53 | 0.009 |
| Peak Respiratory rate, b/m, mean ± SD | 30.5 ± 6.7 | 33.3 ± 7.2 | 0.006 |
| Ventilatory Oscillation, no. (%) | 31 (31) | 19 (19) | 0.07 |
AT: anaerobic threshold; RER: respiratory exchange ratio; SD: standard deviation; VE/VCO2: minute ventilation/carbon dioxide production ratio; VO2: oxygen consumption.
At nominal logistic regression, increase in exercise tolerance (namely, 1-watt increase) was found to be an independent predictor of 6% improvement of VO2 (OR = 1.06; 95% CI: 1.03–1.10; p < 0.0001) at follow-up; a trend towards statistical significance was found with regard to VE/VCO2 slope decrease (OR = 1.02; 95% CI: 0.99–1.04; p = 0.057).
At follow-up, median NT-ProBNP levels decreased from 1344 (IQ range: 439–2191) to 631 pg/mL (298–1554) (p = 0.002).
At follow-up, mean LVEF increased from 27 ± 6 to 29.7 ± 7% (p < 0.0001) and left ventricular end-systolic volume decreased from 153 ± 56 to 145 ± 52 mL (p = 0.030).
3.3. CPET Results Stratified by Sacubitril/Valsartan Dosages
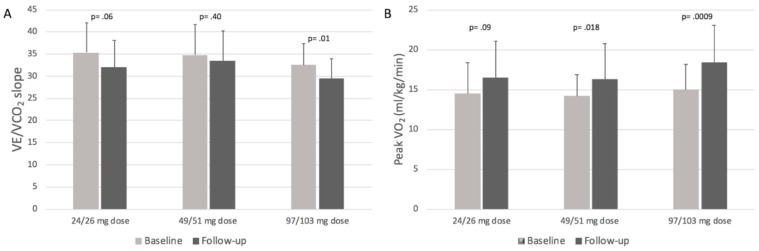
Peak VO2 variation (baseline vs follow-up) was highest in patients taking 97/103 mg of sacubitril/valsartan (∆= +3.4, p = 0.0009), as compared to patients taking low doses (24/26 mg) (∆= +2.0, p = 0.09) and medium doses (49/51 mg) (∆= +2.1, p = 0.018) (full data reported in Table 4). A statistically significant reduction in terms of VE/VCO2 slope was observed at follow-up in the subgroup of patients on the highest dose of sacubitril/valsartan (p = 0.01; Table 4, Figure 2A). Of note, no statistically significant differences were observed among these subgroups in terms of mean follow-up duration and baseline Peak VO2 (Figure 2B).
Table 4.
Cardiopulmonary exercise test parameters stratified by sacubitril/valsartan dosages.
| Baseline | Follow-up | p value | |
|---|---|---|---|
| Peak VO2, mL/kg/min, mean ± SD | |||
| 24/26 mg dose (28 pts) | 14.5 ± 3.9 | 16.5 ± 4.6 | 0.09 |
| 49/51 mg dose (37 pts) | 14.2 ± 2.7 | 16.3 ± 4.5 | 0.018 |
| 97/103 mg dose (34 pts) | 15 ± 3.2 | 18.4 ± 4.7 | 0.0009 |
| Predicted peak VO2, %, mean ± SD | |||
| 24/26 mg dose (28 pts) | 54 ± 12.9 | 62.1 ± 14.1 | 0.029 |
| 49/51 mg dose (37 pts) | 53.8 ± 13.9 | 61.9 ± 16.6 | 0.02 |
| 97/103 mg dose (34 pts) | 53.6 ± 15.6 | 68.6 ± 20.6 | 0.001 |
| VE/VCO2 slope, mean ± SD | |||
| 24/26 mg dose (28 pts) | 35.3 ± 6.8 | 32 ± 6.1 | 0.06 |
| 49/51 mg dose (37 pts) | 34.8 ± 6.9 | 33.4 ± 6.9 | 0.4 |
| 97/103 mg dose (34 pts) | 32.5 ± 4.9 | 29.5 ± 4.5 | 0.01 |
| O2 pulse, ml/beat, mean ± SD | |||
| 24/26 mg dose (28 pts) | 11.4 ± 3.1 | 12.8 ± 4.3 | 0.016 |
| 49/51 mg dose (37 pts) | 11 ± 3.1 | 12.3 ± 3.9 | 0.12 |
| 97/103 mg dose (34 pts) | 12.2 ± 2.8 | 14.9 ± 4.4 | 0.003 |
| ∆VO2/∆work, mL/min/watt, mean ± SD | |||
| 24/26 mg dose (28 pts) | 9.1 ± 1.3 | 9.7 ± 2.2 | 0.24 |
| 49/51 mg dose (37 pts) | 9 ± 1.6 | 9.9 ± 1.8 | 0.028 |
| 97/103 mg dose (34 pts) | 9.3 ± 1.5 | 10.5 ± 1.4 | 0.001 |
SD: standard deviation; VE/VCO2: minute ventilation/carbon dioxide production ratio; VO2: oxygen consumption.
Figure 2.
Panel A, VE/VCO2 slope variations at follow-up in patients stratified by baseline sacubitril/valsartan dosages; Panel B, peak VO2 variations at follow-up in patients stratified by sacubitril/valsartan dosages.
3.4. CPET Results Stratified by Baseline VE/VCO2 Slope Values
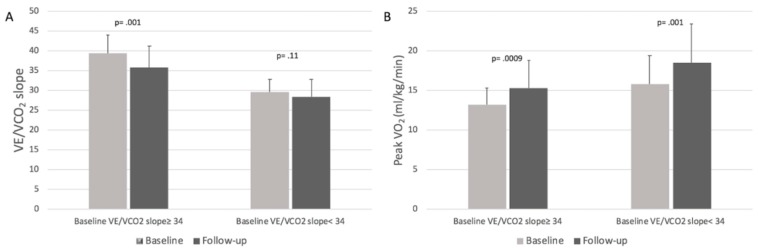
Patients with baseline VE/VCO2 ≥ 34 had a statistically significant decrease in VE/VCO2 slope at follow-up (39.4 ± 4.7 vs. 35.8 ± 5.4, respectively; p = 0.001) together with a significant increase in peak VO2 (13.2 ± 2.1 vs. 15.3 ± 3.5 mL/kg/min, respectively; p = 0.0009); patients with baseline VE/VCO2< 34 had a statistically significant increase in peak VO2 at follow-up (15.8 ± 3.6 vs. 18.5 ± 4.9 mL/kg/min, respectively; p = 0.001) but no significant changes in VE/VCO2 slope (29.6 ± 3.2 vs. 28.4 ± 4.4, respectively; p = 0.11) (Figure 3A,B).
Figure 3.
Panel A, VE/VCO2 slope variations at follow-up in patients stratified by baseline VE/VCO2 slope values. Panel B, Peak VO2 variations at follow-up in patients stratified by baseline VE/VCO2 slope values.
Patients who reached 12-month follow-up showed the greatest reduction in VE/VCO2 Slope (∆= −4.7, p = 0.0006 for baseline versus follow-up), as compared to patients who only had 3- and 6-month follow-up (details of CPET parameters in patients stratified by follow-up duration shown in Table 5).
Table 5.
Cardiopulmonary exercise test parameters in patients stratified by follow-up duration.
| Baseline | Follow-up | p value | |
|---|---|---|---|
| Peak VO2, mL/kg/min, mean ± SD | |||
| 3 months (24 pts) | 15.3 ± 3 | 16.9 ± 4.1 | 0.12 |
| 6 months (40 pts) | 14.8 ± 3.6 | 17.1 ± 5 | 0.02 |
| 12 months (35 pts) | 13.8 ± 3 | 17.3 ± 4.6 | 0.0006 |
| Predicted peak VO2, %, mean ± SD | |||
| 3 months (24 pts) | 54.9 ± 9.5 | 61.3 ± 13.1 | 0.06 |
| 6 months (40 pts) | 56.7 ± 14.5 | 66.9 ± 17.2 | 0.0005 |
| 12 months (35 pts) | 49.7 ± 15.6 | 63.4 ± 20.4 | 0.002 |
| VE/VCO2 slope, mean ± SD | |||
| 3 months (24 pts) | 33.7 ± 6.2 | 33.4 ± 7.8 | 0.9 |
| 6 months (40 pts) | 33.3 ± 6.6 | 31.4 ± 6 | 0.19 |
| 12 months (35 pts) | 35.4 ± 6 | 30.7 ± 4.8 | 0.0006 |
| O2 pulse, ml/beat, mean ± SD | |||
| 3 months (24 pts) | 12.3 ± 3.3 | 13.0 ± 4.2 | 0.52 |
| 6 months (40 pts) | 11.3 ± 3.2 | 13.3 ± 4.3 | 0.023 |
| 12 months (35 pts) | 11.2 ± 2.5 | 13.6 ± 4.5 | 0.007 |
| ∆VO2/∆work, mL/min/watt, mean ± SD | |||
| 3 months (24 pts) | 9.4 ± 1.4 | 10.3 ± 1.7 | 0.09 |
| 6 months (40 pts) | 9.3 ± 1.2 | 10.1 ± 2.1 | 0.042 |
| 12 months (35 pts) | 8.8 ± 1.7 | 9.9 ± 1.7 | 0.007 |
SD: standard deviation; VE/VCO2: minute ventilation/carbon dioxide production ratio; VO2: oxygen consumption
4. Discussion
CPET is a valuable tool to guide clinical decision-making and to derive prognostic information in HF patients [10,14,15,16].
In the PARADIGM-HF trial [1], sacubitril/valsartan reduced the risk of death and hospitalization for HF in patients with HFrEF, as compared to enalapril; however, little is known on how sacubitril/valsartan influences cardiopulmonary function.
To the best of our knowledge, this is the largest observational study prospectively assessing the early effects of sacubitril/valsartan on cardiopulmonary parameters in patients with HFrEF. After initiation of sacubitril/valsartan, we observed a significant improvement in the main prognostically relevant CPET parameters. To date, only one study by Palau et al. [5] showed an improvement in peak VO2 and VE/VCO2 slope in 33 HFrEF patients at 30 days follow-up after sacubitril/valsartan initiation, mostly at low doses. In our study (entailing a larger population, with a longer follow-up and including all available dosages of sacubitril/valsartan) we confirmed the significant improvement in peak VO2 at follow-up (∆= +2.6 mL/kg/min; p < 0.0001); of note, VE/VCO2 slope improvement started at 6 months from sacubitril/valsartan initiation and reached a statistical significant difference only at 12 months (Table 5).
The observed improvement in peak VO2 (+17% versus baseline) and VE/VCO2 slope (−7% versus baseline) at follow-up, might play a clinically and prognostically relevant role in this patient population. Swank et al. [17] reported that for every 6% increase in peak VO2 there is an 8% reduction in cardiovascular mortality or HF hospitalization (hazard ratio [HR] = 0.92; CI = 0.88–0.96; p < 0.001) and a 7% reduction in all-cause mortality (HR = 0.93; CI = 0.90–0.97; p < 0.001). Arena et al. [18] reported worse 1-year event-free survival from cardiac mortality (83.1% vs. 99.2%; p < 0.0001) and worse 1-year event-free survival from cardiac hospitalization (50.6% vs. 84.6%; p < 0.0001) in patients with VE/VCO2 slope ≥34 versus patients with VE/VCO2 slope <34. Furthermore, a large body of evidence confirms the prognostic relevance of VE/VCO2 slope values [19,20,21,22,23,24].
Notably, at follow-up, sicker patients (i.e., patients with baseline VE/VCO2 ≥ 34) improved both oxygen consumption and ventilatory efficiency while healthier patients (i.e., patients with baseline VE/VCO2 < 34) only improved oxygen consumption (Figure 3). Moreover, patients on the highest doses of sacubitril/valsartan were found to be the ones with the best functional improvement (Figure 2). These results are consistent with those reported in the PARADIGM-HF study [1].
A PARADIGM-HF post-hoc analysis by Vardeny et al. [25] demonstrates that lower doses of sacubitril/valsartan confer a similar treatment benefit over enalapril; however, patients taking low doses were associated with a higher risk of the primary events. In our study, patients taking low doses had less improvement of peak VO2 as compared to patients taking the highest dose; this may reflect patient frailty; indeed, patients taking low doses of sacubitril/valsartan showed lower systolic blood pressure (both at baseline and at follow-up), higher levels of NT-proBNP, increased prevalence of NYHA class III, higher furosemide dose use, lower estimated glomerular filtration rate, and a higher VE/VCO2 slope at baseline (details provided in Table 2 and Table 4).
Of note, exercise tolerance (namely, 1-watt increase) was found to be an independent predictor of 6% improvement of VO2 (OR = 1.06; 95% CI: 1.03–1.10; p < 0.0001) at follow-up, and a trend towards statistical significance was found with regard to VE/VCO2 slope decrease (OR = 1.02; 95% CI: 0.99–1.04; p = 0.057). It is likely that the weaker correlation with VE/VCO2 slope decrease might be due to the small sample size of the study population.
Sacubitril/valsartan combines the effects of angiotensin receptor blocker with neprilysin inhibition which amplify the system of natriuretic peptides and other vasoactive peptides [26,27]. However, little is known about the overall effect of vasoactive peptides on heart and lung function. In our study population, we observed an improvement of LVEF and a decrease of left ventricular end-systolic volume at follow-up. We speculate that sacubitril/valsartan might have a synergistically favorable effect on hemodynamics and muscle efficiency through reduced afterload and left ventricular filling pressure. This might result in a net improvement of exercise tolerance and performance. Of note, recent data (a longitudinal and a retrospective study) support an improvement in left ventricular ejection fraction and in left ventricular reverse remodeling after sacubitril/valsartan initiation [28,29].
We also observed an increase in peak ventilatory responses which may be secondary to the improvement of cardiac performance, allowing patients to increase ventilation without increasing the VE/VCO2 slope, although, at the moment, this remains speculative.
5. Study Limitations
This study has a number of limitations. First, we had no control group. However, the patients enrolled were hemodynamically stable and on optimized medical therapy; we may therefore consider patients at first evaluation as their own controls (versus follow-up). Importantly, since the PARADIGM-HF study has already demonstrated a relevant benefit of sacubitril/valsartan over enalapril in this setting and it is now recommended by international guidelines [11,30], denying sacubitril/valsartan to eligible patients in order to have a control group would have raised ethical issues. Conversely, selecting patients not eligible for sacubitril/valsartan as the control group, might have individuated frailer patients (i.e., with systolic arterial hypotension and more advanced chronic renal failure). Secondly, an important limitation of this study is the small sample size; nonetheless, to the best of our knowledge our work currently represents the largest series of HFrEF patients treated with sacubitril/valsartan for whom follow-up CPET parameters have been tested. Unfortunately, no data on diffusing capacity to carbon monoxide are available.
Further studies are necessary to confirm our preliminary results and to understand sacubitril/valsartan influence on cardiopulmonary function. A clinical trial evaluating the effect of sacubitril/valsartan on 6-month Exercise Tolerance in Patients with Heart Failure (NEPRIExTol) is currently ongoing (NCT03190304).
6. Conclusions
In this prospective observational study, administration of sacubitril/valsartan was associated with a significative improvement in exercise tolerance, peak oxygen consumption, and ventilatory efficiency at 6.2 months follow-up. Further studies are necessary to better clarify underlying mechanisms of this functional improvement.
Acknowledgements
We sincerely thank Francesca Rizzo for recruiting and helping to follow up the patients during the study.
Abbreviations List
| AT-VO2 | oxygen consumption at anaerobic threshold |
| CPET | cardiopulmonary exercise test |
| HF | heart failure |
| HFrEF | heart failure with reduced ejection fraction |
| LVEF | left ventricular ejection fraction |
| NYHA | New York Heart Association |
| NT-proBNP | N-terminal pro-brain natriuretic peptides |
| RER | respiratory exchange ratio |
| VE/VCO2 | ventilation/carbon dioxide production relationship |
| VO2 | oxygen consumption |
Author Contributions
F.M.S. and G.V. conceived the study, participated in data collection, analyzed the data, wrote the manuscript, and approved the final submission; G.C., C.N., L.A., S.S. (Salvo Storniolo), S.S. (Silvia Sarullo), V.A., G.R., and G.N. participated in data collection and analysis, revised the manuscript, and approved the final submission; A.D.F. critically revised data analysis and the manuscript and approved the final submission; F.M.S. and F.C. participated to study design, led the entire research group, critically revised data interpretation, revised the manuscript, and approved the final submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.McMurray J.J., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R., Rouleau J.L., Shi V.C., Solomon S., Swedberg K., et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 2.Rodil Fraile R., Malafarina V., Lopez G.T. Sacubitril/valsartan in heart failure and multimorbidity patients. ESC Heart Fail. 2018;5:957–960. doi: 10.1002/ehf2.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sgorbini L., Rossetti A., Galati A. Sacubitril/valsartan: Effect on walking test and physical capability. Cardiology. 2017;138:17–20. doi: 10.1159/000484879. [DOI] [PubMed] [Google Scholar]
- 4.Beltrán P., Palau P., Domínguez E., Faraudo M., Núñez E., Guri O., Mollar A., Sanchis J., Bayés-Genís A., Núñez J. Sacubitril/valsartan and short-term changes in the 6-min walk test: A pilot study. Int. J. Cardiol. 2018;252:136–139. doi: 10.1016/j.ijcard.2017.10.074. [DOI] [PubMed] [Google Scholar]
- 5.Palau P., Mollar A., Dominguez E., Sanchis J., Bayés-Genís A., Núñez J. Early Sacubitril/valsartan-driven benefit on exercise capacity in heart failure with reduced ejection fraction: A pilot study. Rev. Esp. Cardiol. 2019;72:167–169. doi: 10.1016/j.recesp.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Mancini D.M., Eisen H., Kussmaul W., Mull R., Edmunds L.H., Jr., Wilson J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.CIR.83.3.778. [DOI] [PubMed] [Google Scholar]
- 7.Myers J., Gullestad L., Vagelos R., Do D., Bellin D., Ross H., Fowler M.B. Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann. Intern. Med. 1998;129:286–293. doi: 10.7326/0003-4819-129-4-199808150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Myers J., Gullestad L. The role of exercise testing and gas-exchange measurement in the prognostic assessment of patients with heart failure. Curr. Opin. Cardiol. 1998;13:145–155. [PubMed] [Google Scholar]
- 9.Stelken A.M., Younis L.T., Jennison S.H., Miller D.D., Miller L.W., Shaw L.J., Kargl D., Chaitman B.R. Prognostic value of cardiopulmonary exercisetesting using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J. Am. Coll. Cardiol. 1996;27:345–352. doi: 10.1016/0735-1097(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 10.Corrà U., Agostoni P.G., Anker S.D., Coats A.J.S., Crespo Leiro M.G., de Boer R.A., Harjola V.P., Hill L., Lainscak M., Lund L.H. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018;20:3–15. doi: 10.1002/ejhf.1186. [DOI] [PubMed] [Google Scholar]
- 11.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., Gonzalez-Juanately J.R., Harjola V.P., Jankowska E.A., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37:2129–2200. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 12.Piepoli M.F., Corrà U., Adamopoulos S., Benzer W., Bjarnason-Wehrens B., Cupples M., Dendale P., Doherty P., Gaita D., Höfer S., et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery. Eur. J. Prev. Cardiol. 2014;21:664–681. doi: 10.1177/2047487312449597. [DOI] [PubMed] [Google Scholar]
- 13.Wasserman K., Hansen J.E., Sue D.Y., Stringer W., Whipp B.J. Normal Values. In: Weinberg R., editor. Principles of Exercise Testing and Interpretation. 4th ed. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2005. pp. 160–182. [Google Scholar]
- 14.Guazzi M., Arena R., Halle M., Piepoli M.F., Myers J., Lavie C.J. 2016 Focused Update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2016;133:e694–711. doi: 10.1161/CIR.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 15.Contini M. Cardiopulmonary test as a tool to choose therapy in heart failure. Ann. Am. Thorac. Soc. 2017;14:S67–S73. doi: 10.1513/AnnalsATS.201611-887FR. [DOI] [PubMed] [Google Scholar]
- 16.Myers J., Arena R., Cahalin L.P., Labate V., Guazzi M. Cardiopulmonary Exercise Testing in Heart Failure. Curr. Probl. Cardiol. 2015;40:322–372. doi: 10.1016/j.cpcardiol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Swank A.M., Horton J., Fleg J.L., Fonarow G.C., Keteyian S., Goldberg L., Wolfel G., Handberg E.M., Bensimhon D., Illiou M.C., et al. HF-ACTION Investigators. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: Results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ. Heart Fail. 2012;5:579–585. doi: 10.1161/CIRCHEARTFAILURE.111.965186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arena R., Myers J., Aslam S.S., Varughese E.B., Peberdy M.A. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am. Heart J. 2004;147:354–360. doi: 10.1016/j.ahj.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Francis D.P., Shamim W., Davies L.C., Piepoli M.F., Ponikowski P., Anker S.D., Coats A.J. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur. Heart J. 2000;21:154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 20.Robbins M., Francis G., Pashkow F.J., Snader C.E., Hoercher K., Young J.B., Lauer M.S. Ventilatory and heart rate responses to exercise: Better predictors of heart failure mortality than peak oxygen consumption. Circulation. 1999;100:2411–2417. doi: 10.1161/01.CIR.100.24.2411. [DOI] [PubMed] [Google Scholar]
- 21.Corra U., Mezzani A., Bosimini E., Scapellato F., Imparato A., Giannuzzi P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am. Heart J. 2002;143:418–426. doi: 10.1067/mhj.2002.120772. [DOI] [PubMed] [Google Scholar]
- 22.Kleber F.X., Vietzke G., Wernecke K.D., Bauer U., Opitz C., Wensel R., Sperfeld A., Gläser S. Impairment of ventilatory efficiency in heart failure: Prognostic impact. Circulation. 2000;101:2803–2809. doi: 10.1161/01.CIR.101.24.2803. [DOI] [PubMed] [Google Scholar]
- 23.Sarullo F.M., Fazio G., Brusca I., Fasullo S., Paterna S., Licata P., Novo G., Novo S., Di Pasquale P. Cardiopulmonary exercise testing in patients with chronic heart failure: Prognostic comparison from peak VO2 and VE/VCO2 slope. Open Cardiovasc. Med. J. 2010;4:127–134. doi: 10.2174/1874192401004010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacGowan G.A., Janosko K., Cecchetti A., Murali S. Exercise-related ventilatory abnormalities and survival in congestive heart failure. Am. J. Cardiol. 1997;79:1264–1266. doi: 10.1016/S0002-9149(97)00097-0. [DOI] [PubMed] [Google Scholar]
- 25.Vardeny O., Claggett B., Packer M., Zile M.R., Rouleau J., Swedberg K., Teerlink J.R., Desai A.S., Lefkowitz M., Shi V., et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: The PARADIGM-HF trial. Eur. J. Heart Fail. 2016;18:1228–1234. doi: 10.1002/ejhf.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayés-Genís A., Barallat J., Richards A.M. A test in context: Neprilysin: Function, inhibition, and biomarker. J. Am. Coll. Cardiol. 2016;68:639–653. doi: 10.1016/j.jacc.2016.04.060. [DOI] [PubMed] [Google Scholar]
- 27.D’Elia E., Iacovoni A., Vaduganathan M., Lorini F.L., Perlini S., Senni M. Neprilysin inhibition in heart failure: Mechanisms and substrates beyond modulating natriuretic peptides. Eur. J. Heart Fail. 2017;19:710–717. doi: 10.1002/ejhf.799. [DOI] [PubMed] [Google Scholar]
- 28.Martens P., Beliën Dupont M., Vandervoort P., Mullens W. The reverse remodeling response to Sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc. Ther. 2018;36:e12435. doi: 10.1111/1755-5922.12435. [DOI] [PubMed] [Google Scholar]
- 29.Almufleh A., Marbach J., Chih S., Stadnick E., Davies R., Liu P., Mielniczuk L. Ejection fraction improvement and reverse remodeling achieved with Sacubitril/valsartan in heart failure with reduced ejection fraction patients. Am. J. Cardiovasc. Dis. 2017;7:108–113. [PMC free article] [PubMed] [Google Scholar]
- 30.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Colvin M.M., Drazner M.H., Filippatos G.S., Fonarow G.C., Givertz M.M., et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]